Abstract
Transportation of live fish is a common practice among aquaculture facilities. Many studies have previously reported how transport elicits physiological stress responses and increases disease susceptibility in farmed fish. The aim of this work is to investigate the changes that the skin of rainbow trout (Oncorhynchus mykiss) experiences due to stress. Since NaCl is commonly added to transport water as a stress mitigator, the effects of salt addition on the skin mucosa and skin-associated bacteria were also examined. Three experimental groups (Control, post-transport no salt (PTNS) and post-transport with salt (PTS)) were analyzed in a 5-hour transport acute stress model. Results indicate that the skin mucosa and the skin-associated bacteria are affected by transport stress. Total numbers of culturable skin-associated bacteria increased by ~10-fold and ~50-fold in the PTS and PTNS groups, respectively. Compared to controls, MUC2 expression was increased by 5-fold and 2-fold in the PTNS and PTS groups, respectively. Claudin-7, 8d and 12 expression levels were higher in both PTNS and PTS groups whereas antimicrobial peptide gene expression was lower than controls. Expression of the anti-inflammatory cytokine TGF-β but not IL-1β, IL-6 and TNF-α was up-regulated 2-3 fold in both the PTS and PTNS groups. The addition of salt diminished some of the physiological responses measured including the numbers of skin-associated bacteria. The responses recorded here appeared to be efficient at controlling bacterial translocation since stress did not lead to significant presence of bacteria in the liver or spleen of rainbow trout. When examining the ability of skin mucus to inhibit or promote growth of the bacterial pathogen Vibrio anguillarum, the skin mucus of PTS trout was more efficient at inhibiting V. anguillarum growth (20% inhibition) compared to control or PTNS mucus (11-12% inhibition). Our data clearly indicate the skin and skin microbiota of rainbow trout undergo important physiological responses during stress. The reduction in the magnitude of the skin responses recorded when salt was added to the transport water explains a new mechanism by which salt is an effective stress mitigator in some fish species. Aquaculture specialists will benefit from the present study by taking into consideration the importance of skin health during live transport.
Keywords: rainbow trout, skin homeostasis, stress, skin bacteria, mucosal immunity
Introduction
Many aquaculture operations involve transportation of fish from one facility to another or, during restocking practices, from a hatchery to rivers, lakes or ponds. Transportation is known to cause stress to fish and leads to a number of physiological responses such as the release of catecholamines and corticosteroids as well as increased glucose levels in blood (Barton, 2011; Pankhurst, 2011; Pottinger, 2008). Thus, the circulating level of cortisol is commonly used as an indicator of the degree of stress experienced by fish (Barton, 2011; Pankhurst, 2011; Pottinger, 2008).
Studies concerning the effects of stress on the immune system of fish have focused on systemic immune responses; mostly hematological parameters. Furthermore, some studies have shown how stress leads to increased disease susceptibility due to immunesuppression in fish (Iguchi et al., 2003; Tort, 2011; van Kemenade and Chadzinska, 2009). One of the most evident responses of fish to stress is the production of copious amounts of skin and gill mucus (Shephard, 1994; Vatsos et al., 2010). Thus, gross observations of stressed fish indicate that the mucosal barriers of fish are important sensors of stress.
The epidermis defines the physical separation between the internal and external environment of fish and it is a primary defender of internal homeostasis (Shephard, 1994). The skin of fish is an important first barrier against pathogen entry and it is equipped with a mucosal immune system known as SALT (Salinas et al., 2011; Xu et al., 2013). Moreover, fish skin is colonized by a rich and diverse bacterial community known as the microbiota. Disruption of skin barrier homeostasis and dysregulation of skin commensals as a result of stress can potentially explain the increased disease susceptibility thus far reported in stressed fish. The effect of stress on skin homeostasis and skin bacterial communities has yet to be investigated in fish. Our hypothesis is that the skin and skin-associated bacteria sense and respond to stress hormones released by the fish host.
One of the ways and common practices in freshwater fish facilities that mitigate transport stress is the addition of salt to the water. Whereas this practice has shown variable success depending on the fish species of study, it is not yet known why salt can sometimes help freshwater fish species to better respond to stress responses. It has been proposed that salt aids with osmoregulatory disturbances that occur during transport (Crosby et al., 2006; Oyoo-Okoth et al., 2011) but other mechanisms of action may also play a significant role. We hypothesize that the addition of salt to transport water aids with returning to homeostasis thanks to the effects of salt on skin mucosal immunity and the skin microbiota.
In order to test our hypothesis, the present work specifically evaluates the changes that trout skin morphology, epithelial barrier integrity, innate immune gene expression and bacterial communities undergo following a 5-hour transport event in the presence or absence of salt in the transport water. Our results show that despite a clear commensal overgrowth in stressed individuals, bacterial translocation from skin to systemic organs did not occur. Rainbow trout skin maintained homeostasis in a number of ways such as increasing tight junction and mucin gene expression, decreasing anti-microbial peptide expression and inducing an anti-inflammatory phenotype via the TGF-β pathway. Adding salt to transport tanks ameliorated stress parameters and reduced the overgrowth of skin bacteria suggesting that previously beneficial effects of salt addition may be explained by the effects of salt on the fish microbial communities. Our findings have significant implication to the field of aquaculture and underscore the importance of skin mucosal health during transport operations.
Materials and methods
2.1 Animals and live transport experiments
Animals were obtained from Lisboa Springs Hatchery, Pecos, New Mexico. Triploid female rainbow trout (mean weight 200g) were sampled before (control) and after a 5-h transport event. At the hatchery, fish were maintained in concrete rafts using an open circulation system. Water temperature during the experiments ranged from 10-13°C. Fish were fed commercial dry pellets once a day ad libitum using mechanical feeders but starved the days when samplings were conducted. Stressed groups consist of post transport fish named PTNS (post-transport no salt) and post transport group (named PTS), to which a concentration of 5 g NaCl/L was added to the tank water of the transport truck. Transport water was directly obtained from the raft where the fish were held. Fish were not sedated during transport Experiments were conducted three independent times between the months of October and December. Six fish from each experimental group were sampled each time. Sampling began at 9 am for the control fish and 1 pm for the stressed groups. Fish were anesthetized with MS-222 and bled from the caudal vein with a heparinized 3 mL syringe. Plasma samples were collected and stored at −80°C until use. Sampling time per fish was approximately 3 min and performed by the same three researchers every time.
2.2 Glucose and cortisol levels
Blood glucose was measured using the One Touch Ultra2 commercial glucose meter. Plasma cortisol levels were measured by radioimmune assay as explained elsewhere (Kiyma et al., 2004) at New Mexico State University, Las Cruces.
2.3 Skin mucus bacteria isolation and plate counts
Total skin mucus was collected by scraping the body surface of the fish with a sterile cell scraper and collected onto a sterile petri dish. Skin-associated bacteria were isolated by a series of centrifugation steps as explained elsewhere (Xu et al., 2013). PBS and 10 μl of the solution were plated onto Luria Bertani (LB) agar plates and tryptic soy agar (TSA) plates. Plates were incubated for three days at room temperature and colony forming units (cfu) were counted by two different staff members. No further attempts to identify the bacterial isolates were made.
2.5 Scanning electron microscopy and light microscopy
Three skin samples from each experimental group were collected for scanning electron microscopy. Samples were fixed in 1% glutaraldehyde, 1% formaldehyde, 1 M sodium cacodylate solution overnight, dehydrated and critical point dried. Processed samples were examined under a JEOL 5800LV scanning electron microscope. Additionally, three skin samples were fixed in 4% paraformaldehyde for paraffin embedding. Five μm-thick paraffin sections were stained with haematoxylin-eosin as well as with alcian blue/periodic acid-Schiff (AB/PAS stain) at two different pH values (1 and 2.5) in order to reveal the chemical composition of mucosal secretion and visualize different mucins as explained elsewhere (Sarasquete et al., 2001). The mucosal contents of goblet cells were counted under a microscope and scored as blue or magenta. A minimum of three fish per experimental group and six paraffin sections per fish were used for the quantification of mucin types. Light micrographs were acquired in a Zeiss AxioSkop using the AxioVision software.
2.6 Gene expression studies by RT-qPCR
Total RNA was extracted from the skin of 6 control, 6 PTS and 6 PTNS rainbow trout using Trizol. cDNA synthesis was performed using 1 μg of total RNA, which was denatured (65°C, 5 min) in the presence of 1 μl of oligo-dT17, 1 μl dNTP (deoxynucleoside triphosphate mix 10 mM each (Promega) and RNA/DNA free water (Sigma) in a volume of 13 μl. Synthesis was carried out using 1 μl Superscript III enzyme reverse transcriptase (Invitrogen) in the presence of 5 μl of 5x first strand buffer, 1 μl 0.1 M DTT, made up to a final volume of 25 μl with water, and incubated at 55°C for 1 h. The resultant cDNA was stored at −20°C.
The expression of the main tight junction genes in fish (occluding, claudin-7, 8d, 12 and 31), mucin genes (MUC2 and MUC5AC), antimicrobial peptides (DB1, 2, 3 and 4, cathelicidin 1 and 2, hepcidin and LEAP2A) and cytokines (IL-1β, IL-6, TNF-α, TGF-β1a and TGF-β1b) was studied before and after transport by RT-qPCR using specific primers (Table 1). The qPCR was performed using 3 μl of a diluted cDNA template as described in Tacchi et al. 2013 (Tacchi et al., 2013). The relative expression level of the genes was determined using the Pfaffl method (Pfaffl, 2001) as previously described (Tacchi, Larragoite and Salinas, 2013).
Table 1.
Primers used in this study for qPCR
| Gene | Primer | Sequence (5′-3′) |
|---|---|---|
| Occludin | OccludinF | CAGCCCAGTTCCTCCAGTAG |
| OccludinR | GCTCATCCAGCTCTCTGTCC | |
| Claudin-7 | Claudin-7F | CGTCCTGCTGATTGGATCTC |
| Claudin-7R | CAAACGTACTCCTTGCTGCTG | |
| Claudin-8d | Claudin-8dF | GCAGTGTAAAGTGTACGACTCTCTG |
| Claudin-8dR | CACGAGGAACAGGCATCC | |
| Claudin-12 | Claudin-12F | CTTCATCATCGCCTTCATCTC |
| Claudin-12R | GAGCCAAACAGTAGCCCAGTAG | |
| Claudin-31 | Claudin-31F | TCGGCAACAACATCGTGAC |
| Claudin-31R | CGTCCAGCAGATAGGAACCAG | |
| MUC2 | OmMuc2F | CCAGGCACAGAAAAGACAGATGC |
| OmMuc2R | GGATGTAGGAGTGCTTGACC | |
| MUC5AC | OmMuc5ACF | GCTCTGGTCTTCGGACTATCTG |
| OmMuc5ACR | GCTGCTCTTACACAACGACG | |
| DB-1 | omDB-1F | GGTTTTCCTATTGCTTAATGTTGTGG |
| omDB-1R | GACACACAGTTAAGTCATGG | |
| DB-2 | omDB-2F | GCTGACAGCAGTGCAAGCTGATGACAC |
| omDB-2R | GCAAAGCACAGCATCTTAATCTGC | |
| DB-3 | omDB-3F | GCTTGTGGAATACAAGAGTCATCTGC |
| omDB-3R | GCATACATTCGGCCATGTACATCC | |
| DB-4 | omDB-4F | GCAACTCTTCTAAAGAACAGT |
| omDB-4R | CGTGGGCGACACAGCATACAAATCC | |
| Cathelicidin1 | CATH1F | ACCAGCTCCAAGTCAAGACTTTGAA |
| CATH1R | TGTCCGAATCTTCTGCTGCAA | |
| Cathelicidin2 | CATH2F | ACATGGAGGCAGAAGTTCAGAAGA |
| CATH2R | GAGCCAAACCCAGGACGAGA | |
| Hepcidin | HepcidinF | GCTGTTCCTTTCTCCGAGGTGC |
| HepcidinR | GTGACAGCAGTTGCAGCACCA | |
| LEAP2A | LEAP2AF | GGTTCCTGGTGTTTCTGGTGCT |
| LEAP2AR | AGTGGCCACCCCTGCAAAT | |
| IL1β | IL1bF | ACATTGCCAACCTCATCATCG |
| IL1bR | TTGAGCAGGTCCTTGTCCTTG | |
| TNFα | TNFaF | GGGGACAAACTGTGGACTGA |
| TNFaR | GAAGTTCTTGCCCTGCTCTG | |
| IL6 | IL6F | ACTCCCCTCTGTCACACACC |
| IL6R | GGCAGACAGGTCCTCCACTA | |
| TGFβ1a | TGFb1aF | CTCACATTTTACTGATGTCACTTCCTGT |
| TGFb1aR | GGACAACTGCTCCACCTTGTG | |
| TGFβ1b | TGFb1bF | CATGTCCATCCCCCAGAACT |
| TGFb1bR | GGACAACTGTTCCACCTTGTGTT | |
| EF-1α | EF-1aF | CAACGATATCCGTCGTGGCA |
| EF-1aR | ACAGCGAAACGACCAAGAGG |
2.7 Bacterial Translocation experiments
Liver and spleen tissue samples from each fish were collected under sterile condition, weighted and then homogenized using a syringe needle. After homogenization, spleen and liver were separately resuspended in 400 μL of sterile PBS, and 20 μL of the resulting solution was plated onto LB and TSA plates. Plates were incubated at room temperature for two days, and cfu numbers were counted by two different staff members. Bacterial counts were standardized to original weight of the tissue, and final volume of the homogenized solution.
2.8 Vibrio anguillarum growth in the presence of skin mucus
Antibacterial ability was tested for each skin mucus sample, as described by Narvaez et al. (Narvaez et al., 2010). Briefly, V. anguillarum was incubated in Tryptic Soy Broth (TSB) at 37°C until an optical density (OD) of 0.2-0.3 at 620 nm was reached. Antibacterial activity was determined by incubating standard aliquots of 180 μl of a 1:100 dilution of the bacterial suspension in duplicate to which 20 μl of each skin mucus sample were added. After 24 h of incubation at 37°C, absorbance values at 620 nm were measured. Percentage growth inhibition was determined by subtracting the growth values of the bacteria exposed to the mucus from the bacterial growth values in the control wells (PBS only). A maximum growth inhibition control treatment was included by adding the synthetic peptide cecropin A (Anaspec Inc.) at a final concentration of 1 mM; an amount known to fully inhibit Gram negative bacterial growth (McGrath et al., 2013).
2.9 Statistical analysis
Results are expressed as the mean ± standard error (SE). Data analysis was performed in GraphPad Prism version 5.0 including normality tests. All data were normally distributed. Statistically significant differences were considered when p<0.05. The qPCR measurements were analyzed by T-test to identify statistically significant differences between groups. One-way ANOVA and Tukey post-hoc analysis test were performed to identify statistically significant differences among groups.
Results
3.1 Blood Glucose and plasma cortisol levels
Blood glucose was significantly higher in the PTNS group compared to controls. In the PTS group glucose levels were not significantly different than those of control fish (Fig. 1a). With respect to cortisol levels, both PTNS and PTS had significantly higher plasma cortisol values than the control group. Whereas cortisol values were highest in the PTS group, there were no significant differences between PTS and PTNS treatments (Fig. 1b).
Fig. 1.
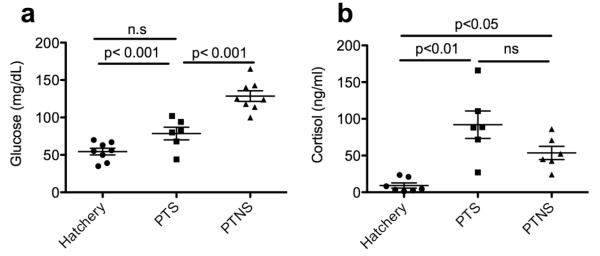
a) Mean blood glucose levels (mg/dL). b) Mean plasma cortisol levels (ng/ml) in control, PTS and PTNS rainbow trout. The p-values for statistically significant differences between groups according to a Tukey’s test are shown. n.s= not significant.
3.2 Skin mucus bacterial counts
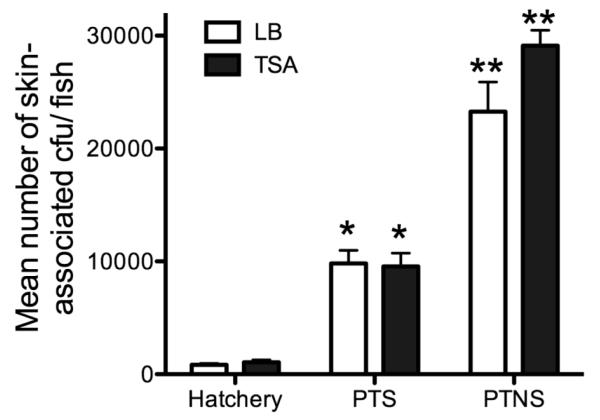
We first evaluated whether skin associated bacteria change prior and after transport from the hatchery to Tingley Beach. Strikingly, the numbers of culturable bacteria in both groups were significantly different and stress caused a ~50-fold increase in total bacterial numbers associated with the skin both in TSA and LB agar plates when no salt was added (Fig. 2). In the presence of salt, bacterial numbers were still significantly higher, but the fold increase was lower than in the absence of salt, about ~10-fold. No differences were found in the numbers recovered on LB or TSA plates,
Fig. 2.
Mean bacterial colony forming units (cfu) recovered on LB or TSA plates from the skin mucus of control, PTS and PTNS rainbow trout. One or two asterisks denote statistically significant differences among the groups according to a Tukey’s test.
3.3 Scanning electron microscopy and light microscopy of rainbow trout skin before and after stress
Stressed fish and other animals are known to secrete greater amounts of mucus. We studied the skin of control and stressed trout by scanning electron microscopy and confirmed these results. The general morphology of the skin surface of control trout (Fig. 3a-b) differed significantly from that of stressed trout in the PTS group (Fig. 3c d) and the PTNS group (Fig. 3e-f). The skin surface of control rainbow trout had an organized appearance characterized by polygonal shaped epithelial cells and presence of some goblet cells showing discrete secretion of mucus and few bacteria cells laying on the surface. In the PTS group a thin but more prominent layer of mucus was observed on the surface. The most obvious feature about PTS skin was the wide opening of goblet cells onto the exterior surface. Greater numbers of bacteria were present compared to controls and in many cases these bacteria were seen in the opening of goblet cells (Fig. 3d). The most prominent changes were found in the skin of PTNS trout. Extensive and thick mucus nets were deposited over the skin surface sometimes masking the polygonal shape of the epithelial cells (Fig. 3e). At a higher magnification, the thick mucus nets contained many bacterial cells trapped within them (Fig. 3f). The wide open goblet cells present in the PTS group were not observed indicating that goblet cell ejection of mucus contents had already reached completion earlier than 5 hours after transport.
Fig. 3.
Scanning electron microscopy images of the skin surface of control (a-b), PTS (c-d) and PTNS (e-f) rainbow trout. Asterisks denote bacterial cells. Muc: mucus; gc: goblet cell, mr: microridges; epc: epithelial cell.
H&E stains of skin paraffin sections show three main features. First, goblet cell numbers and release of mucus contents to the outer layer was evident in both PTS and PTNS groups. Second, the position and morphology of chromatophores changed in response to stress. Thicker and darker clusters of chromatophores were evident in the PTNS and, to a lesser extent in the PTS group. Third, no epithelial tissue damage (break down of epithelial tight junctions, presence of edema or cell death processes) was present in either the PTNS or the PTS trout. Finally, no signs of cell infiltration or inflammatory processes were observed in the PTNS and PTS treatments (Fig. 4).
Fig. 4.
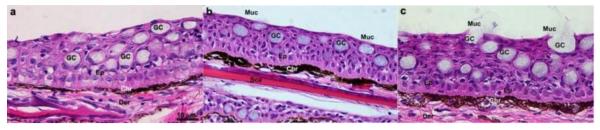
Hematoxylin/eosin stains of skin from control (a), PTS (b) and PTNS (c) trout. Images are representative of n=5 per group. Der: dermis; Ep: epidermis; Sca: scale; Chr: chromatophore; GC: goblet cell; Muc: mucus layer.
3.4 Skin mucosal secretions before and after stress
Stressed trout (PTNS and PTS) had greater proportions (two times higher) of goblet cells that contained mucins with sialic acid as well as greater proportions of goblet cells with sulfated mucins compared to control (Table 2). Addition of salt had an impact in the type of mucosal secretions since less goblet cells appeared to be sulfated in the PTS group compared to the PTNS group (Table 2).
Table 2.
Mean percentages of goblet cells stained blue or magenta using the AB/PAS stain at two different pH values in control, PTS ad PTNS rainbow trout skin (n=3).
| pH=1 | pH=2.5 | |||
|---|---|---|---|---|
| Blue | Magenta | Blue | Magenta | |
|
|
||||
| Control | 7.68% | 92.32% | 14.21% | 85.79% |
| PTS | 11.18% | 88.82% | 23.17% | 76.83% |
| PTNS | 38.90% | 61.10% | 28.31% | 71.69% |
3.5 Bacterial translocation studies
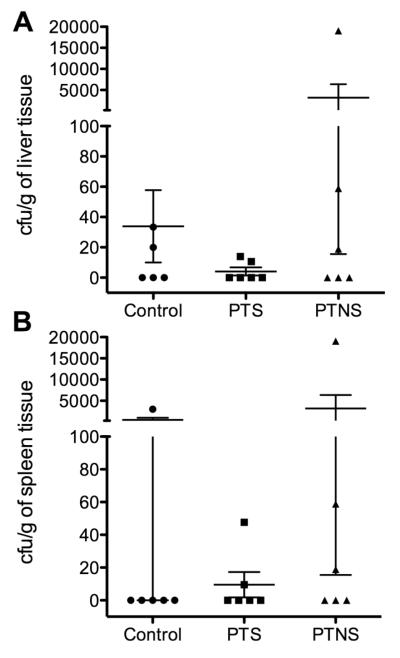
The liver and spleen of control fish contained small numbers of bacterial cfu. In the case of the PTS group, no significant differences were observed with respect to the hatchery control fish. In the PTNS group significant bacterial translocation was only present in one fish (both in the spleen and liver), whereas the other five contained negligible cfu numbers as in the control group (Fig. 5).
Fig. 5.
Bacterial colony forming units (cfu) recovered on TSA plates per gram of liver tissue (a) or spleen tissue (b) of control, PTS and PTNS rainbow trout.
3.6 Expression of tight junction and mucin genes
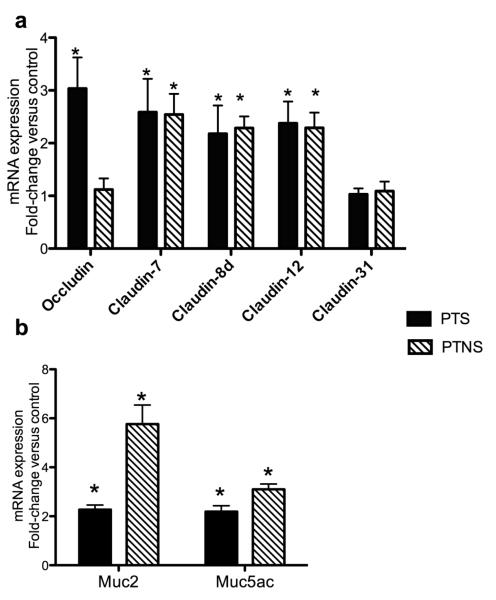
In the PTNS group claudin-7, 8d and 12 expression was significantly up-regulated whereas occludin and claudin-31 expression remained unchanged. In the case of PTS trout skin, the expression of occludin, claudin-7, 8d and 12 was significantly higher than that of controls. Similar to PTNS fish, claudin-31 expression was not affected in the PTS treatment. The increase in expression in all cases was approximately 2-3 fold compared to control trout (Fig. 6a). Mucin genes were significantly up-regulated in the PTS and PTNS groups. In the case of MUC2, a greater up-regulation was recorded in the PTNS group (5-fold) compared to a 2-fold increase in the PTS group (Fig. 6b).
Fig. 6.
Changes in tight junction gene expression (a) and mucin gene expression (b) in PTS and PTNS skin measured by RT-qPCR. Data are expressed as the mean fold-change compared to the control skin group. Bars represent means ± standard error of six fish. Different letters represent statistically significant groups after Tukey’s test (p<0.05).
3.7 Expression of skin antimicrobial peptides and cytokines
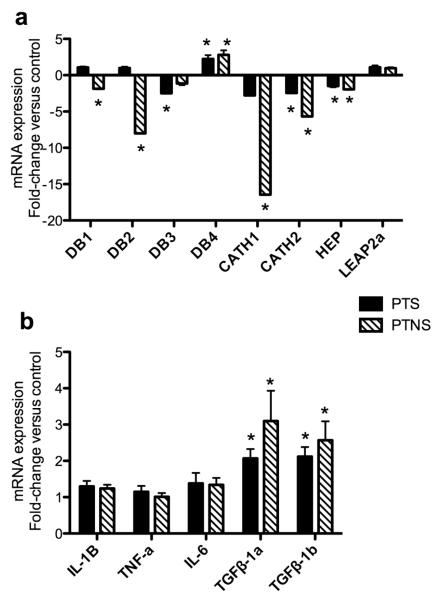
Because significantly higher numbers of cfu were recovered from the skin of trout after transport, we sought to evaluate whether the host expresses higher or lower levels of different antimicrobial peptides in response to the high bacterial numbers. DB1, DB2, cathelicidin1 and 2 and hepcidin were all significantly down-regulated in the PTNS skin. In the case of PTS skin, DB3, cathelicidin2 and hepcidin were down-regulated, whereas DB1, DB2 and cathelicidin1 expression was not significantly affected. Overall, the down-regulation of antimicrobial peptide genes was considerably more dramatic in the PTNS group than in the PTS group, with expression values of 7-17 times lower than controls. No significant modulation of LEAP2A was recorded in any of the stressed treatments. Interestingly, one gene, DB4 displayed a completely different expression pattern than the rest of the antimicrobial peptides here studied. DB4 was up-regulated 2-3 times in both PTS and PTNS group suggesting an specific role of this molecule in response to skin bacteria and stress (Fig. 7a). The expression of pro-inflammatory cytokines such as IL-1β, IL-6 or TNFα remained unchanged. However, the expression of the anti-inflammatory cytokines TGFβ-1a and TGFβ-1b was up-regulated 2-3 fold in both the PTS and PTNS groups, the PTNS trout showing the highest increases (Fig. 7b).
Fig. 7.
Changes in antimicrobial peptide gene expression (a) and cytokine gene expression (b) in PTS and PTNS skin measured by RT-qPCR. Data are expressed as the mean fold-change compared to the control skin group. Bars represent means ± standard error of six fish. Different letters represent statistically significant groups after Tukey’s test (p<0.05).
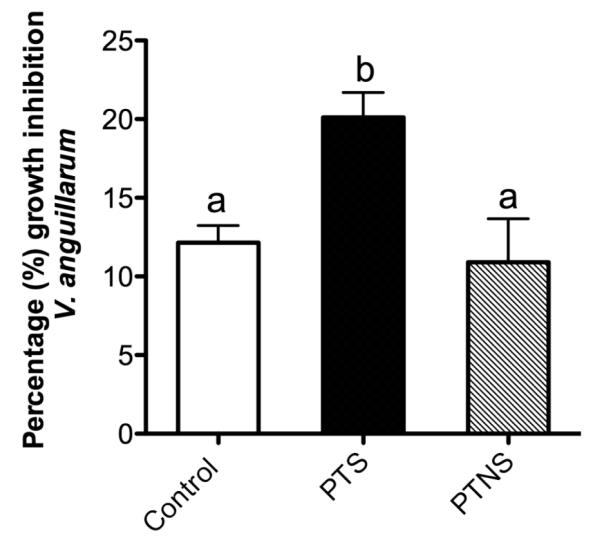
3.8 V. anguillarum growth in the presence of trout skin mucus before and after transport
We used an in vitro killing assay to test whether the skin mucus is capable of killing the bacterial pathogen V. anguillarum. This bacterium is known to enter fish hosts through several mucosal barriers including the gut, gills and skin (Kanno et al., 1989; Muroga and De La Cruz, 1987; Oisson et al., 1996). We incubated the skin mucus of control, stress without salt and stress with salt individuals and we measured the killing activity against V. anguillarum cells. As Fig. 8 shows, after 24 hours, both control and PTNS mucus reduced V. anguillarum growth by ~11-12%. In contrast, the skin mucus of fish from the PTS group significantly inhibited V. anguillarum growth at higher levels (20%) than the control or PTNS mucus samples. The positive control (cecropin A treatment) resulted in 100% inhibition of V. anguillarum growth (not shown).
Fig. 8.
Mean percentage of V. anguillarum growth inhibition by the skin mucus of control, PTS and PTNS rainbow trout after 24 hours in culture. Different letters designate statistically significant groups according to Tukey’s test (p<0.05).
Discussion
The physiological responses of fish to stress are well characterized with regard to endocrine, osmoregulatory, respiratory and behavioral systems (Barton, 2011; Pottinger, 2008). Moreover, the immune response is generally known to be suppressed in stressed fish (Tort, 2011; van Kemenade and Chadzinska, 2009). To date, studies on the effects of stress on immunity have focused on systemic parameters such as blood cell counts and serum innate immune factors but the role of mucosal immune barriers has been overlooked. In the current study, we show for the first time that live transport affects the skin homeostasis in rainbow trout. Using an acute stress model, we investigated how skin mucosal immunity and skin microbiota respond to stress. We compared two different stress groups, a transport treatment without salt and one with the addition of NaCl to the water as a stress mitigator. Both stressed groups had higher cortisol levels than control trout, indicating that addition of salt could not avoid cortisol release. However, blood glucose levels in the PTS group were not different from controls. This suggests that adding salt to the water retards or reduces glucose released into the blood. Previous studies have measured stress markers in the plasma of rainbow trout during different transport conditions (Barton and Peter, 1982).
Increased production of cutaneous mucosal secretions in response to stress had been noted in a number of fish species (Ángeles Esteban, 2012). In the present work, we conducted thorough experiments that compared the two stressed groups (PTS and PTNS). Scanning electron microscopy revealed important differences in both treatments. The mucus nets formed on the skin surface of the PTNS group covered large areas and trapped abundant bacteria. In the PTS group, in turn, mucus formed somehow patchy clusters and bacteria were seen in lower numbers. Moreover, the morphology of the goblet cells in both treatments was clearly different to each other. Goblet cells appeared to be still open to the external surface and therefore still releasing their contents in the PTS group whereas a much tighter semi-closed morphology at their apical pole was observed in the control and PTNS group. Both stressed groups were markedly different to the skin of control unhandled trout. It is know that stress leads to changes in the number of goblet cells and amounts of mucus production in teleost skin (Ángeles Esteban, 2012; Vatsos, Kotzamanis, Henry, Angelidis and Alexis, 2010). Our results suggest that presence of salt in the water may act as a retardant for the release of mucus from goblet cells in response to stress. Transport of euryhaline fish such as eels from freshwater to saltwater appears to decrease goblet cell numbers in the gut (Ciccotti et al., 1993), supporting a link between stress, salinity and goblet cell biology.
Strikingly, we found that the numbers of skin-associated bacteria as measured by plate counts significantly increased in both stressed groups. Stress has been shown to affect the gut microbiota of salmonids (Ringø et al., 2014) but never the skin microbiota. Our results clearly indicate that the skin associated bacterial communities of trout shift in response to stress, at least in numbers; this may due to the better adherence of certain bacterial species to skin mucus produced in response to stress. The glycosylation of individual mucins can change in response to external stimuli such as stress (Bosch et al., 2000). In the present study, AB/PAS staining patterns of skin mucins changed in both stressed groups compared to control. In particular, transport stress results in increased levels of sulfated and sialyated mucins and a decreased in the percentage of carboxylated and neutral mucins. In the case of the PTS group, an intermediate phenotype between control and PTNS group was observed, indicating again the alleviating effect of salt addition to transport water. Sulfaction and sialyation may lead to better adhesion of some bacterial species although this hypothesis requires further investigation. These findings are supported by the increased mucin gene expression observed. Although mucin gene expression was higher in both PTS and PTNS trout compared to the control group, the highest expression levels were detected in the PTNS group. Thus, adding salt to the water has a significant effect on mucus characteristics, goblet cell physiology and mucin gene expression. These effects could be the direct result of water salinity or could possibly be mediated by the numbers of skin-associated bacteria. Since we recorded 50-times more bacteria on the skin mucus of PTNS trout but 10 times only in the PTS group, it is possible that differences in the microbial burden trigger, in turn, different levels of mucin gene expression.
Since adding NaCl resulted in intermediate skin-associated bacterial numbers compared to control and no-salt transport groups, it appears that salinity shapes the bacterial communities that can adhere to the trout skin mucus during transport. It is known that different bacterial species, including symbionts of the human microbiome have different tolerance to NaCl (Culligan et al., 2012). Thus, it is likely that addition of NaCl to transport water favors the growth of certain bacterial groups whereas limits the growth of others. Altogether, our results point to a novel mechanism (i.e skin bacteria) by which salt acts as a stress mitigator in fish.
The mechanisms underlying the overgrowth of skin bacteria as a result of transport may partly involve the overall marked inhibition of skin antimicrobial peptides in the PTS and PTNS groups. In fact, antimicrobial peptide down-regulation in response to chronic stress has been reported in fish in the past as a response (Bui et al., 2010). In the absence of antimicrobial peptides, the skin environment of fish is likely to allow the blooming of skin microorganisms. However, it is possible that other mechanisms are in play including the aforementioned changes in the glycosylation patterns of mucins that may allow for greater bacterial attachment of certain species to the skin mucus.
One of the most interesting findings of the present study is the realization that rainbow trout skin has important homeostatic mechanisms that preserve the integrity of the skin mucosal barrier. Among these mechanisms, it appears that up-regulation of tight junction genes in rainbow trout due to transport stress is critical. Our results are in agreement with previous work in other mucosal surfaces where claudin expression in trout gill was also up-regulated in response to cortisol treatment in vitro (Bui, Bagherie-Lachidan and Kelly, 2010). This is an effective way to protect the epithelial barrier in the face of the abundant bacterial numbers that are found in association with the skin mucus of post-transport fish. Moreover, we found almost no evidence of bacterial translocation to systemic organs such as the liver or the spleen. Bacterial translocation is a common indicator of the breakdown of epithelial barriers and can lead to inflammation (Collins, 2001; Söderholm and Perdue, 2001; Velin et al., 2004). Our results show that the skin epithelial barrier was able to confine the microbiota to the mucosal layers of the body and that transport does not lead to skin leakiness in trout. A second important mechanism appears to be the avoidance of a local inflammatory response in the skin coupled to an induction of anti-inflammatory cytokines such as TGF-β1a and TGF-β1b. It is possible that penetration of skin bacteria into the epithelium led to the induction of TGF-β expression. In any case, the skin epithelium showed no signs of inflammation under histological examination.
Finally we evaluated the ability of a bacteria pathogen, V. anguillarum, to grow in the presence of rainbow trout skin mucus from the three different experimental groups. Surprisingly, the PTS skin mucus elicited different V. anguillarum growth curves compared to the skin mucus collected from the PTNS group. Whereas after 8 hours, PTS mucus allowed greater V. anguillarum growth, after 24 hours, PTS mucus inhibited V. anguillarum growth by 20% compared to the 10% recorded in the PTNS group. Thus, either the presence of salt in the skin mucus or the actual mucus antimicrobial properties of the PTS skin mucus are beneficial against bacterial pathogen growth. This is again, a novel explanation as to why salt can improve fish health during live transportation of fish.
In conclusion the present work sheds new light into the widespread effects that transport can induce on fish physiology and mucosal health. For the first time we have described some of the changes that the skin mucosa undergoes as a result to stress. Importantly, the skin microbiota are also involved. Since our model consisted of a short acute stressor, future studies will have to address how chronic stress affects the skin mucosal responses of fish.
Highlights.
Transport results in high mucus production and increased levels of skin-associated bacteria
Transport stress induces up-regulation of mucin genes, most tight junction genes and anti-inflammatory cytokines and inhibition of most antimicrobial peptide genes in the skin of rainbow trout
Adding salt to the transport water reduces most of the skin mucosal responses caused by the transport event in rainbow trout
Skin mucus from stressed fish transported with salt has a higher ability to inhibit V. anguillarum growth than that of control and stressed no salt fish
Acknowledgements
Authors wish to thanks the Lisboa Springs Hatchery (Pecos, New Mexico) for providing the fish used in this study. We thank Dr. Dennis Hallford for the cortisol analysis and Dr. Debra Millton for the V. anguillarum strain. This work was funded by NIH grant P20GM103452.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Esteban MA. An overview of the immunological defenses in fish skin. ISRN Immunol. 2012 2012. [Google Scholar]
- Barton B. Stress in finfish: past, present and future-a historical perspective. Fish stress and health in aquaculture. 2011;62:1. [Google Scholar]
- Barton B, Peter R. Plasma cortisol stress response in fingerling rainbow trout, Salmo gairdneri Richardson, to various transport conditions, anaesthesia, and cold shock. J. Fish Biol. 1982;20:39–51. [Google Scholar]
- Bosch JA, de Geus EJ, Ligtenberg TJ, Nazmi K, Veerman EC, Hoogstraten J, Amerongen AVN. Salivary MUC5B-mediated adherence (ex vivo) of Helicobacter pylori during acute stress. Psychosom. Med. 2000;62:40–49. doi: 10.1097/00006842-200001000-00006. [DOI] [PubMed] [Google Scholar]
- Bui P, Bagherie-Lachidan M, Kelly SP. Cortisol differentially alters claudin isoforms in cultured puffer fish gill epithelia. Mol. Cell. Endocrinol. 2010;317:120–126. doi: 10.1016/j.mce.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Ciccotti BE, Macchi E, Rossi A, Cataldi E, Cataudella S. Glass eel (Anguilla anguilla) acclimation to freshwater and seawater: morphological changes of the digestive tract. J. Appl Ichthyol. 1993;9:74–81. [Google Scholar]
- Collins SM. IV. Modulation of intestinal inflammation by stress: basic mechanisms and clinical relevance. Am. J. Physiol.-Gastrointestinal and Liver Physiology. 2001;280:G315–G318. doi: 10.1152/ajpgi.2001.280.3.G315. [DOI] [PubMed] [Google Scholar]
- Crosby TC, Hill JE, Watson CA, Yanong RP, Strange R. Effects of tricaine methanesulfonate, Hypno, metomidate, quinaldine, and salt on plasma cortisol levels following acute stress in threespot gourami Trichogaster trichopterus. J. Aquat Anim. health. 2006;18:58–63. [Google Scholar]
- Culligan EP, Sleator RD, Marchesi JR, Hill C. Functional metagenomics reveals novel salt tolerance loci from the human gut microbiome. ISME J. 2012;6:1916–1925. doi: 10.1038/ismej.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi K.i., Ogawa K, Nagae M, Ito F. The influence of rearing density on stress response and disease susceptibility of ayu (Plecoglossus altivelis) Aquaculture. 2003;220:515–523. [Google Scholar]
- Kanno T, Nakai T, Muroga K. Mode of transmission of vibriosis among ayu Plecoglossus altivelis. J. Aquat Anim. health. 1989;1:2–6. [Google Scholar]
- Kiyma Z, Alexander B, Van Kirk E, Murdoch W, Hallford D, Moss G. Effects of feed restriction on reproductive and metabolic hormones in ewes. J. Anim Sci. 2004;82:2548–2557. doi: 10.2527/2004.8292548x. [DOI] [PubMed] [Google Scholar]
- McGrath DM, Barbu EM, Driessen WH, Lasco TM, Tarrand JJ, Okhuysen PC, Kontoyiannis DP, Sidman RL, Pasqualini R, Arap W. Mechanism of action and initial evaluation of a membrane active all-D-enantiomer antimicrobial peptidomimetic. Proc. Natl. Acad. Sci. U.S.A. 2013;110:3477–3482. doi: 10.1073/pnas.1221924110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroga K, De La Cruz MC. Fate and location of Vibrio anguillarum in tissues of artificially infected ayu (Plecoglossus altivelis) Fish Pathol. 1987;22:99–103. [Google Scholar]
- Narvaez E, Berendsen J, Guzmán F, Gallardo JA, Mercado L. An immunological method for quantifying antibacterial activity in Salmo salar (Linnaeus, 1758) skin mucus. Fish Shellfish Immunol. 2010;28:235–239. doi: 10.1016/j.fsi.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Oisson J, Jöborn A, Westerdahl A, Blomberg L, Kjelleberg S, Conway P. Is the turbot, Scophthalmus maximus (L.), intestine a portal of entry for the fish pathogen Vibrio anguillarum? J. Fish. Dis. 1996;19:225–234. [Google Scholar]
- Oyoo-Okoth E, Cherop L, Ngugi CC, Chepkirui-Boit V, Manguya-Lusega D, Ani-Sabwa J, Charo-Karisa H. Survival and physiological response of Labeo victorianus (Pisces: Cyprinidae, Boulenger 1901) juveniles to transport stress under a salinity gradient. Aquaculture. 2011;319:226–231. [Google Scholar]
- Pankhurst NW. The endocrinology of stress in fish: an environmental perspective. General and comparative endocrinology. 2011;170:265–275. doi: 10.1016/j.ygcen.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001:29. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottinger TG. Fish Welfare. Blackwell Publishing Ltd; UK: 2008. The stress response in fish-mechanisms, effects and measurement; pp. 32–48. [Google Scholar]
- Ringø E, Zhou Z, He S, Olsen RE. Effect of stress on intestinal microbiota of Arctic charr, Atlantic salmon, rainbow trout and Atlantic cod: A review. Afr. J. Microbiol. Res. 2014:8. [Google Scholar]
- Salinas I, Zhang YA, Sunyer JO. Mucosal immunoglobulins and B cells of teleost fish. Dev. Comp. Immunol. 2011;35:1346–1365. doi: 10.1016/j.dci.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasquete C, Gisbert E, Ribeiro L, Vieira L, Dinis M. Glyconjugates in epidermal, branchial and digestive mucous cells and gastric glands of gilthead sea bream, Sparus aurata, Senegal sole, Solea senegalensis and Siberian sturgeon, Acipenser baeri development. Eur. J. Histochem. 2001;45:267–278. doi: 10.4081/1637. [DOI] [PubMed] [Google Scholar]
- Shephard KL. Functions for fish mucus. Rev. Fish Biol. fisher. 1994;4:401–429. [Google Scholar]
- Söderholm JD, Perdue MH. II. Stress and intestinal barrier function. American J. Physiol.-Gastrointestinal and Liver Physiology. 2001;280:G7–G13. doi: 10.1152/ajpgi.2001.280.1.G7. [DOI] [PubMed] [Google Scholar]
- Tacchi L, Larragoite E, Salinas I. Discovery of J Chain in African Lungfish (Protopterus dolloi, Sarcopterygii) Using High Throughput Transcriptome Sequencing: Implications in Mucosal Immunity. PLoS One. 2013;8:e70650. doi: 10.1371/journal.pone.0070650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011;35:1366–1375. doi: 10.1016/j.dci.2011.07.002. [DOI] [PubMed] [Google Scholar]
- van Kemenade B, Chadzinska M. The impact of stress on immune regulation. Wszechswiat. 2009 [Google Scholar]
- Vatsos I, Kotzamanis Y, Henry M, Angelidis P, Alexis M. Monitoring stress in fish by applying image analysis to their skin mucous cells. Eur. J. Histochem. 2010;54:e22. doi: 10.4081/ejh.2010.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velin ÅK, Ericson A-C, Braaf Y, Wallon C, Söderholm JD. Increased antigen and bacterial uptake in follicle associated epithelium induced by chronic psychological stress in rats. Gut. 2004;53:494–500. doi: 10.1136/gut.2003.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Parra D, Gómez D, Salinas I, Zhang Y-A, von Gersdorff Jørgensen L, Heinecke RD, Buchmann K, LaPatra S, Sunyer JO. Teleost skin, an ancient mucosal surface that elicits gut-like immune responses. Proc. Natl. Acad. Sci. U.S.A. 2013;110:13097–13102. doi: 10.1073/pnas.1304319110. [DOI] [PMC free article] [PubMed] [Google Scholar]