Abstract
Histone deacetylases (HDACs) constitute a super-family of enzymes grouped into four major classes (Class I–IV) that deacetylate histone tails leading to chromatin condensation and gene repression. Whether stroke-induced oligodendrogenesis is related to the expression of individual HDACs in the oligodendrocyte lineage has not been investigated. We found that 2 days after stroke, oligodendrocyte progenitor cells (OPCs) and mature oligodendrocytes (OLGs) were substantially reduced in the peri-infarct corpus callosum, whereas at 7 days after stroke, a robust increase in OPCs and OLGs was observed. Ischemic brains isolated from rats sacrificed 7 days after stroke were used to test levels of individual members of Class I (1 and 2) and Class II (4 and 5) HDACs in white matter oligodendrocytes during stroke-induced oligodendrogenesis. Double immunohistochemistry analysis revealed that stroke substantially increased the number of NG2+ OPCs with nuclear HDAC1 and HDAC2 immunoreactivity and cytoplasmic HDAC4 which were associated with augmentation of proliferating OPCs, as determined by BrdU and Ki67 double reactive cells after stroke. A decrease in HDAC1 and an increase in HDAC2 immunoreactivity were detected in mature adenomatous polyposis coli (APC) positive OLGs, which paralleled an increase in newly generated BrdU positive OLGs in the peri-infarct corpus callosum. Concurrently, stroke substantially decreased the acetylation levels of histones H3 and H4 in both OPCs and OLGs. Taken together, these findings demonstrate that stroke induces distinct profiles of Class I and Class II HDACs in white matter OPCs and OLGs, suggesting that the individual members of Class I and II HDACs play divergent roles in the regulation of OPC proliferation and differentiation during brain repair after stroke.
Keywords: Stroke, Histone deacetylases, Oligodendrocytes, Epigenetics
Introduction
White matter consists mostly of glial cells and myelinated axons. It comprises about half of the brain volume in humans, and nearly all cases of ischemic stroke involve white matter (Goldberg and Ransom, 2003; Liu et al., 2012). Mature oligodendrocytes (OLGs), the glial cells responsible for CNS myelin formation, are highly vulnerable to ischemic injury mediated by oxidative stress, excitatory amino acids, trophic factor deprivation and apoptosis (Dewar et al., 2003; Pantoni et al., 1996). OLG injury results in demyelination with subsequent impairment of axonal conduction (Franklin and Ffrench-Constant, 2008; McTigue and Tripathi, 2008).
Myelin repair involves the generation of new mature OLGs, since surviving OLGs after injury are incapable of playing a significant role in remyelination (Franklin and Ffrench-Constant, 2008; Keirstead and Blakemore, 1997; McTigue and Tripathi, 2008). New OLGs are derived from non myelinating oligodendrocyte progenitor cells (OPCs) located throughout the grey and white matter of the adult brain (Franklin and Ffrench-Constant, 2008; McTigue and Tripathi, 2008). New OLGs are generated in the peri-infarct area in animal models of stroke (Gregersen et al., 2001; Tanaka et al., 2003; Zhang et al., 2010; Zhang et al., 2011), however, the molecular mechanisms underlying stroke-induced oligodendrogenesis have not been extensively investigated (Zhang et al., 2013).
Histone deacetylases (HDACs) comprise a super-family of enzymes grouped into four major classes (Class I–IV) that deacetylate specific lysine residues in histone tails leading to chromatin condensation and gene repression (Jenuwein and Allis, 2001; Kouzarides, 2007). Numerous studies suggest that the functions and expression profiles of HDAC isoforms in oligodendrocytes dynamically respond to the developmental stage, age and health of the cells. Developmental animal studies revealed that all Class I and Class II HDAC isoforms exist in the corpus callosum at different developmental time points up to 24 days postnatally (Shen et al., 2005). The enzymatic activity of HDACs on nucleosomal histones was found to be essential for embryonic human and rodent OPCs differentiation (Conway et al., 2012; Marin-Husstege et al., 2002; Shen et al., 2005) and systemic administration of valproic acid (VPA), a non-selective HDAC inhibitor, to rat pups prevented the differentiation of developing OPCs and resulted in significant hypomyelination (Shen et al., 2005). However, the deleterious effect of treatment with non-selective HDAC inhibitors on oligodendrocytes in vivo is temporally restricted and takes place up to the first 10 postnatal days (Shen et al., 2005). Other evidence also suggests that the aging process affects histone acetylation in white matter oligodendrocytes. For example, Shen et al. (Shen et al., 2008a) found decreased HDAC enzymatic activity in protein extracts of the corpus callosum of aged mice (8 months old) compared to young mice (8 weeks old) along with a generalized age-dependent decrease of Class I and Class II HDACs expression in OLGs.
Preclinical studies in animal models of stroke showed that inhibition of HDACs provides neuroprotection (Kim et al., 2007; Ren et al., 2004), stimulates neurogenesis and increases white matter repair (Kim et al., 2009; Liu et al., 2012). Treatment of adult stroke rats with VPA increases mature OLG survival by reduction of apoptotic OLG death and augmentation of newly generated oligodendrocytes (Liu et al., 2012). Similarly, two in vitro studies showed that treatment with HDAC inhibitors preserves mature oligodendrocytes and promotes functional recovery in optic nerves from young and aged mice after oxygen and glucose deprivation (OGD) (Baltan, 2012; Baltan et al., 2011). Most of these investigations on HDAC function have been based on non-selective HDAC inhibitors and their effect on white matter after ischemia. The HDAC family has only recently started to be addressed individually, and emerging data suggest that individual HDACs have distinctive biological functions in oligodendrocytes. Single knockouts of Class I HDAC1 or HDAC2 under the control of Olig1 promoter in mice did not yield obvious developmental defects, however, double genetic ablation of HDAC1 and HDAC2 blocked oligodendrocyte differentiation by activation of the Wnt signaling pathway (Ye et al., 2009). In another study, Wei et al. showed that transcription factor Nkx2.2 recruits an HDAC1 complex to repress the myelin basic protein (MBP) promoter in immature oligodendrocytes (Wei et al., 2005). Unlike Class I HDACs, the roles of Class II HDACs in oligodendrocyte development are still not known (Yao and Yang, 2011). Other reports investigated roles of individual Class III and IV HDACs in oligodendrocyte differentiation; Class III HDAC Sirt2 promotes the differentiation of CG4 oligodendrocytes (Ji et al., 2011), and knockdown of the Class IV HDAC (HDAC11) reduces myelin gene expression and arrested oligodendrocyte maturation in the same cell line (Liu et al., 2009).
In the present study, using a rat model of focal cerebral ischemia, we examined the spatial profile of individual Class I and Class II HDACs in OPCs and mature OLGs in the peri-infarct white matter. We found that stroke induces diverse changes in the expression profiles of individual Class I and Class II HDACs in OPCs and OLGs.
Material and methods
All experimental procedures were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee (IACUC) of Henry Ford Health System. All efforts were made to minimize suffering of animals.
Animal model of middle cerebral artery occlusion (MCAO)
Adult male Wistar rats (3–4 months, 270–300 g) were employed in all experiments. The middle cerebral artery (MCA) was permanently occluded via an intraluminal vascular occlusion method modified in our laboratory (Chen et al., 1992; Zhang et al., 2009). Briefly, following anesthesia and neck dissection, a 4-0 surgical nylon suture (18.5–19.5 mm) determined by the animal weight, with its tip expanded by heating near a flame, was advanced from the right external carotid artery (ECA) into the lumen of the internal carotid artery (ICA) to block the origin of the MCA. Rats (n= 6–8/group) were subjected to MCAO and sacrificed at 2, 7 or 14 days after stroke. Sham-operated rats were similarly anesthetized and neck dissection performed without suture advancement.
Bromodeoxyuridine labeling
Bromodeoxyuridine (BrdU), a thymidine analog that is incorporated into cells during DNA synthesis, was used for S-phase labeling. Using a cumulative labeling protocol (Zhang et al., 2001), rats (n= 6–8/group) were injected daily with BrdU (Sigma-Aldrich, St. Louis, MO), intraperitoneally at 50 mg/kg/day for 2 or 7 consecutive days, starting 24 hours after surgery. Animals were sacrificed 2 or 7 days after surgery at 2 hours after the last BrdU injection. Animals sacrificed at 14 days after surgery were injected daily with BrdU for 7 days starting 24 hours after surgery.
Tissue preparation
Brains were fixed by transcardial perfusion with saline, followed by perfusion and immersion in 4% paraformaldehyde. Brains were cut into seven equally spaced (2 mm) coronal blocks and embedded in paraffin. A series of coronal sections (6 μm thick) were obtained at the center of the lesion, corresponding to coronal coordinates for Bregma −1 to +1 mm (Paxinos and Watson, 1986), and used for immunohistochemistry.
Immunohistochemistry
Immunohistochemistry was performed following standard protocols, as previously described (Liu et al., 2012). The following primary antibodies were used: mouse anti-Adenomatous Polyposis Coli (APC [CC-1], 1:20; GWB-D835F1, GenWay, San Diego, CA), a marker for mature OLGs (Bhat et al., 1996), mouse anti-Chondroitin Sulfate Proteoglycan (NG2, 1:1000; 05-710, Millipore, Billerica, MA), a marker of OPCs (Ness et al., 2005), mouse anti-BrdU (1:100; M0744, Dako, Carpinteria, CA) and rabbit anti-Ki67 (1:300; RM-9106, Thermo Scientific, Barrington, IL), markers for proliferating cells. Class I and Class II HDACs were examined using: rabbit anti-HDAC1 (1:2000; ab7028, Abcam, Cambridge, MA), rabbit anti-HDAC2 (1:250; sc-7899, Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-HDAC4 (1:50; sc-11418, Santa Cruz Biotechnology) and rabbit anti-HDAC5 (1:50; sc-11419, Santa Cruz Biotechnology).
Histone acetylation levels were examined using: rabbit anti-Acetyl Histone H3 Lys9/Lys14 (Ac-HH3, 17-615, 1:1200; Millipore) and rabbit anti-Acetyl Histone H4 Lys12 (Ac-HH4, 06-1352, 1:2000; Millipore). The tissues were incubated with the primary antibodies listed above and with Cy3 (Jackson ImmunoResearch, West Groove, PA) or fluorescein isothiocyanate (FITC, Jackson ImmunoResearch) conjugated secondary antibodies. Nuclei were counterstained with 4′, 6′-diamidino-2-phenylindole (DAPI, Vector Laboratories, Burlingame, CA) at 1:10000. Negative controls omitting the primary antibody were used to verify that conditions of the immunostaining did not result in a non-specific background. Negative controls for all double immunohistochemistry experiments produced a negative outcome, ensuring that only specific signals are detected.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
TUNEL for the identification of apoptotic cells was performed using the ApopTag® Fluorescein in Situ Apoptosis Detection Kit (Millipore) following the manufacturer’s manual.
Image acquisition and analysis
Quantification of immunoreactive cells was performed as previously described (Liu et al., 2012). Brain coronal sections were digitized under a 40X objective (BX40; Olympus Optical) using a three-CCD color video camera (Sony DXC-970MD) interfaced with an MCID image analysis system. For each immunostaining, three coronal sections from each rat brain were acquired from the center of the ischemic lesion at 100 μm intervals. From each coronal section, six fields of view from the peri-infarct corpus callosum (within 500μm adjacent to the lesion border) and six fields from the contralateral homologeous area of sham and MCAO operated rats were acquired. Immunoreactive cells were counted on a computer monitor to improve visualization. The total numbers of immunoreactive cells per mm2 area are presented. The number of rats used for each statistical analysis is described as n=x/group in all experiments.
Statistical analysis
Student’s t test was used when comparing two groups. One-way ANOVA with post hoc Bonferroni tests was used when comparing more than two groups. Statistical significance was set a p<0.05. All values are presented as mean ± SE for illustration.
Results
Temporal profile of oligodendrocyte recovery in white matter after stroke
To evaluate the temporal profile of oligodendrocyte recovery in white matter after stroke, we examined the proportions of NG2+ OPCs and APC+ OLGs in the peri-infarct corpus callosum at 2, 7 and 14 days after stroke compared to sham control.
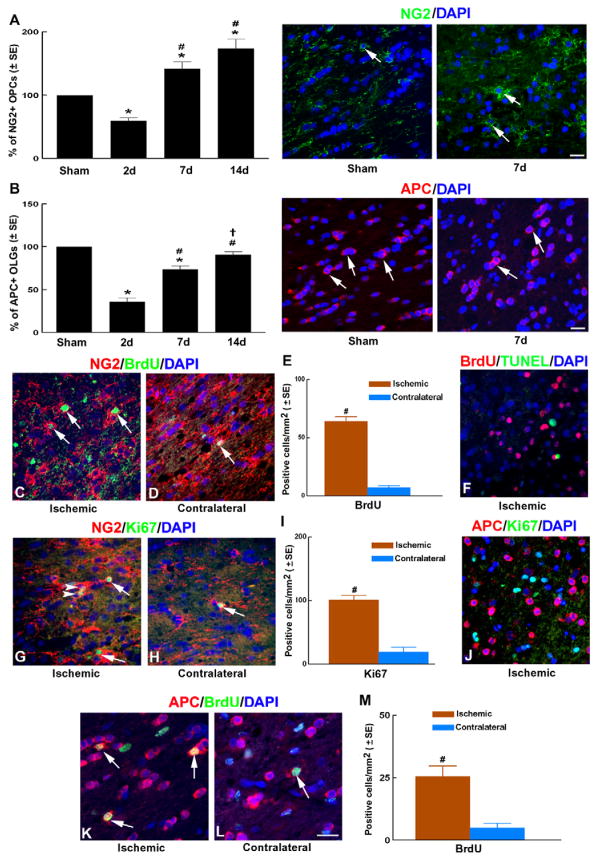
In the normal corpus callosum, NG2+ OPCs had an elongated shape with long processes, while APC+ OLGs had a round body and were found in clusters parallel to the orientation of axons. Compared to sham control, we found a significant reduction of NG2+ OPCs in the ischemic corpus callosum at 2 days after MCAO (down to ~60% of sham), that later increased to 142% and 174% of sham levels at 7 and 14 days after MCAO (Figure 1A). Morphologically, NG2+ OPCs at 2 days after MCAO exhibited short processes, whereas 7 and 14 days after MCAO, NG2+ OPCs displayed multiple and long processes.
Figure 1. OPCs proliferation and OLGs generation in the ischemic white matter.
Panels A–B show quantitative data of NG2+ OPCs (A) and APC+ OLGs (B) in the peri-infarct corpus callosum at 2, 7 and 14 days after MCAO compared to sham control and representative microscopic images of NG2+ OPCs (A) and APC+ OLGs (B) of sham and 7d MCAO groups. n = 6/group. *p<0.05 vs sham group; #p<0.05 vs 2 days group and †p<0.05 vs 7 days group.
Panels C-M show representative microscopic images of double immunofluorescent staining of NG2+ OPCs with BrdU (C to D, arrows) and Ki67 (G to H, arrows) immunoreactivity. Arrowheads in G point at Ki67+ OPCs that appear as doublets. Panels E and I show quantitative data of BrdU (E) and Ki67 (I) immunoreactive OPCs in the ipsilateral and contralateral corpus callosum. Panels K to L show representative microscopic images of APC+ OLGs with BrdU immunoreactivity. Panel M shows quantitative data of BrdU immunoreactive OLGs in the ipsilateral and contralateral corpus callosum. Panels F and J show lack of co-localization of TUNEL+ with BrdU+ cells (F) and APC+ with Ki67+ cells (J) in the peri-infarct corpus callosum. Bar: 20μm. Images (C–M) were taken from the ipsilateral (ischemic) or contralateral (contralateral) corpus callosum. n = 8/group. #p<0.01 and *p<0.05 vs the contralateral corpus callosum.
To examine the effect of stroke on OLGs, we counted the number of APC+ OLGs, because APC is primarily expressed in the cytoplasm of individual mature OLGs and has been previously used as a marker of mature OLGs (Bhat et al., 1996). We found a significant reduction of APC+ OLGs in the ischemic corpus callosum at 2 days after MCAO (down to ~36% of sham), that later recovered to 74% and 91% of sham levels at 7 and 14 days after MCAO (Figure 1B). Together, these data show that a robust recovery of oligodendrocytes occurs in white matter after ischemic injury which can be detected as early as one week after stroke.
Stroke induces OPC proliferation and differentiation in ischemic white matter
We next examined whether OPC proliferation and differentiation lead to increases in OPCs and mature OLGs after stroke. All the following measurements were performed on animals subjected to 7 days of MCAO, because this is the earliest time point among those we tested (2, 7 and 14 days), when an increase in OPCs and OLGs was observed (Figure 1A–B). To examine whether stroke increases proliferating OPCs during brain recovery, ischemic rats (n=8) were injected with BrdU for 7 consecutive days starting 24 hours after MCAO. All rats used for immunohistochemistry were sacrificed 7 days after induction of MCAO and 2 hours after the last BrdU injection. Double immunofluorescent staining showed that the numbers of NG2+/BrdU+ cells significantly increased in peri-infarct corpus callosum at 7 days after stroke (Fig. 1C–E) compared to the contralateral side, suggesting that stroke induces OPC proliferation. Since cells may reenter the cell cycle and incorporate BrdU during apoptotic death (Bauer and Patterson, 2005), we performed TUNEL staining along with BrdU immunostaining to rule out the possibility that apoptotic processes in OLGs induce BrdU incorporation. We found that BrdU+ and TUNEL+ cells were mutually exclusive in the ischemic corpus callosum (Fig. 1F), suggesting that BrdU+ cells represent proliferating cells and not apoptotic cells.
BrdU is incorporated during the S-phase of the cell cycle and can be passed down to daughter cells following division (Wojtowicz and Kee, 2006). Therefore, to examine whether enhanced OPC proliferation continues to actively occur a week after stroke, we measured Ki67 immunoreactivity, a protein that is present during all active phases of the cell cycle (G(1), S, G(2), and mitosis), but is absent from resting cells (G(0)) (Scholzen and Gerdes, 2000). Double immunostaining showed that some Ki67+/NG2+ OPCs in the peri-infarct corpus callosum appeared as doublets (Fig 1G, arrowheads) and quantification revealed that the numbers of NG2+/Ki67+ cells were significantly increased in the peri-infarct corpus callosum (Figure 1G–I) 7 days after stroke. Together, these data indicate that OPCs continue to actively proliferate in situ a week after stroke.
Mature OLGs do not proliferate and cell cycle exit is a prerequisite for oligodendrocyte differentiation (Casaccia-Bonnefil and Liu, 2003). As expected, double immunofluorescent staining showed that none of APC+ cells were Ki67+ (Fig. 1J); however, we observed a significantly higher number of APC+/BrdU+ cells in the peri-infarct corpus callosum (Fig. 1K-M) 7 days after stroke compared to the contralateral side. These data suggest that APC+/BrdU+ cells are newly generated mature OLGs that are likely derived from proliferating OPCs during the period of BrdU labeling. Taken together, our data show that stroke induces enhanced OPC proliferation and differentiation in white matter a week after stroke.
Stroke alters HDAC expression in OPCs and OLGs in peri-infarct white matter
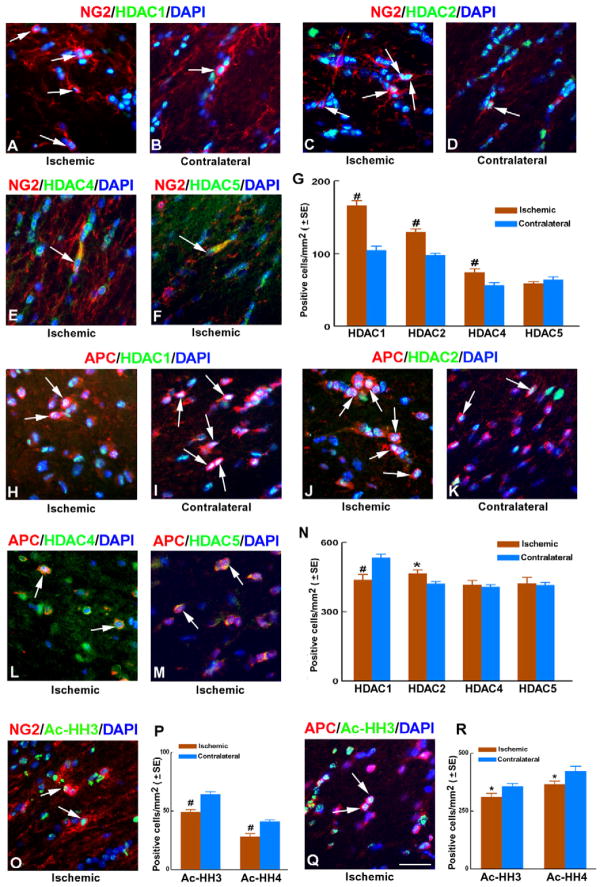
To examine whether HDACs are involved in stroke-induced-oligodendrogensis, we examined protein levels of individual members of Class I (1 and 2) and Class II HDACs (4 and 5) in white matter oligodendrocytes after stroke. We performed double immunofluorescent staining with antibodies specifically against each HDAC and against either NG2 or APC to detect OPCs and OLGs, respectively, in white matter a week after stroke. Immunoreactivity of HDAC1 and HDAC2 was primarily in nuclei, while HDAC4 and HDAC5 were predominantly expressed in the cytoplasm. Double immunostaining revealed that stroke markedly increased the numbers of NG2+ OPCs which co-localized with HDAC1, HDAC2, and HDAC4 immunoreactivity, but did not significantly change the number of HDAC5+/NG2+ cells in the peri-infarct corpus callosum (Fig. 2A–G). However, stroke significantly increased the number of HDAC2+/APC+ cells, whereas the number of HDAC1+/APC+ cells significantly decreased. Stroke did not significantly alter the numbers of HDAC4+ and HDAC5+/APC+ cells (Fig. 2H–N). These data reveal that stroke induces diverse changes in the expression profiles of individual Class I and Class II HDACs in OPCs and OLGs.
Figure 2. HDAC expression in OPCs and OLGs of ischemic white matter.
Representative microscopic images of double immunofluorescent staining (A to F) show NG2+ OPCs with nuclear Class I HDAC immunoreactivity (A to D, arrows) and cytoplasmic Class II HDAC immunoreactivity (E, F, arrows). Panel G shows quantitative data of individual HDAC immunoreactive OPCs in the ipsilateral and contralateral corpus callosum. Panels H to M show representative microscopic images of APC+ OLGs with nuclear Class I HDAC immunoreactivity (H to K, arrows) and cytoplasmic Class II HDAC immunoreactivity (L, M, arrows). Panel N is quantitative data of individual HDAC immunoreactive OLGs in the ipsilateral and contralateral corpus callosum. Panels O and Q show representative microscopic images of NG2+ OPCs and APC+ OLGs with nuclear Ac-HH3 immunoreactivity, respectively. Panels P and R show quantitative data of Ac-HH3 and Ac-HH4 immunoreactive OPCs (P) and OLGs (R) in the ipsilateral and contralateral corpus callosum. Bar: 50μm. All images were taken from the ipsilateral (ischemic) or contralateral (contralateral) corpus callosum. n = 8/group. #p<0.01 and *p<0.05 vs the contralateral corpus callosum.
Stroke alters histone acetylation levels in OPCs and OLGs in peri-infarct white matter
HDAC activity catalyzes the removal of an acetyl group from lysine residues of histone tails within nuclear chromatin (Kouzarides, 2007). To investigate whether the observed changes in expression levels of HDAC isoforms in OPCs and OLGs after stroke are accompanied by changes in HDAC activity, histone acetylation levels were assessed. Using double immunofluorescent staining, we quantified co-localization of acetylated histone H3 (Ac-HH3) and acetylated histone H4 (Ac-HH4) immunoreactivity in NG2+ OPCs and APC+ OLGs. Stroke substantially decreased the numbers of Ac-HH3 and Ac-HH4 positive OPCs, as well as decreased the numbers of Ac-HH3 and Ac-HH4 positive OLGs (Fig. 2O-R) in the peri-infarct corpus callosum. The observed higher density of Ac-HH3+ and Ac-HH4+ OLGs versus Ac-HH3+ and Ac-HH4+ OPCs (Fig. 2O-R) is most likely due to the larger number of OLGs in white matter compared to OPCs. Together, these data suggest that histone acetylation levels in both OPCs and OLGs in the white matter are reduced after ischemia.
Discussion
In the present study, we show that stroke-induced oligodendrogenesis in white matter is associated with distinct profiles of Class I (1–2) and Class II (4–5) HDACs in OPCs and OLGs, suggesting that the individual members of Class I and II HDACs could play divergent roles in OPCs and OLGs in response to ischemia during white matter remodeling. HDAC activity catalyzes the removal of an acetyl group (deacetylation) from lysine residues of histone tails within nuclear chromatin (Kouzarides, 2007) and favors compaction of chromatin leading to gene repression (Roth and Allis, 1996). A crosstalk between extrinsic signals, transcription factors and chromatin modifiers (such as HDACs), determine if a progenitor cell would proliferate and differentiate in response to injury (Li et al., 2009). Stroke induces oligodendrogenesis in peri-infarct regions by augmentation of OPC proliferation and promoting OPC differentiation into mature OLGs (McIver et al., 2010; Tanaka et al., 2001; Tanaka et al., 2003; Zhang et al., 2010; Zhang et al., 2011). Whether stroke-induced oligodendrogenesis is related to expression of HDACs has not been examined. The present study shows that stroke induces diverse changes in the expression profiles of individual Class I and Class II HDACs in OPCs and OLGs. The identification via epiflurescent microscopy of Class I HDACs as primarily nuclear, while Class II HDACs as mainly cytoplasmic in OPCs and OLGs of the peri-infarct white matter, suggest that Class I HDACs are more likely to participate in epigenetic regulation, while Class II HDACs are more likely to deacetylate cytoplasmic substrates. However, additional 3D confocal imaging is required to verify these observations. Class II HDACs had a lower expression levels and showed less dramatic changes in white matter OLGs after stroke. Therefore, Class I HDACs could be more actively involved than Class II HDACs in mediating stroke-induced oligodendrogenesis. The importance of histone deacetylation for OPC differentiation has previously been demonstrated by a large series of experiments (Dewar et al., 2003; Marin-Husstege et al., 2002). In vitro, selective silencing of HDAC1 or HDAC2 was found to impair the timely differentiation of primary OPCs (Shen et al., 2008b). Mice with ablations of both HDAC1 and HDAC2 genes, but not an individual gene, in the OLG lineage suppress OPC differentiation into mature OLGs, consequently leading to animal death at postnatal week 2, indicating that these HDACs play redundant roles in OPC differentiation during development (Ye et al., 2009). Our study shows an association between substantial increases in Class I HDACs in OPCs and augmentation of proliferating OPCs as determined by BrdU and Ki67 double positive cells after stroke, suggesting a possible role for these HDACs in regulating OPC proliferation after injury. In addition, our data show mixed patterns of decrease in HDAC1 and increase in HDAC2 in OLGs, which were associated with an increase in mature OLGs after stroke. We also found that stroke increased BrdU positive OLGs that were out of the cell cycle, as determined by negative Ki67 immunoreactivity. Given that mature OLGs do not proliferate, these BrdU positive OLGs derive from OPCs. Thus, our data suggest that in addition to OPC proliferation, different levels of HDAC1 and HDAC2 mediate OPC differentiation. Indeed, by downregulation of Sox2, these HDACs promote OPCs to differentiate into myelinating OLGs (Shen et al., 2008b). HDAC1 and HDAC2 also negatively regulate Wnt and Notch signaling that inhibit OPC differentiation (Ren et al., 2004; Shen et al., 2008b). Recruitment of HDAC1 to the myelin basic protein (MBP) promoter leads to repression of the MBP expression (Wei et al., 2005). MBP is exclusively expressed by mature OLGs and its expression is mainly regulated at the transcriptional level (Wei et al., 2003). Together along with our data, we speculate that upregulation of MBP expression mediated by a decrease in HDAC1, and suppression of Sox2 and Wnt and Notch signaling in OPCs mediated by an increase in HDAC2, lead to enhancement of OPC differentiation to mature OLGs after stroke.
Recent studies have primarily focused on the use of non-selective HDAC inhibitors such as VPA (Liu et al., 2012) to promote white matter recovery after stroke. These compounds target multiple classes of HDACs. The present study revealed that individual HDACs in OLGs and OPCs may play distinctive roles in inducing oligodendrogenesis during white matter remodeling after stroke. Therefore, additional mechanistic studies are warranted to facilitate the development of a more specific and effective HDAC-targeting drug therapy for stroke.
Highlights.
Stroke-induced oligodendrogenesis is associated with distinct profiles of HDACs.
Stroke increases HDAC1, HDAC2 and HDAC4 positive OPCs.
A decrease in HDAC1 and increase in HDAC2 is detected in mature OLGs after stroke.
Stroke decreases the acetylation levels of histones H3 and H4 in OPCs and OLGs.
Class I and II HDACs could play divergent roles in OPCs and OLGs after ischemia.
Acknowledgments
This work was supported by National Institutes of Health Grants, RO1 AG037506 (MC), and RO1 NS075156 (ZGZ) and AHA Scientist Development Grant 10SDG2790012 (XL). The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
The authors wish to thank Qinge Lu and Sutapa Santra for technical assistance.
List of abbreviations
- Ac-HH
acetylated histone H
- APC
adenomatous polyposis coli
- BrdU
bromodeoxyuridine
- DAPI
4,6′-diamidino-2-phenylindole
- HDAC
histone deacetylase
- MBP
myelin basic protein
- MCAO
middle cerebral artery occlusion
- NG2
chondroitin sulfate proteoglycan
- OLGs
mature oligodendrocytes
- OPCs
oligodendrocyte progenitor cells
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baltan S. Histone deacetylase inhibitors preserve function in aging axons. J Neurochem. 2012;123(Suppl 2):108–115. doi: 10.1111/j.1471-4159.2012.07949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltan S, Murphy SP, Danilov CA, Bachleda A, Morrison RS. Histone deacetylase inhibitors preserve white matter structure and function during ischemia by conserving ATP and reducing excitotoxicity. J Neurosci. 2011;31:3990–3999. doi: 10.1523/JNEUROSCI.5379-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S, Patterson PH. The cell cycle-apoptosis connection revisited in the adult brain. J Cell Biol. 2005;171:641–650. doi: 10.1083/jcb.200505072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat RV, Axt KJ, Fosnaugh JS, Smith KJ, Johnson KA, Hill DE, Kinzler KW, Baraban JM. Expression of the APC tumor suppressor protein in oligodendroglia. Glia. 1996;17:169–174. doi: 10.1002/(SICI)1098-1136(199606)17:2<169::AID-GLIA8>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Casaccia-Bonnefil P, Liu A. Relationship between cell cycle molecules and onset of oligodendrocyte differentiation. J Neurosci Res. 2003;72:1–11. doi: 10.1002/jnr.10565. [DOI] [PubMed] [Google Scholar]
- Chen H, Chopp M, Zhang ZG, Garcia JH. The effect of hypothermia on transient middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1992;12:621–628. doi: 10.1038/jcbfm.1992.86. [DOI] [PubMed] [Google Scholar]
- Conway GD, O’Bara MA, Vedia BH, Pol SU, Sim FJ. Histone deacetylase activity is required for human oligodendrocyte progenitor differentiation. Glia. 2012;60:1944–1953. doi: 10.1002/glia.22410. [DOI] [PubMed] [Google Scholar]
- Dewar D, Underhill SM, Goldberg MP. Oligodendrocytes and ischemic brain injury. J Cereb Blood Flow Metab. 2003;23:263–274. doi: 10.1097/01.WCB.0000053472.41007.F9. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Goldberg MP, Ransom BR. New light on white matter. Stroke. 2003;34:330–332. doi: 10.1161/01.str.0000054048.22626.b9. [DOI] [PubMed] [Google Scholar]
- Gregersen R, Christensen T, Lehrmann E, Diemer NH, Finsen B. Focal cerebral ischemia induces increased myelin basic protein and growth-associated protein-43 gene transcription in peri-infarct areas in the rat brain. Exp Brain Res. 2001;138:384–392. doi: 10.1007/s002210100715. [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Ji S, Doucette JR, Nazarali AJ. Sirt2 is a novel in vivo downstream target of Nkx2.2 and enhances oligodendroglial cell differentiation. J Mol Cell Biol. 2011;3:351–359. doi: 10.1093/jmcb/mjr009. [DOI] [PubMed] [Google Scholar]
- Keirstead HS, Blakemore WF. Identification of post-mitotic oligodendrocytes incapable of remyelination within the demyelinated adult spinal cord. J Neuropathol Exp Neurol. 1997;56:1191–1201. doi: 10.1097/00005072-199711000-00003. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Leeds P, Chuang DM. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J Neurochem. 2009;110:1226–1240. doi: 10.1111/j.1471-4159.2009.06212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Rowe M, Ren M, Hong JS, Chen PS, Chuang DM. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J Pharmacol Exp Ther. 2007;321:892–901. doi: 10.1124/jpet.107.120188. [DOI] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Li H, He Y, Richardson WD, Casaccia P. Two-tier transcriptional control of oligodendrocyte differentiation. Curr Opin Neurobiol. 2009;19:479–485. doi: 10.1016/j.conb.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Hu Q, D’Ercole AJ, Ye P. Histone deacetylase 11 regulates oligodendrocyte-specific gene expression and cell development in OL-1 oligodendroglia cells. Glia. 2009;57:1–12. doi: 10.1002/glia.20729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Chopp M, Kassis H, Jia LF, Hozeska-Solgot A, Zhang RL, Chen C, Cui YS, Zhang ZG. Valproic acid increases white matter repair and neurogenesis after stroke. Neuroscience. 2012;220:313–321. doi: 10.1016/j.neuroscience.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Husstege M, Muggironi M, Liu A, Casaccia-Bonnefil P. Histone deacetylase activity is necessary for oligodendrocyte lineage progression. J Neurosci. 2002;22:10333–10345. doi: 10.1523/JNEUROSCI.22-23-10333.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIver SR, Muccigrosso M, Gonzales ER, Lee JM, Roberts MS, Sands MS, Goldberg MP. Oligodendrocyte degeneration and recovery after focal cerebral ischemia. Neuroscience. 2010;169:1364–1375. doi: 10.1016/j.neuroscience.2010.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem. 2008;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- Ness JK, Valentino M, McIver SR, Goldberg MP. Identification of oligodendrocytes in experimental disease models. Glia. 2005;50:321–328. doi: 10.1002/glia.20206. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27:1641–1646. doi: 10.1161/01.str.27.9.1641. discussion 1647. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2. Academic Press; Sydney ; Orlando: 1986. [Google Scholar]
- Ren M, Leng Y, Jeong M, Leeds PR, Chuang DM. Valproic acid reduces brain damage induced by transient focal cerebral ischemia in rats: potential roles of histone deacetylase inhibition and heat shock protein induction. J Neurochem. 2004;89:1358–1367. doi: 10.1111/j.1471-4159.2004.02406.x. [DOI] [PubMed] [Google Scholar]
- Roth SY, Allis CD. Histone acetylation and chromatin assembly: a single escort, multiple dances? Cell. 1996;87:5–8. doi: 10.1016/s0092-8674(00)81316-1. [DOI] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Shen S, Li J, Casaccia-Bonnefil P. Histone modifications affect timing of oligodendrocyte progenitor differentiation in the developing rat brain. J Cell Biol. 2005;169:577–589. doi: 10.1083/jcb.200412101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Liu A, Li J, Wolubah C, Casaccia-Bonnefil P. Epigenetic memory loss in aging oligodendrocytes in the corpus callosum. Neurobiol Aging. 2008a;29:452–463. doi: 10.1016/j.neurobiolaging.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Sandoval J, Swiss VA, Li J, Dupree J, Franklin RJ, Casaccia-Bonnefil P. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat Neurosci. 2008b;11:1024–1034. doi: 10.1038/nn.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Nogawa S, Ito D, Suzuki S, Dembo T, Kosakai A, Fukuuchi Y. Activation of NG2-positive oligodendrocyte progenitor cells during post-ischemic reperfusion in the rat brain. Neuroreport. 2001;12:2169–2174. doi: 10.1097/00001756-200107200-00025. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Nogawa S, Suzuki S, Dembo T, Kosakai A. Upregulation of oligodendrocyte progenitor cells associated with restoration of mature oligodendrocytes and myelination in peri-infarct area in the rat brain. Brain Res. 2003;989:172–179. doi: 10.1016/s0006-8993(03)03317-1. [DOI] [PubMed] [Google Scholar]
- Wei Q, Miskimins WK, Miskimins R. Cloning and characterization of the rat myelin basic protein gene promoter. Gene. 2003;313:161–167. doi: 10.1016/s0378-1119(03)00675-9. [DOI] [PubMed] [Google Scholar]
- Wei Q, Miskimins WK, Miskimins R. Stage-specific expression of myelin basic protein in oligodendrocytes involves Nkx2.2-mediated repression that is relieved by the Sp1 transcription factor. J Biol Chem. 2005;280:16284–16294. doi: 10.1074/jbc.M500491200. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM, Kee N. BrdU assay for neurogenesis in rodents. Nat Protoc. 2006;1:1399–1405. doi: 10.1038/nprot.2006.224. [DOI] [PubMed] [Google Scholar]
- Yao YL, Yang WM. Beyond histone and deacetylase: an overview of cytoplasmic histone deacetylases and their nonhistone substrates. J Biomed Biotechnol. 2011;2011:146493. doi: 10.1155/2011/146493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F, Chen Y, Hoang T, Montgomery RL, Zhao XH, Bu H, Hu T, Taketo MM, van Es JH, Clevers H, Hsieh J, Bassel-Duby R, Olson EN, Lu QR. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat Neurosci. 2009;12:829–838. doi: 10.1038/nn.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li Y, Zhang ZG, Lu M, Borneman J, Buller B, Savant-Bhonsale S, Elias SB, Chopp M. Bone marrow stromal cells increase oligodendrogenesis after stroke. J Cereb Blood Flow Metab. 2009;29:1166–1174. doi: 10.1038/jcbfm.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chopp M, Zhang RL, Wang L, Zhang J, Wang Y, Toh Y, Santra M, Lu M, Zhang ZG. Erythropoietin amplifies stroke-induced oligodendrogenesis in the rat. PLoS One. 2010;5:e11016. doi: 10.1371/journal.pone.0011016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Chopp M, Zhang ZG. Oligodendrogenesis after cerebral ischemia. Front Cell Neurosci. 2013;7:201. doi: 10.3389/fncel.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Roberts C, Jia L, Wei M, Lu M, Wang X, Pourabdollah S, Zhang ZG. Ascl1 lineage cells contribute to ischemia-induced neurogenesis and oligodendrogenesis. J Cereb Blood Flow Metab. 2011;31:614–625. doi: 10.1038/jcbfm.2010.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]