Abstract
Adult liver transplant (LT) recipients commonly develop advanced kidney disease. However, burden of end-stage kidney disease (ESKD) after pediatric LT has not been well-described. We performed a retrospective cohort study of pediatric LTs in the US from 1990–2010. Multivariable Cox regression models were fit to determine risk factors for ESKD and death. 8976 children received LTs. During median follow-up of 7.8 years, 2005 (22%) subjects died (mortality rate 26.1 cases/1000 person-years); 167 (2%) developed ESKD (incidence rate 2.2 cases/1000 person-years). Risk factors for ESKD included older age at LT (highest risk age>15 vs <5 years, HR=4.94, p<0.001), hepatitis C (HR 2.79, p=0.004), liver re-transplant (HR 2.67, p<0.001), eGFR pre-LT <60 vs ≥60 (HR 2.37, p<0.001), hepatitis B (HR 2.25, p=0.027), black race (HR 1.46, p=0.046), and male sex (HR 1.44, p=0.022). LT recipients with ESKD had increased risk of mortality (HR 2.37, p<0.001). Among pediatric LT recipients, rate of ESKD was lower than among adults and far exceeded by rate of death, however follow-up time in this study may underestimate lifetime burden of ESKD. Although uncommon, ESKD was highly associated with mortality. Pediatric LT recipients should be routinely monitored for kidney disease, particularly those at highest risk of ESKD.
Keywords: End-stage renal disease, liver transplantation, pediatric liver transplant, mortality, Scientific Registry of Transplant Recipients
Introduction
End-stage kidney (ESKD) disease occurs commonly after adult liver transplantation (LT) but has seldom been studied among children (1–3). ESKD is an important complication of LT because of the increased mortality seen in adult LT recipients (3) as well as the significant morbidities associated with chronic dialysis. The incidence of and risk factors for ESKD among pediatric LT recipients may differ from adults given unique causes of pediatric end-stage liver disease, lower baseline rates of chronic kidney disease (CKD) in children, and lower prevalence of comorbidities such as hypertension and diabetes. Identification of pediatric LT recipients at high risk of ESKD is essential to ensure appropriate monitoring for earlier stages of CKD and implementation of renoprotective measures which may slow progression of kidney damage.
ESKD post-LT is likely caused by a multitude of factors, most notably long-term exposure to calcineurin inhibitor (CNI)-based immunosuppression, including cyclosporine and tacrolimus, which leads to chronic nephrotoxicity characterized by interstitial fibrosis, tubular atrophy, glomerulosclerosis, and, in some cases, thrombotic microangiopathy (4–7). Additional factors which may contribute to ESKD include duration and degree of impaired pre-LT kidney function, peri-transplant acute kidney injury, and hypertension (8–16).
Existing studies of kidney dysfunction after pediatric LT have been limited in assessing the outcome of ESKD due to single-center experiences or insufficient follow-up time. In order to address these limitations, we examined a national cohort of pediatric LT recipients to determine the incidence of ESKD, to identify risk factors for death and ESKD, and to determine the impact of ESKD on mortality in this population.
Materials and Methods
Sources of data
This study used a linked dataset from the Scientific Registry of Transplant Recipients (SRTR) and the United States Renal Data System (USRDS). The SRTR includes demographic and clinical data on all donors, wait-listed candidates, and transplant recipients in the US. Outcomes of death in SRTR are determined through center reports as well as through linkage to the Social Security Death Master File. ESKD outcomes were ascertained through SRTR data on kidney transplantation and Centers for Medicare and Medicaid Services (CMS) form 2728 for chronic dialysis submitted to USRDS. The Institutional Review Board at the Children’s Hospital of Philadelphia deemed this study exempt under provisions of the Code of Federal Regulations 45 CFR 46.101, category 4.
Study Subjects
The study population included children aged ≤18 years who received a first LT in the US between January 1, 1990 and March 1, 2010. The SRTR datafile was obtained in March 2011. Therefore, March 2010 was chosen as the end date for inclusion of subjects to allow for lags in center reporting to SRTR and CMS data in USRDS. We excluded subjects who received a combined organ transplant (liver in combination with kidney, heart, lung, intestine, and/or pancreas) as these patients may have a different risk of ESKD related to immunosuppressive regimen or comorbidities of their primary disease. We also excluded subjects if they had ESKD prior to LT.
Analytic Approach
We assessed baseline demographic and clinical characteristics of the study population using median and interquartile (IQR) ranges for continuous variables and distributions for categorical variables. The primary endpoints were death and ESKD, defined as initiation of chronic dialysis or receipt of a kidney transplant. Date of ESKD was considered the first date reported on CMS form 2728 submitted to USRDS or date of kidney transplant reported in SRTR, whichever occurred first. ESKD was categorized as pre-emptive kidney transplant if the subject had no dialysis prior to receipt of kidney transplant, kidney transplant after dialysis, or chronic dialysis if the subject remained on dialysis without receiving a kidney transplant at death or last follow-up. Subjects were followed from the date of LT until ESKD, death, or March 1, 2011, whichever occurred first. The last ESKD event captured in this dataset was December 22, 2010 and the last death event was February 22, 2011.
We performed a competing risks regression to estimate the cumulative incidence of ESKD, treating death as a competing risk for ESKD. We fit separate multivariable Cox regression models for the outcomes of ESKD and death. We inspected graphical displays and statistical tests of proportionality of hazards to confirm that the proportional hazards assumption was satisfied. On the basis of prior studies about risks for ESKD and clinical experience, we a priori identified independent variables for these models. Recipient variables included age at transplant (categorized as <5 years, 5 years to <10 years, 10 years to <15 years, and 15 years to ≤18 years), era of transplant (categorized in 5- or 6-year intervals from 1990 to 2010), sex, race, presence of underlying liver diseases that are often associated with concomitant kidney disease or injury (defined as Alagilles syndrome, alpha-1 anti-trypsin deficiency, congenital hepatic fibrosis, cystic fibrosis, glycogen storage diseases, primary hyperoxaluria, tyrosinemia, or Wilsons disease), type of donor (living or deceased), transplant center volume (categorized in tertiles of median yearly volume), hepatitis B serostatus (categorized as documented hepatitis B if there was a positive hepatitis B core antibody or a primary diagnosis code for acute or chronic hepatitis B), hepatitis C serostatus (categorized as documented hepatitis C if there was a positive hepatitis C serology available or a primary diagnosis code for acute or chronic hepatitis C), type of immunosuppressive therapy at transplant discharge (categorized as cyclosporine-based, tacrolimus-based, or other), and estimated GFR (eGFR) at the time of transplant, calculated using the bedside CKiD formula (0.413*height/serum creatinine) with the creatinine reported in SRTR at the time of LT (17). We also treated liver re-transplant as a time-varying covariate in the analysis of risk factors for ESKD. Subjects who received a second liver transplant during the follow-up period were treated as exposed to a single liver from the time of first transplant until time of second transplant, and then exposed to a repeat liver from the time of second transplant until ESKD, death, or end of follow-up. Independent variables with a p value <0.2 in univariable analyses were entered into the final multivariable models.
Baseline eGFR at the time of LT was a priori identified as an important variable to include in assessing risk factors for ESKD. However, 1410 (16%) subjects lacked data on either creatinine at the time of LT or height, which are both required to calculate eGFR using the bedside CKiD formula. A minority of subjects lacked data on creatinine at the time of LT (n=239, 3%). These subjects were categorized as “missing eGFR” in the primary analysis. An additional 1247 (14%) subjects lacked data on height alone. The LMS method was used to assign a sex- and age-based standard deviation score (SDS) to every subject with reported height (18). The median reported height SDS was -1.23, corresponding to a median height at approximately the 10th percentile. Therefore, for subjects with no reported height, the 10th percentile for age and sex was used to impute height using Centers for Disease Control 2000 growth reference data (19). In primary analyses, we used the imputed 10th percentile heights to calculate eGFR values for those with missing height. In a sensitivity analysis, we repeated the analyses imputing missing heights with the 25th percentile and 50th percentile for age and sex. We also repeated the analyses with missing height data as “missing eGFR”.
In a secondary analysis, we created Kaplan-Meier plots to examine the effect of ESKD on mortality after LT. In this analysis, subjects were excluded if they died during the first year after LT, as these deaths were thought to be primarily driven by peri-transplant complications, such as infection and graft dysfunction, rather than by kidney disease.
Analyses were conducted using Stata 12.0 (Stata Corporation, College Station, TX). All reported p values are two-sided, and a p value <0.05 was the threshold for statistical significance.
Results
Baseline Characteristics
There were 8976 pediatric LTs performed at 126 transplant centers during the study period. The baseline demographic and clinical characteristics of the cohort are summarized in Table 1. The median age at LT was 2.3 years [interquartile range (IQR) 0.8, 10.3]. Biliary atresia was the most common cause of liver failure (39%). Almost 14% of subjects had underlying liver disease that is often associated with concomitant kidney disease or injury. Only 2% of subjects had documented hepatitis B status, and 2% had documented hepatitis C status. Most subjects were treated with tacrolimus-based immunosuppression at the time of LT (n=5724, 64%). Cyclosporine-based immunosuppression was used in 2353 (26%) subjects. There were only 11 (0.1%) subjects treated with sirolimus without a CNI at the time of transplant discharge. There were an additional 73 (0.8%) subjects treated with a combination of a CNI and sirolimus, and these subjects were grouped with the respective CNI category. The vast majority of children had normal kidney function by eGFR at the time of transplant, with a median eGFR of 106.7 [79.8, 139.4] ml/min/1.73m2. Only 13% of children had an eGFR<60 ml/min/1.73m2 at the time of LT. There were 1333 (15%) subjects who received a second liver transplant during the follow-up period.
Table 1.
Baseline Characteristics of Pediatric Liver Transplant Recipients, 1990–2010
| Characteristic | N=8976 |
|---|---|
|
| |
| Age in years at liver transplant, median [IQR] | 2.3 [0.8, 10.3] |
|
| |
| Male sex, n(%) | 4318 (48) |
|
| |
| Race, n(%) | |
| White | 5145 (57) |
| Black | 1621 (18) |
| Asian | 369 (4) |
| Other | 1841 (21) |
|
| |
| Year of liver transplant, n(%) | |
| 1990–1994 | 2118 (24) |
| 1995–1999 | 2209 (25) |
| 2000–2004 | 2287 (26) |
| 2005–2010 | 2362 (26) |
|
| |
| Primary Cause of Liver Failure, n(%) | |
| Biliary atresia | 3453 (39) |
| Other cholestatic | 1193 (13) |
| Fulminant liver failure | 1269 (14) |
| Metabolic disease* | 1240 (14) |
| Cirrhosis | 830 (9) |
| Tumor | 476 (5) |
| Other | 515 (6) |
|
| |
| Underlying liver disease with potential kidney involvement**, n(%) | 1222 (14) |
|
| |
| Living donor, n(%) | 1210 (14) |
|
| |
| Location at time of liver transplant, n(%) | |
| Hospitalized, intensive care unit | 2427 (27) |
| Hospitalized, non-intensive care unit | 1637 (18) |
| Not hospitalized | 4905 (55) |
|
| |
| Immunosuppressive therapy at time of transplant, n(%) | |
| Cyclosporine-based | 2353 (26) |
| Tacrolimus-based | 5724 (64) |
| Other | 209 (2) |
| Missing | 690 (8) |
|
| |
| Documented hepatitis B, n(%) | 174 (2) |
|
| |
| Documented hepatitis C, n(%) | 169 (2) |
|
| |
| Pre-transplant serum creatinine mg/dL, median [IQR] | 0.4 [0.2, 0.5] |
|
| |
| Pre-transplant eGFR ml/min/1.73 m2, median [IQR]*** | 106.7 [79.8, 139.4] |
|
| |
| Pre-transplant eGFR ml/min/1.73 m2, n(%) | |
| ≥90 | 5683 (63) |
| 60–89 | 1921 (21) |
| 30–59 | 811 (9) |
| <30 | 318 (4) |
| Missing | 243 (3) |
|
| |
| Last MELD/PELD score before transplant, median [IQR] | 18 [11, 27] |
Alpha-1 anti-trypsin deficiency, cystic fibrosis, hemochromatosis, homozygous hypercholesterolemia, glycogen storage diseases, maple syrup urine disease, primary hyperoxaluria, tyrosinemia, Wilsons disease, other metabolic
Alagilles syndrome, alpha-1 anti-trypsin deficiency, congenital hepatic fibrosis, cystic fibrosis, glycogen storage diseases, primary hyperoxaluria, tyrosinemia, Wilsons disease
Using bedside CKiD formula: eGFR=0.413*height (cm)/serum creatinine
Incidence of ESKD and Death
During a median follow-up time of 7.8 years [IQR 2.9, 13.5], 167 (2%) subjects developed ESKD, with an incidence rate of 2.2 cases per 1000 person-years. Pre-emptive kidney transplantation was performed in 49 (29%) subjects. Dialysis was initiated in the remaining 118 (71%) subjects; 58 of these subjects eventually received a kidney transplant, and 60 subjects remained on chronic dialysis until death or last follow-up. The median time from LT to ESKD was 9.0 years [IQR 4.4, 13.4]. Figure 1 shows the cumulative incidence of ESKD, with death treated as a competing risk.
Figure 1.
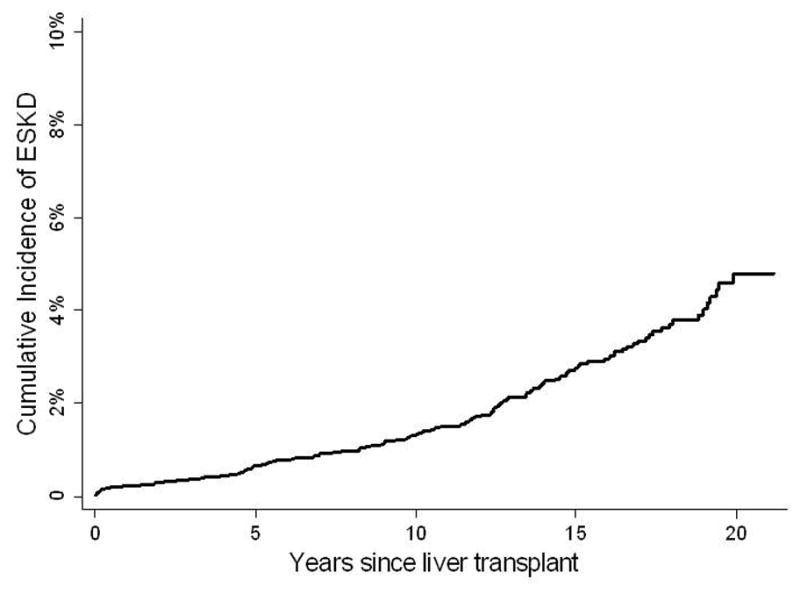
Cumulative incidence of ESKD
During the same follow-up time, 2005 (22%) subjects died, with a mortality rate of 26.1 cases per 1000 person-years. The majority of deaths occurred early after LT, with a median time to death of only 0.6 years [IQR 0.1, 3.8]. Of the 2005 deaths in this cohort, 953 (48%) were reported by both the Social Security Death Master File and the transplant center, 984 (49%) were missed by the Death Master File but captured by the center, and only 68 (3%) were reported in the Death Master File but not by the center.
Risk Factors for ESKD and Mortality
Table 2 shows recipient risk factors associated with the development of ESKD in univariable and multivariable Cox regression. Older age at transplant compared to age<5 years was associated with ESKD in an age-dependent manner, with the highest risk in subjects older than age 15 years (HR=4.94, 95% CI 3.29–7.42, p<0.001). Male sex (HR 1.44, 95% CI 1.05–1.96, p=0.022), black compared to non-black race (HR 1.46, 95% CI 1.01–2.13, p=0.046), documented hepatitis B (HR 2.25, 95% CI 1.10–4.62, p=0.027), documented hepatitis C (HR 2.79, 95% CI 1.40–5.58, p=0.004), and eGFR pre-transplant <60 compared to eGFR ≥60 ml/min/1.73m2 (HR 2.37, 95% CI 1.66–3.37, p<0.001) were all independently associated with the development of ESKD after LT. Receiving a second liver transplant, treated as a time-varying covariate, was also independently associated with ESKD (HR 2.67, 95% CI 1.88–3.80, p<0.001), adjusted for all other variables. Era of transplant, liver disease with the potential for primary kidney involvement, living versus deceased donor, center volume, and type of immunosuppressive therapy at the time of transplant were not associated with the development of ESKD.
Table 2.
Risk Factors for ESKD after Pediatric Liver Transplantation: Results of Univariable and Multivariable Cox Regression
| Variable | Unadjusted Hazard Ratio for ESKD* (95% conf int) | p value | Adjusted Hazard Ratio for ESKD* (95% conf int) | p value |
|---|---|---|---|---|
|
| ||||
| Age at liver transplant | ||||
| 0 to <5 years | Reference | Reference | ||
| 5 to <10 years | 2.64 (1.66–4.19) | <0.001 | 2.75 (1.72–4.40) | <0.001 |
| 10 to <15 years | 4.30 (2.83–6.52) | <0.001 | 3.93 (2.57–6.00) | <0.001 |
| 15 to 18 years | 5.48 (3.67–8.18) | <0.001 | 4.94 (3.29–7.42) | <0.001 |
|
| ||||
| Male sex | 1.43 (1.05–1.94) | 0.023 | 1.44 (1.05–1.96) | 0.022 |
|
| ||||
| Black vs non-black race | 1.42 (0.99–2.05) | 0.059 | 1.46 (1.01–2.13) | 0.046 |
|
| ||||
| Era of transplant | ||||
| 1990–1994 | Reference | Reference | ||
| 1995–1999 | 0.63 (0.42–0.94) | 0.024 | 0.67 (0.44–1.00) | 0.050 |
| 2000–2004 | 0.83 (0.51–1.36) | 0.467 | 0.87 (0.53–1.42) | 0.573 |
| 2005–2010 | 0.96 (0.49–1.88) | 0.905 | 1.11 (0.56–2.17) | 0.765 |
|
| ||||
| Liver disease with potential primary kidney involvement** | 1.41 (0.97–2.04) | 0.070 | 1.30 (0.89–1.91) | 0.175 |
|
| ||||
| Hepatitis B | 3.09 (1.52–6.29) | 0.002 | 2.25 (1.10–4.62) | 0.027 |
|
| ||||
| Hepatitis C | 4.16 (2.12–8.16) | <0.001 | 2.79 (1.40–5.58) | 0.004 |
|
| ||||
| eGFR pre-LT <60 vs ≥60, ml/min/1.73m2 | 2.31 (1.63–3.28) | <0.001 | 2.37 (1.66–3.37) | <0.001 |
|
| ||||
| Liver re-transplant*** | 2.78 (1.96–3.94) | <0.001 | 2.67 (1.88–3.80) | <0.001 |
Multivariable Cox regression for ESKD is censored at death and adjusted for other variables in table
Alagilles syndrome, alpha-1 anti-trypsin deficiency, congenital hepatic fibrosis, cystic fibrosis, glycogen storage diseases, primary hyperoxaluria, tyrosinemia, Wilsons disease
Liver re-transplant is treated as a time-varying covariate
Table 3 shows recipient risk factors associated with death in univariable and multivariable Cox regression. There was a stepwise decrease in the risk of death with each subsequent era compared to LT during 1990–1994, with the lowest risk in the most recent era of 2005–2010 (HR 0.45, 95% CI 0.39–0.53, p<0.001). Black compared to non-black race (HR 1.30, 95% CI 1.17–1.44, p<0.001) and documented hepatitis C (HR 1.84, 95% CI 1.44–2.35, p<0.001) were also independently associated with death after LT. Subjects categorized as having liver disease with the potential for primary kidney involvement had a decreased risk of death compared to all other diagnoses (HR 0.84, 95% CI 0.74–0.96, p=0.01). In addition to being independently associated with the development of ESKD post-LT, eGFR pre-transplant <60 compared to eGFR ≥60 ml/min/1.73m2 was also independently associated with mortality (HR 1.51, 95% CI 1.35–1.70, p<0.001).
Table 3.
Risk Factors for Death after Pediatric Liver Transplantation: Results of Univariable and Multivariable Cox Regression
| Variable | Unadjusted Hazard Ratio for Death (95% conf int) | p value | Adjusted Hazard Ratio for Death (95% conf int)* | p value |
|---|---|---|---|---|
|
| ||||
| Age at liver transplant | ||||
| 0 to <5 years | Reference | Reference | ||
| 5 to <10 years | 0.89 (0.77–1.02) | 0.101 | 0.92 (0.80–1.06) | 0.256 |
| 10 to <15 years | 1.06 (0.93–1.21) | 0.399 | 1.05 (0.92–1.21) | 0.441 |
| 15 to 18 years | 1.48 (1.32–1.67) | <0.001 | 1.43 (1.27–1.61) | <0.001 |
|
| ||||
| Male sex | 0.99 (0.91–1.09) | 0.895 | N/A | |
|
| ||||
| Black vs non-black race | 1.34 (1.21–1.49) | 0.059 | 1.30 (1.17–1.44) | <0.001 |
|
| ||||
| Era of transplant | ||||
| 1990–1994 | Reference | Reference | ||
| 1995–1999 | 0.79 (0.71–0.88) | <0.001 | 0.79 (0.71–0.89) | <0.001 |
| 2000–2004 | 0.64 (0.57–0.72) | <0.001 | 0.63 (0.55–0.71) | <0.001 |
| 2005–2010 | 0.45 (0.39–0.52) | <0.001 | 0.45 (0.39–0.53) | <0.001 |
|
| ||||
| Liver disease with potential primary kidney involvement** | 0.86 (0.76–0.99) | 0.029 | 0.84 (0.74–0.96) | 0.010 |
|
| ||||
| Hepatitis B | 1.09 (0.80–1.48) | 0.592 | N/A | |
|
| ||||
| Hepatitis C | 1.98 (1.55–2.51) | <0.001 | 1.84 (1.44–2.35) | <0.001 |
|
| ||||
| eGFR pre-LT <60 vs ≥60, ml/min/1.73m2 | 1.62 (1.44–1.82) | <0.001 | 1.51 (1.35–1.70) | <0.001 |
Adjusted for other variables in table
Alagilles syndrome, alpha-1 anti-trypsin deficiency, congenital hepatic fibrosis, cystic fibrosis, glycogen storage diseases, primary hyperoxaluria, tyrosinemia, Wilsons disease
Effect of ESKD on Mortality
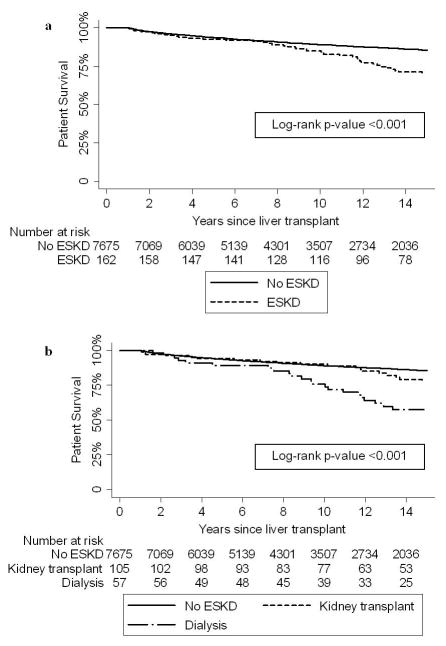
There were 7837 (87%) subjects who survived the first year after LT and were included in the analysis of the impact of ESKD on mortality. The unadjusted HR for death was 2.37 (95% CI 1.80–3.12, p<0.001) for those with ESKD versus those without ESKD (Figure 2). Adjusted for age at LT, sex, and race, the HR for death was 1.82 (95% CI 1.38–2.40, p<0.001) for those with ESKD versus no ESKD. Adjusted for age at LT, sex, and race, the HR for death was not significantly different among those subjects who received a kidney transplant, either preemptively or after a period on dialysis, compared to subjects without ESKD (HR 1.41, 95% CI 0.94–2.10, p=0.09). However, the adjusted HR for death was significantly higher among those subjects who remained on dialysis compared to those without ESKD (HR 2.43, 95% CI 1.67–3.55, p<0.001).
Figure 2.
Kaplan-Meier estimates of death by presence of ESKD
In the sensitivity analysis using original height data and coding the 16% of subjects with missing height and/or creatinine as “missing GFR”, we found a similar effect of pre-LT eGFR on ESKD and mortality. Pre-LT eGFR<60 vs ≥60 ml/min/1.73m2 was associated with increased risk of ESKD (HR 2.45, 95% CI 1.68–3.58, p<0.001). Those with missing eGFR data were not significantly different compared to eGFR ≥60 ml/min/1.73m2 (HR 0.94, 95% CI 0.58–1.52, p=0.81) with respect to ESKD risk. Compared to eGFR ≥60 ml/min/1.73m2, lower pre-LT eGFR was also associated with increased risk of mortality (HR 1.50, 95% CI 1.32–1.70, p<0.001). Those with missing eGFR data were not significantly different compared to eGFR ≥60 ml/min/1.73m2 (HR 1.10, 95% CI 0.97–1.24, p=0.13) with respect to death risk. The results of the secondary analyses using 25th and 50th percentile imputed heights were similar to our primary results and not shown.
Discussion
In this study, we report on the long-term mortality rates and risk factors for ESKD in a 20 year national cohort of pediatric LT recipients. ESKD was uncommon and far exceeded by the rate of death, but the risk of death was more than twice as high in individuals developing ESKD after LT. Our analysis highlights a number of potential targets for intervention to decrease morbidity and mortality in children receiving LTs.
We found a rate of post-LT ESKD of 2.2 cases per 1000 person-years, much lower than the reported rate of 14.5 cases per 1000 person-years in adult LT recipients (3). This lower rate of ESKD among pediatric LT recipients may be due to the low prevalence of comorbidities in this population including baseline impaired kidney function, hypertension, diabetes, and hepatitis. Consistent with studies assessing risk factors for ESKD in adults, we identified male sex, black race, hepatitis, and lower pre-LT eGFR as significant predictors of post-LT ESKD (3). In addition, we identified risk factors unique to the pediatric population including a stepwise increase in the risk of ESKD with increasing age at LT, with the highest risk in those older than 15 years, and liver re-transplant. Also consistent with previous reports in adult LT recipients, we found that lower pre-LT eGFR was independently associated with mortality (9, 12).
Although we found a lower rate of ESKD in the pediatric LT population, it is important to consider that the potential person-years of risk encountered by pediatric patients is much greater than among adults. Although this study included a cohort of children receiving LTs over a 20 year period, the median follow-up time was only approximately 8 years; therefore, we may be underestimating the lifetime risk of ESKD in this population. We also know that ESKD represents only a small proportion of all cases of CKD, and the low ESKD incidence rate reported in this study should not diminish the importance of detecting and treating long-term kidney dysfunction in this population, as the burden of earlier stages of disease is certainly much higher. This prevalence of CKD has been described in multiple small studies of pediatric LT recipients, with the rate of CKD reported as high as 31%, depending on the definition of CKD and duration of follow-up (10, 20–25). Additionally, non-transplant studies consistently show that the natural history of CKD in children is characterized by a steady decline in kidney function over time. For example, in the Chronic Kidney Disease in Children (CKiD) multicenter prospective cohort study of children with CKD, there was a median decline in GFR of -4.3 ml/min/1.73m2 and -1.5 ml/min/1.73m2 per year in children with glomerular and nonglomerular diagnoses, respectively (26), so with longer follow-up time, it is likely that some subjects in this cohort with earlier stages of CKD would progress to ESKD. Although we can not necessarily determine the lifetime burden of ESKD with this data, this study still provides the largest assessment of risk of ESKD in pediatric LT recipients.
We report a 1.8-fold higher risk of death in subjects with ESKD overall versus those without ESKD. CKD and ESKD have previously been shown to have a significant effect on mortality after LT in adults (1, 3), similar to the increased risk of mortality from ESKD in the general population (27–29). The impact on survival in this cohort seems to be primarily driven by the increased mortality among those subjects who remained on chronic dialysis without ever receiving a kidney transplant. Kidney transplantation is associated with improved patient survival compared to dialysis (30); however, it is important to consider that the increased mortality seen in dialysis patients may be confounded by selection of healthier subjects for kidney transplantation. Because there are very few absolute contraindications for kidney transplantation in pediatric patients, it is likely that those children in our cohort who remained on dialysis had a medical condition or complication which both precluded kidney transplantation and carried a high risk for death.
Identifying patients at highest risk of ESKD post-LT is critical, as implementation of renoprotective measures at earlier stages of CKD may prevent progressive kidney damage. Use of CNI-sparing immunosuppressive protocols (31–34), close monitoring for hypertension and strict blood pressure control (35, 36), use of agents that target the renin-angiotensin-aldosterone system (37, 38), and avoidance of potentially nephrotoxic medications (39) are all modifiable and may potentially slow progression of CKD.
This study has a number of limitations. First, as we analyzed registry data, missing data were common and misclassification of important exposures was possible. For example, 14% of subjects had missing height data, which is necessary to estimate pre-LT GFR. Thus, we imputed height based on the 10th percentile for age and sex. Analyses with and without this imputation yielded similar results. We also used the bedside CKiD formula to estimate GFR at the time of LT. Traditional creatinine-based estimates of GFR have been shown to overestimate measured kidney function in LT patients (40, 41). This is likely because chronic illness, malnutrition, and decreased muscle mass in LT patients lead to lower concentrations of serum creatinine, independent of kidney function.(42). The bedside CKiD formula was developed in a cohort of children with CKD (17). While it performs more accurately than other estimates of GFR in a CKD population, it has been shown to potentially underestimate kidney function in a non-CKD population (43). Given that the majority of children had a pre-transplant eGFR>90 ml/min/1.73 m2 in this cohort, it is possible that we have underestimated true GFR among some members of the cohort. However, if this were true, this would only serve to underestimate the impact of pre-transplant eGFR on post-transplant ESKD. As this is a retrospective study using previously collected data, we are unable to perform a measured GFR study, which would be a more accurate way of categorizing pre-LT kidney function.
Additionally, we had no information about important risks for kidney disease after LT, such as long-term choice of immunosuppressive regimen and trough concentrations of CNI’s over time. We did examine for an effect of immunosuppressive therapy at the time of LT, which was not associated with an increased risk of post-LT ESKD. The relationship between immunosuppressive therapy and ESKD should be further assessed in prospective studies. An additional risk in analyses of administrative data is the possibility of misclassification of covariates, but this misclassification would likely be non-differential and tend to bias the result toward the null, whereas we identified a number of important risk factors that were significantly associated with ESKD.
Finally, although we chose objective, well-captured outcomes of ESKD and death, it is possible that some outcomes were missed. Some subjects receiving chronic dialysis could be missed in the USRDS data system if CMS form 2728 was not submitted. We should have complete capture of post-LT kidney transplant outcomes, as we obtained these data through SRTR kidney transplant data. Mortality could be missed in the Social Security Death Master File for pediatric subjects without a Social Security number or if no claims were made for Social Security benefits. However, by supplementing Death Master File data with center mortality reporting, we believe we should have near complete capture of mortality outcomes.
In conclusion, in a 20 year national cohort of pediatric LT recipients, the rate of ESKD was much lower than adults and was far exceeded by the rate of death. However, the burden of earlier stages of CKD and the lifetime risk of ESKD are likely higher. Important recipient risk factors for post-LT ESKD included older age at LT, male sex, black race, hepatitis B seropositivity, hepatitis C seropositivity, liver re-transplant and lower eGFR pre-LT. ESKD was associated with a significantly increased risk of mortality in pediatric LT recipients, particularly for those children who remained on chronic dialysis. Screening for earlier stages of CKD with routine assessments of creatinine, estimated GFR, or ideally measured GFR as well as monitoring for hypertension and proteinuria are important aspects of post-LT care, especially for those children with risk factors for ESKD identified in this study. Future studies will focus on the effectiveness of renoprotective measures in slowing progression to or preventing ESKD in pediatric LT recipients.
Acknowledgments
Funding sources:
Dr. Reese is supported by NIH grant K23 - DK078688-01
Dr. Furth is supported by K24DK078737 and U01 DK066174
The SRTR comprises information on all donors, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration, U.S. Department of Health and Human Services, provides oversight to the activities of the OPTN and SRTR contractors. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Abbreviations
- CKD
chronic kidney disease
- CMS
Centers for Medicare and Medicaid Services
- CNI
calcineurin inhibitor
- ESKD
end-stage kidney disease
- GFR
glomerular filtration rate
- HR
hazard ratio
- LT
liver transplant
- OPTN
Organ Procurement and Transplantation Network
- SRTR
Scientific Registry of Transplant Recipients (SRTR)
- USRDS
United States Renal Data Systems
Footnotes
Conflicts of interest: The authors have no financial conflicts of interest to disclose as defined by the American Journal of Transplantation.
References
- 1.Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349(10):931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 2.Gonwa TA, Mai ML, Melton LB, Hays SR, Goldstein RM, Levy MF, et al. End-stage renal disease (ESRD) after orthotopic liver transplantation (OLTX) using calcineurin-based immunotherapy: risk of development and treatment. Transplantation. 2001;72(12):1934–1939. doi: 10.1097/00007890-200112270-00012. [DOI] [PubMed] [Google Scholar]
- 3.Sharma P, Schaubel DE, Guidinger MK, Goodrich NP, Ojo AO, Merion RM. Impact of MELD-based allocation on end-stage renal disease after liver transplantation. Am J Transplant. 2011;11(11):2372–2378. doi: 10.1111/j.1600-6143.2011.03703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baran DA, Galin ID, Gass AL. Calcineurin inhibitor-associated early renal insufficiency in cardiac transplant recipients: risk factors and strategies for prevention and treatment. Am J Cardiovasc Drugs. 2004;4(1):21–29. doi: 10.2165/00129784-200404010-00003. [DOI] [PubMed] [Google Scholar]
- 5.Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4(2):481–508. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- 6.Pillebout E, Nochy D, Hill G, Conti F, Antoine C, Calmus Y, et al. Renal histopathological lesions after orthotopic liver transplantation (OLT) Am J Transplant. 2005;5(5):1120–1129. doi: 10.1111/j.1600-6143.2005.00852.x. [DOI] [PubMed] [Google Scholar]
- 7.Tonshoff B, Hocker B. Treatment strategies in pediatric solid organ transplant recipients with calcineurin inhibitor-induced nephrotoxicity. Pediatr Transplant. 2006;10(6):721–729. doi: 10.1111/j.1399-3046.2006.00577.x. [DOI] [PubMed] [Google Scholar]
- 8.Campbell MS, Kotlyar DS, Brensinger CM, Lewis JD, Shetty K, Bloom RD, et al. Renal function after orthotopic liver transplantation is predicted by duration of pretransplantation creatinine elevation. Liver Transpl. 2005;11(9):1048–1055. doi: 10.1002/lt.20445. [DOI] [PubMed] [Google Scholar]
- 9.Gonwa TA, McBride MA, Anderson K, Mai ML, Wadei H, Ahsan N. Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: where will MELD lead us? Am J Transplant. 2006;6(11):2651–2659. doi: 10.1111/j.1600-6143.2006.01526.x. [DOI] [PubMed] [Google Scholar]
- 10.Harambat J, Ranchin B, Dubourg L, Liutkus A, Hadj-Haissa A, Rivet C, et al. Renal function in pediatric liver transplantation: a long-term follow-up study. Transplantation. 2008;86(8):1028–1034. doi: 10.1097/TP.0b013e318187748f. [DOI] [PubMed] [Google Scholar]
- 11.McLin VA, Anand R, Daniels SR, Yin W, Alonso EM. Blood pressure elevation in long-term survivors of pediatric liver transplantation. Am J Transplant. 2012;12(1):183–190. doi: 10.1111/j.1600-6143.2011.03772.x. [DOI] [PubMed] [Google Scholar]
- 12.Nair S, Verma S, Thuluvath PJ. Pretransplant renal function predicts survival in patients undergoing orthotopic liver transplantation. Hepatology. 2002;35(5):1179–1185. doi: 10.1053/jhep.2002.33160. [DOI] [PubMed] [Google Scholar]
- 13.O’Riordan A, Wong V, McCormick PA, Hegarty JE, Watson AJ. Chronic kidney disease post-liver transplantation. Nephrol Dial Transplant. 2006;21(9):2630–2636. doi: 10.1093/ndt/gfl247. [DOI] [PubMed] [Google Scholar]
- 14.Pawarode A, Fine DM, Thuluvath PJ. Independent risk factors and natural history of renal dysfunction in liver transplant recipients. Liver Transpl. 2003;9(7):741–747. doi: 10.1053/jlts.2003.50113. [DOI] [PubMed] [Google Scholar]
- 15.Velidedeoglu E, Bloom RD, Crawford MD, Desai NM, Campos L, Abt PL, et al. Early kidney dysfunction post liver transplantation predicts late chronic kidney disease. Transplantation. 2004;77(4):553–556. doi: 10.1097/01.tp.0000114609.99558.41. [DOI] [PubMed] [Google Scholar]
- 16.Gonwa TA. Hypertension and renal dysfunction in long-term liver transplant recipients. Liver Transpl. 2001;7(11 Suppl 1):S22–26. doi: 10.1053/jlts.2001.28511. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44(1):45–60. [PubMed] [Google Scholar]
- 19.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109(1):45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 20.Arora-Gupta N, Davies P, McKiernan P, Kelly DA. The effect of long-term calcineurin inhibitor therapy on renal function in children after liver transplantation. Pediatr Transplant. 2004;8(2):145–150. doi: 10.1046/j.1399-3046.2003.00132.x. [DOI] [PubMed] [Google Scholar]
- 21.Mention K, Lahoche-Manucci A, Bonnevalle M, Pruvot FR, Declerck N, Foulard M, et al. Renal function outcome in pediatric liver transplant recipients. Pediatr Transplant. 2005;9(2):201–207. doi: 10.1111/j.1399-3046.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 22.Campbell KM, Yazigi N, Ryckman FC, Alonso M, Tiao G, Balistreri WF, et al. High prevalence of renal dysfunction in long-term survivors after pediatric liver transplantation. J Pediatr. 2006;148(4):475–480. doi: 10.1016/j.jpeds.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Kivela JM, Raisanen-Sokolowski A, Pakarinen MP, Makisalo H, Jalanko H, Holmberg C, et al. Long-term renal function in children after liver transplantation. Transplantation. 2011;91(1):115–120. doi: 10.1097/tp.0b013e3181fa94b9. [DOI] [PubMed] [Google Scholar]
- 24.Campbell K, Ng V, Martin S, Magee J, Goebel J, Anand R, et al. Glomerular filtration rate following pediatric liver transplantation--the SPLIT experience. Am J Transplant. 2010;10(12):2673–2682. doi: 10.1111/j.1600-6143.2010.03316.x. [DOI] [PubMed] [Google Scholar]
- 25.Anastaze Stelle K, Belli DC, Parvex P, Girardin E, Giroud A, Wildhaber B, et al. Glomerular and tubular function following orthotopic liver transplantation in children treated with tacrolimus. Pediatr Transplant. 2012;16(3):250–256. doi: 10.1111/j.1399-3046.2011.01625.x. [DOI] [PubMed] [Google Scholar]
- 26.Furth SL, Abraham AG, Jerry-Fluker J, Schwartz GJ, Benfield M, Kaskel F, et al. Metabolic abnormalities, cardiovascular disease risk factors, and GFR decline in children with chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(9):2132–2140. doi: 10.2215/CJN.07100810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins AJFR, Herzog C, et al. Excerpts from the US Renal Data System 2009 Annual Data Report. Am J Kidney Dis. 2010;55(S1) doi: 10.1053/j.ajkd.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chertow GM, Johansen KL, Lew N, Lazarus JM, Lowrie EG. Vintage, nutritional status, and survival in hemodialysis patients. Kidney Int. 2000;57(3):1176–1181. doi: 10.1046/j.1523-1755.2000.00945.x. [DOI] [PubMed] [Google Scholar]
- 29.McDonald SP, Craig JC. Long-term survival of children with end-stage renal disease. N Engl J Med. 2004;350(26):2654–2662. doi: 10.1056/NEJMoa031643. [DOI] [PubMed] [Google Scholar]
- 30.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 31.Herlenius G, Felldin M, Norden G, Olausson M, Backman L, Gustafsson B, et al. Conversion from calcineurin inhibitor to either mycophenolate mofetil or sirolimus improves renal function in liver transplant recipients with chronic kidney disease: results of a prospective randomized trial. Transplant Proc. 2010;42(10):4441–4448. doi: 10.1016/j.transproceed.2010.09.113. [DOI] [PubMed] [Google Scholar]
- 32.Barkmann A, Nashan B, Schmidt HH, Boker KH, Emmanouilidis N, Rosenau J, et al. Improvement of acute and chronic renal dysfunction in liver transplant patients after substitution of calcineurin inhibitors by mycophenolate mofetil. Transplantation. 2000;69(9):1886–1890. doi: 10.1097/00007890-200005150-00025. [DOI] [PubMed] [Google Scholar]
- 33.Orlando G, Baiocchi L, Cardillo A, Iaria G, De Liguori Carino N, De Luca L, et al. Switch to 1. 5 grams MMF monotherapy for CNI-related toxicity in liver transplantation is safe and improves renal function, dyslipidemia, and hypertension. Liver Transpl. 2007;13(1):46–54. doi: 10.1002/lt.20926. [DOI] [PubMed] [Google Scholar]
- 34.Sindhi R, Webber S, Venkataramanan R, McGhee W, Phillips S, Smith A, et al. Sirolimus for rescue and primary immunosuppression in transplanted children receiving tacrolimus. Transplantation. 2001;72(5):851–855. doi: 10.1097/00007890-200109150-00019. [DOI] [PubMed] [Google Scholar]
- 35.Appel LJ, Wright JT, Jr, Greene T, Agodoa LY, Astor BC, Bakris GL, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363(10):918–929. doi: 10.1056/NEJMoa0910975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wuhl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361(17):1639–1650. doi: 10.1056/NEJMoa0902066. [DOI] [PubMed] [Google Scholar]
- 37.Artz MA, Hilbrands LB, Borm G, Assmann KJ, Wetzels JF. Blockade of the renin-angiotensin system increases graft survival in patients with chronic allograft nephropathy. Nephrol Dial Transplant. 2004;19(11):2852–2857. doi: 10.1093/ndt/gfh462. [DOI] [PubMed] [Google Scholar]
- 38.Wright JT, Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288(19):2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 39.Patzer L. Nephrotoxicity as a cause of acute kidney injury in children. Pediatr Nephrol. 2008;23(12):2159–2173. doi: 10.1007/s00467-007-0721-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonwa TA, Jennings L, Mai ML, Stark PC, Levey AS, Klintmalm GB. Estimation of glomerular filtration rates before and after orthotopic liver transplantation: evaluation of current equations. Liver Transpl. 2004;10(2):301–309. doi: 10.1002/lt.20017. [DOI] [PubMed] [Google Scholar]
- 41.Berding G, Geisler S, Melter M, Marquardt P, Luhr A, Scheller F, et al. Estimation of glomerular filtration rate in liver-transplanted children: comparison of simplified procedures using 51Cr-EDTA and endogenous markers with Sapirstein’s method as a reference standard. Pediatr Transplant. 2010;14(6):786–795. doi: 10.1111/j.1399-3046.2010.01342.x. [DOI] [PubMed] [Google Scholar]
- 42.Borrows R, Cockwell P. Measuring renal function in solid organ transplant recipients. Transplantation. 2007;83(5):529–531. doi: 10.1097/01.tp.0000255566.42513.00. [DOI] [PubMed] [Google Scholar]
- 43.Fadrowski JJ, Neu AM, Schwartz GJ, Furth SL. Pediatric GFR estimating equations applied to adolescents in the general population. Clin J Am Soc Nephrol. 2011;6(6):1427–1435. doi: 10.2215/CJN.06460710. [DOI] [PMC free article] [PubMed] [Google Scholar]