Abstract
Background
Initial shortening of stem vowels in three-word derivational paradigms (e.g., zip, zipper, zippering) was studied in persons with Parkinson’s disease (PWPD) with and without deep brain stimulation (DBS), and in normal speakers.
Method
Seven PWPD without DBS, 7 PWPD with DBS ON (DBSN) or OFF (DBSF), and 6 healthy control (CON) persons were studied. Stimuli were 7 three-word paradigms consisting of a stem word and two derived longer forms created by adding the suffixes er (+1), and er+ing (+2).
Results
Vowel durations decreased across word forms of increasing length (initial shortening) for DBSF, DBSN, PWPD, and CON. Vowel shortening did not interact with group. For each word form, CON vowel duration was shorter than those for PWPD, DBSN and DBSF but word duration did not differ between groups. DBS did not have a significant effect on either vowel or word duration.
Conclusion
These results agree with previous findings for a PWPD with accelerated speech and faster rates of speech in DBS-ON. Observations that vowel duration patterns are maintained in subcortical and cerebellar but not left hemisphere damage suggest that cortical control factors play a primary role in relational timing.
Keywords: timing in speech, basal ganglia disease, deep brain stimulation
Background
Rate and timing in the speech of persons with Parkinson’s disease (PWPD) often present clinically as abnormal (Blanchet & Snyder, 2009). Abnormally rapid speech, up to 250 words per minute, has been reported (Canter, 1963). In a recent study of articulatory rate in 121 PWPD, acceleration in the course of speaking was a significant factor (Skodda & Schlegel, 2008). Rapid rate, short rushes, and progressive acceleration within phrases and overall in utterances characterize persons with Parkinson’s disease (Darley, Aronson, & Brown, 1975). These rate and timing abnormalities have been reported to occur during syllable repetition, reading, repetition, and spontaneous speech. Occasionally the speech rate exceeds what can be done voluntarily, suggesting the engagement of a mode of neuromotor activity beyond the speaker’s control.
An opportunity to study speech timing contingencies in subcortical disease arises in the phenomenon initial shortening. In normal speech, syllables, words and phrases become shortened when embedded in an increasing longer phrase; conversely, syllables and words are relatively lengthened in final position when compared to their durations in phrase-medial or phrase-initial positions (Gaitenby, 1965; Kreiman & Sidtis, 2011). Initial shortening is observed in single words when embedded longer forms. In word paradigms, such as “zip, zipper, zippering,” the stem vowel is longest in “zip,” shorter in “zipper,” and shortest in “zippering” (Lehiste, 1972). This temporal pattern was reported to be unaffected in a PWPD with accelerated speech (Canter & Van Lancker, 1985) and in persons with cerebellar ataxia (Connaghan et al., 1994). In contrast, disturbance to this temporal pattern has been observed in nonfluent aphasia (Van Lancker Sidtis et al., 2010).
We extended the study of initial shortening to individuals treated by deep brain stimulation (DBS) of the subthalamic nucleus. DBS-ON has been associated with increased pausing (Sidtis et al., 2012), so that comparison of the ON and OFF states in these persons could provide further information about motor control of speech rate.
Method
Subjects
Fourteen right-handed participants diagnosed with Parkinson’s disease (PD), ages 49–73 (mean =61.23) and education 14–20 years (mean =16.15) volunteered for the study. The duration of PD was 7–16 yrs (mean=11.30). Seven of the 14 PWPD were treated with bilateral deep brain stimulation of the subthalamic nucleus (DBS). The number of months having had the surgery ranged from 2–56 months (mean =23.87). For comparison, 6 healthy control speakers (CON) matched for age and education with no history of speech or hearing deficits, confounding neurological or psychiatric disease, were studied. All participants were native speakers of English. PWPD-DBS subjects were studied twice during both on (DBSN) and off (DBSF) stimulation at separate sessions. Stimulators were turned off at least two hours prior to the study for DNSF. The PWPD without DBS subjects were tested once. Anti-parkinsonian medication was withheld for twelve hours prior to the study for all PWPD.
Procedure
The stimulus set originally consisting of the 4 three-word paradigms from the procedure developed by Lehiste (1972) so that a comparison could be made to existing data (Canter & Van Lancker, 1985), plus the words pot, tot, and cot. The derived forms incrementally added the monosyllabic suffixes –er, and –ing, yielding, for example, zip, zipper, zippering. The words “zip”, “jab”, “flat”, “thick”, “pot”, “tot”, and “cot” were used. The three forms of each target word were presented by the examiner in a repetition format using the expression, “Say ___”. Speech samples were recorded using a head-worn microphone (Shure model SM10A) and a Marantz digital recorder (Model PMD 660) with a mouth-to-microphone distance of two inches.
Results
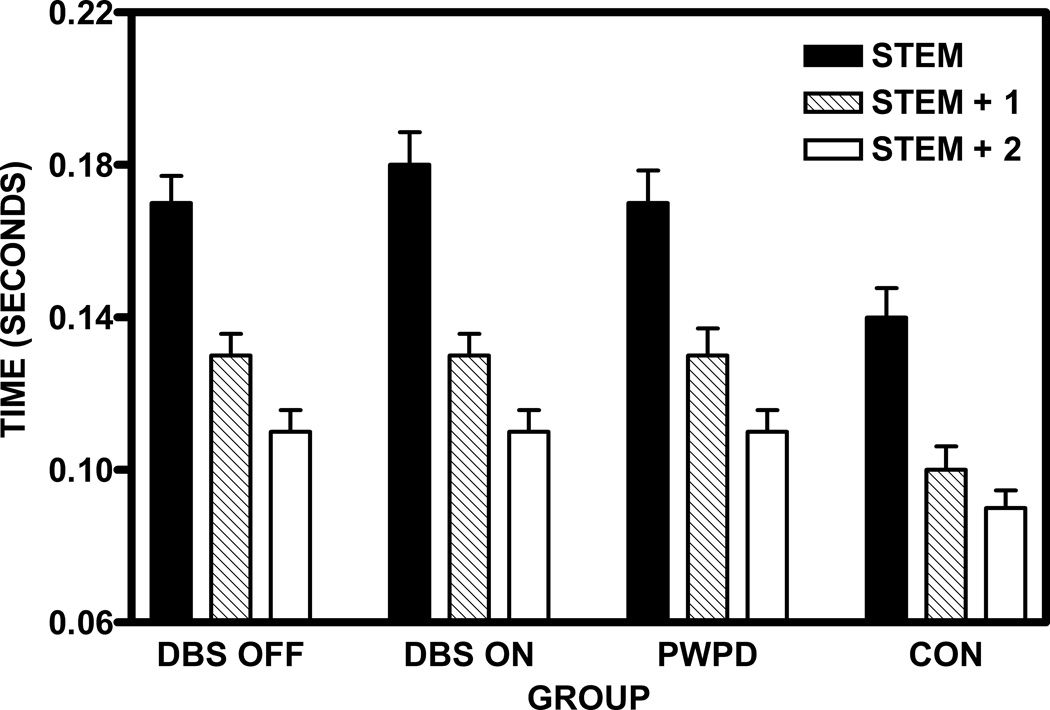
Initial shortening across word forms was found comparing DBSF, PWPD, and CON [F(2,276)=136.5; p < 0.001], and comparing DBSN, PWPD, and CON [F(2,276)=65.9; p < 0.001]. Initial shortening did not interact with group for either comparison. These relationships are presented in Figure 1. Compared to CON, vowel duration was longer for DBSF for each word form [stem: t(89)=3.1; p=0.003; stem+1: t(89)=2.9; p=0.005; stem+2: t(89)=2.6; p=0.01]. This was true for DBSN [stem: t(89)=3.7; p<0.001; stem+1: t(89)=2.9; p=0.005; stem+2: t(89)=3.3; p=0.002], and PWPD [stem: t(89)=2.9; p=0.005; stem+1: t(89)=2.7; p=0.008; stem+2: t(89)=2.9; p=0.005] as well. In contrast, the groups did not differ on word duration for any form. DBS did not have an effect on vowel or word durations for any word forms.
Figure 1.
Group mean stem vowel durations (seconds) for stem, stem+1, and stem+2 word forms (error bars are standard errors of the mean). Comparable results were obtained when vowel durations are expressed as a percentage of word duration.
Discussion
Initial shortening was preserved in PWPD with and without DBS. Despite well documented deficits in overall speech rate, relational timing in these paradigms, involving a morphological expansion, was preserved in all the PWPD speakers. This effect was not altered by DBS. Interestingly, while vowel durations were greater than normal in PWPD with and without DBS, word durations did not show group effects.
Preserved initial shortening was reported for a single case study of a PWPD of postencephalitic etiology with greatly accelerated speech following bilateral thalamotomies. In this single case, despite the extreme overall rate increase, the temporal relations at both word (derivational paradigm) and phrase level were preserved (Canter & Van Lancker, 1985). That is, initial shortening in the phrase “Why don’t you get tickets for tomorrow night’s performance” in all stages of expansion was retained, despite a tendency toward short rushes in the middle of the phrase.
Speech rate abnormalities are also seen in persons with ataxic disturbance pursuant to cerebellar disease (Sidtis et al, 2006). Slowed rate with prolongation of speech elements as well as excessive and equal stress on speech elements are commonly observed (Darley, Aronson & Brown, 1975; Duffy, 1995; Kent et al., 2000; 1997). Abnormal timing relationships as well as high variability in performance are characteristic of cerebellar disease (Ackermann & Hertrich, 1995; Ziegler & Wessel, 1996). In spite of these changes in ataxic speech, initial shortening is preserved, at least in several genotypes of spino-cerebellar ataxia (Connaghan et al., 1994).
The preservation of these timing relations leads to a proposal about cerebral control of temporal parameters. Speech timing and rhythmic relations have been consistently associated with left hemispheric function (Alcock et al., 2000). Disruption of timing relations has been reported in left-hemisphere damage associated with dysarthria and apraxia of speech (DiSimoni & Darley, 1977; Kent & Rosenbek, 1982; Collins, Rosenbek, & Wertz, 1983). The diagnostic criterion ‘impaired melody of speech’ associated with left hemisphere damage in the Boston Aphasia Diagnostic system (Goodglass & Kaplan, 1972) involves primary disruption of temporal integrity in spoken output. Absence of prepausal lengthening in phrases has been reported (Danly & Shapiro, 1982; Seddoh, 2004), appearing mostly in nonfluent aphasia, but also seen in fluent aphasia (Shah, Baum, & Dwivedi, 2006). More specifically, disturbance to initial shortening have been observed in nonfluent aphasia (Van Lancker Sidtis et al., 2010).
In contrast to observations in persons with aphasia following left hemisphere damage and similar to persons with ataxia, these studies indicated that the temporal speech problems observed in PWPD are confined to rate control abnormalities during production of sequential words and phrases. In PWPD, rate difficulties appear to arise from initiation, execution, and monitoring of the overall speech utterance. Internal timing relations in morphological and phrasal paradigms are not affected by the rate disorder. These observations lead to the proposal that relational speech timing within words and phrases, and speech rate, specifically, rapid rate, short rushes, and progressive acceleration within phrases and overall in utterances, are subserved by different control systems. Relational speech timing appears to emanate from left hemisphere structures, while rate and fluency are modulated by subcortical systems.
Acknowledgements
This work was supported by NINCD R01 DC007658 and ARRA supplement. The contribution of Lisa Bonura is gratefully acknowledged.
References
- Ackermann H, Hertrich I. Speech rate and rhythm in cerebellar dysarthria: An acoustic analysis of syllabic timing. Folia Phoniatrica et Logopaedica. 1994;46:70–78. doi: 10.1159/000266295. [DOI] [PubMed] [Google Scholar]
- Alcock KJ, Wade D, Anslow P, Passingham RE. Pitch and timing abilities in adult left-hemisphere dysphasic and right-hemisphere subjects. Brain and Language. 2000;75:47–65. doi: 10.1006/brln.2000.2324. [DOI] [PubMed] [Google Scholar]
- Blanchet PG, Snyder GJ. Speech rate deficit in individuals with Parkinson’s disease: A reviews of the literature. Journal of Medical Speech-Language Pathology. 2009;17:1–7. [Google Scholar]
- Canter GJ, Van Lancker D. Disturbances of the temporal organization of speech following bilateral thalamic surgery in a patient with Parkinson’s disease. Journal of Communication Disorders. 1985;18:329–349. doi: 10.1016/0021-9924(85)90024-3. [DOI] [PubMed] [Google Scholar]
- Canter GJ. Speech characteristics of patients with Parkinson’s disease. I. Intensity, pitch, and duration. J. Speech Hear. Dis. 1963;28:221–229. doi: 10.1044/jshd.2803.221. [DOI] [PubMed] [Google Scholar]
- Collins M, Rosenbek JC, Wertz RT. Spectrographic analysis of vowel and word duration in apraxia of speech. J. Speech Hear. Res. 1983;26:217–224. doi: 10.1044/jshr.2602.224. [DOI] [PubMed] [Google Scholar]
- Connaghan K, Liss JM, Sidtis JJ. Seventh Biennial Conference on Motor Speech. Sedona, AZ: 1994. Relational timing in ataxic dysarthria. [Google Scholar]
- Danly M, Shapiro B. Speech prosody in Broca’s aphasia. Brain and Language. 1982;16:171–190. doi: 10.1016/0093-934x(82)90082-7. [DOI] [PubMed] [Google Scholar]
- Darley FL, Aronson AE, Brown JR. Motor Speech Disorders. Philadelphia: WB: Saunders; 1975. [Google Scholar]
- DiSimoni FG, Darley FL. Effect on phoneme duration control of three utterance-length conditions in an apractic patient. J. Speech Hear. Dis. 1977;42:257–264. doi: 10.1044/jshd.4202.257. [DOI] [PubMed] [Google Scholar]
- Duffy JR. Motor speech disorders. Philadelphia: Mosby; 1995. [Google Scholar]
- Gaitenby J. The elastic word. Haskins Laboratories Status Report on Speech Research SR-2. 1965:3.1–3.12. [Google Scholar]
- Goodglass H, Kaplan E. The assessment of aphasia and related disorders. Philadelphia: Lea and Febiger; 1972. [Google Scholar]
- Kent RD, Rosenbek J. Prosodic disturbance and neurologic lesion. Brain and Language. 1982;15:259–291. doi: 10.1016/0093-934x(82)90060-8. [DOI] [PubMed] [Google Scholar]
- Kent RD, Kent JF, Duffy JR, Thomas JE, Weismer G, Stuntebeck S. Ataxic dysarthria. J. Speech Lang., Hear. Res. 2000;43:1275–1289. doi: 10.1044/jslhr.4305.1275. [DOI] [PubMed] [Google Scholar]
- Kent RD, Kent JE, Rosenbek JC, Vorperian HK, Weismer G. A speaking task analysis of the dysarthria in cerebellar disease. Folia Phoniatrica et Logopaedica. 1997;49:63–82. doi: 10.1159/000266440. [DOI] [PubMed] [Google Scholar]
- Kreiman J, Sidtis D. Foundations of Voice Studies: An Interdisciplinary Approach to Voice Production and Perception. Boston: Wiley-Blackwell; 2011. [Google Scholar]
- Lehiste I. The timing of utterances and linguistic boundaries. J. Acoust. Soc. Am. 1972;51:2018–2024. [Google Scholar]
- Seddoh SAK. Prosodic disturbance in aphasia: speech timing versus intonation production. Clinical Linguistics & Phonetics. 2004;18:17–38. doi: 10.1080/0269920031000134686. [DOI] [PubMed] [Google Scholar]
- Shah AP, Baum SR, Dwivedi VD. Neural substrates of linguistic prosody: evidence from syntactic disambiguation in the productions of brain-damaged patients. Brain and Language. 2006;96:78–89. doi: 10.1016/j.bandl.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Sidtis JJ, Gomez C, Naoum A, Strother SC, Rottenberg DA. Mapping cerebral blood flow during speech production in hereditary ataxia. NeuroImage. 2006;31:246–254. doi: 10.1016/j.neuroimage.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Sidtis JJ, Tagliati M, Alterman R, Sidtis D, Dhawan V, Eidelberg D. Therapeutic high frequency stimulation of the subthalamic nucleus in Parkinson’s Disease produces global increases cerebral blood flow. Journal of Cerebral Blood Flow and Metabolism. 2012;32:41–49. doi: 10.1038/jcbfm.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skodda S, Schlegel U. Speech rate and rhythm in Parkinson’s Disease. Movement Disorders. 2008;23:985–992. doi: 10.1002/mds.21996. [DOI] [PubMed] [Google Scholar]
- Van Lancker Sidtis D, Kempler D, Jackson C, Metter EJ. Prosodic changes in aphasic speech: timing. Journal of Clinical Linguistics and Phonetics. 2010;24:155–167. doi: 10.3109/02699200903464439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler W, Wessel K. Speech timing in ataxic disorders. Neurology. 1996;47:208–214. doi: 10.1212/wnl.47.1.208. [DOI] [PubMed] [Google Scholar]