Abstract
Thymic stromal lymphopoietin (TSLP) is an epithelial-derived cytokine similar to IL- 7, whose gene is located on chromosome 5q22.1 and it exerts its biological function through the TSLP-Receptor (TSLP-R). TSLP is expressed primarily by epithelial cells at barrier surfaces such as the skin, gut and lung in response to danger signals. Since it was cloned in 1994, there has been accumulating evidence that TSLP is crucial for the maturation of antigen presenting cells and hematopoietic cells. TSLP genetic variants and its dysregulated expression have been linked to atopic diseases such as atopic dermatitis, asthma, allergic rhinitis and eosinophilic esophagitis.
Keywords: asthma, atopy, eczema, Eosinophilic esophagitis, TSLP, TSLP-R
Thymic stromal lymphopoietin (TSLP) is an epithelial derived cytokine that exerts its biological function through TSLP receptor (TSLPR), and it plays an important role in many atopic diseases [1–3]. Since it was cloned in 1994, there has been accumulating evidence that TSLP is crucial for the maturation of APCs, and for skewing a T-helper immune response toward the Th2 phenotype, typical of allergic inflammation (Figure 1) [2,3]. TSLP genetic variants and its dysregulated expression have been linked to atopic diseases such as atopic dermatitis (AD), asthma, allergic rhinoconjunctivitis (AR) and eosinophilic esophagitis (EoE), but also to other immune-mediated diseases such as cancer [4–6], rheumatoid arthritis [7] and immune defense against helminth [8]. In the present article, we will review TSLP biology and regulation and its role in atopic diseases.
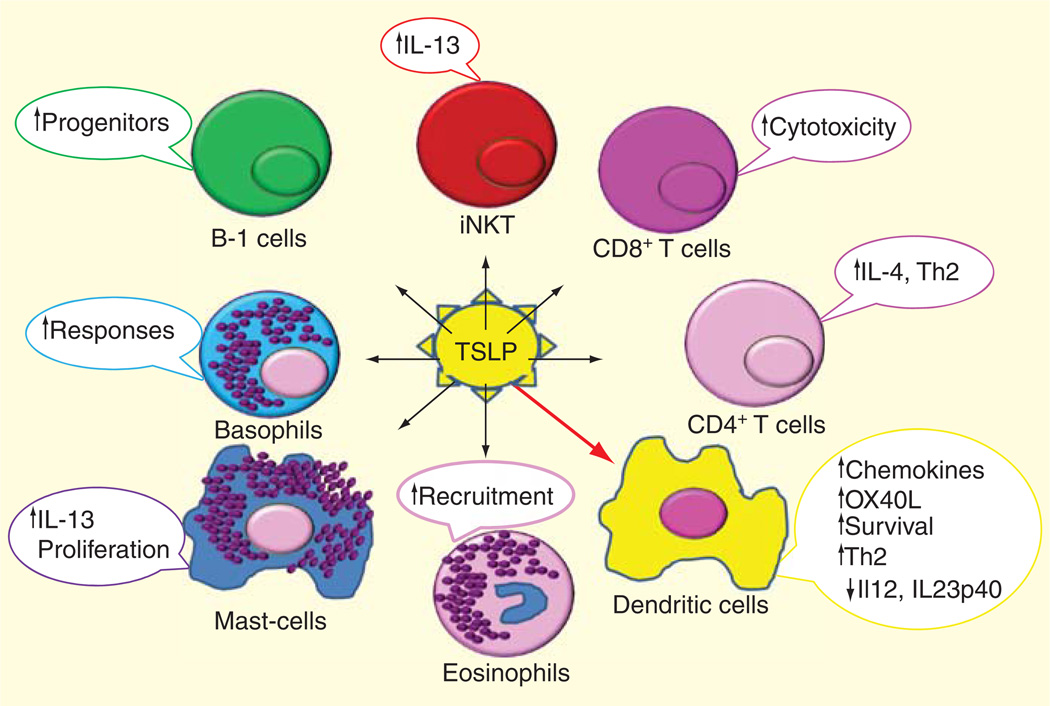
Figure 1. TSLP effect on different immune cell types to enhance Th2 response.
iNKT: Invariant natural killer T cells; TSLP: Thymic stromal lymphopoietin.
Atopy
To better understand the role of TSLP in allergic inflammation, it is important to briefly review the major key immunological features of atopy.
Atopy is an inflammatory process that occurs due to an abnormal immunological reaction against environmental and food allergens, and it can be IgE mediated, non-IgE mediated (i.e., cell mediated) or mixed IgE and non-IgE mediated [9–14]. Atopic inflammation causes diseases such as asthma, AR, AD, food allergies and EoE [9–14]. It is due to a T-helper type 2 (Th2) inflammation that drives hyperrespon-siveness to stimuli and remodeling of tissues. Th2 inflammation is driven by Th2-type cytokines, such as IL-4, IL-5 and IL-13, secreted by the Th2 subtype of Th2 cells and by many other cells belonging to the innate immunity such as eosinophils, basophils, mast cells, macrophages, invariant natural killer T cells (iNKTs) and innate helper type 2 cells (ILC2) [9–16].
Th2 cells develop from naïve T-helper cells that are primed by an antigen presented by APCs in the presence of IL-4, and this development is driven by the activation of GATA-3, a transcription factor that drives Th2 differentiation by potentiating transcription of the linked, Il4, Il13 and Il5 genes [17]. APCs (i.e., Langerhans cells (LCs), macrophages, dendritic cells and B cells) are central to the development of Th2 immunity because antigen presentation is required to initiate responses. Substantial evidence demonstrates that reciprocal cytokine interactions involving APCs regulate the balance between Th1 and Th2 response patterns, for example, APCs secrete the Th2-associated cytokine IL-10 [18], which inhibits APC IL-12 production and thereby drives IL-4 production and GATA3 expression [17,19]. However, the underlying mechanisms leading to the decision as to whether a Th1 or Th2 cytokine pattern predominates in a given response are still not clearly defined.
A novel subset of monocytes called innate helper type 2 cells (ILC2, also known nuocytes, natural helper cells) are novel Th2 cytokine-producing cells that may play a major role in allergic disease. First described in 2001 by Fort et al. [20] as non-B/non-T cells that produced IL-5 and IL-13 in response to IL-25 and expressed MHC class IIhigh and CD11cdull, these cells have now been better defined by expression of many surface markers such as the prostaglandin D2 (PGD2) receptor CRTH2, CD7, CD25, CD62L, CD127, CD161, CRTH2, ST2 (IL-33R), ALX, CMKLR1, NKG2D, c-kit and DR3 [21–25]. Human ILC2 are present in the gastrointestinal tract, lung, nasal polyp tissue and peripheral blood and have been shown to be activated not only by IL-25 and IL-33 as initially reported but also by a number of cytokines and other inflammatory mediators such as TSLP [22], TNF superfamily member TL1A [25] and IL-9 [26], eicosanoids, arachidonic acid-derived lipid mediators (i.e., PGD2 [24,27], leukotrien D4 [28]). ILC2s are good producers of Th2 cytokines, such as IL-13, IL-5, IL-9, IL-6 and the EGFR ligand amphiregulin [27,29–31]. Not surprisingly, ILC2 highly expresses the master Th2 cytokine transcription factor GATA3 that is required for ILC2 Th2 cytokine production [21,22,32,33]. Even if both conventional Th2 cells and ILC2 express GATA3, ILC2 already express GATA3 in the bone marrow, suggesting they are primed for Th2 cytokine production without the peripheral differentiation that is necessary for Th2 development [21,33]. Overall, these reports suggest that the variable cytokine production by ILC2 may play distinct roles depending on the inflammatory context.
Most of the inflammatory cells are recruited through epithelial-derived chemokines such as Rantes and Eotaxin, which are secreted in response to Th2 cytokines or epithelial damage in genetically predisposed individuals [34,35]. Enzymes and cytotoxic products secreted by inflammatory cells such as eosinophils, mast cells, CD8+ cytotoxic T cell and iNKTs lead to tissue damage. Th2 cytokines, especially IL-13, favor repair characterized by excessive fibrosis and tissue remodeling, causing permanent and long-lasting damage in tissue affected by Th2 inflammation [9–14].
IgE-mediated classic allergic reactions are due to the presence of allergen-specific IgEs, which bind to its high-affinity receptor (FcεRI) expressed on mast cells and basophils. When specific antigens engage the IgE linked to the FcεRI, they establish receptor cross-linking and the consequent release of mediators [12,36]. Even if initially it was thought that mast cells were the principal effectors cells in IgE-mediated acute reactions, further studies have shown that basophils also play a major role [37,38]. Typical examples of IgE-mediated reactions are acute food allergy reactions, or acute asthma or allergic rhinitis episodes after exposure to environmental allergens. IgE responses can also initiate a delayed chronic inflammation typical of the IgE and non-IgE mixed reactions observed in some forms of chronic asthma, AD and some subtypes of EoE [9–14]. The non-IgE-mediated allergic reactions represent the minority of immunologic responses to environmental and food allergens. They are caused by T-cell and eosinophil activation, and they develop in the absence of demonstrable allergen-specific IgE antibodies in the skin or serum [36,39,40].
Allergens are non-dangerous antigens that usually do not elicit any immune response or otherwise elicit a tolerogenic one through activation of a subtype of T cell called Treg characterized by the presence of transcription factor forkhead box 3 [41–47].
Tolerance to an allergen often depends on an intact and immunologically active epithelial barrier (skin, respiratory or gastrointestinal). This barrier includes epithelial cells joined by tight junctions and a thick mucus layer, which create a physical blockade of the antigen, as well as luminal and brush border enzymes, bile salts and extremes of pH, which contribute to make antigens less immunogenic. In addition, innate (natural killer cells, polymorphonuclear leukocytes, macrophages, epithelial cells and toll-like receptors) and adaptive immunity (intraepithelial and lamina propria lymphocytes, Peyer’s patches, IgA and cytokines) provide an active barrier to foreign antigens that are able to go through an intact epithelial barrier and contribute to elicit a tolerotogenic Treg response. In contrast, damaged tissue lets more intact antigen pass through the barrier and when in contact with the immune system an antigen presented through such inflamed tissue causes antigenic sensitization instead of tolerance [48–51]. This is believed to be the basis of the atopic march observed in children with AD, where food allergens first and environmental allergens second are presented by inflamed skin to the immune system inducing a sequential sensitization to allergens [51,52].
TSLP & TSLPR
TSLP is a four-helix bundle cytokine that it is closely related to IL-7, a member of the hematopoietin family of cytokines. Its name is derived from the fact that TSLP was initially isolated from a mouse thymic stromal cell line and was found to be a growth factor for B lymphocytes [53,54]. A TSLP human homolog was subsequently isolated [55], which was 43% identical at the amino acid level with conserved glycosylation sites and cysteine residuals [56]. TSLP binds to its receptor (TSLP-R) to exert its biological activities. TSLP-R is a heterodimeric receptor that consists of the IL-7 receptor alpha-chain (IL-7Rα) and the TSLP receptor alpha chain 1 (TSLPRα, also known as CRL2, TSLPR and CRLF2Y), which is closely related to the common receptor-γ chain (γc) that is found in IL-2, IL-4, IL-9 and IL-15 receptor complexes. The functional TSLPR is mainly expressed in hematopoietic cells (dendritic cells [DC], T cells, B cells, natural killer (NK) cells, iNKT, monocytes, basophils, mast cells and eosinophils), liver, brain, skeletal muscle, kidney, spleen and thymus [50,57–59].
The human TSLP gene is located on chromosome 5q22.1 next to the atopic cytokine (IL-4, IL5, IL13, IL3) cluster of chromosome 5q31 [56,60]. The mouse gene is located on chromosome 18 [2]. IL-7Rα (CD127) is located on gen 5q.13 and TSLPRα is encoded on Xp22.3; Yp11.3. Two transcript variants encoding different isoforms have been found for this gene [61].
TSLP regulation
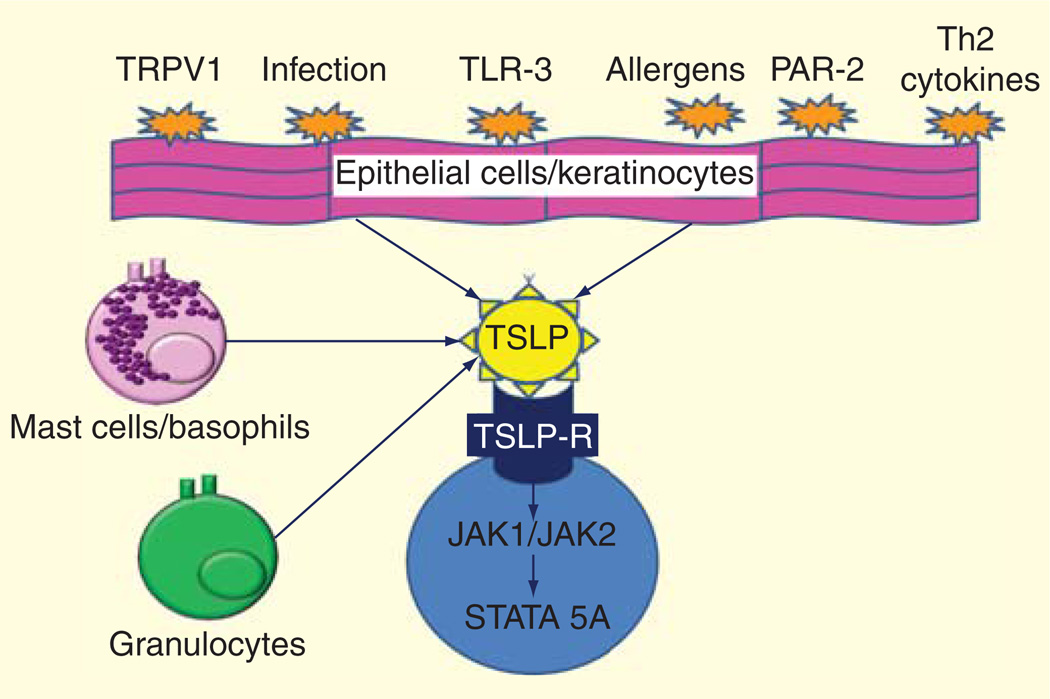
Despite the initial identification of TSLP in the thymus, TSLP is expressed primarily by epithelial cells at barrier surfaces such as the skin, gut and lung [55,56,62,63]. A variety of danger signals and cytokines are able to activate TSLP production (Figure 2). More specifically, infection agents and their products such as respiratory viruses, bacterial peptidoglycan, lipoteichoic acid, double-stranded RNA (dsRNA), as well as cytokines such as IL-4, IL-13, TNF-α and IL-1 and trauma, air pollutants and allergens have been shown to induce TSLP expression by lung-derived parenchymal cells, skin cells and immune cells [64–72]. Specifically, trigger stimuli for TSLP can exert their function by the activation of toll-like receptor 3 [35,61], protease-activated receptor-2 (PAR-2) [69,73] and the transient receptor potential vanilloid type 1 (TRPV1). Toll-like receptor 3 is activated by viral dsRNA and has been shown to activate TSLP in respiratory and esophageal epithelial cells [35,61]. Proteases (such as trypsin and papain) from airborne allergens like Alternaria induce TSLP production in human airway epithelial cells via PAR-2-a G protein-coupled receptor [69]. Finally, TRPV1 is a temperature- and ligand-sensitive Ca2+-permeable ion channel activated by pungent extracts, proton, heat and membrane depolarization [73]. TRPV1 is highly expressed in dorsal root ganglia nociceptor neurons, but it is also found on non-sensory tissues including the human epidermal keratinocyte and airway. It has been shown to be able to activate TSLP release from epithelial respiratory cells [73].
Figure 2. Cell source of TSLP and stimuli that are able to activate TSLP production.
TSLP: Thymic stromal lymphopoietin.
To date, the molecular mechanisms that control the expression and release of TSLP are still not completely understood, but monogenetic diseases and mouse models have helped to understand the transcription regulation of TSLP. It appears that TSLP transcription is dependent to a certain extent on Ca2+, nuclear factor active T cells and nuclear factor κB (NF-κB) activation [64,73]. For example, a mouse model has demonstrated that TSLP is secreted after activation of the ORAI1/nuclear factor active T cells calcium signaling pathway [74]. Moreover, TSLP is highly overexpressed in Netherton syndrome, a severe skin disease characterized by AD-like lesions. Netherton syndrome is a result of a defect barrier, caused by mutations in the serine peptidase inhibitor Kazal-type 5 (SPINK5) gene, which encodes the serine protease inhibitor lymphoepithelial Kazal-type-related inhibitor [75]. In SPINK5 knockout (SPINK5−/−) mice, the absence of lymphoepithelial Kazal-type-related inhibitor results in unrestrained activity of the serine protease kalli-krein 5, which directly activates PAR-2 and induces NF-κB-mediated overexpression of TSLP without contribution from the adaptive immune system [69,75]
TSLP-R signaling
TSLPR has low affinity for TSLP, but in combination with IL-7Rα generates a high-affinity binding site for TSLP and triggers signaling through signal transducer and activator of transcription STAT1, STAT3, STAT5 and JAK1 and JAK2 [76]. In approximately 10–60% of patients with B-cell acute lymphoblastic leukemias (ALL) and in some with T-ALL, investigators have described somatic gain-of-function mutations in TSLP-R associated with the aberrant expression of TSLPRα and mutant IL-7R proteins have formed a functional receptor TSLP. In addition, a subset of TSLPRα-overexpressing B-ALLs have a gain-of-function TSLPRα F232C mutation or activating mutations in JAK2 and JAK1. These data confirm that signaling from the TSLPR activates STAT5 by phosphorylation of JAK1 and JAK2 [4–6].
TSLP & Th2 inflammation
TSLP is a hematopoietic factor that was originally purified as a B-cell stimulatory factor as it promotes the proliferation and differentiation of committed murine B220+/IgM+ B cells progenitors; however, the role of TSLP in normal B-cell development or during inflammatory responses remains undefined [77]. On the contrary, in the last several years, it has been clearly demonstrated that the major role of TSLP in both humans and mice is to induce a Th2 cellular adaptive response [55]. Indeed, if TSLP-stimulated CD11c(+)dendritic cells (DCs) are primed in an antigen-specific manner (e.g., in an allogeneic culture) in the absence of IL-12, they express OX40 ligand (OX40L-CD134) and therefore promote the development of Th2 differentiated cells [71,78]. Moreover TSLP-activated DCs further favor Th2 inflammation by polarizing CRTH2+ Th2 effector memory cells [79] and by hindering the production and/or maintenance of FOXP3+ Tregs in vivo in airway allergic inflammation [80,81].
TSLP can also directly promote naïve CD4+ and CD8+ T cells to develop into Th2 cells because TSLPR activation induces IL-4 gene transcription, which in turn further up-regulates TSLPR on CD4+ T cells, resulting in a positive feedback loop [82–84]. Naïve human CD8+ T cells express low, if any, TSLPR; however, following activation, TSLPR expression is up-regulated [85]. Moreover, TSLP stimulation up-regulates the survival protein Bcl-2 in an STAT5-dependent manner in both CD4+ and CD8+ T cells [82,85,86].
TSLP not only is able to induce a Th2 adaptive immune response, but in the last few years it appears to play a major role in favoring the development of Th2 innate immune cells. For example, ILC2, mast cells, NKT cells, basophils and eosinophils express the TSLPR. They respond to TSLP with enhanced Th2 cytokine production, contributing significantly to the Th2 inflammation in AD, asthma and EoE [87–90].
In the last 2 years, there have been several studies focusing on the role of TSLP in ILC2 cells. Indeed these innate cells, which are good producers of Th2 cytokines, appear to be strongly induced by TSLP. In particular, TSLP is able to enhance IL-4, IL-5 and IL-13 expression in IL-33-stimulated human ILC2 purified from peripheral blood and nasal polyps [22]. TSLP has also been shown to activate mouse lung and skin ILC2 [91,92]. Indeed, even if they were initially described to produce high levels of IL-5 and IL-13 and very low levels of IL-4 in response to IL-33, when stimulated with TSLP and leukotriene D4, they are able to produce IL-4, suggesting that ILC2 can also be a good source of IL-4 and therefore could be important in creating the right IL-4-rich environment able to make Th2 development possible when APCs present the antigen to naïve T cells [22,28]. Importantly, a recent study demonstrated that TSLP further enhances GATA3 expression in human ILC2 and thus may be one mechanism of ILC2 Th2 cytokine production induced by TSLP [22].
Finally, TSLP has been shown to promote activity and chemotaxis of eosinophils [90]. Indeed, human eosinophils constitutively expressed functional TSLPR. In vitro, in a concentration-dependent manner, TSLP can significantly delay eosinophil apoptosis, up-regulate cell surface expression of adhesion molecule CD18 and intercellular adhesion molecule-1, but down-regulate L-selectin, enhance eosinophil adhesion onto fibronectin and induce the release of inflammatory cytokine IL-6 and chemokines CXCL8, CXCL1 [90]. Hence, TSLP can significantly induce eosinophilic inflammation.
TSLP & atopic dermatitis
Several lines of evidence support the strong role that TSLP has in AD development.
TSLP has been linked with AD by the association with SNPs in the TSLP gene and its receptor [93–95]. AD had been shown to be significantly associated with four TSLP-SNPs (rs1898671, rs11466749, rs10043985 and rs2289276AD) [93,96] and with two IL7R-SNPs (rs12516866; rs1053496) (Table 1) [93]. This is not surprising as there are many clinical data that point to a strong genetic component in AD. Indeed, family history of atopy is frequently positive in children with AD [93–95].
Table 1.
SNP in TSLP and TSLP receptor associated with major atopic diseases.
| TSLP SNP | TSLP-R SNP | |
|---|---|---|
| Atopic dermatitis | rs11466749 rs10043985 rs2289276 |
rs12516866 rs1053496 |
| Asthma | rs3806933, rs1837253, rs1837253, rs2289276 | |
| EoE | rs3806932 | rs36133495 |
EoE: Eosinophilic esophagitis; TSLP: Thymic stromal lymphopoietin; TSLP-R: TSLP-Receptor.
From an immunological point of view, increased expression of TSLP is recognized to be a pivotal pathogenetic factor in AD development. TSLP protein is undetectable in non-lesional skin in healthy individuals and in AD patients [63,71]; however, TSLP is highly expressed in acute and chronic AD lesions [71,97]. In particular, it is overexpressed in the skin stratum corneum and it correlates with severity scoring of AD index and epidermal barrier function, such as stratum corneum hydration and transepidermal water loss [97]. Interestingly, moisturizer application results in reduced skin TSLP levels and reduced AD symptoms and scores [97]. In a mouse model, overexpression of skin TSLP was enough to induce a disease phenotype similar to AD [98].
One of the major questions is why TSLP is dysregulated in AD. One possible mechanism is that TSLP is increased due to skin injury (chronic itch in AD or underlying skin defect) and/or Notch signaling impairment, which is common in AD. Indeed, AD epidermis has a marked deficiency of Notch receptors [99], which has been linked to increased TSLP expression. In a mouse model, Notch signaling defects in keratinocyte cause severe epidermal differentiation defects (dry skin, signs of scratching, skin barrier abnormalities, increased transepidermal water loss) and high systemic levels of TSLP with associated Th2 cell-mediated immunological changes [100]. In addition, Notch signals are critically involved in the differentiation of Treg cells, in the feedback inhibition of activated innate immunity, and in late epidermal differentiation associated with filaggerin- and stratum corneum barrier lipid processing [99]. Specifically, Notch signaling regulates the homeostasis of aqua-porin 3 and of the tight junction component claudin-1 and Notch1 is a repressor of activator protein-1, which is up-regulated in AD epidermis and leads to increased IL-31 [99]. However, TSLP expression may be due to skin barrier defects that are known to directly induce TSLP [101,102] rather than resulting directly from the loss of keratinocyte-specific Notch [100]. Regardless, a reduced Notch signaling may act synergistically with TSLP to increase Th2 inflammation.
Excessive vitamin D levels may also cause an increase in TSLP levels, as TSLP in the skin is negatively regulated by retinoid X receptors (RXRs). In mice, keratinocyte-specific ablation of the RXR (RXRα and RXRβ) resulted in the up-regulation of TSLP and development of AD-like skin inflammation [103]. RXRs are inhibited by vitamin D as it binds vitamin D receptor, and indeed vitamin D or its analogs induce TSLP expression and result in AD development [104,105].
Demethylation of TSLP promoter may also play a role to determine increased TSLP expression in eczema lesions in children with AD. mRNA and protein levels of TSLP measured in lesional skin samples from 10 children with AD and 10 healthy controls showed that levels were increased in skin lesions from patients with AD compared with healthy controls. Such levels were associated with promoter hypomethylation of the TSLP gene in skin lesions from patients with AD. Reversing methylation level by treating keratinocytes with 5-azacytidine, a DNA methyltransferase inhibitor, caused an increase in TSLP. Therefore, in keratinocytes, DNA demethylation of a specific regulatory region of the TSLP gene may contribute to TSLP overexpression in skin lesions in patients with AD [106].
Elevated TSLP activates several positive feedback loops that contribute to AD chronicity and its severity. TSLP and IL-31, another cytokine elevated in AD, stimulate sensory cutaneous neurons involved in the induction of itch. Recently, it has been shown that in skin keratinocytes, TSLP acts directly on a subset of TRPA1-positive sensory neurons to trigger itch [74]. This phenomenon may cause a positive feedback mechanism as skin mechanical injuries such as tape stripping have been shown to up-regulate TSLP levels in the skin [101,102]. Moreover, TSLP in AD potentiates TH2 inflammation by acting directly on Th2-secreting immune cells belonging to both the innate and the adaptive immune system or indirectly by inducing APCs to favor a Th2 response and Th2 cytokines, which are known stimuli for TSLP [38,67,77,82].
TSLP has been shown to act directly on T cells, iNKT, eosinophils, basophils and mast cells to potentiate TH2 cytokine production (Table 1) [65,89,101,107]. In mice, it has also been found that TSLP can also induce the proliferation and differentiation of mast cells from bone marrow progenitors in a STAT6-dependent manner. TSLP-deficient mice have significantly reduced populations and maturation of mast cells and reduced expression of STAT6. TSLP-induced mast cell proliferation was also abolished by depletion of STAT6. These observations suggest that TSLP is a factor for mast cell development in mice and that it may aggravate mast cell-mediated immune responses [108]
In addition, TSLP has been shown to favor LC migration, maturation and activation as well as DC polarization, which elicit an in situ Th2 response in human AD skin lesions [71,102]. TSLP appears also to be important in mediating skin fibrosis through IL-13-induced collagen production [109]. Given such broad direct and indirect effects on the immune system, it does not surprise that in the setting of chronic high TSLP expression, skin inflammation can also occur in the absence of T cells [98].
What is less clear is whether TSLP influences the initiation and/or progression of allergic skin inflammation [110,111]. In addition, clarification is needed to define how crucial TSLP is in the phenomenon referred to as the atopic march, which describes the increased likelihood of individuals with AD of developing food allergy, AR and asthma later in life [13,52]. Several mouse models that had artificially high systemic levels of TSLP expression typical of a patient with AD seem to suggest a role of TSLP for the progression from AD to subsequent allergic airway inflammation [112–115]. More recently, intradermal administration of TSLP, which more loosely mimics human AD in TSLP expression, triggers progression from eczema to asthma in the absence of systemic TSLP. In such a model, TSLP was only needed during sensitization as the airway response to antigen challenge was TSLP independent [116]. Similarly, in a model of AD, sensitization to food allergens through an atopic dermatitis-like skin lesion was associated with the development of intestinal food allergy, through an expansion of TSLP-elicited basophils in the skin, a stronger specific antigen-specific Th2 cytokine responses, increased antigen-specific serum IgE levels and accumulation of mast cells in the intestine [51]. The disruption of TSLP responses or depletion of basophils reduced the susceptibility to intestinal food allergy, whereas transfer of TSLP-elicited basophils into intact skin promoted disease, suggesting that both TSLP and basophils were essential to promote food sensitization through the skin [51]. These data suggest that TSLP is important for at least initiation of the atopic march and possibly also for its progression.
TSLP, asthma & allergic rhinitis
Extensive research in humans and mice support a role of TSLP in asthma and AR development. Several genetic studies (genome-wide and single polymorphism studies) have shown multiple SNPs at the TSLP genomic locus associated with increased asthma susceptibility (rs3806933 [117], rs1837253 [118,119]) or protection (rs1837253 [120], rs2289276 [121]) in different ethnic backgrounds, gender or age. The SNP described by Harada et al. [117] in the genomic TSLP locus (rs3806933 (−847C/T) creates a novel activator protein-1 transcription factor-binding site that could potentially lead to increased TSLP transcription (Table 1).
TSLP mRNA is expressed in human lung fibroblasts, bronchial epithelial and smooth muscle cells [71]. TSLP expression both at mRNA and at protein levels appear to be increased in asthmatic patients and correlates directly with Th2 cytokine and chemokine expression and inversely with lung function [122,123]. Similar results were observed in COPD patients, suggesting that epithelial damage may play a role in driving TSLP expression in the lung in asthma [123]. Increased expression of TSLP in the nasal epithelium has also been found in biopsies from patients with AR and nasal polyps. As for asthma, increased levels of TSLP were associated with Th2-type inflammation [124–126].
Several mouse models support the importance of TSLP in human asthma. If TSLP is overexpressed, because TSLP production either is constitutively activated (i.e., in surfactant protein C (SPC)-TSLP mice, where TSLP in the lung epithelium is under control of the SPC promoter) [127] or is administrated intranasally in conjunction with antigen [128,129], mice develop an asthma-like disease associated with significant Th2 inflammation. CD4+ T cells, Th2 cytokines and antigens played crucial roles in such models, whereas disease symptoms were significantly reduced in the absence of TSLPR or by blocking TSLP activity by antibody or recombinant TSLPR protein [105,127,130–132]. Similar results were obtained in a mouse model of allergic rhinitis [133].
Similar to AD, TSLP seems to induce TH2 inflammation by modulating DC function, promoting Th2 cytokine production from T cells and by inhibiting Treg. The primary target of TSLP in human asthma and mouse models are DCs; hence it appears to influence mostly the sensitization stage of asthma. TSLP-stimulated DCs increase OX40L expression and production of TH2 chemokines, such as CCL17 and CCL21, leading to the priming of CD4+ TH2 cell development and mast cell production of Th2-associated cytokines [65,66,71,105,127,132]. However, TSLP may also influence the challenge stage of allergic airway disease by directly inducing Th2 CD4+ T-cell cytokine production [101,105,130–133]. Finally, TSLP may significantly impair Treg development. Indeed in an allergic airway disease model, TSLP inhibited Treg function and specific antigen Treg development in vivo [81,134]. This phenomenon could be mediated trough nucleotide-binding oligomerization domain-containing protein 2 and Nod1 stimulation, which induce TSLP, OX40L and TH2 cytokine expression and inhibit antigen-specific FOXP3+ T cells and ovalbumin (OVA) tolerance [80]. Nod stimulation and other triggers of TSLP expression such as peptidoglycan, lipoteichoic acid, dsRNA, respiratory viruses, air pollutants and allergens most likely act via the NF-κB pathway [64–69,71,72,135]. On the other hand, TSLP transcription in airway epithelial cells is negatively regulated by 9-cis-retinoic acid via RXRs [136]. The regulation of TSLP is a balancing act between negative and positive signals that might affect Treg function.
TSLP & eosinophilic esophagitis
Many studies have now demonstrated that TSLP is as an important factor for EoE pathogenesis [1,61]. The first indication of TSLP importance in EoE came from the discovery of an association between a SNP in the TSLP gene and risk for EoE. In collaboration with the Center for Applied Genomics at The Children’s Hospital of Philadelphia and Cincinnati Children’s Hospital, we identified SNP rs3806932 in the promoter region of the TSLP gene. The protective minor allele (G) is present in a higher percentage of control subjects (45.8%) compared to EoE subjects (31.2%) [1]. Individuals homozygous for the TSLP risk allele (AA) have increased TSLP expression and basophil infiltration in the esophageal epithelium compared to those carrying heterozygous (AG) risk allele and homozygous (GG) protective minor alleles [1]. In addition, Sherrill et al. [61] also identified a significant association between a SNP in the TSLP receptor (TSLPR) and male EoE subjects, which is encoded by Xp22.3; Yp11.3 and SNP on X or Y chromosome may affect males specifically (Table 1) [61].
As with other atopic diseases, TSLP is increased in the esophageal biopsies of EoE subjects compared to non-EoE subjects, especially in those that do not carry the protective SNP [1,137,138]. Recent studies from Dr Artis’s group [51,137,138] showed that TSLP may promote Th2 inflammation in EoE through basophils. Basophils are known players in type I allergic responses secondary to the surface expression of high-affinity receptor for IgE, FcεRI and their ability to secrete histamine and Th2 cytokines such as IL-4 and IL-13 in an IgE-dependent or -independent manner [16]. Hence recent studies have shown that basophils may play a significant role in non-IgE-mediated allergic conditions such as EoE. Siracusa et al. [138] described a sub-population of basophils that developed in the presence of TSLP independently from IL-3. Such a population is overexpressed in allergic disorders, including EoE, and is able to produce significant Th2 cytokines (IL-4, IL-6, CCL3, CCL4 and CCL12). Critically, basophils isolated from EoE subjects exhibited similarities to in vitro TSLP-elicited basophils. Noti et al. [137] recently described a novel mouse model of EoE in which the development of EoE-like features was dependent upon both TSLP and basophils, but independent of IgE responses. Wild-type and IgE-deficient mice developed similar levels of esophageal inflammation upon antigen challenge, while on the other hand TSLP- or basophil-blocking antibodies ameliorated the EoE-like disease when administered after the onset of disease. Together, these studies show that TSLP-mediated basophil responses might play an important role in the pathogenesis of human EoE and may suggest that targeting the TSLP–basophil axis may lead to potential therapeutic treatments for EoE. Other potential targets of TSLP in EoE are DC, T cells, iNKTs and Treg. TSLP, as previously discussed, promotes the maturation and activation of DCs, which secrete factors involved in the migration and differentiation of naïve CD4+ T cells into Th2 cells [71]. The currently accepted hypothesis of immune responses in EoE is that antigens penetrate through a dysfunctional esophageal epithelial barrier and are processed by professional APCs such as LCs which in turn promote the polarization of Th2 T cells. TSLP may polarize DCs in genetically predisposed individuals to amplify Th2 responses. However, it is unclear how important the role of esophageal DCs in EoE is, as LCs are present in the esophageal epithelium, but their density is similar in EoE and non-EoE populations [139]. Furthermore, there is clinical knowledge that patients receiving enteral nutrition via post-pyloric feeding tubes can develop EoE, suggesting that direct contact between food antigens and the esophageal epithelium is not a requirement for disease pathogenesis. TSLP could, however, directly influence T cells and iNKTs to produce Th2 cytokines. Indeed, intraepithelial T lymphocytes are the single most prominent infiltrating cell type in EoE. In EoE patients, T cells are Th2-skewed and secrete Th2 cytokines: IL-4, IL-5 and IL-13 in both the peripheral blood and active esophageal biopsies [14,140]. In addition, mice deficient in T cells do not develop EoE, demonstrating the importance of T cells in EoE development [141]. Jyonouchi et al. [35] recently demonstrated that iNKTs, which are a subset of T cells specialized in their ability to recognize self and foreign lipids, may provide a functional link between cow milk allergy with EoE. Children with active EoE compared to children with inactive EoE and healthy controls had higher numbers of iNKTs, which appeared to be recruited in the esophagus via RANTES. iNKT cells from EoE subjects produced higher levels of IL-13 in response to milk sphingolipid stimulation when compared to non-EoE controls. iNKTs have been shown to have TSLPRs and, in AD, to be more Th2-skewed in the presence of TSLP. These observations suggested that IL-13 production derived from iNKT cells migrating to the esophageal epithelium during active inflammation in EoE could be amplified in the presence of TSLP. An increased number of Treg has been reported in the esophageal epithelium of EoE subjects compared to GERD and healthy controls [142,143]. However, the levels of interleukin-10, an anti-inflammatory cytokine secreted by Tregs, were found to be decreased in EoE subjects. TSLP has been shown to inhibit Treg function, so this could explain the apparent paradox mechanisms in EoE, in which increased Tregs in EoE subjects may be an immune compensatory mechanism to curb inflammation.
Potential therapeutic benefits
Anti-TSLP antibodies have been shown to be beneficial in various murine models of atopy. In an EoE murine model, anti-TSLP has been shown to block the development of esophageal eosinophilia and food impactions [137]. In models of asthma or allergic rhinitis, TSLP antibodies or antibodies that inhibit TSLPR block symptoms of and CD4 Th2 development [105,127,130–132]. For example, in an asthma model of allergic asthma, animals were sensitized with OVA intraperitoneally, then challenged for 7 days with either aerosolized OVA or intranasally; animals who were pretreated with anti-TSLP neutralizing antibodies or anti-TSLPR antibodies administered before each OVA sensitization showed eosinophilic airway inflammation, goblet cell hyperplasia and Th2 cytokine productions [105,131]. In both models, the alleviating effects of TSLP-blocking were achieved by inhibition of maturation and migration of airway DCs, as well as their ability to initiate CD4+T-cell responses [105,131].
AMG 157, a fully human anti-TSLP monoclonal antibody that specifically binds human TSLP, preventing interaction with its receptor, has been tested in stable adult asthmatic patients. In a double-blind, placebo-controlled study, 31 patients received three monthly doses of AMG 157 (700 mg) or placebo intravenously. The allergen challenges on days 42 and 84 showed an attenuated allergen-induced bronchocon-striction in both early and late asthmatic responses. In the group receiving the antibody, there were also reduced markers of systemic and airway inflammation. Although this was only a proof-of-concept study, which did not determine whether anti-TSLP therapeutics will have clinical impact, these findings are consistent with the animal model findings and confirm that TSLP has a key role in allergic asthma [144]. One of the mechanisms that could be acted upon is that TSLP antibody, by decreasing TSLP, may reduce eosinophilic infiltration, as TSLP is a potent chemoattractant for eosinophils [90].
Conclusion
TSLP appears to be a specific promoter of atopic inflammation, and it often acts with a positive feedback loop amplifying and leading to the chronicity of Th2 inflammatory responses. Many genetic variants that influence its expression have been described in the last few years. Therefore, several aspecific triggers such as infectious agents may cause its release and initiate a chronic Th2 inflammation in predisposed individuals. Given its important specific role in chronic atopic diseases, TSLP is a promising pharmacological target.
Five-year view
As the first positive clinical trial for anti-TSLP was shown in asthma, [118], Phase II and III clinical trials will examine the role of TSLP in asthma, atopic dermatitis and EoE. From a mechanistic standpoint, comparison of the different roles of IL33, IL31 and TSLP as danger signals in the epithelium will be elicited.
Expert commentary
TSLP has been cloned from murine and human cell lines, indicating a broad biologic role. It is an epithelial-derived cytokine that is induced via various signals (trauma, infections or injuries), suggesting that it is key molecule for the start of various atopic diseases. In addition, it is induced by a broad range of factors, which further indicates an important role for the cytokine. In fact, TSLP appears to be a key cytokine for the progression of the atopic march (atopic dermatitis to asthma) and development of EoE in multiple murine models. In these murine models, inhibition of TSLP has shown great promise with almost complete inhibition of disease. The first published human trial in asthma shows similar promise. This indicates that TSLP might be a key molecule in the development of allergic disease, and treatment with anti-TSLP compounds will have important clinical effects.
Key issues.
Thymic stromal lymphopoietin (TSLP) is an epithelial derived cytokine, related to IL7 and interacts with TSLP-receptor.
TSLP has an important role in the maturation and activation of many blood-derived cells.
It promotes Th2 cell development via IL4 activation.
It is linked to Asthma, atopic dermatitis and eosinophilic esophagitis based on genetic analysis.
Murine models identify TSLP as key molecule for the atopic march.
TSLP has been identified as an essential molecule in the development of eosinophilic esophagitis in murine models (likely in human disease as well).
It activates mast cells, eosinophils and basophils for allergic inflammation.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Rothenberg ME, Spergel JM, Sherrill JD, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet. 2010;42:289–291. doi: 10.1038/ng.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He R, Geha RS. Thymic stromal lymphopoietin. Ann N Y Acad Sci. 2010;1183:13–24. doi: 10.1111/j.1749-6632.2009.05128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziegler SF, Roan F, Bell BD, et al. The biology of thymic stromal lymphopoietin (TSLP) Adv Pharmacol. 2013;66:129–155. doi: 10.1016/B978-0-12-404717-4.00004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shochat C, Tal N, Bandapalli OR, Palmi C, Ganmore I, te Kronnie G, et al. Gain-of-function mutations in interleukin-7 receptor-alpha (IL7R) in childhood acute lymphoblastic leukemias. J Exp Med. 2011;208:901–908. doi: 10.1084/jem.20110580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Bodegom D, Zhong J, Kopp N, et al. Differences in signaling through the B-cell leukemia oncoprotein CRLF2 in response to TSLP and through mutant JAK2. Blood. 2012;120:2853–2863. doi: 10.1182/blood-2012-02-413252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tasian SK, Doral MY, Borowitz MJ, et al. Aberrant STAT5 and PI3K/mTOR pathway signaling occurs in human CRLF2-rearranged B-precursor acute lymphoblastic leukemia. Blood. 2012;120:833–842. doi: 10.1182/blood-2011-12-389932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pathak K. The expanding role of IL-7 and thymic stromal lymphopoietin as therapeutic target for rheumatoid arthritis. Expert Opin Ther Targets. 2014;18:581–594. doi: 10.1517/14728222.2014.893295. [DOI] [PubMed] [Google Scholar]
- 8.Maizels RM, Hewitson JP, Smith KA. Susceptibility and immunity to helminth parasites. Curr Opin Immunol. 2012;24:459–466. doi: 10.1016/j.coi.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 10.Robinson D, Hamid Q, Bentley A, et al. Activation of CD4+ T cells, increased TH2-type cytokine mRNA expression, and eosinophil recruitment in bronchoalveolar lavage after allergen inhalation challenge in patients with atopic asthma. J Allergy Clin Immunol. 1993;92:313–324. doi: 10.1016/0091-6749(93)90175-f. [DOI] [PubMed] [Google Scholar]
- 11.Robinson DS, Hamid Q, Jacobson M, et al. Evidence for Th2-type T helper cell control of allergic disease in vivo. Springer Semin Immunopathol. 1993;15:17–27. doi: 10.1007/BF00204623. [DOI] [PubMed] [Google Scholar]
- 12.Sampson HA. Food allergy. Part 1: immunopathogenesis and clinical disorders. J Allergy Clin Immunol. 1999;103:717–728. doi: 10.1016/s0091-6749(99)70411-2. [DOI] [PubMed] [Google Scholar]
- 13.Spergel JM. Atopic march: link to upper airways. Curr Opin Allergy Clin Immunol. 2005;5:17–21. doi: 10.1097/00130832-200502000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Straumann A, Bauer M, Fischer B, et al. Idiopathic eosinophilic esophagitis is associated with a T(H)2-type allergic inflammatory response. J Allergy Clin Immunol. 2001;108:954–961. doi: 10.1067/mai.2001.119917. [DOI] [PubMed] [Google Scholar]
- 15.Kim EY, Battaile JT, Patel AC, et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med. 2008;14:633–640. doi: 10.1038/nm1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siracusa MC, Kim BS, Spergel JM, Artis D. Basophils and allergic inflammation. J Allergy Clin Immunol. 2013;132:789–801. doi: 10.1016/j.jaci.2013.07.046. quiz 788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanhere A, Hertweck A, Bhatia U, et al. T-bet and GATA3 orchestrate Th1 and Th2 differentiation through lineage-specific targeting of distal regulatory elements. Nat Commun. 2012;3:1268. doi: 10.1038/ncomms2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Andrea A, Aste-Amezaga M, Valiante NM, et al. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tripp CS, Wolf SF, Unanue ER. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci USA. 1993;90:3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fort MM, Cheung J, Yen D, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 21.Doherty TA, Khorram N, Chang JE, et al. STAT6 regulates natural helper cell proliferation during lung inflammation initiated by Alternaria. Am J Physiol Lung Cell Mol Physiol. 2012;303:L577–L588. doi: 10.1152/ajplung.00174.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mjosberg J, Bernink J, Golebski K, et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Mjosberg JM, Trifari S, Crellin NK, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 24.Barnig C, Cernadas M, Dutile S, et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3004812. 174ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu X, Pappu R, Ramirez-Carrozzi V, et al. TNF superfamily member TL1A elicits type 2 innate lymphoid cells at mucosal barriers. Mucosal Immunol. 2014;7:730–740. doi: 10.1038/mi.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner JE, Morrison PJ, Wilhelm C, et al. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J Exp Med. 2013;210:2951–2965. doi: 10.1084/jem.20130071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang JE, Doherty TA, Baum R, Broide D. Prostaglandin D2 regulates human type 2 innate lymphoid cell chemotaxis. J Allergy Clin Immunol. 2014;133:899–901. doi: 10.1016/j.jaci.2013.09.020. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doherty TA, Khorram N, Lund S, et al. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013;132:205–213. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doherty TA, Khorram N, Sugimoto K, et al. Alternaria induces STAT6-dependent acute airway eosinophilia and epithelial FIZZ1 expression that promotes airway fibrosis and epithelial thickness. J Immunol. 2012;188:2622–2629. doi: 10.4049/jimmunol.1101632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moro K, Yamada T, Tanabe M, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 31.Wilhelm C, Hirota K, Stieglitz B, et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 2011;12:1071–1077. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klein Wolterink RG, Serafini N, van Nimwegen M, et al. Essential, dose-dependent role for the transcription factor Gata3 in the development of IL-5+ and IL-13+ type 2 innate lymphoid cells. Proc Natl Acad Sci USA. 2013;110:10240–10245. doi: 10.1073/pnas.1217158110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoyler T, Klose CS, Souabni A, et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37:634–648. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blanchard C, Durual S, Estienne M, et al. Eotaxin-3/CCL26 gene expression in intestinal epithelial cells is up-regulated by interleukin-4 and interleukin-13 via the signal transducer and activator of transcription 6. Int J Biochem Cell Biol. 2005;37:2559–2573. doi: 10.1016/j.biocel.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Jyonouchi S, Smith CL, Saretta F, et al. Invariant natural killer T cells in children with eosinophilic esophagitis. Clin Exp Allergy. 2014;44:58–68. doi: 10.1111/cea.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee LA, Burks AW. Food allergies: prevalence, molecular characterization, and treatment/prevention strategies. Annu Rev Nutr. 2006;26:539–565. doi: 10.1146/annurev.nutr.26.061505.111211. [DOI] [PubMed] [Google Scholar]
- 37.Sampson HA, Broadbent KR, Bernhisel-Broadbent J. Spontaneous release of histamine from basophils and histamine-releasing factor in patients with atopic dermatitis and food hypersensitivity. N Engl J Med. 1989;321:228–232. doi: 10.1056/NEJM198907273210405. [DOI] [PubMed] [Google Scholar]
- 38.Sampson HA, Mendelson L, Rosen JP. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N Engl J Med. 1992;327:380–384. doi: 10.1056/NEJM199208063270603. [DOI] [PubMed] [Google Scholar]
- 39.Yan BM, Shaffer EA. Primary eosinophilic disorders of the gastrointestinal tract. Gut. 2009;58:721–732. doi: 10.1136/gut.2008.165894. [DOI] [PubMed] [Google Scholar]
- 40.Gray HC, Foy TM, Becker BA, Knutsen AP. Rice-induced enterocolitis in an infant: TH1/TH2 cellular hypersensitivity and absent IgE reactivity. Ann Allergy Asthma Immunol. 2004;93:601–605. doi: 10.1016/S1081-1206(10)61270-7. [DOI] [PubMed] [Google Scholar]
- 41.Chehade M, Mayer L. Oral tolerance and its relation to food hypersensitivities. J Allergy Clin Immunol. 2005;115:3–12. doi: 10.1016/j.jaci.2004.11.008. quiz 3. [DOI] [PubMed] [Google Scholar]
- 42.Heyman M. Symposium on ‘dietary influences on mucosal immunity’. How dietary antigens access the mucosal immune system. Proc Nutr Soc. 2001;60:419–426. [PubMed] [Google Scholar]
- 43.Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 44.Dahan S, Roth-Walter F, Arnaboldi P, et al. Epithelia: lymphocyte interactions in the gut. Immunol Rev. 2007;215:243–253. doi: 10.1111/j.1600-065X.2006.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 46.Hayakawa Y, Berzins SP, Crowe NY, et al. Antigen-induced tolerance by intrathymic modulation of self-recognizing inhibitory receptors. Nat Immunol. 2004;5:590–596. doi: 10.1038/ni1069. [DOI] [PubMed] [Google Scholar]
- 47.Bacchetta R, Gambineri E, Roncarolo MG. Role of regulatory T cells and FOXP3 in human diseases. J Allergy Clin Immunol. 2007;120:227–235. doi: 10.1016/j.jaci.2007.06.023. quiz 36-7. [DOI] [PubMed] [Google Scholar]
- 48.Spergel JM, Mizoguchi E, Brewer JP, et al. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614–1622. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spergel JM, Mizoguchi E, Oettgen H, et al. Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J Clin Invest. 1999;103:1103–1111. doi: 10.1172/JCI5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010;11:289–293. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noti M, Kim BS, Siracusa MC, et al. Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin-basophil axis. J Allergy Clin Immunol. 2014;133:1390–1399. doi: 10.1016/j.jaci.2014.01.021. 9 e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112:S118–S127. doi: 10.1016/j.jaci.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 53.Friend SL, Hosier S, Nelson A, et al. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol. 1994;22:321–328. [PubMed] [Google Scholar]
- 54.Sims JE, Williams DE, Morrissey PJ, et al. Molecular cloning and biological characterization of a novel murine lymphoid growth factor. J Exp Med. 2000;192:671–680. doi: 10.1084/jem.192.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reche PA, Soumelis V, Gorman DM, et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. 2001;167:336–343. doi: 10.4049/jimmunol.167.1.336. [DOI] [PubMed] [Google Scholar]
- 56.Quentmeier H, Drexler HG, Fleckenstein D, et al. Cloning of human thymic stromal lymphopoietin (TSLP) and signaling mechanisms leading to proliferation. Leukemia. 2001;15:1286–1292. doi: 10.1038/sj.leu.2402175. [DOI] [PubMed] [Google Scholar]
- 57.Park LS, Martin U, Garka K, et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med. 2000;192:659–670. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ziegler SF. The role of thymic stromal lymphopoietin (TSLP) in allergic disorders. Curr Opin Immunol. 2010;22:795–799. doi: 10.1016/j.coi.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reardon C, Lechmann M, Brustle A, et al. Thymic stromal lymphopoetin-induced expression of the endogenous inhibitory enzyme SLPI mediates recovery from colonic inflammation. Immunity. 2011;35:223–235. doi: 10.1016/j.immuni.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loots GG, Locksley RM, Blankespoor CM, et al. Identification of a coordinate regulator of interleukins 4, 13, and 5 by cross-species sequence comparisons. Science. 2000;288:136–140. doi: 10.1126/science.288.5463.136. [DOI] [PubMed] [Google Scholar]
- 61.Sherrill JD, Gao PS, Stucke EM, et al. Variants of thymic stromal lymphopoietin and its receptor associate with eosinophilic esophagitis. J Allergy Clin Immunol. 2010;126:160–165. doi: 10.1016/j.jaci.2010.04.037. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taylor BC, Zaph C, Troy AE, et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med. 2009;206:655–667. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Volpe E, Pattarini L, Martinez-Cingolani C, et al. Thymic stromal lymphopoietin links keratinocytes and dendritic cell-derived IL-23 in patients with psoriasis. J Allergy Clin Immunol. 2014;164(2):373–381. doi: 10.1016/j.jaci.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 64.Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc Natl Acad Sci USA. 2007;104:914–919. doi: 10.1073/pnas.0607305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Allakhverdi Z, Comeau MR, Jessup HK, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bleck B, Tse DB, Gordon T, et al. Diesel exhaust particle-treated human bronchial epithelial cells upregulate Jagged-1 and OX40 ligand in myeloid dendritic cells via thymic stromal lymphopoietin. J Immunol. 2010;185:6636–6645. doi: 10.4049/jimmunol.1000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kashyap M, Rochman Y, Spolski R, et al. Thymic stromal lymphopoietin is produced by dendritic cells. J Immunol. 2011;187:1207–1211. doi: 10.4049/jimmunol.1100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kato A, Schleimer RP. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Curr Opin Immunol. 2007;19:711–720. doi: 10.1016/j.coi.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kouzaki H, O’Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 2009;183:1427–1434. doi: 10.4049/jimmunol.0900904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smelter DF, Sathish V, Thompson MA, et al. Thymic stromal lymphopoietin in cigarette smoke-exposed human airway smooth muscle. J Immunol. 2010;185:3035–3040. doi: 10.4049/jimmunol.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soumelis V, Reche PA, Kanzler H, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 72.Zhang K, Shan L, Rahman MS, et al. Constitutive and inducible thymic stromal lymphopoietin expression in human airway smooth muscle cells: role in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L375–L382. doi: 10.1152/ajplung.00045.2007. [DOI] [PubMed] [Google Scholar]
- 73.Jia X, Zhang H, Cao X, et al. Activation of TRPV1 mediates thymic stromal lymphopoietin release via the Ca/NFAT pathway in airway epithelial cells. FEBS Lett. 2014;588(17):3047–3054. doi: 10.1016/j.febslet.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 74.Wilson SR, Bautista DM. Role of transient receptor potential channels in acute and chronic itch. In: Carstens E, Akiyama T, editors. Itch: mechanisms and treatment. Boca Raton (FL): CRC Press; 2014. Chapter 16. [PubMed] [Google Scholar]
- 75.Briot A, Deraison C, Lacroix M, et al. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med. 2009;206:1135–1147. doi: 10.1084/jem.20082242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Borowski A, Vetter T, Kuepper M, et al. Expression analysis and specific blockade of the receptor for human thymic stromal lymphopoietin (TSLP) by novel antibodies to the human TSLPRalpha receptor chain. Cytokine. 2013;61:546–555. doi: 10.1016/j.cyto.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 77.Ray RJ, Furlonger C, Williams DE, Paige CJ. Characterization of thymic stromal-derived lymphopoietin (TSLP) in murine B cell development in vitro. Eur J Immunol. 1996;26:10–16. doi: 10.1002/eji.1830260103. [DOI] [PubMed] [Google Scholar]
- 78.Ito T, Wang YH, Duramad O, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang YH, Ito T, Homey B, et al. Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity. 2006;24:827–838. doi: 10.1016/j.immuni.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 80.Duan W, Mehta AK, Magalhaes JG, et al. Innate signals from Nod2 block respiratory tolerance and program T(H)2-driven allergic inflammation. J Allergy Clin Immunol. 2010;126:1284–1293. doi: 10.1016/j.jaci.2010.09.021. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lei L, Zhang Y, Yao W, et al. Thymic stromal lymphopoietin interferes with airway tolerance by suppressing the generation of antigen-specific regulatory T cells. J Immunol. 2011;186:2254–2261. doi: 10.4049/jimmunol.1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kitajima M, Lee HC, Nakayama T, Ziegler SF. TSLP enhances the function of helper type 2 cells. Eur J Immunol. 2011;41:1862–1871. doi: 10.1002/eji.201041195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol. 2007;178:1396–1404. doi: 10.4049/jimmunol.178.3.1396. [DOI] [PubMed] [Google Scholar]
- 84.Rochman I, Watanabe N, Arima K, et al. Cutting edge: direct action of thymic stromal lymphopoietin on activated human CD4+ T cells. J Immunol. 2007;178:6720–6724. doi: 10.4049/jimmunol.178.11.6720. [DOI] [PubMed] [Google Scholar]
- 85.Rochman Y, Leonard WJ. The role of thymic stromal lymphopoietin in CD8+ T cell homeostasis. J Immunol. 2008;181:7699–7705. doi: 10.4049/jimmunol.181.11.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rochman Y, Leonard WJ. Thymic stromal lymphopoietin: a new cytokine in asthma. Curr Opin Pharmacol. 2008;8:249–254. doi: 10.1016/j.coph.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: the ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol. 2007;179:2051–2054. doi: 10.4049/jimmunol.179.4.2051. [DOI] [PubMed] [Google Scholar]
- 88.Nagata Y, Kamijuku H, Taniguchi M, et al. Differential role of thymic stromal lymphopoietin in the induction of airway hyperreactivity and Th2 immune response in antigen-induced asthma with respect to natural killer T cell function. Int Arch Allergy Immunol. 2007;144:305–314. doi: 10.1159/000106319. [DOI] [PubMed] [Google Scholar]
- 89.Wu WH, Park CO, Oh SH, et al. Thymic stromal lymphopoietin-activated invariant natural killer T cells trigger an innate allergic immune response in atopic dermatitis. J Allergy Clin Immunol. 2010;126:290–299. doi: 10.1016/j.jaci.2010.05.024. 9 e1–4. [DOI] [PubMed] [Google Scholar]
- 90.Wong CK, Hu S, Cheung PF, Lam CW. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: implications in allergic inflammation. Am J Respir Cell Mol Biol. 2010;43:305–315. doi: 10.1165/rcmb.2009-0168OC. [DOI] [PubMed] [Google Scholar]
- 91.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 92.Kim BS, Siracusa MC, Saenz SA, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3005374. 170ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gao PS, Rafaels NM, Mu D, et al. Genetic variants in thymic stromal lymphopoietin are associated with atopic dermatitis and eczema herpeticum. J Allergy Clin Immunol. 2010;125:1403–1407. doi: 10.1016/j.jaci.2010.03.016. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Beck LA, Boguniewicz M, Hata T, et al. Phenotype of atopic dermatitis subjects with a history of eczema herpeticum. J Allergy Clin Immunol. 2009;124:260–269. doi: 10.1016/j.jaci.2009.05.020. 9 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tay YK, Khoo BP, Goh CL. The epidemiology of atopic dermatitis at a tertiary referral skin center in Singapore. Asian Pac J Allergy Immunol. 1999;17:137–141. [PubMed] [Google Scholar]
- 96.Margolis DJ, Kim B, Apter AJ, et al. Thymic stromal lymphopoietin variation, filaggrin loss of function, and the persistence of atopic dermatitis. JAMA Dermatol. 2014;150:254–259. doi: 10.1001/jamadermatol.2013.7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sano Y, Masuda K, Tamagawa-Mineoka R, et al. Thymic stromal lymphopoietin expression is increased in the horny layer of patients with atopic dermatitis. Clin Exp Immunol. 2013;171:330–337. doi: 10.1111/cei.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yoo J, Omori M, Gyarmati D, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Melnik BC. The potential role of impaired notch signalling in atopic dermatitis. Acta Derm Venereol. 2014 doi: 10.2340/00015555-1898. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 100.Demehri S, Liu Z, Lee J, et al. Notch-deficient skin induces a lethal systemic B-lymphoproliferative disorder by secreting TSLP, a sentinel for epidermal integrity. PLoS Biol. 2008;6:e123. doi: 10.1371/journal.pbio.0060123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.He R, Oyoshi MK, Garibyan L, et al. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc Natl Acad Sci USA. 2008;105:11875–11880. doi: 10.1073/pnas.0801532105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oyoshi MK, Larson RP, Ziegler SF, Geha RS. Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J Allergy Clin Immunol. 2010;126:976–984. doi: 10.1016/j.jaci.2010.08.041. 84 e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li M, Messaddeq N, Teletin M, et al. Retinoid X receptor ablation in adult mouse keratinocytes generates an atopic dermatitis triggered by thymic stromal lymphopoietin. Proc Natl Acad Sci USA. 2005;102:14795–14800. doi: 10.1073/pnas.0507385102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li M, Hener P, Zhang Z, et al. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci USA. 2006;103:11736–11741. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li YL, Li HJ, Ji F, et al. Thymic stromal lymphopoietin promotes lung inflammation through activation of dendritic cells. J Asthma. 2010;47:117–123. doi: 10.3109/02770900903483816. [DOI] [PubMed] [Google Scholar]
- 106.Luo Y, Zhou B, Zhao M, et al. Promoter demethylation contributes to TSLP overexpression in skin lesions of patients with atopic dermatitis. Clin Exp Dermatol. 2014;39:48–53. doi: 10.1111/ced.12206. [DOI] [PubMed] [Google Scholar]
- 107.Jessup HK, Brewer AW, Omori M, et al. Intradermal administration of thymic stromal lymphopoietin induces a T cell- and eosinophil-dependent systemic Th2 inflammatory response. J Immunol. 2008;181:4311–4319. doi: 10.4049/jimmunol.181.6.4311. [DOI] [PubMed] [Google Scholar]
- 108.Han NR, Oh HA, Nam SY, et al. TSLP Induces Mast Cell Development and Aggravates Allergic Reactions through the Activation of MDM2 and STAT6. J Invest Dermatol. 2014;134(10):2521–2530. doi: 10.1038/jid.2014.198. [DOI] [PubMed] [Google Scholar]
- 109.Oh MH, Oh SY, Yu J, et al. IL-13 induces skin fibrosis in atopic dermatitis by thymic stromal lymphopoietin. J Immunol. 2011;186:7232–7242. doi: 10.4049/jimmunol.1100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boehme SA, Franz-Bacon K, Chen EP, et al. A small molecule CRTH2 antagonist inhibits FITC-induced allergic cutaneous inflammation. Int Immunol. 2009;21:81–93. doi: 10.1093/intimm/dxn127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Larson RP, Zimmerli SC, Comeau MR, et al. Dibutyl phthalate-induced thymic stromal lymphopoietin is required for Th2 contact hypersensitivity responses. J Immunol. 2010;184:2974–2984. doi: 10.4049/jimmunol.0803478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Demehri S, Morimoto M, Holtzman MJ, Kopan R. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7:e1000067. doi: 10.1371/journal.pbio.1000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Leyva-Castillo JM, Hener P, Jiang H, Li M. TSLP produced by keratinocytes promotes allergen sensitization through skin and thereby triggers atopic march in mice. J Invest Dermatol. 2013;133:154–163. doi: 10.1038/jid.2012.239. [DOI] [PubMed] [Google Scholar]
- 114.Jiang H, Hener P, Li J, Li M. Skin thymic stromal lymphopoietin promotes airway sensitization to inhalant house dust mites leading to allergic asthma in mice. Allergy. 2012;67:1078–1082. doi: 10.1111/j.1398-9995.2012.02857.x. [DOI] [PubMed] [Google Scholar]
- 115.Zhang Z, Hener P, Frossard N, et al. Thymic stromal lymphopoietin overproduced by keratinocytes in mouse skin aggravates experimental asthma. Proc Natl Acad Sci USA. 2009;106:1536–1541. doi: 10.1073/pnas.0812668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Han H, Xu W, Headley MB, et al. Thymic stromal lymphopoietin (TSLP)-mediated dermal inflammation aggravates experimental asthma. Mucosal Immunol. 2012;5:342–351. doi: 10.1038/mi.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Harada M, Hirota T, Jodo AI, et al. Functional analysis of the thymic stromal lymphopoietin variants in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2009;40:368–374. doi: 10.1165/rcmb.2008-0041OC. [DOI] [PubMed] [Google Scholar]
- 118.Torgerson DG, Ampleford EJ, Chiu GY, et al. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet. 2011;43:887–892. doi: 10.1038/ng.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ferreira MA, Matheson MC, Tang CS, et al. Genome-wide association analysis identifies 11 risk variants associated with the asthma with hay fever phenotype. J Allergy Clin Immunol. 2014;133:1564–1571. doi: 10.1016/j.jaci.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bunyavanich S, Melen E, Wilk JB, et al. Thymic stromal lymphopoietin (TSLP) is associated with allergic rhinitis in children with asthma. Clin Mol Allergy. 2011;9:1. doi: 10.1186/1476-7961-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hunninghake GM, Soto-Quiros ME, Avila L, et al. TSLP polymorphisms are associated with asthma in a sex-specific fashion. Allergy. 2010;65:1566–1575. doi: 10.1111/j.1398-9995.2010.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shikotra A, Choy DF, Ohri CM, et al. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol. 2012;129:104–111. doi: 10.1016/j.jaci.2011.08.031. e1–9. [DOI] [PubMed] [Google Scholar]
- 123.Ying S, O’Connor B, Ratoff J, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181:2790–2798. doi: 10.4049/jimmunol.181.4.2790. [DOI] [PubMed] [Google Scholar]
- 124.Kamekura R, Kojima T, Koizumi J, et al. Thymic stromal lymphopoietin enhances tight-junction barrier function of human nasal epithelial cells. Cell Tissue Res. 2009;338:283–293. doi: 10.1007/s00441-009-0855-1. [DOI] [PubMed] [Google Scholar]
- 125.Kimura S, Pawankar R, Mori S, et al. Increased expression and role of thymic stromal lymphopoietin in nasal polyposis. Allergy Asthma Immunol Res. 2011;3:186–193. doi: 10.4168/aair.2011.3.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mou Z, Xia J, Tan Y, et al. Overexpression of thymic stromal lymphopoietin in allergic rhinitis. Acta Otolaryngol. 2009;129:297–301. doi: 10.1080/00016480802225884. [DOI] [PubMed] [Google Scholar]
- 127.Zhou B, Comeau MR, De Smedt T, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 128.Miazgowicz MM, Headley MB, Larson RP, Ziegler SF. Thymic stromal lymphopoietin and the pathophysiology of atopic disease. Expert Rev Clin Immunol. 2009;5:547–556. doi: 10.1586/eci.09.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Seshasayee D, Lee WP, Zhou M, et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J Clin Invest. 2007;117:3868–3878. doi: 10.1172/JCI33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Al-Shami A, Spolski R, Kelly J, et al. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shi L, Leu SW, Xu F, et al. Local blockade of TSLP receptor alleviated allergic disease by regulating airway dendritic cells. Clin Immunol. 2008;129:202–210. doi: 10.1016/j.clim.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 132.Zhang F, Huang G, Hu B, et al. A soluble thymic stromal lymphopoietin (TSLP) antagonist, TSLPR-immunoglobulin, reduces the severity of allergic disease by regulating pulmonary dendritic cells. Clin Exp Immunol. 2011;164:256–264. doi: 10.1111/j.1365-2249.2011.04328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Miyata M, Hatsushika K, Ando T, et al. Mast cell regulation of epithelial TSLP expression plays an important role in the development of allergic rhinitis. Eur J Immunol. 2008;38:1487–1492. doi: 10.1002/eji.200737809. [DOI] [PubMed] [Google Scholar]
- 134.Nguyen KD, Vanichsarn C, Nadeau KC. TSLP directly impairs pulmonary Treg function: association with aberrant tolerogenic immunity in asthmatic airway. Allergy Asthma Clin Immunol. 2010;6:4. doi: 10.1186/1710-1492-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Magalhaes JG, Rubino SJ, Travassos LH, et al. Nucleotide oligomerization domain-containing proteins instruct T cell helper type 2 immunity through stromal activation. Proc Natl Acad Sci USA. 2011;108:14896–14901. doi: 10.1073/pnas.1015063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee HC, Headley MB, Iseki M, et al. Cutting edge: inhibition of NF-kappaB-mediated TSLP expression by retinoid X receptor. J Immunol. 2008;181:5189–5193. doi: 10.4049/jimmunol.181.8.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Noti M, Wojno ED, Kim BS, et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat Med. 2013;19(8):1005–1013. doi: 10.1038/nm.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Siracusa MC, Saenz SA, Hill DA, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477:229–233. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yen EH, Hornick JL, Dehlink E, et al. Comparative analysis of FcepsilonRI expression patterns in patients with eosinophilic and reflux esophagitis. J Pediatr Gastroenterol Nutr. 2010;51:584–592. doi: 10.1097/MPG.0b013e3181de7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Blanchard C, Stucke EM, Rodriguez-Jimenez B, et al. A striking local esophageal cytokine expression profile in eosinophilic esophagitis. J Allergy Clin Immunol. 2011;127:208–217. doi: 10.1016/j.jaci.2010.10.039. 17 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mishra A, Schlotman J, Wang M, Rothenberg ME. Critical role for adaptive T cell immunity in experimental eosinophilic esophagitis in mice. J Leukoc Biol. 2007;81:916–924. doi: 10.1189/jlb.1106653. [DOI] [PubMed] [Google Scholar]
- 142.Tantibhaedhyangkul U, Tatevian N, Gilger MA, et al. Increased esophageal regulatory T cells and eosinophil characteristics in children with eosinophilic esophagitis and gastroesophageal reflux disease. Ann Clin Lab Sci. 2009;39:99–107. [PubMed] [Google Scholar]
- 143.Fuentebella J, Patel A, Nguyen T, et al. Increased number of regulatory T cells in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2010;51:283–289. doi: 10.1097/MPG.0b013e3181e0817b. [DOI] [PubMed] [Google Scholar]
- 144.Gauvreau GM, O’Byrne PM, Boulet LP, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014;370:2102–2110. doi: 10.1056/NEJMoa1402895. [DOI] [PubMed] [Google Scholar]