Abstract
Doxorubicin is a widely used chemotherapeutic drug that intercalates between DNA base-pairs and poisons Topoisomerase II, although the mechanistic basis for cell killing remains speculative. Doxorubicin and related anthracycline compounds have been shown to increase nucleosome turnover and/or eviction around promoters, which suggests that the resulting enhanced exposure of DNA might underlie cell killing. Previously, we showed that low doses of anthracyclines increase nucleosome turnover around active gene promoters, which suggests that loss of nucleosomes might contribute to cancer cell killing. Here we apply a genome-wide method to precisely map DNA double-strand breaks (DSBs) in cancer cells. We find that spontaneous DSBs occur preferentially around promoters of active genes, and that both anthracyclines and etoposide, a Topoisomerase II poison, increase DSBs around promoters, although CpG islands are conspicuously protected from DSBs. We propose that torsion-based enhancement of nucleosome turnover by anthracyclines exposes promoter DNA, ultimately causing DSBs around promoters.
Keywords: DNA double-strand breaks, Doxorubicin, Etoposide, Nucleosome turnover, Squamous cell carcinoma
1. Introduction
Doxorubicin (also called Adriamycin) is one of the most effective anti-cancer compounds, although exactly how it kills dividing cells has been a matter of debate [1, 2]. Doxorubicin and related anthracyclines consist of flat aromatic moieties that intercalate between DNA bases, each anchored tightly by one or more sugars in the minor groove [3]. Intercalation pushes apart the neighboring bases, which results in bidirectional transmission of positive torsion [3]. The resulting alterations in DNA structure can inhibit enzymes, including topoisomerases [4, 5]. Doxorubicin can also trap Topoisomerase II (TopoII) in the double-strand cleavage form and prevent ligation, and so one model for cell killing is the direct introduction of a double-strand break (DSB) caused by TopoII poisoning [4]. However, whether the primary anti-cancer action of Doxorubicin is by trapping TopoII in its double-strand cleaved form, or by inhibiting TopoII with the consequent failure to relieve the positive torsion, or by some other mechanism, is uncertain.
We previously showed that sublethal doses of Doxorubicin (<0.5 μM) nevertheless enhance nucleosome turnover around promoters in mouse squamous cell carcinoma (SCC) cell lines [6], raising the possibility that cell killing at chemotherapeutic doses is a downstream consequence of the increased exposure of DNA when nucleosomes are disrupted. Indeed, Pang et al. [7] showed that histones were evicted around promoters using 9 μM of Doxorubicin or a related anthracycline, Daunarubicin. In both studies, Aclarubicin, an anti-cancer anthracycline compound that does not poison TopoII, likewise evicted nucleosomes around promoters at similar doses. Etoposide, a TopoII poison that does not intercalate into DNA, but rather covalently traps TopoII preferentially at induced DNA single-strand breaks [8], did not evict histones at therapeutic doses [7]. Taken together, these observations suggest that anthracycline intercalation enhances nucleosome depletion around promoters, perhaps by increasing torsion [2].
If anthracycline drugs kill cancer cells by their preferential action at mammalian promoters, then we might expect them to also cause DSBs at promoters. Here, we tested this hypothesis by applying a genome-wide method for sensitive detection of DSBs. Consistent with this prediction, we find that regions around active promoters are hotspots for DSBs caused by Doxorubicin, Aclarubicin and Etoposide.
2. Materials and Methods
2.1. Tissue culture, drug treatment and lysis
Mouse squamous cell carcinoma cell line MSCC-CK1 [6] was cultured in Dulbecco's Modified Eagle Medium (DMEM) media (Cat# 11965-092, Invitrogen) with 10% fetal bovine serum and 1X Antibiotic-Antimycotic at 37 °C. For drug treatment, MSCC-CK1 cells were treated with the indicated drugs for 24 hours to maximize turnover with minimal toxicity based on our published finding that nucleosome turnover around promoters increases gradually up to 24 hours when using low concentrations of anthracyclines [6]. We chose a clinically relevant concentration of 3 μM Doxorubicin based on two published sources: Namur et al. [9] who measured liver tissue dosages around drug-eluting beads in capillaries at 5 μM, and Goodman and Gilman's Pharmacological Basis of Therapeutics [10] which listed 1.7 μM as the peak plasma concentration during standard chemotherapy. Our high concentration of 3 μM was chosen to lie in between these two values. During chemotherapy, plasma concentration rapidly declines over 24 hours to less than 0.05 μM. Therefore, our low concentration of 0.3 μM can be considered low relative to either high-dose standard but is equivalent to concentrations used in other in vitro experiments (e.g., [11]). As 0.3 μM anthracycline does not induce significant toxicity, these low-concentration experiments probably underestimate the degree of DSBs that occur during chemotherapy.
2.2 BLESS
BLESS (direct in situ breaks labeling, enrichment on streptavidin and next-generation sequencing) was performed as described by Crosetto et al. [12]. Drug-treated or untreated mouse SCC cells were cross-linked for 30 min at room temperature with 2% formaldehyde, followed by 5 min incubation with 125 mM glycine. The cells were then incubated with lysis buffer [10 mM Tris-HCl, 10 mM NaCl, 1 mM EDTA, 1mM EGTA, 0.2% NP-40, 1mM DTT, and protease inhibitors (Roche cOmplete ULTRA)] for 90 min at 4 °C and nuclear break buffer (10mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 0.3% SDS, and 1 mM DTT) for 45 min at 37 °C for nuclei isolation.
For yeast BLESS, yeast cells (W303) were grown in YPD and harvested at OD600 = 0.6–0.8. The cells were cross-linked and quenched as described above and yeast nuclei were isolation as previously described [13].
The mouse nuclei or yeast nuclei were quickly digested by proteinase K (100 μg/ml) at 37 °C for 4 min, and then washed twice with NEB Restriction Buffer 2 and once with quick blunting buffer followed by end polishing using the quick blunting kit (New England Biolabs). The nuclei were then washed and subject to ligation with biotin labeled proximal linker or nonbiotin labeled proximal linker at 16 °C for 18 hours. After ligation, genomic DNA was isolated and digested with HaeIII, followed by streptavidin bead capture, ligation of distal linker, digestion with I-SceI, PCR amplification, and XhoI digestion before illumina sequencing. Illumina libraries were prepared as previously described [14]. For quantitative analysis (Fig. 4B), the same volume of mouse BLESS sample was mixed with 6 ng yeast BLESS DNA followed by library preparation as described [14]. The samples were sequenced using illumina 50-bp paired-end sequencing.
2.3. Data analysis
After Illumina paired-end sequencing, each read in a pair was treated separately. Adaptors were removed using the program trim_galore (http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/). Then, those reads containing the sequence 5’...TCGAGGTAGTA-3’ derived from the BLESS first hairpin adapter sequence were selected and the sequence following it was retained. The selected reads were mapped as single-end reads against the MM9 release of the mouse genome using Bowtie2 (http://bowtiebio.sourceforge.net/bowtie2/index.shtml). For the mapped reads, the cleavage site was determined as the first base pair for reads mapped to the forward strand and the last base pair for reads mapped to the reverse strand. Cleavage points were normalized by first aggregating them into 25-bp intervals and then multiplying the fraction of total counts in each interval by the size of the mouse genome. Ends analysis, k-mean clustering, and heat map construction were performed as previously described [6]. CpG island coordinates from Mus musculus MM9 were downloaded from the UCSC Genome table (mm9_cpgisland.bed.gz).
For the spike-in samples, selected reads were also mapped to yeast release sacCer3 and yeast-specific cleavage points from all samples were pooled before normalized counts were calculated using the yeast genome size. The greater yield of DNA for the more heavily cleaved drug-treated mouse samples will result in proportionally fewer yeast reads than in the untreated control. Multiplication by the spike-in untreated/treated ratio corrects for the fact that normalization equalizes the genome-wide average. Accordingly, to calibrate a mouse sample, each 25-bp interval value was multiplied by the total number of cleavage points over the yeast genome for the untreated mouse sample and divided by that for the drug-treated sample.
3. Results
3.1. Spontaneous DSBs occur preferentially around promoters
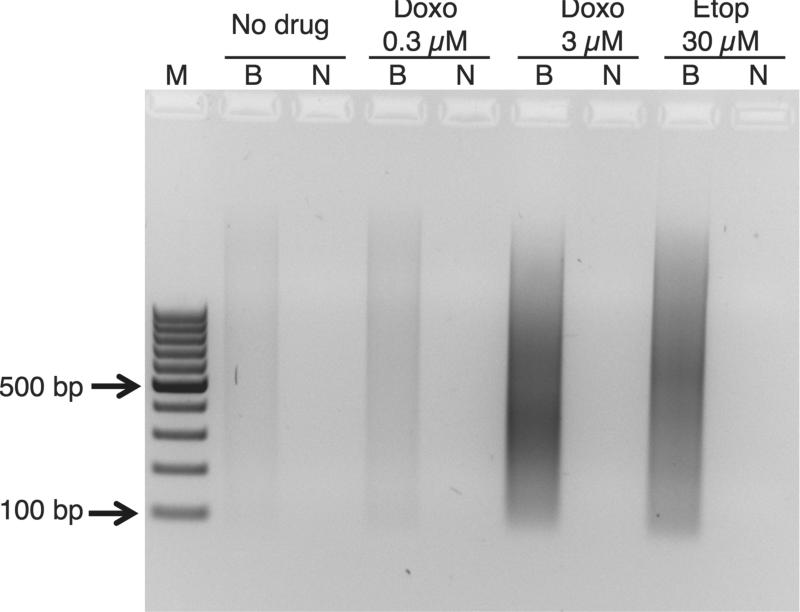
Control cells and cells treated for 24 hr with anthracyclines or Etoposide were fixed with formaldehyde to stabilize chromatin and prevent artificial DSBs. The BLESS (direct in situ Breaks Labeling, Enrichment on Streptavidin and next-generation Sequencing) method was used for mapping DNA DSBs genome-wide. After brief Proteinase K digestion, ends were repaired and ligated to a biotinylated hairpin linker in situ, total DNA was extracted, cleaved with a 4-cutter restriction enzyme, and the new ends were ligated to a second hairpin linker. Cleavage of restriction sites within the hairpin linkers yielded DNA duplexes in which the DSB end is continuous with the first adapter, and these were purified using streptavidin beads. As a negative control, we used a non-biotinylated first linker. Agarose gel electrophoresis of the duplexes extracted from the streptavidin beads revealed a very low level of cleavage for the biotinylated sample from untreated cells (Fig. 1). Treatment with 0.3 μM Doxorubicin resulted in a slightly higher concentration of fragments, suggesting a barely detectable increase in genome-wide cleavage frequency. Treatment with either 3 μM Doxorubicin or 30 μM Etoposide (therapeutic levels) resulted in high levels of cleavage using this assay.
Fig. 1. Doxorubicin and etoposide cause DNA double-stand breaks in mouse SCC cells.
Agarose gel electrophoresis of BLESS fragments from a representative experiment is shown, where equal aliquots of DNA were loaded from samples prepared in parallel. BLESS DNA library samples derived from mouse SCC cells before and after Doxorubicin (Doxo) or Etoposide (Etop) treatment. M, 100 bp ladder DNA marker; B, biotin-labeled linker; N, nonbiotin linker.
BLESS libraries were subjected to Illumina sequencing and fragment ends corresponding to the DSB were mapped to the mouse genome. Mouse genes were aligned at their transcriptional start sites (TSSs) and divided into three equal groups based on level of expression [6] and averaged. In the absence of drug, genes in the medium and high expression categories showed increases in BLESS signal on either side of the TSS, peaking around −1 kb and +1 kb, then decreasing and leveling off by −2 and +2 kb (Fig. 2A). In contrast, genes in the low expression category showed only a slightly elevated BLESS signal at TSSs. As the low expression category is likely to be dominated by silenced genes, and the high expression category shows higher overall BLESS signal than the middle expression category, we interpret these patterns as reflecting transcription-mediated DNA damage. This transcription-mediated damage is a feature specific to regions around promoters, because no patterns were seen around transcriptional end sites (TESs, Fig. S1A).
Fig. 2. DNA DSB level correlates with gene expression level in mouse SCC cells with and without drug treatment.
(A) No drug treatment control. Average signal of DNA DSB ±5 kb surrounding the transcription start site (TSS, arrow) is shown for each gene group based on gene expression level (from highest to lowest). Data were plotted using a 100-bp sliding window. (B) Same as A, except that SCC cells were treated with 0.3 μM Doxorubicin. (C) Same as B, except that treatment was with 3 μM Doxorubicin. (D) Same as B, except that treatment was with 0.4 μM Aclarubicin. (E) Same as B except that treatment was with 30 μM Etoposide.
3.2. Drug-induced DSBs occur preferentially around promoters
We next examined the effect of drug treatment on the distribution of DNA DSBs. We observed qualitatively similar BLESS patterns after treatment with 0.3 μM Doxorubicin, 3 μM Doxorubicin, 0.4 μM Aclarubicin and 30 μM Etoposide (Fig. 2B-E). Drug-treated cells showed better definition of the −1 kb and +1 kb peaks and TSS valleys, presumably because of the more robust signal resulting from the higher density of DNA breaks than in the untreated controls. The similar profiles of genome-normalized BLESS patterns at TSSs (Fig. 2) but not at TESs (Fig. S1) with or without drug treatment suggests that drug treatment causes transcription-coupled DNA damage to the same regions that are subject to spontaneous DNA damage.
Whereas the −1 kb and +1 kb peaks were of similar height in the untreated sample, all four drug treatments resulted in +1 kb peaks higher than −1 kb peaks (Fig. 2). As we have previously shown in Drosophila cells using a high-resolution method for measuring torsion, inhibition of topoisomerases increases positive torsion downstream and negative torsion upstream [15], consistent with the classical twin supercoil domain model [16]. These torsional changes, in turn, drive increases in nucleosome turnover downstream and decreases upstream [15]. Thus, the drug-induced increase in DSBs at +1 kb relative to that at −1 kb (Fig. 2) is consistent with the possibility that these drugs induce torsion and drive nucleosome turnover, thus making DNA more susceptible to double-strand breakage.
3.3. CpG islands are protected from DSBs
Mammalian promoters are typically embedded in “CpG islands”, which are CG-rich regions that are conspicuously unmethylated. We next asked if there is any difference in DSBs between CpG island and non-CpG island genes. Because CpG islands are of different lengths and span intra- and inter-genic regions in addition to promoters, we first aligned BLESS data around the starts and ends of annotated CpG islands. Strikingly, we observed that CpG islands showed reduced BLESS signal with prominent boundaries that corresponded almost precisely to the annotated island junctions (Fig. 3A). Very similar profiles were seen for the untreated sample and for all drug treatment samples, although the 3 μM Doxorubicin treatment showed a much lower BLESS signal over CpG islands than the other samples. This reduction in CpG island DSB frequency cannot be attributed to possible discrimination against C+G-rich DNA by the BLESS procedure, because the base composition of DSB sites was more C+G-rich in drug-treated samples (43-54% C+G) than in either untreated mouse (40% C+G) or yeast (27% C+G) samples (Table S1). Rather, it would appear that some feature of CpG islands protects DNA from spontaneous and drug-induced DSBs. Base composition has a strong effect on DNA shape, including minor groove width [17], and so the strongly biased base composition of CpG islands might cause them to resist spontaneous breakage and interfere with intercalation of anthracyclines or anchoring of sugars in the minor groove. The approximate similarity of these TSS patterns to CATCH-IT nucleosome turnover patterns in the same cell type (Fig. S2) provides support for the possibility that nucleosome turnover results in elevated DSB damage.
We next asked whether protection of DNA from double-strand breaks could account for the “dip” in the BLESS signal over the TSS (Fig. 2). By separating promoters depending on whether or not they were associated with a CpG island, we observed a clear distinction, in which the CpG island promoters showed a prominent dip, whereas the non-CpG island promoters showed at most a barely perceptible promoter dip with or without drug treatment (Fig. 3B). We conclude that the distribution of genome-wide DSB patterns represents the superimposition of transcription-coupled DNA breakage and CpG island protection from breakage.
Fig. 3. CpG islands are conspicuously protected from DNA DSBs.
(A) Average signal of DNA DSB ±1 kb surrounding the 5’ end or 3’ end of CpG island is shown in SCC cells before and after treatment with Doxorubicin, Etoposide, and Aclarubicin. (B) DNA DSB signal before and after drug treatment in SCC cells is presented as heat maps of the ±3 kb surrounding the TSS using Java TreeView. Genes were separated into two groups, CpG island genes and Non-CpG island genes. There were 16,026 CpG island genes that have at least one CpG island ±500 bp surrounding the TSS.
3.4 Anthracycline- and Etoposide-induced DSBs occur at sub-lethal concentrations
Our qualitative BLESS patterns appear to be similar in magnitude between treatment groups because read densities are normalized to the total number of reads in the genome. To obtain a quantitative comparison of BLESS library samples with one another, it was necessary to include an appropriate spike-in calibration control. Spike-in calibration is needed to account for the increased number of reads generated by the increased number of overall DSBs caused by drug treatment (See Methods). Accordingly, we prepared a BLESS library in parallel from the budding yeast, Saccharomyces cerevisiae. By mixing a fixed amount of yeast DNA with each of the mouse samples, which were derived from the same number of SCC cells, we could use the total yeast DNA BLESS abundance in the sample to calibrate the mouse BLESS data.
In addition to serving as a calibration control, the yeast BLESS spike-in DNA also served as a positive control for the BLESS procedure in a genome that is ~200x smaller than that of mouse (~12.5 Mb versus ~2800 Mb), providing much higher coverage per base-pair of spontaneous DSBs. A heat map of pooled yeast BLESS data shows that the nucleosome-depleted regions (NDRs) upstream and downstream of yeast coding sequences [18] are subject to a much higher level of double-strand breakage than are the nucleosome-rich coding regions (Fig. 4). Preferential spontaneous DSBs at NDRs are consistent with the mapping of TopoII to yeast NDRs [19] and the observation that NDRs are also preferentially mutated when cytidine deaminases are introduced into yeast [20]. We might interpret the sensitivity of yeast NDRs, which are hotspots of nucleosome turnover [21], to their low occupancy of nucleosomes, which would otherwise protect DNA from damage [22].
Fig. 4. Spontaneous DSBs occur preferentially at NDRs in budding yeast.
Heat map of DNA DSBs ±500 bp surrounding the translation start site (TSS) and translational end site (TES) of all genes in the budding yeast genome. Genes were ordered by expression level. Spontaneous DNA DSBs correspond to nucleosome-depleted regions of the yeast genome.
When mouse DSB profiles were calibrated using the yeast spike-in DNA, a drug-dependent increase in overall levels of DSBs became evident (Fig. 5). Moreover, there were some prominent quantitative differences between treatments. Treatment with anthracyclines (Fig. 5 upper right), but not with Etoposide (lower right), resulted in a DSB peak directly over the TES, which is relatively AT-rich owing to alignment of poly(A)-addition sites [23]. As expected, increasing the dose of Etoposide from 3 μM to 30 μM caused a corresponding increase in BLESS signal throughout the −5 kb to +5 kb interval around TSSs (Fig. 5 lower left). This result is consistent with recent findings using different methodologies that Etoposide induces both single- and double-strand breaks at mammalian promoters [8]. No increase in BLESS signal was seen around promoters when the dosage of Doxorubicin was increased from 0.3 μM to 3 μM, which we attribute to the greater protection from DSB damage over CpG islands at the 3 μM dose (Fig. 5 upper left). Aclarubicin at 0.4 μM showed the highest BLESS signal overall, with elevated levels throughout the −5 kb to +5 kb interval. Thus, all three of the drugs showed high levels of DSBs around promoters, even at low levels that do not kill SCC cells [6]. The fact that drug-induced DSB patterns are similar to patterns of nucleosome turnover enhancement observed previously, even at sub-lethal concentrations, strongly suggests that nucleosome turnover and eviction results in DSBs.
Fig. 5. Doxorubicin, Etoposide, and Aclarubicin enhance DNA DSBs.
Average signal of DNA DSB ±5 kb surrounding the TSS and TES in SCC cells before (No drug) and after drug treatment is shown. The DNA DSB signal of each mouse BLESS sample was normalized to its corresponding budding yeast BLESS signal (see Methods). Solid lines indicate means and dots above and below indicate 95% confidence intervals.
Discussion
In previous work, we showed that low-dose anthracycline drug treatment enhances nucleosome turnover around promoters of active genes in mouse SCC cells [6]. Similarly Pang and coworkers showed that high chemotherapeutic doses of anthracyclines leads to nucleosome eviction [7]. These findings raised the possibility that acute loss of nucleosomes increases DSBs, which is in turn responsible for cancer cell killing. Here, we have determined the distribution of anthracycline drug-induced DSBs genome-wide. We find that indeed anthracycline drug treatments result in DSBs around promoters, consistent with the hypothesis that nucleosome turnover and eviction increases exposure of DNA to processes that can lead to toxic DNA DSBs. Spontaneous and drug-induced breakage patterns are similar (Fig. 2), which suggests that drug treatments increase the degree of DNA exposure to ambient clastogenic processes. Transcription-coupled nucleosome turnover is highest adjacent to active gene promoters in animal cells [24-26] and at NDRs in yeast [21, 27], which is where we also observe the highest levels of spontaneous and drug-induced DSBs.
Enhancement of DSBs around mouse promoters by anthracyclines is similar to or greater in magnitude than enhancement by Etoposide, which specifically targets the active site of TopoII. Although Doxorubicin can act as a TopoII poison and trap the enzyme in its double-strand cleaved conformation [4], Aclarubicin is an intercalating anthracycline that does not poison TopoII, and yet causes levels of DSBs around promoters that are even higher than levels caused by concentrations of Etoposide that kill cells. Therefore, DNA damage caused by anthracyclines is not likely entirely attributable to TopoII poisoning, but rather to DNA intercalation, which is a general feature of anthracycline compounds. The very stable intercalation of anthracyclines into DNA led to their early use as fluorescent stains for “D-banding” of chromosomes [28]. Stable DNA intercalation leading to DNA structural change is thought to be responsible for the inhibition of topoisomerases by anthracyclines [4], thus protecting against poisoning in the enzymatically cleaved state [5].
Previously Pang et al. [7] showed that both Etoposide and Doxorubicin caused DNA breaks, but Aclarubicin did not, which would at first seem to contradict our findings. However, the concentrations used by Pang et al. were much higher than in our study, and in the case of Aclarubicin, 50-fold higher (20 μM versus 0.4 μM). We found that levels of Aclarubicin much above 0.4 μM result in death of SCC cells during the 24 hr period over which we observed the maximum turnover effect [6]. It therefore seems possible that catalytic inhibition of TopoII and/or the poisoning of Topoisomerase I that has been attributed to Aclarubicin [5] kills cells before maximum turnover is reached.
It might seem surprising that all three drugs showed similar patterns of DSBs, despite the fact that Aclarubicin intercalates into DNA but does not poison TopoII, Etoposide poisons TopoII and causes primarily single-strand breaks [8] but does not intercalate into DNA, whereas Doxorubicin both intercalates into DNA and poisons TopoII [4]. To explain their similar effects around promoters, we suggest that the common denominator is torsion driven by transcription. We have previously shown that in Drosophila cells, positive torsion drives nucleosome turnover in the bodies of active genes, and negative torsion reduces turnover [15]. Torsion increased when either Topoisomerase I or TopoII was specifically inhibited with drugs [15], suggesting that nucleosomes are destabilized by RNA polymerase transit downstream and stabilized upstream [29]. Although both Etoposide and Doxorubicin trap TopoII in the cleaved form, the topological constraints imposed by the trapped TopoII-DNA covalent complex would not be expected to allow relief of the torsion [30], as would be the case for single or double-strand breaks, where free ends are able to swivel. Therefore, the common action of all three drugs in causing DSBs can be explained by their effects on torsion: Aclarubicin pushes the base pairs apart upon intercalation to create positive torsion and alter DNA structural properties that might inhibit topoisomerases and other enzymes. In contrast, Etoposide covalently traps TopoII and prevents it from relieving the torsion. Doxorubicin does both in a concentration-dependent manner [4], and as we have shown here cells are especially well-protected from DSBs at CpG-island promoters at therapeutic doses of Doxorubicin. These distinct effects of Doxorubicin and related anthracyclines on the chromatin landscape might contribute to the fact that even after 40 years in the clinic, these drugs are still among the most commonly used and most broadly effective anti-cancer agents [31].
Supplementary Material
Highlights.
DNA double-strand (ds) breaks occur preferentially around promoters
Anthracyclines and etoposide enhance ds breaks at sub-lethal concentrations
Ds breaks around promoters correlate with gene expression level
CpG islands are protected from ds breaks
Torsion-based enhancement of nucleosome turnover by anthracyclines might expose DNA
Acknowledgements
The authors thank Christine Codomo for Solexa library construction, Jorja Henikoff for data processing, the FHCRC Genomic Shared Resource for Illumina sequencing and Srinivas Ramachandran and Paul Talbert for comments on the manuscript. This work was supported by a grant from NIH (R01 ES020116). Illumina sequencing data have been submitted to GEO (GSE62927).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol. 1999;57:727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 2.Yang F, Teves SS, Kemp CJ, Henikoff S, Doxorubicin DNA. torsion and chromatin dynamics. BBA - Reviews on Cancer, 1845. 2014 doi: 10.1016/j.bbcan.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frederick CA, Williams LD, Ughetto G, van der Marel GA, van Boom JH, Rich A, Wang AH. Structural comparison of anticancer drug-DNA complexes: adriamycin and daunomycin. Biochemistry (Mosc) 1990;29:2538–2549. [PubMed] [Google Scholar]
- 4.Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010;17:421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hajji N, Mateos S, Pastor N, Dominguez I, Cortes F. Induction of genotoxic and cytotoxic damage by aclarubicin, a dual topoisomerase inhibitor. Mutat. Res. 2005;583:26–35. doi: 10.1016/j.mrgentox.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Yang F, Kemp CJ, Henikoff S. Doxorubicin enhances nucleosome turnover around promoters. Curr. Biol. 2013;23:782–787. doi: 10.1016/j.cub.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pang B, Qiao X, Janssen L, Velds A, Groothuis T, Kerkhoven R, Nieuwland M, Ovaa H, Rottenberg S, van Tellingen O, Janssen J, Huijgens P, Zwart W, Neefjes J. Drug-induced histone eviction from open chromatin contributes to the chemotherapeutic effects of doxorubicin. Nat Commun. 2013;4:1908. doi: 10.1038/ncomms2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baranello L, Kouzine F, Wojtowicz D, Cui K, Przytycka TM, Zhao K, Levens D. DNA break mapping reveals topoisomerase II activity genome-wide. International journal of molecular sciences. 2014;15:13111–13122. doi: 10.3390/ijms150713111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Namur J, Citron SJ, Sellers MT, Dupuis MH, Wassef M, Manfait M, Laurent A. Embolization of hepatocellular carcinoma with drug-eluting beads: doxorubicin tissue concentration and distribution in patient liver explants. J. Hepatol. 2011;55:1332–1338. doi: 10.1016/j.jhep.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 10.Brunton L. Goodman & Gilman's Pharmacological Basis of Therapeutics. McGraw-Hill; 2010. [Google Scholar]
- 11.Attardi LD, de Vries A, Jacks T. Activation of the p53-dependent G1 checkpoint response in mouse embryo fibroblasts depends on the specific DNA damage inducer. Oncogene. 2004;23:973–980. doi: 10.1038/sj.onc.1207026. [DOI] [PubMed] [Google Scholar]
- 12.Crosetto N, Mitra A, Silva MJ, Bienko M, Dojer N, Wang Q, Karaca E, Chiarle R, Skrzypczak M, Ginalski K, Pasero P, Rowicka M, Dikic I. Nucleotide-resolution DNA double-strand break mapping by next-generation sequencing. Nat Methods. 2013;10:361–365. doi: 10.1038/nmeth.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furuyama S, Biggins S. Centromere identity is specified by a single centromeric nucleosome in budding yeast. Proc. Natl. Acad. Sci. USA. 2007;104:14706–14711. doi: 10.1073/pnas.0706985104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henikoff JG, Belsky JA, Krassovsky K, Macalpine DM, Henikoff S. Epigenome characterization at single base-pair resolution. Proc. Natl. Acad. Sci. U. S. A. 2011;108:18318–18323. doi: 10.1073/pnas.1110731108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teves SS, Henikoff S. Transcription-generated torsional stress destabilizes nucleosomes. Nat Struct Mol Biol. 2014;21:88–94. doi: 10.1038/nsmb.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu LF, Wang JC. Supercoiling of the DNA template during transcription. Proc. Natl. Acad. Sci. U. S. A. 1987;84:7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou T, Yang L, Lu Y, Dror I, Dantas Machado AC, Ghane T, Di Felice R, Rohs R. DNAshape: a method for the high-throughput prediction of DNA structural features on a genomic scale. Nucleic Acids Res. 2013;41:W56–62. doi: 10.1093/nar/gkt437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan GC, Liu YJ, Dion MF, Slack MD, Wu LF, Altschuler SJ, Rando OJ. Genome-scale identification of nucleosome positions in S. cerevisiae. Science. 2005;309:626–630. doi: 10.1126/science.1112178. [DOI] [PubMed] [Google Scholar]
- 19.Sperling AS, Jeong KS, Kitada T, Grunstein M. Topoisomerase II binds nucleosome-free DNA and acts redundantly with topoisomerase I to enhance recruitment of RNA Pol II in budding yeast. Proc. Natl. Acad. Sci. U. S. A. 2011;108:12693–12698. doi: 10.1073/pnas.1106834108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor BJ, Wu YL, Rada C. Active RNAP pre-initiation sites are highly mutated by cytidine deaminases in yeast, with AID targeting small RNA genes. eLife. 2014;3 doi: 10.7554/eLife.03553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dion M, Kaplan T, Friedman N, Rando OJ. Dynamics of replication-independent histone turnover in budding yeast. Science. 2007;315:1405–1408. doi: 10.1126/science.1134053. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Chen Z, Chen H, Su Z, Yang J, Lin F, Shi S, He X. Nucleosomes suppress spontaneous mutations base-specifically in eukaryotes. Science. 2012;335:1235–1238. doi: 10.1126/science.1217580. [DOI] [PubMed] [Google Scholar]
- 23.Tian B, Hu J, Zhang H, Lutz CS. A large-scale analysis of mRNA polyadenylation of human and mouse genes. Nucleic Acids Res. 2005;33:201–212. doi: 10.1093/nar/gki158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teves SS, Weber CM, Henikoff S. Transcribing through the nucleosome. Trends Biochem. Sci. 2014;39:577–586. doi: 10.1016/j.tibs.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Huang C, Zhang Z, Xu M, Li Y, Li Z, Ma Y, Cai T, Zhu B. H3.3-h4 tetramer splitting events feature cell-type specific enhancers. PLoS Genet. 2013;9:e1003558. doi: 10.1371/journal.pgen.1003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banaszynski LA, Wen D, Dewell S, Whitcomb SJ, Lin M, Diaz N, Elsasser SJ, Chapgier A, Goldberg AD, Canaani E, Rafii S, Zheng D, Allis CD. Hira-dependent histone H3.3 deposition facilitates PRC2 recruitment at developmental loci in ES cells. Cell. 2013;155:107–120. doi: 10.1016/j.cell.2013.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rufiange A, Jacques PE, Bhat W, Robert F, Nourani A. Genome-wide replication-independent histone H3 exchange occurs predominantly at promoters and implicates H3 K56 acetylation and Asf1. Mol. Cell. 2007;27:393–405. doi: 10.1016/j.molcel.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Lin CC, Van de Sande JH. Differential fluorescent staining of human chromosomes with daunomycin and adriamycin--the d-bands. Science. 1975;190:61–63. doi: 10.1126/science.52193. [DOI] [PubMed] [Google Scholar]
- 29.Lee MS, Garrard WT. Transcription-induced nucleosome ‘splitting’: an underlying structure for DNase I sensitive chromatin. EMBO J. 1991;10:607–615. doi: 10.1002/j.1460-2075.1991.tb07988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu CC, Li TK, Farh L, Lin LY, Lin TS, Yu YJ, Yen TJ, Chiang CW, Chan NL. Structural basis of type II topoisomerase inhibition by the anticancer drug etoposide. Science. 2011;333:459–462. doi: 10.1126/science.1204117. [DOI] [PubMed] [Google Scholar]
- 31.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.