Abstract
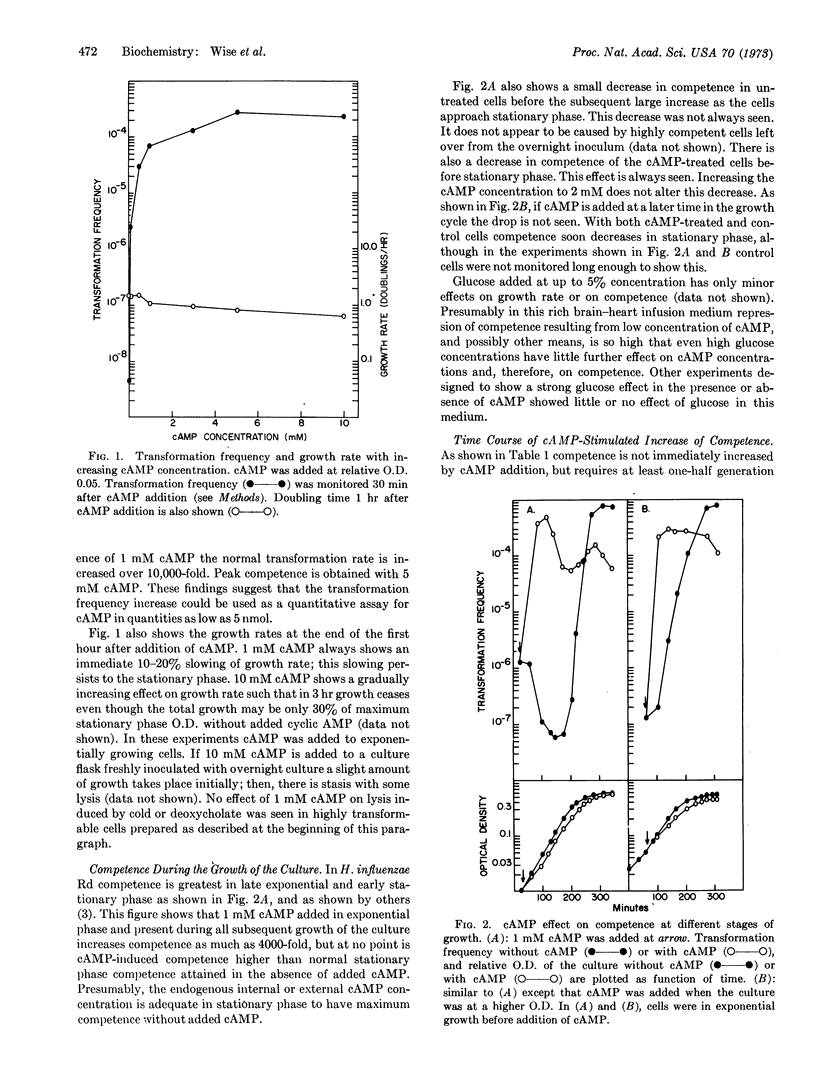
Adenosine 3′:5′-cyclic monophosphate added to exponentially growing cells of Hemophilus influenzae strain Rd increases competence for transformation 100- to 10,000-fold. Cyclic AMP added to near-stationary or stationary cells does not increase competence over the high level normally found in the early stationary phase.
Keywords: Hemophilus influenzae Rd, cyclic-AMP assay
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER H. E., LEIDY G. Determination of inherited traits of H. influenzae by desoxyribonucleic acid fractions isolated from type-specific cells. J Exp Med. 1951 Apr 1;93(4):345–359. doi: 10.1084/jem.93.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Barnhart B. J. Competence-stimulating activity in sterile filtrates from Hemophilus influenzae. Biochim Biophys Acta. 1967 Jul 18;142(2):465–474. doi: 10.1016/0005-2787(67)90627-2. [DOI] [PubMed] [Google Scholar]
- Dobrogosz W. J., Hamilton P. B. The role of cyclic AMP in chemotaxis in Escherichia coli. Biochem Biophys Res Commun. 1971 Jan 22;42(2):202–207. doi: 10.1016/0006-291x(71)90088-x. [DOI] [PubMed] [Google Scholar]
- Elmerich C., Aubert J. P. Role of glutamine synthetase in the repression of bacterial sporulation. Biochem Biophys Res Commun. 1972 Jan 31;46(2):892–897. doi: 10.1016/s0006-291x(72)80225-0. [DOI] [PubMed] [Google Scholar]
- Eron L., Arditti R., Zubay G., Connaway S., Beckwith J. R. An adenosine 3':5'-cyclic monophosphate-binding protein that acts on the transcription process. Proc Natl Acad Sci U S A. 1971 Jan;68(1):215–218. doi: 10.1073/pnas.68.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODGAL S. H., HERRIOTT R. M. Studies on transformations of Hemophilus influenzae. I. Competence. J Gen Physiol. 1961 Jul;44:1201–1227. doi: 10.1085/jgp.44.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzicker T., Arditti R. R., Eisen H. Establishment of repression by lambdoid phage in catabolite activator protein and adenylate cyclase mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Feb;69(2):366–370. doi: 10.1073/pnas.69.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood J., Smith D. H. Catabolite repression of chloramphenicol acetyl transferase synthesis in E. coli K12. Biochem Biophys Res Commun. 1971 Jan 8;42(1):57–62. doi: 10.1016/0006-291x(71)90361-5. [DOI] [PubMed] [Google Scholar]
- Hempfling W. P., Beeman D. K. Release of glucose repression of oxidative phosphorylation in Escherichia coli B by cyclic adenosine 3',5'-monophosphate. Biochem Biophys Res Commun. 1971 Nov;45(4):924–930. doi: 10.1016/0006-291x(71)90426-8. [DOI] [PubMed] [Google Scholar]
- Hirata M., Hayaishi O. Adenyl cyclase of Brevibacterium liquefaciens. Biochim Biophys Acta. 1967 Nov 21;149(1):1–11. doi: 10.1016/0005-2787(67)90685-5. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Smith G. R., Ames B. N. Adenosine 3':5'-cyclic monophosphate concentration in the bacterial host regulates the viral decision between lysogeny and lysis. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2258–2262. doi: 10.1073/pnas.68.9.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss R. D., Gabor M. Bacterial transformation, with special reference to recombination process. Annu Rev Genet. 1970;4:193–224. doi: 10.1146/annurev.ge.04.120170.001205. [DOI] [PubMed] [Google Scholar]
- Khandelwal R. L., Hamilton I. R. Purification and properties of adenyl cyclase from Streptococcus salivarius. J Biol Chem. 1971 May 25;246(10):3297–3304. [PubMed] [Google Scholar]
- Kuwano M., Schlessinger D. Binding of adenosine 3':5'-cyclic phosphate to G factor of Escherichia coli, and its effects on GTPase, RNase V, and protein synthesis. Proc Natl Acad Sci U S A. 1970 May;66(1):146–152. doi: 10.1073/pnas.66.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAKMAN R. S., SUTHERLAND E. W. ADENOSINE 3',5'-PHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1965 Mar;240:1309–1314. [PubMed] [Google Scholar]
- Monard D., Janecek J., Rickenberg H. V. The enzymic degradation of 3',5' cyclic AMP in strains of E. Coli sensitive and resistant to catobolite repression. Biochem Biophys Res Commun. 1969 May 22;35(4):584–591. doi: 10.1016/0006-291x(69)90388-x. [DOI] [PubMed] [Google Scholar]
- Nakazawa A., Tamada T. Stimulation of colicin E 1 synthesis by cyclic 3', 5'-adenosine monophosphate in mitomycin C-induced Escherichia coli. Biochem Biophys Res Commun. 1972 Jan 31;46(2):1004–1010. doi: 10.1016/s0006-291x(72)80241-9. [DOI] [PubMed] [Google Scholar]
- Nealson K. H., Eberhard A., Hastings J. W. Catabolite repression of bacterial bioluminescence: functional implications. Proc Natl Acad Sci U S A. 1972 May;69(5):1073–1076. doi: 10.1073/pnas.69.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKABAYASHI T., YOSHIMOTO A., IDE M. OCCURRENCE OF NUCLEOTIDES IN CULTURE FLUIDS OF MICROORGANISMS. V. EXCRETION OF ADENOSINE CYCLIC 3',5'-PHOSPHATE BY BREVIBACTERIUM LIQUEFACIENS SP. N. J Bacteriol. 1963 Nov;86:930–936. doi: 10.1128/jb.86.5.930-936.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Glucose and the metabolism of adenosine 3':5'-cyclic monophosphate in Escherichia coli. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2794–2798. doi: 10.1073/pnas.68.11.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Perlman R. L., Pastan I. Regulation of lac messenger ribonucleic acid synthesis by cyclic adenosine 3',5'-monophosphate and glucose. J Biol Chem. 1970 May 10;245(9):2259–2267. [PubMed] [Google Scholar]