Abstract
OBJECTIVE
The purpose of this study was to establish the prognostic utility in human papillomavirus (HPV)–positive stage III and IV oropharyngeal squamous cell carcinoma (SCC) of the 18F-FDG parameters maximal, mean, and peak standardized uptake value (SUVmax, SUVmean, and SUVpeak, respectively); metabolic tumor volume (MTV); and total lesion glycolysis (TLG).
MATERIALS AND METHODS
We included 70 patients in the present study who had a biopsy-proven HPV-positive (by in situ hybridization) stage III and IV oropharyngeal SCC and had a baseline PET/CT examination at our institution. Outcome endpoint was event-free survival (EFS), which included recurrence-free and overall survival. Cox proportional hazards multivariate regression analyses were performed. Survival analysis was performed using Kaplan-Meier survival curves.
RESULTS
In Cox regression proportional hazard univariate analysis, total MTV (hazard ratio [HR], 1.02; p = 0.008), primary-tumor MTV (HR, 1.02; p = 0.024), neck nodal MTV (HR, 1.03; p = 0.006), neck nodal TLG (HR, 1.01; p = 0.006), and neck node status (HR, 4.45; p = 0.03) showed a statistically significant association with EFS. There was no statistically significant association of EFS with SUVmax, SUVmean, SUVpeak, and primary-tumor or overall TLG. In Cox regression proportional hazard multivariate model I, total MTV remained an independent prognostic marker for EFS when adjusted for every other variable individually in the model; in model II, primary-tumor MTV, neck node status, and SUVpeak are independent prognostic markers for EFS. The Kaplan-Meier survival curves using optimum cut point of 41 mL of total MTV were not significant (p = 0.09).
CONCLUSION
Total MTV and primary-tumor MTV are associated with survival outcomes in patients with HPV-positive stage III and IV oropharyngeal SCC.
Keywords: human papillomavirus (HPV), metabolic tumor volume, oropharyngeal SCC, outcome, PET/CT
The prevalence of human papillomavirus (HPV)–positive oropharyngeal squamous cell carcinoma (SCC) has increased over the past decade [1, 2], and this subgroup of oropharyngeal cancers are reported to have improved survival compared with HPV-negative oropharyngeal SCC [3–5]. Intensified strategies combining chemotherapy and radiotherapy have been increasingly employed as organ-preserving protocols, which have been associated with decreased rates of disease progression and increased survival when compared with radiotherapy alone [6–8]. The recent trend is to deintensify treatment combinations to reduce toxicity while maintaining efficacy in this group of patients.
There are multiple clinical factors that determine candidacy of a patient for relevant treatment options to deintensify, such as careful evaluation of tumor size and stage, lymph node involvement, and anatomic sub-site. Identification of imaging-related prognostic factors that potentially predict long-term survival may allow the development of individualized strategies. Several 18F-FDG PET/CT parameters—such as maximum, mean, and peak standardized uptake value (SUVmax, SUVmean, and SUVpeak, respectively); metabolic tumor volume (MTV); and total lesion glycolysis (TLG)—have been established as useful prognostic markers in various human solid tumors and in head and neck tumors [9–11]. However, value of FDG PET/CT–based parameters for prognosis of HPV-positive advanced oropharyngeal SCC is not well established. Hence, the primary objective of this study is to evaluate the prognostic significance of SUVmax, SUVmean, SUVpeak, MTV, and TLG in HPV-positive stage III and IV oropharyngeal SCC.
Materials and Methods
Study Population
We conducted a retrospective study of patients with proven HPV-positive (by in situ hybridization) advanced (American Joint Committee on Cancer [AJCC] stages III and IV) oropharyngeal SCC who underwent FDG PET/CT between 2004 and 2011 at our institution [12]. Our institutional review board approved this HIPAA-compliant study, and informed consent was waived. All patients with a biopsy-proven HPV-positive (i.e., in situ hybridization positive) stage III or IV oropharyngeal SCC who underwent a baseline PET/CT examination at our institution were included in the study. All patients who had undergone local or systemic therapy or surgical intervention before the baseline PET/CT examination were excluded from this study. All surviving patients had at least a 6-month follow-up. Seventy patients (60 men and 10 women; age range, 29–78 years) were eligible and included in the study.
PET/CT Protocol
All PET/CT studies were performed using a Discovery LS (2D) or a Discovery VCT (3D) scanner (GE Healthcare), according to standard institutional clinical protocol. Twenty-one studies were performed using the Discovery LS, and the other 49 studies were performed using the Discovery VCT. All patients were scanned using a dedicated head and neck protocol. Patients were scanned skull vertex to mid thighs in two separate acquisitions, starting from mid thigh to chin and then from carina to skull vertex. Head and neck images were acquired with the arms down, and body images were obtained with arms up. After at least a 4-hour fast and serum glucose measurement, patients were administered FDG IV. The mean dose of injected FDG was 18.1 mCi. Injection to scan time for head and neck acquisition was 78.1 ± 18.4 minutes. Mean ± SD serum glucose was 98.9 ± 13.4 mg/dL.
The ordered-subsets expectation maximization algorithm was used to reconstruct all PET images. The 2D implementation on the Discovery LS used two iterations, 28 subsets, a 5.5-mm postreconstruction gaussian filter, and 3.9-mm pixels. The 3D implementation on the Discovery VCT (RX) used two iterations, 21 subsets, a 3.0-mm postreconstruction gaussian filter, and 4.7-mm pixels. All PET data were reconstructed with and without CT-based attenuation correction.
Helical CT images for attenuation correction and anatomic correlation (CTAC) were also obtained in two acquisitions—head and neck; and whole body—covering the same regions as PET. Both CTAC acquisitions were obtained with a matrix of 512 × 512. X-ray source voltage was fixed at 120 kVp. Current intensity was modulated via automatic exposure control on the GE scanner with a minimum of 20 mA and a maximum of 200 mA. Beam collimation was 10 mm with a pitch of 0.984, with a rotation speed of 0.5 seconds per revolution. Reconstruction slice thickness was 3.75 mm. Noise index was fixed at 20 for both head and neck and whole-body acquisitions.
PET/CT Image Analysis
All PET/CT studies were electronically retrieved and reviewed on a MIMvista workstation (version 5.2, MIM Software) by a board-certified nuclear medicine physician who was a National Institutes of Health T32 postresidency fellow. PET, CT, and fused PET/CT images were displayed in axial, coronal, and sagittal planes. For this study, the relevant imaging biomarker measurements included SUVmax (i.e., the maximum value within the tumor normalized to lean body mass), SUVmean (i.e., the average value within the tumor segmented from the background FDG uptake, normalized to lean body mass), SUVpeak (i.e., the average value in a group of voxels within 10-mm diameter surrounding the voxel with the highest activity), MTV, and TLG segmented from the PET images. MTV was defined as the volume of tumor showing FDG uptake. TLG was defined as the product of MTV by SUVmean.
All parameters were measured from the tumor volume segmented by a gradient-based method. MIMvista software analysis suite (MIM Software) includes a contouring PET/CT suite. The gradient segmentation method of volume measurement available in MIMvista software relies on an operator-defined starting point near the center of the lesion. As the operator drags the cursor from the center of the lesion, six axes extend outward, providing visual feedback for the starting point of gradient segmentation. Spatial gradients are calculated along each axis interactively, and the length of an axis is restricted when a large gradient is detected along that axis. The six axes define an ellipsoid that is then used as an initial bounding region for gradient detection [13]. Once the primary target was segmented, SUVmax, SUVmean, SUVpeak, MTV and TLG were calculated by MIMvista software. For nodal metastases, we considered lymph nodes larger than 1 cm in long axis with a minimal SUVmax cut point of 1.5 (greater than liver SUVmean) as positive lymph nodes and segmented the nodes. The MTV from 16 patients were derived only from primary sites, whereas for the 54 patients with suspected neck nodes, the MTV was derived from both primary and neck nodes.
Outcome Endpoints
The primary endpoints were to establish whether the imaging markers—SUVmax, SUVmean, SUVpeak, MTV, and TLG—added prognostic information for event-free survival (EFS), which included recurrence-free and overall survival. An event can be either recurrence of disease at the primary site, at regional nodes, or at distant metastatic sites or overall mortality of the patient. EFS is defined as the time from baseline PET/CT to the first documented recurrence or death. The data were censored to most recent inpatient or outpatient follow-up through June 30, 2012. Electronic medical records, imaging records, office visits at our institution, and a web-based mortality database (Ancestry.com) were used to establish the recurrence (all confirmed by tissue diagnosis) and death.
Statistical Methods
We present our summary statistics as mean ± SD for continuous variables or frequency and as percentages for categoric variables. The association between clinical variables, imaging parameters, and survival was examined with Cox proportional hazards regression analysis. Crude and adjusted Cox regression relative risks were estimated. Multicollinearity between variables was established using Pearson correlation coefficient (r > 0.70). Kaplan-Meier curves with median cut points for total MTV were generated for survival analysis and compared using the Mantel-Cox log-rank and Gehan-Breslow-Wilcoxon tests. We used the Prism MAC (version 5.0, GraphPad Software) and SPSS (version 20, SPSS) statistical packages for all analyses. All hypothesis tests were two sided, with a significance level of 0.05.
Results
Patient Characteristics
Seventy patients met the eligibility criteria. Sixty (85.7%) patients were men and 10 (14.3%) were women; the average age of patients was 58.8 years (range of 29–78 y). The mean and median follow-up was 31 and 25 months, respectively (range, 3–97 months). Twelve (17%) patients had stage III and 58 (82.9%) had stage IV HPV-positive oropharyngeal SCC. Fifty-three (75.7%) patients had concurrent chemoradiation, nine (12.9%) had surgery followed by chemoradiation, four (5.7%) had chemoradiation followed by surgery, three (4.3%) had radiation alone, and one (1.4%) had no treatment of oropharyngeal cancer. A total of nine (12.9%) patients had an event (death or recurrence) during the follow-up period (Table 1).
TABLE 1.
Stage, 18F-FDG Parameters, and Outcome for Patients With an Event
| Patient No. | Stagea | Maximum Standardized Uptake Value | Total Metabolic Tumor Volumeb (mL) | Eventc |
|---|---|---|---|---|
|
| ||||
| 1 | IV | 14.6 | 160.4 | Death |
| 2 | IV | 17.0 | 48.8 | Death |
| 3 | III | 5.7 | 5.4 | Death |
| 4 | IV | 10.0 | 14.0 | Death |
| 5 | IV | 9.2 | 42.4 | Recurrence |
| 6 | IV | 6.8 | 4.2 | Recurrence |
| 7 | IV | 8.0 | 164.5 | Death |
| 8 | IV | 5.4 | 3.2 | Recurrence |
| 9 | IV | 14.4 | 18.1 | Death |
Stages III and IV describe advanced cancers in the classification of the American Joint Committee on Cancer [12].
Derived from primary tumor only in three patients who died (patients 4, 7, and 9) and two patients who had recurrence (patient 6 and 8) and from primary tumor and suspected nodal metastases in other three patients who died (patients 1, 2, and 3) and one patient who had recurrence (patient 5).
Death indicates overall mortality, and recurrences were confirmed by biopsy.
FDG PET Parameters
The median ± SD SUVmax, SUVpeak, MTV, and TLG of the primary tumor were 11.3 ± 4.4, 10.3 ± 3.5, 15.4 ± 23.6 mL, and 94.2 ± 126 g, respectively. The median primary-tumor MTV and TLG, neck nodal MTV and TLG, and total MTV and TLG of lesions in patients with an event were higher than the corresponding values of lesions in patients without an event, without statistical significance (Table 2 and Fig. 1).
TABLE 2.
18F-FDG Parameters for Patients With an Event, Without an Event, and Entire Study Population
| Parameter | Patients With an Event (n = 9) | Patients Without an Event (n = 61) | Entire Study Population (n = 70) |
|---|---|---|---|
|
| |||
| SUVmax | 10.1 (4.2) | 11.5 (4.5) | 11.3 (4.40) |
| SUVmean | 5.3 (2.7) | 5.6 (2.0) | 5.6 (2.12) |
| SUVpeak | 8.9 (4.5) | 10.5 (3.3) | 10.3 (3.50) |
| Total MTV (mL) | 51.2 (65.1) | 26.1 (2 0.1) | 29.4 (30.2) |
| Primary-tumor MTV (mL) | 27.5 (52.1) | 13.6 (15.9) | 15.4 (23.6) |
| Lymph node MTV (mL) | 53.2 (64.9) | 15.4 (12.1) | 18.1 (21.8) |
| Total TLG (g) | 242.4 (291.7) | 149.2 (147.3) | 161.2 (172.4) |
| Primary-tumor TLG (g) | 115.6 (141.2) | 91.1 (12 4. 6) | 94.2 (126.0) |
| Lymph node TLG (g) | 285.4 (357.4) | 70.9 (71.7) | 86.8 (123.3) |
Note—Data are given in the form of mean (SD) throughout. There was no significant difference (p < 0.05) between the group of patients who had an event and those who did not have an event (either recurrence of disease at the primary site, at regional nodes, or at distant metastatic sites or overall patient mortality). SUVmax = maximum standardized uptake value, SUVmean = mean standardized uptake value, SUVpeak = peak standardized uptake value, MTV = metabolic tumor volume, TLG = total lesion glycolysis.
Fig. 1.

Primary-tumor 18F-FDG parameters in patients who had event versus those who had no event.
A–D, Box-and-whisker graphs show comparisons between groups with each FDG parameter. There was no statistically significantdifference in mean values between groups for maximum standardized uptake value (SUVmax) (p = 0.75) (A), peak standardized uptake value (SUVpeak) (p = 0.3) (B), metabolic tumor volume (MTV) (p = 0.57) (C), or total lesion glycolysis (TLG) (p = 0.18) (D).
Cox Proportional Hazards Univariate Analysis
Cox proportional hazards regression analysis was performed to assess the impact that clinical and imaging parameters had on the likelihood of predicting EFS for patients with HPV-positive oropharyngeal SCC. The initial model contained seven clinical (age, sex, race, smoking status, AJCC stage, lymph node status, and treatment) and nine imaging (SUVmax, SUVmean, SUVpeak, total MTV [primary tumor plus lymph nodes], total TLG [primary tumor plus lymph nodes], primary-tumor MTV, primary-tumor TLG, lymph node MTV, and lymph node TLG) variables. In univariate analyses, lymph node status was the only statistically significant clinical variable associated with EFS (hazard ratio [HR], 4.45; p = 0.026). Among the imaging variables, total MTV (HR, 1.02; p = 0.008), primary-tumor MTV (HR, 1.02; p = 0.024), nodal MTV (HR, 1.03; p = 0.006), and nodal TLG (HR, 1.01; p = 0.006) showed a statistically significant association with EFS (Table 3). There was no statistically significant association of EFS with SUVmax (HR, 0.95; p = 0.51), SUVmean (HR, 0.95; p = 0.77), SUVpeak (HR 0.89; p = 0.361), and total TLG (HR, 1.002; p = 0.101) or any of the clinical variables other than lymph node status (Table 3).
TABLE 3.
Cox Univariate Analysis
| Parameter | Event-Free Survivala
|
p | |
|---|---|---|---|
| Hazard Ratio | 95% CI | ||
|
| |||
| Age(per y) | 1.080 | 0.99–1.17 | 0.064 |
| Sex(male vs female) | 0.310 | 0.08–1.24 | 0.098 |
| Race | 0.364 | 0.09–1.46 | 0.154 |
| Smoking (smoker vs nonsmoker) | 1.333 | 0.33–5.33 | 0.685 |
| Stageb (III vs IV) | 1.918 | 0.24–15.35 | 0.539 |
| Nodal status (positive vs negative) | 4.449 | 1.19–16.58 | 0.026 |
| Treatment (CRT vs no CRT) | 1.275 | 0.27–6.14 | 0.762 |
| SUVmax | 0.946 | 0.81–1.11 | 0.506 |
| SUVmean | 0.950 | 0.67–1.34 | 0.772 |
| SUVpeak | 0.892 | 0.69–1.14 | 0.361 |
| Total MTV (per mL) | 1.019 | 1.01–1.03 | 0.008 |
| Primary-tumor MTV (per mL) | 1.021 | 1.00–1.04 | 0.024 |
| Lymph node MTV (per mL) | 1.030 | 1.01–1.05 | 0.006 |
| Total TLG (per g) | 1.002 | 1.00–1.01 | 0.101 |
| Primary-tumor TLG (per g) | 1.002 | 1.00–1.01 | 0.423 |
| Lymph node TLG (per g) | 1.005 | 1.00–1.01 | 0.006 |
Note—SUVmax = maximum standardized uptake value, SUVmean = mean standardized uptake value, SUVpeak = peak standardized uptake value, MTV = metabolic tumor volume, TLG = total lesion glycolysis, CRT = chemoradiation therapy.
Includes recurrence-free and overall survival.
Stages III and IV describe advanced cancers in the classification of the American Joint Committee on Cancer [12].
Cox Proportional Hazards Multivariate Analysis
Because the total MTV and primary-tumor MTV were associated with EFS in the univariate Cox regression analysis and there were only nine events in the study population and multicollinearity between total and primary-tumor MTV, we tested the associations between total MTV and EFS and between primary-tumor MTV and EFS, while controlling for each covariate individually in two separate Cox multivariate regression models. Total MTV remained an independent prognostic marker for EFS when adjusted for every other variable in the model (Table 4) individually. Primary-tumor MTV also remained an independent prognostic marker for EFS when adjusted for other variables, except for neck lymph node status and SUVpeak (Table 5). Though nodal MTV and nodal TLG were associated with EFS in the univariate Cox regression analysis, only four events were associated with these parameters in the patients who had neck nodal metastases; therefore, we did not perform multivariate modeling for these parameters.
TABLE 4.
Cox Multivariate Analysis for Total Metabolic Tumor Volume (MTV)—Model I
| Parameter | Event-Free Survivala
|
p | |
|---|---|---|---|
| Hazard Ratio | 95% CI | ||
|
| |||
| Total MTV (per mL) | 1.024 | 1.01–1.04 | 0.002 |
| Age (per y) | 1.108 | 1.02–1.21 | 0.022 |
| Total MTV (per mL) | 1.023 | 1.01–1.04 | 0.002 |
| Sex (male vs female) | 0.177 | 0.04–0.82 | 0.027 |
| Total MTV (per mL) | 1.020 | 1.01–1.04 | 0.006 |
| Race | 0.322 | 0.08–1.34 | 0.119 |
| Total MTV (per mL) | 1.019 | 1.00–1.03 | 0.012 |
| Smoking (smoker vs nonsmoker) | 1.022 | 0.24–4.41 | 0.976 |
| Total MTV (per mL) | 1.019 | 1.00–1.03 | 0.012 |
| Stageb (III vs IV) | 0.928 | 0.11–8.21 | 0.946 |
| Total MTV (per mL) | 1.022 | 1.01–1.04 | 0.002 |
| Nodal status (positive vs negative) | 6.240 | 1.58–24.59 | 0.009 |
| Total MTV (per mL) | 1.020 | 1.01–1.03 | 0.007 |
| Treatment (CRT vs no CRT) | 1.666 | 0.33–8.36 | 0.535 |
| Total MTV (per mL) | 1.022 | 1.01–1.04 | 0.002 |
| SUVmax | 0.877 | 0.72–1.06 | 0.181 |
| Total MTV (per mL) | 1.020 | 1.01–1.04 | 0.004 |
| SUVmean | 0.864 | 0.62–1.22 | 0.402 |
| Total MTV (per mL) | 1.018 | 1.00–1.03 | 0.018 |
| SUVpeak | 0.832 | 0.64–1.08 | 0.170 |
Note—Total MTV remained significant when adjusted for other clinical and imaging parameters. SUVmax = maximum standardized uptake value, SUVmean = mean standardized uptake value, SUVpeak = peak standardized uptake value, CRT = chemoradiation therapy.
Includes recurrence-free and overall survival.
Stages III and IV describe advanced cancers in the classification of the American Joint Committee on Cancer [12].
TABLE 5.
Cox Multivariate Analysis for Primary-Tumor Metabolic Tumor Volume (MTV)—Model II
| Parameter | Event-Free Survivala
|
p | |
|---|---|---|---|
| Hazard Ratio | 95% CI | ||
|
| |||
| Primary-tumor MTV(per mL) | 1.020 | 1.00–1.04 | 0.031 |
| Age (per y) | 1.083 | 0.99–1.18 | 0.069 |
| Primary-tumor MTV (per mL) | 1.024 | 1.01–1.04 | 0.012 |
| Sex (male vs female) | 0.248 | 0.06–1.05 | 0.058 |
| Primary-tumor MTV (per mL) | 1.024 | 1.01–1.04 | 0.011 |
| Race | 0.247 | 0.06–1.17 | 0.181 |
| Primary-tumor MTV (per mL) | 1.021 | 1.00–1.04 | 0.028 |
| Smoking (smoker vs nonsmoker) | 1.214 | 0.30–4.96 | 0.787 |
| Primary-tumor MTV (per mL) | 1.020 | 1.00–1.04 | 0.034 |
| Stageb (III vs IV) | 0.690 | 0.08–5.74 | 0.731 |
| Primary-tumor MTV (per mL) | 1.017 | 1.00–1.04 | 0.076 |
| Nodal status (positive vs negative) | 3.891 | 1.01–14.99 | 0.048 |
| Primary-tumor MTV (per mL) | 1.022 | 1.00–1.04 | 0.019 |
| Treatment (CRT vs no CRT) | 1.711 | 0.34–8.70 | 0.517 |
| Primary-tumor MTV (per mL) | 1.022 | 1.01–1.04 | 0.013 |
| SUVmax | 0.918 | 0.78–1.09 | 0.322 |
| Primary-tumor MTV (per mL) | 1.021 | 1.00–1.04 | 0.022 |
| SUVmean | 0.929 | 0.68–1.28 | 0.649 |
| Primary-tumor MTV (per mL) | 1.017 | 1.00–1.04 | 0.078 |
| SUVpeak | 0.885 | 0.70–1.13 | 0.320 |
Note—Primary-tumor MTV remained significant when adjusted for other clinical and imaging parameters, except for neck nodal status and SUVpeak. SUVmax = maximum standardized uptake value, SUVmean = mean standardized uptake value, SUVpeak = peak standardized uptake value, CRT = chemoradiation therapy.
Includes recurrence-free and overall survival.
Stages III and IV describe advanced cancers in the classification of the American Joint Committee on Cancer [12].
Kaplan-Meier Survival Analysis and Event-Free Survival
We further investigated whether dichotomizing the total MTV and primary-tumor MTV using median cut point and optimum cut point would predict the EFS. The Kaplan-Meier survival curves using median cut point of 23.3 mL for total MTV were not statistically different (HR, 1.12 [95% CI, 0.3–4.15]; log-rank p = 0.86, and Gehan-Breslow-Wilcoxon test p = 0.9). We further identified an optimum cut point of 41 mL (sensitivity, 44.4%, specificity, 80.3%; and likelihood ratio, 2.26) using ROC analysis. The Kaplan-Meier survival curves using optimum cut point of 41 mL for total MTV also were not statistically significant (HR, 2.87 [95% CI, 0.8–18.6]; log-rank p = 0.10, and Gehan-Breslow-Wilcoxon test p = 0.09) (Fig. 2). Figures 3 and 4 illustrate the patient outcomes and the value of high and low total MTV at the same overall stage of disease.
Fig. 2.
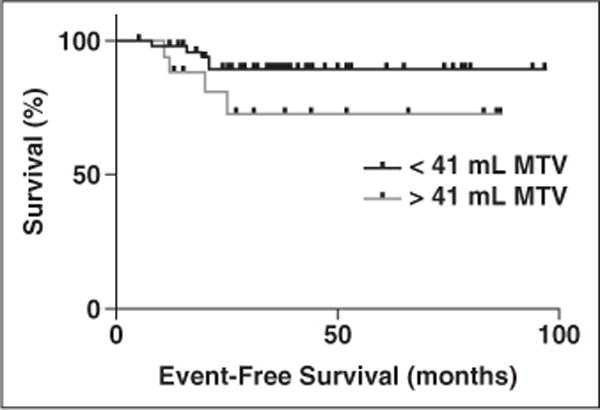
Kaplan-Meier survival curves. With use of optimum cut point of 41 mL for total MTV, there was trending toward statistical significance (hazard ratio, 2.87 [95% CI, 0.8–18.6]; log-rank p = 0.10, and Gehan-Breslow-Wilcoxon test p = 0.09).
Fig. 3.

61-year-old man with history of stage IV (T2N2bM0) human papillomavirus–positive squamous cell carcinoma of right tonsil.
A, Axial fused PET/CT baseline image yielded high total metabolic tumor volume of 43 mL. Primary right-sided oropharyngeal tumor is outlined in green; larger right level IIA node is outlined in magenta; and smaller right level IIA node is outlined in yellow.
B, Axial fused PET/CT image after treatment with definitive chemoradiation (cisplatin and radiotherapy to total dose of 70 Gy with excellent response).
C, Subsequent fused PET/CT image at 24-month follow-up showed recurrence in large necrotic right level III lymph node.
Fig. 4.
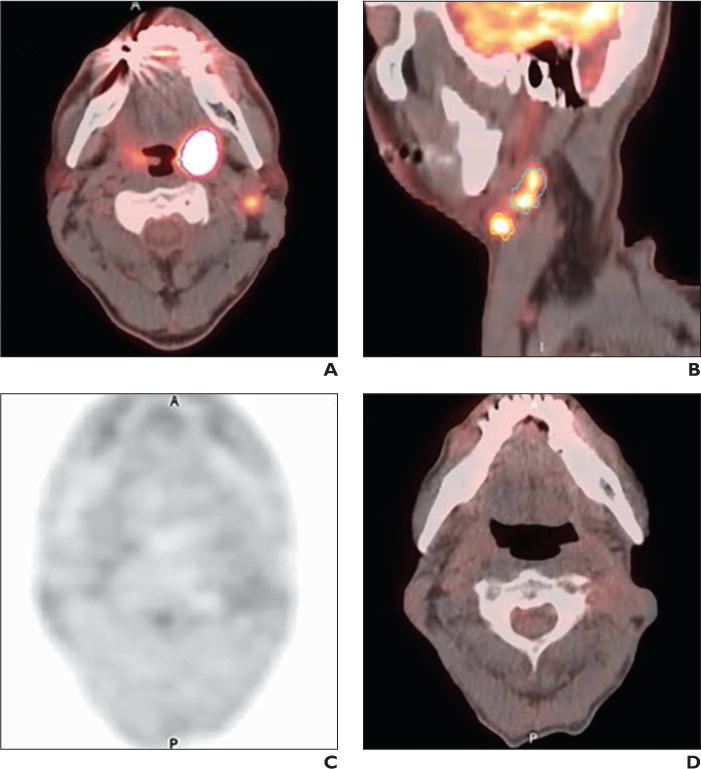
55-year-old man with history of stage IV (T2N2bM0) human papillomavirus–positive squamous cell carcinoma of left tonsil and left level IIA nodes.
A and B, Axial (A) and sagittal (B) fused PET/CT baseline images yielded low total metabolic tumor volume of 18 mL. Left tonsillar primary is outlined in magenta; larger left IIA node is outlined in green; and smaller left IIA node is outlined in yellow.
C and D, No recurrence is evident on subsequent PET (C) and fused PET/CT (D) images at 48-month follow-up after treatment with definitive chemoradiation (cisplatin and radiotherapy to total dose of 70 Gy with good response).
A = anterior; P = posterior.
Discussion
The goal of our study was to establish the prognostic value of FDG PET parameters in HPV-positive stage III and IV oropharyngeal SCC. Our study shows that total and primary-tumor MTV are prognostic indicators of survival in patients with HPV-positive stage III and IV oropharyngeal SCC, adjusted for other variables in the models. There was no statistically significant association of EFS with SUVmax, SUVmean, SUVpeak, and TLG.
Previous studies have shown that MTV is a potential prognostic indicator of survival in head and neck cancers [14–17]. Except for the study by Xie et al. [15] on nasopharyngeal cancer, all other studies included patients with cancers from different sub-sites of head and neck, which introduces heterogeneity. In the present study, we included only patients with oropharyngeal SCC whose tumors were at an advanced stage (stages III and IV), to minimize the effect of heterogeneity. Most of the previous studies concluded that primary-tumor MTV was a significant predictor of survival in head and neck SCC. In the study by Tang et al. [16], both primary-tumor and nodal MTV were considered, and it was shown that primary-tumor MTV predicted survival whereas nodal MTV did not. In our study, both primary-tumor MTV and nodal MTV were significant predictors of EFS.
We further minimized the heterogeneity by including only patients who had HPV-positive oropharyngeal SCC tested by in situ hybridization. p16 is a biomarker of infection with HPV in oropharyngeal SCC. The expression of p16 is not limited to HPV-positive tumors. The specificity of p16 in screening for HPV-positive tumors ranged from 79% to 93% in two different studies [18, 19]. Hence, including patients who had an in situ hybridization HPV positive tumor, rather than a p16 positive, decreased the heterogeneity.
Some studies have shown that SUVmax predicts survival [20–25], whereas others did not support this association [14, 15, 26, 27]. These inconsistencies could be a result of the heterogeneity of treatment modalities, tumor stage, tumor sites, and use of several outcome endpoints, such as local failure or disease-free or overall survival. In our study, SUVmax was not associated with EFS in patients with HPV-positive oropharyngeal SCC. The prognostic utility of SUVpeak in head and neck SCC has not been previously established, and our study showed no association between SUVpeak and EFS.
Several clinical trials (e.g., RTOG-1016, ECOG-1308) are investigating treatment deintensification approaches in patients with HPV-positive oropharyngeal SCC [28]. The approach for deintensification should be considered cautiously. Recently, O’Sullivan et al. [29] showed that primary-tumor and nodal staging can identify HPV-positive patients with low risk of distant metastasis, and deintensification strategies can be most optimally used in such subgroups. Our study also suggests that lymph node status (positive vs negative) has a significant association with EFS. Unlike the prior study, overall stage was not associated with EFS in this study, most likely because we included only stage III and IV patients.
Our study results need to be interpreted in the context of the study design. The follow-up period was limited (median, 25 months; mean, 31 months), which resulted in a limited number of events (recurrence or death). For this reason, we combined the recurrence-free and overall survival into one outcome parameter, namely EFS. A follow-up study with more patients and longer follow-up would increase the number of events and may confirm our initial results and support their significance. We did not include any volumetric parameters from CT in our analysis, because it is difficult to segment the tumors and lymph nodes without IV contrast material and because of the lack of availability of robust segmentation programs for CT in head and neck.
In conclusion, total MTV and primary-tumor MTV may have superior prognostic utility in patients with HPV-positive stage III and IV oropharyngeal SCC relative to other FDG PET parameters. These results need to be validated in a prospective study and can be incorporated with clinical parameters in risk stratification for therapy deintensification strategies in patients with HPV-positive stage III and IV oropharyngeal SCC.
Acknowledgments
A. K. Tahari was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health (grant T32EB006351).
References
- 1.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus–related and –unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus–positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 5.Rischin D, Young RJ, Fisher R, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol. 2010;28:4142–4148. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pignon JP, le Maitre A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Calais G, Alfonsi M, Bardet E, et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst. 1999;91:2081–2086. doi: 10.1093/jnci/91.24.2081. [DOI] [PubMed] [Google Scholar]
- 8.Denis F, Garaud P, Bardet E, et al. Final results of the 94-01 French head and neck oncology and radiotherapy group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol. 2004;22:69–76. doi: 10.1200/JCO.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Paidpally V, Chirindel A, Lam S, Agrawal N, Quon H, Subramaniam RM. FDG-PET/CT imaging biomarkers in head and neck squamous cell carcinoma. Imaging Med. 2012;4:633–647. doi: 10.2217/iim.12.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romesser PB, Qureshi MM, Shah BA, et al. Superior prognostic utility of gross and metabolic tumor volume compared to standardized uptake value using PET/CT in head and neck squamous cell carcinoma patients treated with intensity-modulated radiotherapy. Ann Nucl Med. 2012;26:527–534. doi: 10.1007/s12149-012-0604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim R, Eaton A, Lee NY, et al. 18F-FDG PET/CT metabolic tumor volume and total lesion glycolysis predict outcome in oropharyngeal squamous cell carcinoma. J Nucl Med. 2012;53:1506–1513. doi: 10.2967/jnumed.111.101402. [DOI] [PubMed] [Google Scholar]
- 12.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7. New York: Springer; 2010. [Google Scholar]
- 13.Werner-Wasik M, Nelson AD, Choi W, et al. What is the best way to contour lung tumors on PET scans? Multiobserver validation of a gradient-based method using a NSCLC digital PET phantom. Int J Radiat Oncol Biol Phys. 2012;82:1164–1171. doi: 10.1016/j.ijrobp.2010.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.La TH, Filion EJ, Turnbull BB, et al. Metabolic tumor volume predicts for recurrence and death in head and neck cancer. Int J Radiat Oncol Biol Phys. 2009;74:1335–1341. doi: 10.1016/j.ijrobp.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie P, Yue JB, Zhao HX, et al. Prognostic value of 18F-FDG PET-CT metabolic index for nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2010;136:883–889. doi: 10.1007/s00432-009-0729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang C, Murphy JD, Khong B, et al. Validation that metabolic tumor volume predicts outcome in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2012;83:1514–1520. doi: 10.1016/j.ijrobp.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dibble EH, Lara Alvarez AC, Truong MT, Mercier G, Cook EF, Subramaniam RM. 18F-FDG metabolic tumor volume and total glycolytic activity of oral cavity and oropharyngeal squamous cell cancer: adding value to clinical staging. J Nucl Med. 2012;53:709–715. doi: 10.2967/jnumed.111.099531. [DOI] [PubMed] [Google Scholar]
- 18.Pannone G, Rodolico V, Santoro A, et al. Evaluation of a combined triple method to detect causative HPV in oral and oropharyngeal squamous cell carcinomas: p16 immunohistochemistry, consensus PCR HPV-DNA, and in situ hybridization. Infect Agent Cancer. 2012;7:4. doi: 10.1186/1750-9378-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas J, Primeaux T. Is p16 immunohistochemistry a more cost-effective method for identification of human papilloma virus–associated head and neck squamous cell carcinoma? Ann Diagn Pathol. 2012;16:91–99. doi: 10.1016/j.anndiagpath.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Allal AS, Dulguerov P, Allaoua M, et al. Standardized uptake value of 2-[18F] fluoro-2-deoxy-D-glucose in predicting outcome in head and neck carcinomas treated by radiotherapy with or without chemotherapy. J Clin Oncol. 2002;20:1398–1404. doi: 10.1200/JCO.2002.20.5.1398. [DOI] [PubMed] [Google Scholar]
- 21.Allal AS, Slosman DO, Kebdani T, Allaoua M, Lehmann W, Dulguerov P. Prediction of outcome in head-and-neck cancer patients using the standardized uptake value of 2-[18F]fluoro-2-deoxy-D-glucose. Int J Radiat Oncol Biol Phys. 2004;59:1295–1300. doi: 10.1016/j.ijrobp.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 22.Torizuka T, Tanizaki Y, Kanno T, et al. Prognostic value of 18F-FDG PET in patients with head and neck squamous cell cancer. AJR. 2009;192:W156–W160. doi: 10.2214/AJR.08.1429. [web] [DOI] [PubMed] [Google Scholar]
- 23.Schwartz DL, Rajendran J, Yueh B, et al. FDG-PET prediction of head and neck squamous cell cancer outcomes. Arch Otolaryngol Head Neck Surg. 2004;130:1361–1367. doi: 10.1001/archotol.130.12.1361. [DOI] [PubMed] [Google Scholar]
- 24.Kubicek GJ, Champ C, Fogh S, et al. FDG-PET staging and importance of lymph node SUV in head and neck cancer. Head Neck Oncol. 2010;2:19. doi: 10.1186/1758-3284-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao CT, Chang JT, Wang HM, et al. Pretreatment primary tumor SUVmax measured by FDG-PET and pathologic tumor depth predict for poor outcomes in patients with oral cavity squamous cell carcinoma and pathologically positive lymph nodes. Int J Radiat Oncol Biol Phys. 2009;73:764–771. doi: 10.1016/j.ijrobp.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Chang KP, Tsang NM, Liao CT, et al. Prognostic significance of 18F-FDG PET parameters and plasma Epstein-Barr virus DNA load in patients with nasopharyngeal carcinoma. J Nucl Med. 2012;53:21–28. doi: 10.2967/jnumed.111.090696. [DOI] [PubMed] [Google Scholar]
- 27.Schinagl DA, Span PN, Oyen WJ, Kaanders JH. Can FDG PET predict radiation treatment outcome in head and neck cancer? Results of a prospective study. Eur J Nucl Med Mol Imaging. 2011;38:1449–1458. doi: 10.1007/s00259-011-1789-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quon H, Richmon JD. Treatment deintensification strategies for HPV-associated head and neck carcinomas. Otolaryngol Clin North Am. 2012;45:845–861. doi: 10.1016/j.otc.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 29.O’Sullivan B, Huang SH, Perez-Ordonez B, et al. Outcomes of HPV-related oropharyngeal cancer patients treated by radiotherapy alone using altered fractionation. Radiother Oncol. 2012;103:49–56. doi: 10.1016/j.radonc.2012.02.009. [DOI] [PubMed] [Google Scholar]


