Abstract
The mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase that exists in two complexes (mTORC1 and mTORC2) and integrates extracellular and intracellular signals to act as a master regulator of cell growth, survival, and metabolism. The PI3K/AKT/mTOR pro-survival pathway is often dysregulated in multiple sarcoma subtypes. First-generation allosteric inhibitors of mTORC1 (rapalogues) have been extensively tested with great pre-clinical promise, but have had limited clinical utility. Here we report that MLN0128, a second-generation, ATP-competitive, pan-mTOR kinase inhibitor, acts on both mTORC1 and mTORC2, and has potent in vitro and in vivo anti-tumor activity in multiple sarcoma subtypes. In vitro, MLN0128 inhibits mTORC1/2 targets in a concentration dependent fashion, and shows striking anti-proliferative effect in rhabdomyosarcoma (RMS), Ewing sarcoma (ES), malignant peripheral nerve sheath tumor, synovial sarcoma, osteosarcoma, and liposarcoma. Unlike rapamycin, MLN0128 inhibits phosphorylation of 4EBP1 and NDRG1 as well as prevents the reactivation of pAKT that occurs via negative feedback release with mTORC1 inhibition alone. In xenograft models, MLN0128 treatment results in suppression of tumor growth with two dosing schedules (1 mg/kg daily and 3 mg/kg BID TIW). At the 3 mg/kg dosing schedule, MLN0128 treatment results in significantly better tumor growth suppression than rapamycin in RMS and ES models. Additionally, MLN0128 induces apoptosis in models of RMS both in vitro and in vivo. Results from our study strongly suggest that MLN0128 treatment should be explored further as potential therapy for sarcoma.
Keywords: MLN0128, mTOR, Rapamycin
Introduction
Mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase that exists in two distinct protein complexes (mTORC1 and mTORC2) that regulates metabolism, homeostasis, survival, and proliferation (1–3), and is often dysregulated in multiple sarcoma subtypes (4–6). Abnormal signals both upstream and downstream of the mTOR kinase lead to aberrant activity in sarcomas, and its dysregulation has been well documented by elevated phosphorylation of multiple components of the pathway (7–10). This deregulated activity of mTOR and its surrounding axis has been shown to correlate with poor clinical outcomes in sarcomas (11–13), as well as other tumor types (14). Upstream, overexpression or constitutive activation of platelet derived growth factor receptor (PDGFR) (15), insulin-like growth factor 1 receptor (IGF1R)(16, 17), vascular endothelial growth factor receptor (VEGFR) (18) and fibroblast growth factor receptor (FGFR)(19), have been demonstrated to play a role, while S6 kinase (S6K), and eukaryotic initiation binding factor 4E (eIF4E) (11) are implicated downstream. Other critical pathways, such as the mitogen-activated protein kinase (MAPK) pathway, have been shown to interact with mTOR in sarcoma, and cross talk between them is implicated in treatment-mediated resistance (20, 21). Most recently, large-scale genomic sequencing projects have revealed distinct mutations clustered in and around the phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR axis (22–24), which further verify mTOR as a valid anti-tumor target in the treatment of sarcomas.
The importance of the PI3K/AKT/mTOR axis in sarcoma has driven interest in therapies targeted to mTOR, and led to a focus on rapamycin and a group of roughly equivalent structural analogues (25) termed rapalogues. Despite significant pre-clinical enthusiasm, the utility of these first-generation agents has been limited due to the allosteric inhibition of only one of the two mTOR complexes, mTORC1 (26). This inhibition of mTORC1 only leaves the downstream effectors of mTORC2 unchecked, and contributes to the unwanted reactivation of AKT, which occurs via release of negative feedback in an IGF1-R dependent fashion (27). Taken together, these limitations suggest that rapalogues do not comprehensively exploit the antitumor potential of mTOR inhibition, and have driven development of second-generation agents (28).
MLN0128 (Millennium/Takeda Pharmaceuticals) is a selective, highly potent, and orally bioavailable adenosine triphosphate (ATP) competitor of both mTORC1 and mTORC2, which is currently in phase I and II clinical trials as a single agent in patients with advanced solid malignancies (NCT01899053, NCT01058707, NCT01351350, NCT0133183, NCT02091531), in combination with bevacizumab in patients with glioblastoma multiforme or advanced solid tumors (NCT02142803), in combination with MLN1117 (PI3K inhibitor) in patients with advanced non-hematologic malignancies (NCT01899053), in combination with ziv-afilbercept in recurrent solid tumors (NCT02159989), and breast cancer (NCT02049957). An additional dose escalation study in relapsed or refractory Multiple Myeloma or Waldenstrom-Macroglobulinemia has recently been completed (NCT01118689). Pre-clinically, MLN0128 has been shown to have antitumor activity in prostate cancer (29), B-cell leukemia (30), breast cancer (31, 32), and renal cell carcinoma (33). In the current study we describe the preclinical characterization of MLN0128 in bone and soft tissue sarcomas, evaluate its in vitro and in vivo effects, and demonstrate its anti-tumor properties superior to those of its first-generation rapalogue predecessors.
Materials and Methods
Chemicals and Drugs
MLN0128 was provided by Millennium/Takeda Pharmaceuticals. Rapamycin was purchased from EMD chemicals. MLN0128 and rapamycin were dissolved in dimethyl sulfoxide (DMSO) and stored at −20°C.
Cell culture and reagents
Cells were cultured in Roswell Park Memorial Institute (RPMI) media with 10% fetal bovine serum, 100 U/mL penicillin, and 100 mg/mL streptomycin, maintained at 37°C in 5% CO2, and passaged for no more than four months. Initial stocks of all cell lines were received from their sources within the past 3 years. Malignant peripheral nerve sheath tumor (MPNST, ST8814) and rhabdomyosarcoma cell line RMS-559 were supplied by Dr. Jonathan Fletcher (Dana Farber Cancer Institute, Boston, MA). RMS-559 (22), MPNST and ST8814 (34) cell lines were authenticated as previously described. Ewing Sarcoma (CHP100, A673) cell lines were obtained from Dr. Melinda S. Merchant (Center for Cancer Research, NCI/NIH, Bethesda, MD). De-differentiated liposarcoma cell lines (LS141, DDLS) were obtained from Dr. Samuel Singer (Memorial Sloan Kettering Cancer Center (MSKCC), New York, NY), and were authenticated by gene expression profiling prior to distribution (35). Synovial sarcoma cell lines (SYO-1 and HSSY-II) were obtained from Dr. Marc Ladanyi (MSKCC). Rhabdomyosarcoma cell lines Rh28, Rh30, RD, SMS-CTR and Ewing sarcoma cell lines TE-381, TC32, TC71, and CHLA9 were obtained from Dr. Timothy Triche (University of Southern California, Los Angeles, CA). SK-RMS -3 and SK-RMS -4 were derived from patient tissues and provided by Dr. Christine Pratilas (Johns Hopkins Kimmel Comprehensive Cancer Center, Baltimore, MD). SK-RMS -3 and SK-RMS -4 were derived from patient tumors and use of patients’ tumor material was conducted under an MSKCC IRB approved protocol for the use of human bio-specimen (IRB 10-130) and with patient authorization for research use (IRB 06-107). Osteosarcoma cell line (SaOS2) was obtained from American Type Culture Collection (ATCC). Rhabdomyosarcoma cell lines generously provided by Drs. Pratilas and Triche were not independently authenticated unless otherwise mentioned. Cell lines TC32, TC71, CHP100, A673, and CHLA9 were authenticated using reverse transcription-polymerase chain reaction (RT-PCR), and found to have their expected characteristic chromosomal translocations. SYO-1 and HSSY cell lines were authenticated by confirming the expression of the pathognomonic SYT-SSX fusion gene by RT-PCR. All cell lines were determined to be mycoplasma free via testing in the MSKCC Monoclonal Antibody Core Facility using biochemical assay MycoAlertTM.
Cell viability assays
Cell viability assays were carried out using the Dojindo Molecular Technologies (CCK-8) kit as per manufacturer’s instructions. Briefly, 2,000 to 5,000 cells were plated in 96-well plates, allowed to grow overnight, and then treated with the indicated drugs for 72 hours. Media was replaced with 100μL of media with 10% serum and 10% CCK-8 solution (Dojindo Molecular Technologies Kit). After 1 hour, the optical density was read at 450nm using a Spectra Max 340 PC (Molecular Devices Corporation) to determine viability. Background values from negative control wells without cells were subtracted for final sample quantification. Survival is expressed as a percentage of untreated cells. Half maximal inhibitory concentrations (IC50) were extrapolated from cell viability data using CompuSyn software according to the manufacturer’s instructions.
Western Immunoblotting
Cells and tissues were lysed with radioimmunoprecipitation assay (RIPA) buffer supplemented with protease inhibitor cocktail tablets (Roche Diagnostics) and 1mmol/L Na3VO4. Equal amounts (20–30μg) of protein were electrophoresed onto 4% to 12% gradient gels (Life Technologies) and transferred onto polyvinylidene difluoride (PVDF) or 0.45-micron nitrocellulose membranes. Membranes were blocked with 5% non-fat dried milk and probed with primary antibodies. All named antibodies were obtained from Cell Signaling Technology (See Supplemental Table 1 for a complete list of antibodies with catalog numbers). Ku70 (E-5) antibody was obtained from Santa Cruz Biotechnology (Catalog # sc-17789). Bound antibodies were detected with horseradish peroxidase secondary antibodies (GE Healthcare) and visualized by Enhanced Chemiluminescence Reagent (GE Healthcare).
Xenograft Studies
Approximately eight-week old athymic female mice were injected with 10–15 million cells in MatriGelTM of the cell line of interest and allowed to grow until tumors reached 100 mm3 in volume prior to treatment with either vehicle, rapamycin, or MLN0128. Tumors were measured every 2 to 3 days using calipers and volumes calculated using the formula p/6 x (large diameter) x (small diameter). Animal weights were measured every 2 to 3 days as a surrogate marker for overall toxicity. Animals were sacrificed 4 hours after the final dose of treatment week 1, or 4 hours after the final dose of treatment (week 3 or week 5 as indicated). Tumors were extracted from surrounding tissue and either flash-frozen in liquid nitrogen for western immunoblotting or placed in formalin or paraformaldehyde for immunohistochemical studies. Flash-frozen tissue was ground in RIPA lysis buffer and resin as per the manufacturer’s instructions using Sample Grinding Kit (GE Healthcare) and analyzed via western immunoblotting (WB) as described above. Formalin fixed tissues were sectioned and stained with the indicated antibodies following standard protocols in the Molecular Cytology Core Facility at MSKCC.
Statistical Analysis
All in vitro experiments were carried out at least three times unless otherwise indicated. P-values were calculated using Student’s T-test, with values of ≤0.05 determined to be statistically significant. Standard error was calculated as the standard deviation divided by the square root of the number of samples tested.
Results
MLN0128 exhibits potent anti-proliferative activity in multiple sarcoma cell lines and blocks mTORC1/2 targets in a concentration dependent fashion in vitro
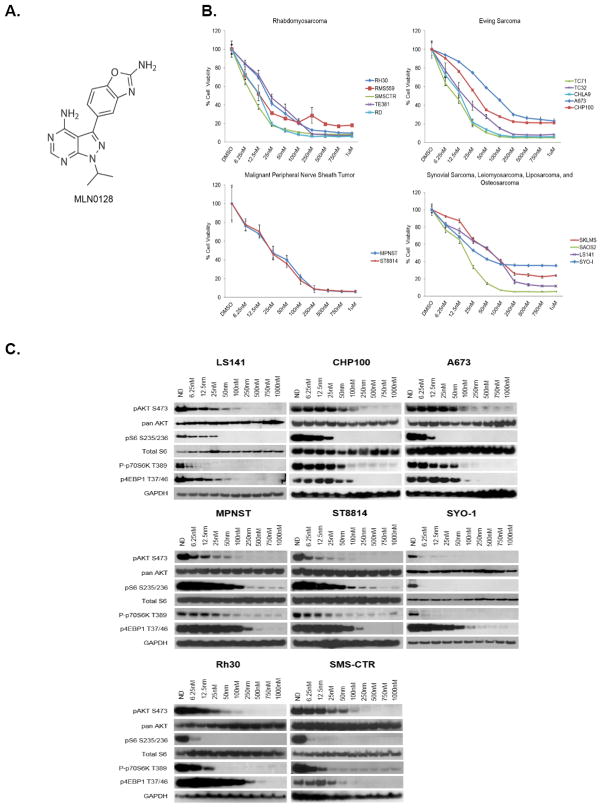
MLN0128 (Figure 1A) was tested against a broad panel of bone and soft tissue sarcoma cell lines following three days of exposure to increasing concentrations of the drug to determine its anti-proliferative activity. MLN0128 was found to inhibit proliferation of all sarcoma subtypes tested in a dose-dependent fashion at low nanomolar concentrations (Figure 1B) with IC50 values ranging from 2 to 130 nM (Table 1).
Figure 1. MLN0128 is a potent, ATP-competitive, mTOR kinase inhibitor.
A, Chemical structure of MLN0128. B, Indicated cell lines were plated in 96-well plates and treated in 6 wells per condition with increasing doses of MLN0128 for 72 hours. Cell viability was measured using Dojindo Cell Counting Kit 8. Dose-response curves were generated as a % of the no drug control. C, Indicated cell lines were grown to 60% confluency in 60mm plates and treated for 6 hours using indicated drugs. 20–30μg of RIPA lysates were then loaded on SDS/PAGE and immunoblotted using indicated antibodies.
Table 1.
Approximate IC50 values for MLN0128 in 20 bone and soft tissue sarcoma subtypes are shown.
| RHABDOMYOSARCOMA | |
|---|---|
| A204 | 2 nM |
| SMS-CTR | 4 nM |
| SK-RMS -3 | 6 nM |
| RD | 8 nM |
| Rh28 | 9 nM |
| RMS-559 | 15 nM |
| Rh30 | 28 nM |
| TE-381 | 30 nM |
| SK-RMS -4 | 70 nM |
| EWING SARCOMA | |
| TC-71 | 6 nM |
| CHLA9 | 7 nM |
| CHP100 | 64 nM |
| TC-32 | 17 nM |
| A673 | 130 nM |
| MALIGNANT PERIPHERAL NERVE SHEATH TUMOR | |
|---|---|
| MPNST | 25 nM |
| ST8814 | 25 nM |
| LEIOMYOSARCOMA | |
| SKLMS | 29 nM |
| OSTEOSARCOMA | |
| SaOS-2 | 14 nM |
| LIPOSARCOMA | |
| LS141 | 53 nM |
| SYNOVIAL SARCOMA | |
| SYO-1 | 82 nM |
At concentrations ranging from 6.25nM to 1μM, the target inhibition profile of MLN0128 was examined using western immunoblotting (Figure 1C). Inhibition of mTORC1 activity was determined using the well-characterized mTORC1 substrates P70-S6K1 and 4EBP1 (36). Phosphorylation of S6 ribosomal protein was used as an additional marker of mTORC1 activity given its direct phosphorylation by S6 kinase 1 (S6K1). Inhibition of mTORC2 activity was assessed via phosphorylation of AKT at Ser473 (pAKTS473). In all sarcoma subtypes tested, MLN0128 potently inhibited each of these anticipated targets in a dose-dependent fashion (Figure 1C). Phosphorylation of S6 and P70-S6K1 was inhibited significantly with nearly complete blockade at low nanomolar concentrations (Figure 1C). Inhibition of phosphorylation of 4EBP1 and pAKTS473 was also observed at low nanomolar concentrations (approximately at 100 and 50nM respectively, Figure 1C). AKT phosphorylation at Thr308 was, however, limited due to weak signal and detection of non-specific proteins in many of the sarcoma cell lines studied, but when detectable, MLN0128 inhibited this PDK1 phosphorylation site (Supplemental Figure 1A).
MLN0128 demonstrates superior down-regulation of mTORC1/2 substrates when compared with rapamycin
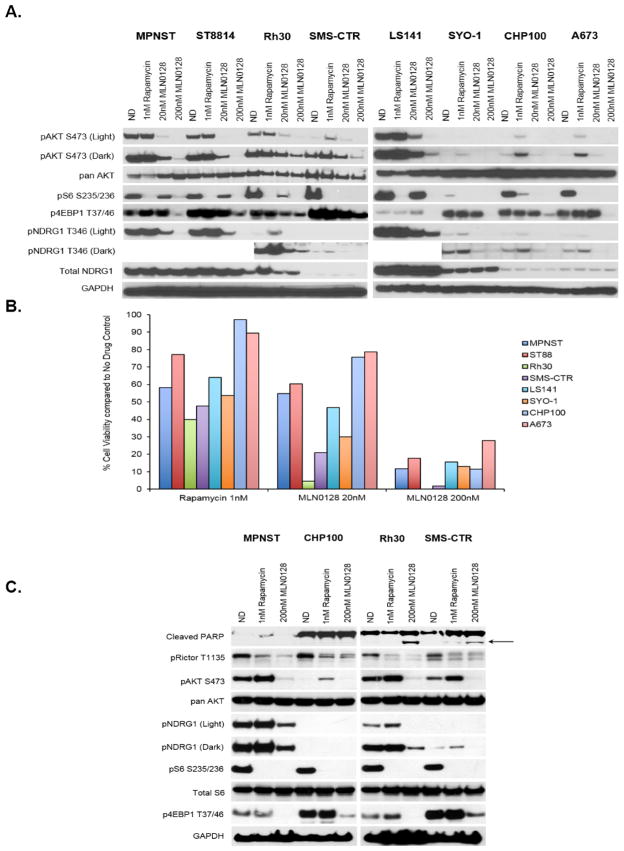
Rapamycin allosterically inhibits mTORC1 kinase activity via binding of the FKBP12-rapamycin (FR) complex to the FR binding domain of mTORC1 (37), while MLN0128 provides direct, ATP-competitive kinase inhibition of both mTORC1 and mTORC2 (29). Although there is evidence for molecular interplay between mTORC2 and rapamycin (38, 39), this second complex is largely unresponsive to rapamycin and related rapalogues. Western immunoblotting was therefore used to further investigate the variances in molecular signaling between rapamycin and MLN0128. Phosphorylation of serum/glucocorticoid-regulated kinase 1 (SGK1), one of three isoforms activated by insulin and other growth factors, is regulated by mTORC2, but not mTORC1, as evidenced by absence of activity in mTORC2 knockout fibroblasts (40). Since SGK1 was not readily detectable by western immunoblotting (data not shown), its direct substrate, N-myc downstream regulated gene 1 (NDRG1), whose activity is also ablated in mTORC2 knockout fibroblasts (40) was used in addition to pAKTS473 as a marker of mTORC2 activity.
Similar to rapamycin treatment, MLN0128 treatment resulted in concentration-dependent inhibition of phosphorylation of S6K1 and S6. MLN0128, however, also inhibited phosphorylation of 4EBP1 in all sarcoma subtypes suggesting more comprehensive mTORC1 targeting when compared with rapamycin (Figure 2A). In addition to complete blockade of the mTORC1 targets pS6K1, pS6 and p4EBP1, MLN0128 treatment also inhibited phosphorylation of NDRG1 at Thr346 indicating distinct mTORC2 inhibition. In contrast, rapamycin had no effect on NDRG1 phosphorylation, supporting its primarily mTORC1 effects (Figure 2A). Importantly, simultaneous inhibition of both mTOR complexes by MLN0128 prevented feedback reactivation of pAKT, whereas rapamycin led to an increase in pAKTS473 (Figure 2A) as has been previously reported (27, 41). Taken together, these data indicate that MLN0128 provides more comprehensive inhibition of the mTOR axis, including superior mTORC1 inhibition when compared with that of rapamycin as evidenced by down-regulation of p4EBP1 in addition to pS6K1 and pS6, effective targeting of the mTORC2 complex as illustrated by inhibition of pNDRG1, as well as attenuation of the release of negative feedback observed with rapamycin treatment.
Figure 2. MLN0128 has a molecular profile distinct from that of rapamycin.
A, Eight cell lines representing a broad range of bone and soft tissue sarcoma subtypes were treated with indicated concentrations of rapamycin and MLN0128 for 6 hours. 20–30μg of RIPA lysates were then loaded on SDS/PAGE and immunoblotted using indicated antibodies. B, Cell lines examined in Fig. 2A were treated with MLN0128 for 72 hours to compare the anti-proliferative effect of rapamycin and MLN0128. Cell viability was measured using Dojindo Cell Counting Kit 8 and shown as a % of the no drug control. Note: Error bars representing standard error mean have been added but are too small to be seen. C, Representative cell lines from Fig. 2A and 2B were treated with indicated concentrations of rapamycin or MLN0128 for 6 hours. 20–30μg of RIPA lysates were then loaded on SDS/PAGE and immunoblotted using indicated antibodies.
In order to determine if the broader molecular profile of MLN0128 also translated into superior anti-proliferative effect in vitro when compared with rapamycin, concentrations based upon complete blockade of downstream targets were chosen for testing. Figure 2B illustrates that MLN0128 treatment resulted in superior anti-proliferative effect compared with that of rapamycin (p<0.04 and p<0.000006 when comparing rapamycin at 20nM and 200nM MLN0128 respectively) across the broad spectrum of cell lines tested.
MLN0128, but not rapamycin, induces apoptosis in models of alveolar and embryonal rhabdomyosarcoma
MLN0128 was next compared with rapamycin in its ability to induce cleavage of poly-ADP ribose polymerase (PARP). In models of alveolar (ARMS, Rh30) and embryonal (ERMS, SMS-CTR) rhabdomyosarcoma, MLN0128 treatment at 200nM induced cleavage of poly-ADP ribose polymerase (PARP) at time points as early as 6 hours (Figure 2C). Induction of PARP cleavage was also seen in two other rhabdomyosarcoma cell lines RMS-559 as well as SK-RMS -3 only when treated with MLN0128 but not with rapamycin (Supplemental Figure 1B). MLN0128, however, was unable to induce cleavage of PARP in an MPNST and Ewing sarcoma model (MPNST, CHP100, Figure 2C) as well as in liposarcoma, leiomyosarcoma, or synovial sarcoma (data not shown). Treatment with rapamycin, using concentrations established in Figure 2A, was unable to induce cleavage of PARP in any of the cell lines tested (Figure 2C, Supplemental Figure 1B, and data not shown).
Both MLN0128 and rapamycin were able to inhibit phosphorylation of Rictor, a component of mTORC2, at Thr1135 (Figure 2C) as has been shown in previously published studies (39, 42).
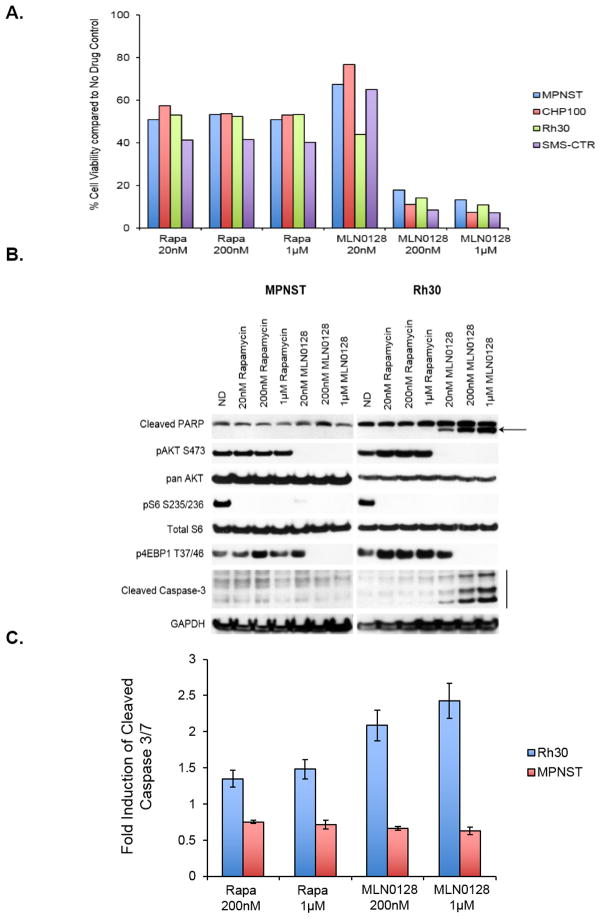
To confirm superior in vitro effects of MLN0128 treatment when compared with those of rapamycin, and that increased concentrations of rapamycin would not improve the anti-proliferative potency or induce apoptotic effects in the cell lines tested, we next tested equivalent concentrations of both rapamycin and MLN0128 up to 1μM. Figure 3A indicates that rapamycin exerts its maximal anti-proliferative effects at low concentrations, and continued dose escalation does not potentiate these effects. As observed previously, MLN0128 exhibited superior anti-tumor effect when compared with rapamycin in all cell lines tested.
Figure 3. Increasing concentrations of MLN0128 but not rapamycin inhibit cell proliferation and phosphorylation of downstream targets, and induce apoptosis in rhabdomyosarcoma cells.
A, Representative cell lines were exposed to indicated concentrations of rapamycin and MLN0128 for 72 hours. Cell viability was measured using Dojindo Cell Counting Kit 8 and shown as a % of the no drug control. Note: Error bars representing standard error mean have been added but are too small to be seen.
B, Equimolar concentrations established in Figure 3A were used to treat indicated cell lines for 6 hours. 20–30μg of RIPA lysates were loaded on SDS/PAGE and immunoblotted using indicated antibodies. C, Indicated cell lines were treated with rapamycin or MLN0128 for 6 hours. Induction of Caspase 3/7 activity was determined using CaspaseGlo (Promega) assay according to manufacturer’s instructions.
To further compare the molecular signaling profiles of rapamycin and MLN0128 at higher and equivalent doses, two cell lines, MPNST and Rh30, were chosen for additional analysis. Despite using concentrations as high as 1μM, rapamycin treatment was unable to effectively inhibit pAKTS473 or p4EBP1T37/46. Similar to earlier results (Figure 2C), neither rapamycin nor MLN0128 treatment resulted in induction of cleaved PARP in MPNST, however, treatment of Rh30 cells with MLN0128 but not with rapamycin resulted in dose dependent induction of cleaved PARP as well as cleaved caspase-3 (Figure 3B) strongly indicating induction of apoptosis.
To further confirm apoptosis in rhabdomyosarcoma models, an in vitro assay (Caspase Glo) was carried out to determine induction of cleaved caspase 3/7 after 6 hours of treatment with rapamycin or MLN0128 (Figure 3C). As seen earlier (Figure 3B), induction of cleaved caspase 3/7 activity was observed only in the RMS model, but not in the MPNST model (Rh30=p<0.0008 and p<0.0006 and MPNST=p<0.1 and p<0.09 when comparing 200 nM and 1μM respectively). Overall, it is particularly notable that markers of apoptosis (cleavage of PARP by WB, induction of cleaved caspase 3/7 by WB, induction of cleaved caspase by in vitro assay) consistently occurred in rhabdomyosarcoma cell lines (Rh30 in Figure 2C and 3B, SMS-CTR in Figure 2C, RMS-559 and SK-RMS -3 in Supplementary Figure 1B), but not in other sarcoma subtypes. These apoptotic markers occurred at relatively low and clinically achievable dosing levels (20 and 200 nM), and were not noted when equivalent and even higher doses of up to 1μM of rapamycin were used to treat the same cell lines. While the exact mechanism warrants further investigation, these data are highly suggestive of MLN0128’s ability to induce apoptosis in rhabdomyosarcoma models.
MLN0128 inhibits tumor growth in vivo and is superior to rapamycin in xenograft models of Ewing Sarcoma (CHP100) and Rhabdomyosarcoma (Rh30)
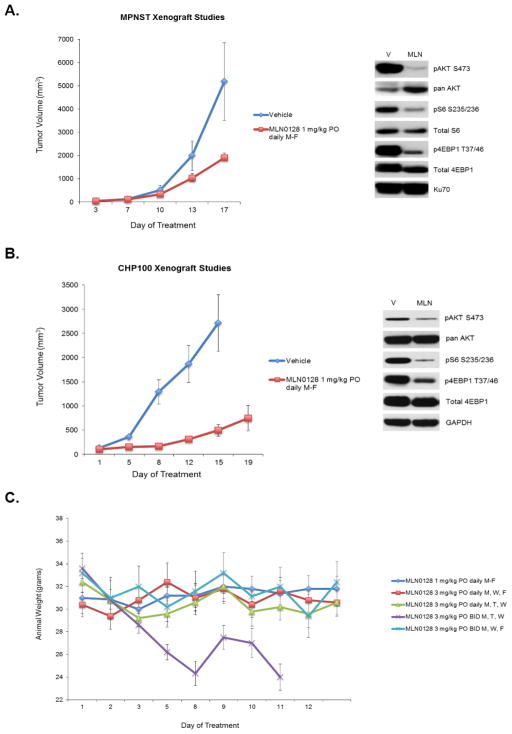
The efficacy of MLN0128 was next tested in multiple tumor xenograft models. A 1mg/kg daily dosing schedule had been previously analyzed and found to be tolerable in other tumor types (32, 33). Treatment of MPNST and CHP100 xenografts with MLN0128 resulted in suppression of tumor volume (Figure 4A–B) compared to vehicle control. Pharmacodynamic analysis of tumors harvested 4 hours following last dose showed inhibition of expected molecular targets pS6, p4EBP1, and pAKT473 (Figure 4A–B).
Figure 4. MLN0128 inhibits tumor growth in sarcoma xenograft models in vivo.
A and B, MPNST (MPNST) and Ewing sarcoma (CHP100) xenograft models were treated with either vehicle control or MLN0128 1 mg/kg by oral gavage daily Monday through Friday and tumor volumes were measured serially. A subset of tumors was harvested 4 hours following the last dose for western blot analysis. 30–50μg of RIPA lysates obtained using sample grinding kit (GE healthcare) from xenograft were loaded on SDS/PAGE and immunoblotted using indicated antibodies. V=Vehicle; MLN=MLN0128 1 mg/kg PO daily M-F. C, 5 different dosing schedules of MLN0128 were tested in athymic mice without tumors to determine tolerability of higher dosing schedules than those tested in Figure 4A and B.
While MLN0128 activity in these models was confirmed at a dosing schedule of 1 mg/kg orally daily, only tumor growth suppression but not tumor stability or regression was noted. Additionally, molecular targets were effectively inhibited but not comprehensively ablated (Figure 4A–B) at the tested dose. Given these observations, and the testing of higher dosing schedules including 3 mg/kg three times weekly in other tumor models (32), we next completed a toxicity study to determine which dosing schedules that provided higher overall dose intensity were likely to be tolerated (Figure 4C). This study indicated that a high-dose, intermittent schedule of 3 mg/kg twice daily on Mondays, Wednesdays, and Fridays was tolerable, although 3 mg/kg twice daily on three consecutive days, i.e., Mondays, Tuesdays, and Wednesdays was not tolerated and resulted in high toxicity.
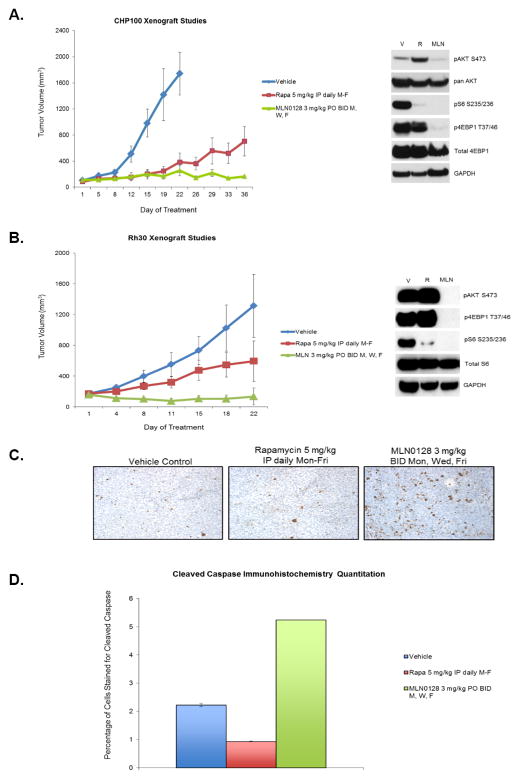
Given the tolerability of a 3 mg/kg twice daily Mondays, Wednesdays, and Fridays dosing schedule, and our hypothesis that this higher intermittent dosing might provide more comprehensive inhibition of downstream targets and therefore tumor growth, this dosing schedule was next chosen for comparison against rapamycin. Treatment of CHP100 (ES) tumor xenografts with MLN0128 resulted in significantly enhanced tumor suppression when compared with rapamycin (Figure 5A) at the end of 5 weeks of treatment (p<0.01). As anticipated, western immunoblotting analysis (Figure 5A) showed inhibition of downstream targets such as pAKT and pS6. Similar to in vitro studies, only MLN0128 but not rapamycin treatment resulted in inhibition of p4EBP1 in vivo (Figure 5A)
Figure 5. MLN0128 is superior to rapamycin in xenograft models of Ewing sarcoma and rhabdomyosarcoma.
A and B, Ewing sarcoma (CHP100) and rhabdomyosarcoma (Rh30) xenograft models were treated with vehicle control, rapamycin 5 mg/kg IP daily Monday-Friday, and MLN0128 3 mg/kg oral gavage twice daily on Monday, Wednesday, and Friday, and tumor volumes were measured serially. Tumors harvested 4 hours following the last dose were analyzed by western blot. 30–50μg of RIPA lysates obtained using sample grinding kit (GE healthcare) from xenograft were loaded on SDS/PAGE and immunoblotted using indicated antibodies. V=Vehicle; R= Rapamycin 5mg/kg IP daily M-F, MLN=MLN0128 3 mg/kg PO daily Mondays, Wednesdays, Fridays. C and D Immunhistochemistry for cleaved caspase was performed at the end of drug treatment on paraformaldehyde fixed tumors harvested 4 hours after the last dose. Cleaved caspase signal (brown staining) was quantitated using MetaMorph image analysis software (Molecular Devices). Quantitation of cleaved caspase signal obtained from 3–5 animals per treatment condition is shown. Error bars represent standard error.
Given the apoptotic potential of MLN0128 in RMS model in vitro (Figure 3B–C), we next investigated MLN0128’s in vivo anti-tumor effects in Rh30 xenografts. MLN0128 resulted in significant tumor suppression when compared to rapamycin (Figure 5B, p<0.001) as well as resulted in significant inhibition of downstream targets such as pS6 and p4EBP1 (Figure 5B). In concordance with the apoptosis observed in vitro in the Rh30 cell line, immunohistochemical analysis of Rh30 xenograft tissue (Figure 5C and 5D) revealed increased cleaved caspase staining (p<0.0001).
Discussion
The present study reports the in vitro and in vivo pre-clinical characterization of MLN0128, a potent and orally bioavailable pan-mTOR kinase inhibitor, in a broad range of sarcoma subtypes. MLN0128 exerts potent anti-proliferative effect and has a molecular signaling profile distinct from that of rapamycin in vitro and in vivo. MLN0128 induces apoptosis in models of rhabdomyosarcoma, and provides superior anti-tumor effect when compared with rapamycin in in vivo rhabdomyosarcoma and Ewing sarcoma models.
Considerable pre-clinical and clinical data using first-generation allosteric mTORC1 inhibitors have validated mTOR as an anticancer target, and there is a robust threefold rationale for its specific use in the treatment of multiple sarcoma subtypes. First, hyper-phosphorylation of multiple components of the mTOR pathway suggests increased activity in numerous models likely occurring in an autocrine/paracrine fashion (5, 7–11). Second, aberrant upstream signals from PDGFR (15), IGF1R (16, 17), VEGFR (18), FGFR (19, 43) pathways and others have been shown to contribute to dysregulation of the PI3K/AKT/mTOR axis in various sarcoma subtypes. Lastly, more recently discovered discrete genetic mutations (22–24) in or clustered around the PI3K/AKT/mTOR axis, document vulnerabilities in the genetic landscapes of sarcoma that can be exploited via mTOR inhibition. The first-generation rapamycin and its analogues have had limited clinical utility despite robust pre-clinical rationale, thought to be at least partly a result of release of the negative feedback from S6K1 to insulin receptor substrate 1 (IRS1), leading to an unwanted increase in the pro-survival pAKT. Additionally, rapalogues do not inhibit 4EBP1 (44), which allows cap dependent translation to proceed unchecked, and have essentially no effect on mTORC2 or its downstream effectors.
MLN0128 potently inhibits proliferation of all sarcoma cell lines tested in a dose-dependent fashion with IC50 values in the low nanomolar range (2–130 nM). While differences in sensitivity between various cell lines tested are not well explained by the biomarkers used in this study (Figure 1C), we can hypothesize that related pathway components, phosphatase and tensin homolog (PTEN) or v-raf murine sarcoma viral oncogene homolog B (BRAF) status, for example A673 which carries a BRAF V600E mutation, could play a role in sensitivity to anti-proliferative effects of the drug. Future investigation into such differential responses to mTOR inhibition is certainly warranted. In contrast, rapamycin does not exhibit significant dose dependency despite escalation to doses as high as 1μM, and achieves its maximal anti-proliferative effect at very low concentrations. This threshold effect is consistent with the effective down-regulation of P-p70-S6K at 1nM noted in the current study, as well as the early (10–20 minutes) feedback reactivation of AKT-S473 that has been documented following treatment with 1nM rapamycin (27). These anti-proliferative and molecular signaling events justify the unequal concentrations used to compare MLN0128 and rapamycin in the current work, although we have additionally shown that equivalent concentrations up to 1μM of each agent do not alter outcomes. In all conditions tested in vitro, MLN0128 provides profound anti-proliferative effect that is superior to that of rapamycin.
In all sarcoma subtypes tested, MLN0128 successfully inhibited both of the major regulators of protein synthesis downstream of mTORC1, p70-S6K1 (45) and 4EBP1 (46) in a dose-dependent fashion. In contrast, rapamycin inhibited only P-p70-S6K as observed in previously published studies (44). In other tumor types, there is emerging evidence that 4EBP1 is critical to the response to both rapalogues and mTOR kinase inhibition (47), making MLN0128’s effective down-regulation of particular importance. The exact role that 4EBP1 plays in mediating the anti-proliferative effects of MLN0128 and other mTOR kinase inhibitors in sarcomas remains under investigation, and will be indispensable to better understand the role of mTOR inhibition in sarcoma.
MLN0128 potently inhibited phosphorylation of AKT at Ser473 in all sarcoma subtypes tested. Analysis of phosphorylation on AKT at Thr308 was limited due to weak signal and detection of non-specific proteins in many of the sarcoma cell lines studied, but when detectable, MLN0128 did inhibit this PDK1 phosphorylation site (Supplemental Figure 1A). This data is consistent with that of other mTOR kinase inhibitors and is likely at least in part due to decreased ability of PDK to phosphorylate AKT at Thr308 when Ser473 is fully inhibited (48, 49). Genetic knockout of SIN1 or RICTOR, however, is noted to completely suppress Ser473, but not Thr308 phosphorylation (50), suggesting that MLN0128 may have additional signaling effects not mediated through mTORC2 that down-regulate phosphorylation of AKT at Thr308.
SGK1 is phosphorylated by mTORC2, but not mTORC1, as SGK1 activity is ablated in fibroblasts possessing mTORC1 activity, but lacking the mTORC2 components RICTOR, Sin1, and mlST8 (40). The physiologic substrate of SGK1, NDRG1 is similarly affected making the phosphorylation of NDRG1 a ready surrogate marker for mTORC2 activity. MLN0128 inhibited the phosphorylation of NDRG1 in a dose-dependent fashion in all cell lines tested, corroborating the anticipated mTORC2 kinase inhibition. As expected, rapamycin had no effect on NDRG1 recapitulating its lack of interaction with mTORC2.
MLN0128 was well-tolerated in vivo and resulted in dose-dependent growth inhibition in a broad range of sarcoma xenograft models. Pharmacodynamic analysis revealed down-regulation of expected targets in vivo, in a fashion consistent with in vitro data. At a dosing schedule of 3 mg/kg BID by oral gavage three times weekly, MLN0128 provided significantly better tumor suppression when compared with rapamycin in tumor xenograft models of rhabdomyosarcoma (p < 0.001) and Ewing sarcoma (p<0.01). Additionally, MLN0128 was able to induce apoptosis seen as increased cleaved caspase both in vitro and in vivo model of rhabdomyosarcoma (Rh30). Further studies are warranted to determine the exact mechanism by which apoptosis is induced in this tumor subtype but not others.
In summary, MLN0128 is a potent and orally bioavailable pan-mTOR kinase inhibitor with effects on both mTORC1 and mTORC2 and their downstream effectors, and has a molecular signaling profile distinct from that of rapamycin. MLN0128 induces dose-dependent inhibition of tumor growth superior to that of rapamycin in a broad range of sarcoma models in vitro. In models of rhabdomyosarcoma, MLN0128 induces apoptosis. Finally, in in vivo models of rhabdomyosarcoma and Ewing sarcoma, MLN0128 provides superior anti-tumor effect when compared with that of rapamycin. MLN0128 warrants further evaluation as a potential therapy for sarcoma.
Supplementary Material
Acknowledgments
Financial Support: NIH (National Institutes of Health) Grant R01CA140331-04 to G.K. Schwartz
The authors would like to thank Dr. Rachael Brake from Millennium/Takeda Pharmaceuticals for her valuable input and providing MLN0128, and Dr. Lee Spraggon for his suggestions and critical reading of the manuscript.
Footnotes
The authors have no conflicts to disclose in relation to this work.
References
- 1.Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes & development. 2002;16:1472–87. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohanna M, Sobering AK, Lapointe T, Lorenzo L, Praud C, Petroulakis E, et al. Atrophy of S6K1(−/−) skeletal muscle cells reveals distinct mTOR effectors for cell cycle and size control. Nature cell biology. 2005;7:286–94. doi: 10.1038/ncb1231. [DOI] [PubMed] [Google Scholar]
- 3.Dancey J. mTOR signaling and drug development in cancer. Nature reviews Clinical oncology. 2010;7:209–19. doi: 10.1038/nrclinonc.2010.21. [DOI] [PubMed] [Google Scholar]
- 4.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nature reviews Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 5.Dobashi Y, Suzuki S, Sato E, Hamada Y, Yanagawa T, Ooi A. EGFR-dependent and independent activation of Akt/mTOR cascade in bone and soft tissue tumors. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2009;22:1328–40. doi: 10.1038/modpathol.2009.104. [DOI] [PubMed] [Google Scholar]
- 6.Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006;25:6416–22. doi: 10.1038/sj.onc.1209888. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi Y, Kohashi K, Yamada Y, Endo M, Setsu N, Ishii T, et al. Activation of the Akt/mammalian target of rapamycin pathway in myxofibrosarcomas. Human pathology. 2014;45:984–93. doi: 10.1016/j.humpath.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Setsu N, Yamamoto H, Kohashi K, Endo M, Matsuda S, Yokoyama R, et al. The Akt/mammalian target of rapamycin pathway is activated and associated with adverse prognosis in soft tissue leiomyosarcomas. Cancer. 2012;118:1637–48. doi: 10.1002/cncr.26448. [DOI] [PubMed] [Google Scholar]
- 9.Hernando E, Charytonowicz E, Dudas ME, Menendez S, Matushansky I, Mills J, et al. The AKT-mTOR pathway plays a critical role in the development of leiomyosarcomas. Nature medicine. 2007;13:748–53. doi: 10.1038/nm1560. [DOI] [PubMed] [Google Scholar]
- 10.Italiano A, Chen CL, Thomas R, Breen M, Bonnet F, Sevenet N, et al. Alterations of the p53 and PIK3CA/AKT/mTOR pathways in angiosarcomas: a pattern distinct from other sarcomas with complex genomics. Cancer. 2012;118:5878–87. doi: 10.1002/cncr.27614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petricoin EF, 3rd, Espina V, Araujo RP, Midura B, Yeung C, Wan X, et al. Phosphoprotein pathway mapping: Akt/mammalian target of rapamycin activation is negatively associated with childhood rhabdomyosarcoma survival. Cancer research. 2007;67:3431–40. doi: 10.1158/0008-5472.CAN-06-1344. [DOI] [PubMed] [Google Scholar]
- 12.Setsu N, Kohashi K, Fushimi F, Endo M, Yamamoto H, Takahashi Y, et al. Prognostic impact of the activation status of the Akt/mTOR pathway in synovial sarcoma. Cancer. 2013;119:3504–13. doi: 10.1002/cncr.28255. [DOI] [PubMed] [Google Scholar]
- 13.Endo M, Yamamoto H, Setsu N, Kohashi K, Takahashi Y, Ishii T, et al. Prognostic significance of AKT/mTOR and MAPK pathways and antitumor effect of mTOR inhibitor in NF1-related and sporadic malignant peripheral nerve sheath tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:450–61. doi: 10.1158/1078-0432.CCR-12-1067. [DOI] [PubMed] [Google Scholar]
- 14.Mueller S, Phillips J, Onar-Thomas A, Romero E, Zheng S, Wiencke JK, et al. PTEN promoter methylation and activation of the PI3K/Akt/mTOR pathway in pediatric gliomas and influence on clinical outcome. Neuro-oncology. 2012;14:1146–52. doi: 10.1093/neuonc/nos140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho AL, Vasudeva SD, Lae M, Saito T, Barbashina V, Antonescu CR, et al. PDGF receptor alpha is an alternative mediator of rapamycin-induced Akt activation: implications for combination targeted therapy of synovial sarcoma. Cancer research. 2012;72:4515–25. doi: 10.1158/0008-5472.CAN-12-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie Y, Skytting B, Nilsson G, Brodin B, Larsson O. Expression of insulin-like growth factor-1 receptor in synovial sarcoma: association with an aggressive phenotype. Cancer research. 1999;59:3588–91. [PubMed] [Google Scholar]
- 17.Dilling MB, Dias P, Shapiro DN, Germain GS, Johnson RK, Houghton PJ. Rapamycin selectively inhibits the growth of childhood rhabdomyosarcoma cells through inhibition of signaling via the type I insulin-like growth factor receptor. Cancer research. 1994;54:903–7. [PubMed] [Google Scholar]
- 18.Wan X, Shen N, Mendoza A, Khanna C, Helman LJ. CCI-779 inhibits rhabdomyosarcoma xenograft growth by an antiangiogenic mechanism linked to the targeting of mTOR/Hif-1alpha/VEGF signaling. Neoplasia. 2006;8:394–401. doi: 10.1593/neo.05820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor JGt, Cheuk AT, Tsang PS, Chung JY, Song YK, Desai K, et al. Identification of FGFR4-activating mutations in human rhabdomyosarcomas that promote metastasis in xenotransplanted models. The Journal of clinical investigation. 2009;119:3395–407. doi: 10.1172/JCI39703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guenther MK, Graab U, Fulda S. Synthetic lethal interaction between PI3K/Akt/mTOR and Ras/MEK/ERK pathway inhibition in rhabdomyosarcoma. Cancer letters. 2013;337:200–9. doi: 10.1016/j.canlet.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Renshaw J, Taylor KR, Bishop R, Valenti M, De Haven Brandon A, Gowan S, et al. Dual blockade of the PI3K/AKT/mTOR (AZD8055) and RAS/MEK/ERK (AZD6244) pathways synergistically inhibits rhabdomyosarcoma cell growth in vitro and in vivo. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:5940–51. doi: 10.1158/1078-0432.CCR-13-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shukla N, Ameur N, Yilmaz I, Nafa K, Lau CY, Marchetti A, et al. Oncogene mutation profiling of pediatric solid tumors reveals significant subsets of embryonal rhabdomyosarcoma and neuroblastoma with mutated genes in growth signaling pathways. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:748–57. doi: 10.1158/1078-0432.CCR-11-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohsaka S, Shukla N, Ameur N, Ito T, Ng CK, Wang L, et al. A recurrent neomorphic mutation in MYOD1 defines a clinically aggressive subset of embryonal rhabdomyosarcoma associated with PI3K-AKT pathway mutations. Nature genetics. 2014;46:595–600. doi: 10.1038/ng.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shern JF, Chen L, Chmielecki J, Wei JS, Patidar R, Rosenberg M, et al. Comprehensive genomic analysis of rhabdomyosarcoma reveals a landscape of alterations affecting a common genetic axis in fusion-positive and fusion-negative tumors. Cancer discovery. 2014;4:216–31. doi: 10.1158/2159-8290.CD-13-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartford CM, Ratain MJ. Rapamycin: something old, something new, sometimes borrowed and now renewed. Clinical pharmacology and therapeutics. 2007;82:381–8. doi: 10.1038/sj.clpt.6100317. [DOI] [PubMed] [Google Scholar]
- 26.Gibbons JJ, Abraham RT, Yu K. Mammalian target of rapamycin: discovery of rapamycin reveals a signaling pathway important for normal and cancer cell growth. Seminars in oncology. 2009;36 (Suppl 3):S3–S17. doi: 10.1053/j.seminoncol.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 27.O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer research. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nature reviews Drug discovery. 2011;10:868–80. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012;485:55–61. doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janes MR, Vu C, Mallya S, Shieh MP, Limon JJ, Li LS, et al. Efficacy of the investigational mTOR kinase inhibitor MLN0128/INK128 in models of B-cell acute lymphoblastic leukemia. Leukemia. 2013;27:586–94. doi: 10.1038/leu.2012.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Garcia C, Ibrahim YH, Serra V, Calvo MT, Guzman M, Grueso J, et al. Dual mTORC1/2 and HER2 blockade results in antitumor activity in preclinical models of breast cancer resistant to anti-HER2 therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:2603–12. doi: 10.1158/1078-0432.CCR-11-2750. [DOI] [PubMed] [Google Scholar]
- 32.Gokmen-Polar Y, Liu Y, Toroni RA, Sanders KL, Mehta R, Badve S, et al. Investigational drug MLN0128, a novel TORC1/2 inhibitor, demonstrates potent oral antitumor activity in human breast cancer xenograft models. Breast cancer research and treatment. 2012;136:673–82. doi: 10.1007/s10549-012-2298-8. [DOI] [PubMed] [Google Scholar]
- 33.Ingels A, Zhao H, Thong AE, Saar M, Valta MP, Nolley R, et al. Preclinical trial of a new dual mTOR inhibitor, MLN0128, using renal cell carcinoma tumorgrafts. International journal of cancer Journal international du cancer. 2014;134:2322–9. doi: 10.1002/ijc.28579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patwardhan PP, Surriga O, Beckman MJ, de Stanchina E, Dematteo RP, Tap WD, et al. Sustained inhibition of receptor tyrosine kinases and macrophage depletion by PLX3397 and rapamycin as a potential new approach for the treatment of MPNSTs. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:3146–58. doi: 10.1158/1078-0432.CCR-13-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singer S, Socci ND, Ambrosini G, Sambol E, Decarolis P, Wu Y, et al. Gene expression profiling of liposarcoma identifies distinct biological types/subtypes and potential therapeutic targets in well-differentiated and dedifferentiated liposarcoma. Cancer research. 2007;67:6626–36. doi: 10.1158/0008-5472.CAN-07-0584. [DOI] [PubMed] [Google Scholar]
- 36.Nojima H, Tokunaga C, Eguchi S, Oshiro N, Hidayat S, Yoshino K, et al. The mammalian target of rapamycin (mTOR) partner, raptor, binds the mTOR substrates p70 S6 kinase and 4E-BP1 through their TOR signaling (TOS) motif. The Journal of biological chemistry. 2003;278:15461–4. doi: 10.1074/jbc.C200665200. [DOI] [PubMed] [Google Scholar]
- 37.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–9. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 38.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Molecular cell. 2006;22:159–68. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 39.Julien LA, Carriere A, Moreau J, Roux PP. mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling. Molecular and cellular biology. 2010;30:908–21. doi: 10.1128/MCB.00601-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia-Martinez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) The Biochemical journal. 2008;416:375–85. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- 41.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer research. 2005;65:7052–8. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 42.Boulbes D, Chen CH, Shaikenov T, Agarwal NK, Peterson TR, Addona TA, et al. Rictor phosphorylation on the Thr-1135 site does not require mammalian target of rapamycin complex 2. Molecular cancer research : MCR. 2010;8:896–906. doi: 10.1158/1541-7786.MCR-09-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ganti R, Skapek SX, Zhang J, Fuller CE, Wu J, Billups CA, et al. Expression and genomic status of EGFR and ErbB-2 in alveolar and embryonal rhabdomyosarcoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2006;19:1213–20. doi: 10.1038/modpathol.3800636. [DOI] [PubMed] [Google Scholar]
- 44.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:17414–9. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown EJ, Beal PA, Keith CT, Chen J, Shin TB, Schreiber SL. Control of p70 s6 kinase by kinase activity of FRAP in vivo. Nature. 1995;377:441–6. doi: 10.1038/377441a0. [DOI] [PubMed] [Google Scholar]
- 46.Gingras AC, Kennedy SG, O’Leary MA, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes & development. 1998;12:502–13. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cope CL, Gilley R, Balmanno K, Sale MJ, Howarth KD, Hampson M, et al. Adaptation to mTOR kinase inhibitors by amplification of eIF4E to maintain cap-dependent translation. Journal of cell science. 2014;127:788–800. doi: 10.1242/jcs.137588. [DOI] [PubMed] [Google Scholar]
- 48.Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, Critchlow SE, et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer research. 2010;70:288–98. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- 49.Bhagwat SV, Gokhale PC, Crew AP, Cooke A, Yao Y, Mantis C, et al. Preclinical characterization of OSI-027, a potent and selective inhibitor of mTORC1 and mTORC2: distinct from rapamycin. Molecular cancer therapeutics. 2011;10:1394–406. doi: 10.1158/1535-7163.MCT-10-1099. [DOI] [PubMed] [Google Scholar]
- 50.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–37. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.