Abstract
Context
Nonpsychotic siblings of patients with childhood-onset schizophrenia (COS) share cortical gray matter abnormalities with their probands at an early age; these normalize by the time the siblings are aged 18 years, suggesting that the gray matter abnormalities in schizophrenia could be an age-specific endophenotype. Patients with COS also show significant white matter (WM) growth deficits, which have not yet been explored in nonpsychotic siblings.
Objective
To study WM growth differences in non-psychotic siblings of patients with COS.
Design
Longitudinal (5-year) anatomic magnetic resonance imaging study mapping WM growth using a novel tensor-based morphometry analysis.
Setting
National Institutes of Health Clinical Center, Bethesda, Maryland.
Participants
Forty-nine healthy siblings of patients with COS (mean [SD] age, 16.1[5.3] years; 19 male, 30 female) and 57 healthy persons serving as controls (age, 16.9[5.3] years; 29 male, 28 female).
Intervention
Magnetic resonance imaging.
Main Outcome Measure
White matter growth rates.
Results
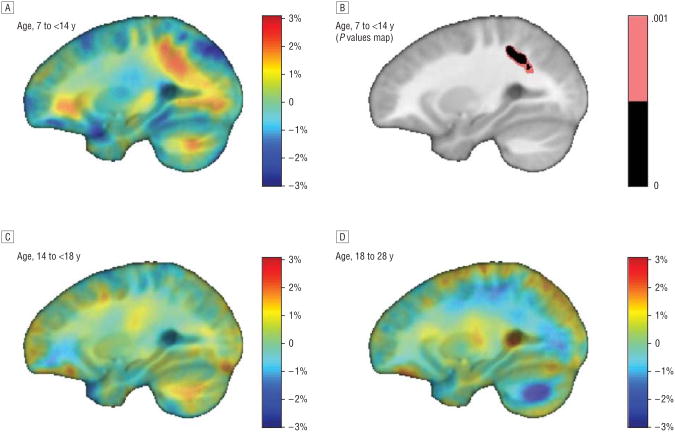
We compared the WM growth rates in 3 age ranges. In the youngest age group (7 to <14 years), we found a significant difference in growth rates, with siblings of patients with COS showing slower WM growth rates in the parietal lobes of the brain than age-matched healthy controls (false discovery rate, q = 0.05; critical P = .001 in the bilateral parietal WM; a post hoc analysis identified growth rate differences only on the left side, critical P =.004). A growth rate difference was not detectable at older ages. In 3-dimensional maps, growth rates in the siblings even appeared to surpass those of healthy individuals at later ages, at least locally in the brain, but this effect did not survive a multiple comparisons correction.
Conclusions
In this first longitudinal study of nonpsychotic siblings of patients with COS, the siblings showed early WM growth deficits, which normalized with age. As reported before for gray matter, WM growth may also be an age-specific endophenotype that shows compensatory normalization with age.
Schizophrenia is a complex psychiatric disorder with a strong genetic predisposition.1 Childhood-onset schizophrenia (COS) is neurobiologically continuous with the adult disorder but is usually more clinically severe, with onset of psychosis before age 13 years.2,3 Psychotic symptoms found in COS are complex, variable, and difficult to summarize, and it is important to seek reliable brain imaging measures to quantify disease severity and assess risk for developing the illness. Brain imaging studies consistently show enlarged lateral ventricles, progressive loss of cortical gray matter (GM), and GM deficits in medial temporal lobe structures.4-7 The GM abnormalities, particularly the loss observed during childhood and early adolescence, are typically greater in COS relative to patients with the adult-onset disorder.2,3
Brain measures obtained using structural magnetic resonance imaging (MRI) may also serve as targets for genetic analysis.8,9 Nonpsychotic first-degree relatives— siblings who share risk genes with the patients but remain healthy—offer a unique opportunity to identify brain measures associated with heightened genetic risk. These siblings share up to 50% of their genetic polymorphisms (and their environment in many cases) with the patients. Nonpsychotic siblings of patients with adult-onset schizophrenia show inconsistent findings: most studies10 do not detect cortical GM deficits in the nonpsychotic siblings. Studies of patients with COS provide a unique opportunity to study very young nonpsychotic siblings, allowing earlier prospective studies of brain development than previously carried out for adult schizophrenia. The brain can therefore be assessed, in some cases even longitudinally, well before the typical age at onset for schizophrenia near late adolescence. In some cases, the brain can be examined both before and after disease onset.11
In earlier studies, young nonpsychotic siblings of patients with COS showed significant GM deficits in the prefrontal and temporal cortices at earlier ages that, interestingly, normalized by the time the siblings were 18 years old.12 These findings were recently replicated in a non-overlapping sample of 43 nonpsychotic siblings of patients with COS (68 scans) and 86 matched healthy controls (136 scans).13 This allowed us to identify the location and duration of derailed brain development that was associated with increased genetic liability. The GM deficits seen in relatives of patients with COS may therefore be age specific; currently unknown protective factors (environmental or genetic) may help to normalize these deficits with age in individuals who remain nonpsychotic.14
White matter (WM) abnormalities in schizophrenia are relatively underexplored. Compared with controls, smaller and larger volumes of lobar WM have been reported in groups of patients with schizophrenia.15 Similarly, abnormal WM connectivity16,17 or integrity18-20 has been reported in schizophrenia, as measured by diffusion tensor imaging (DTI). However, only one study has assessed WM integrity in populations with early-onset schizophrenia. A DTI study21 in adolescent-onset schizophrenia showed widespread areas of lower fractional anisotropy (FA) in the brain. In another study22 using tensor-based morphometry, the only longitudinal study of COS patients to date, we found slower WM growth rates in COS patients relative to the typical WM development in controls during the adolescent years. Recent studies of WM volume in twins discordant for schizophrenia indicate genetic influences on decreased WM volume in schizophrenia,23 with no clear influences on WM changes.24,25 Another pertinent study is a cross-sectional DTI analysis from Utrecht, the Netherlands, in which the arcuate fasciculus exhibited significantly different FA values in full siblings of patients with schizophrenia compared with matched healthy controls. Healthy siblings had higher FA in this tract compared with controls, perhaps suggesting a compensatory increase.26 White matter abnormalities may also be markers of genetic risk for the illness. We hypothesized that, similar to the GM findings, WM growth rate abnormalities would be detectable in siblings at earlier ages but would normalize as the siblings became older.
In this study, we examined longitudinal brain development in nonpsychotic siblings of COS patients and compared them with matched healthy controls, using tensor-based morphometry to compute growth rates in different parts of the brain.27 We hypothesized that nonpsychotic siblings would show WM developmental abnormalities compared with healthy controls at earlier ages. To study age effects, we divided the sample into 3 age groups: pre-adolescence (age, 7 to <14 years; included only children who had not passed their 14th birthday), postpuberal/adolescence (age, 14 to <18 years), and young adult (age, 18-28 years).
Methods
All participants were recruited as part of an ongoing National Institute of Mental Health study of COS and normal brain development. Forty-nine healthy siblings of patients with COS (19 male, 30 female) and 57 healthy individuals serving as controls (29 male, 28 female), matched for age, sex, and scan interval, were evaluated prospectively during a 5-year longitudinal study.
Participants
All available full biological siblings of patients with COS participated in the study and were scanned prospectively every 2 years along with the patients. From this data set, there were 49 healthy siblings with at least 2 usable scans acquired 1 to 4 years apart. The midinterval mean (SD) age for the group was 16.1 (5.3) years. Siblings were evaluated using structured psychiatric interviews for Axis I (using either the Schedule for Affective Disorders and Schizophrenia or the Schedule for Affective Disorders and Schizophrenia for School-age Children)28 and Axis II (using the Structured Interview for the DSM-IV-TR Personality Disorders)29 diagnoses, and their Global Assessment Scale scores were estimated by a child psychiatrist. Siblings were considered healthy if they were free of any psychotic or schizophrenia spectrum disorder (which included schizophrenia, schizoaffective disorder on Axis I, or paranoid, schizotypal, schizoid, or avoidant personality disorders on Axis II). Other Axis I and Axis II diagnoses are reported in the Table. Most common among these is depressive illness at some point in life, often attributable to the stress of having a schizophrenic proband in the family. Additionally, symptom counts for all Axis II disorders were calculated for individual disorders and cumulatively for schizophrenia spectrum diagnoses. As expected, none of the siblings was receiving antipsychotic or mood stabilizer medications, and only 3 siblings had taken a selective serotonin reuptake inhibitor or stimulant medications either for anxiety/depression or attentional issues, respectively.
Table. Demographics.
| Characteristic | Healthy Controls (n = 57) | Nonpsychotic Siblings of COS Patients (n = 49)a | Test Statistic With df | P Value | |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 28 | 30 |
|
.21 | |
| Male | 29 | 19 | |||
| Handedness | |||||
| Left | 4 | 2 |
|
.52 | |
| Right | 53 | 47 | |||
| Race | |||||
| Asian | 1 | 1 |
|
.32 | |
| Black/African American | 6 | 8 | |||
| Hispanic | 5 | 4 | |||
| Other/mixed race | 4 | 9 | |||
| White | 41 | 27 | |||
| Socioeconomic status, mean (SD)b | 41.9 (20.3) | 51.4 (24.7) | t104 = 0.77 | .03 | |
| Vocabulary score, mean (SD) | 11.5 (2.1) | 10.9 (2.9) | t97 = 1.12 | .26 | |
| Age at scan, y | |||||
| Overall, mean (SD) | 16.9 (5.3) | 16.1 (5.3) | t210 = 1.06 | .44 | |
| Median (range) | 16.3 (8.3-28.3) | 16.1 (7.8-27.2) | |||
| Time 1, mean (SD) | 15.7 (5.2) | 14.8 (5.1) | t104 = 0.93 | .35 | |
| Median (range) | 15.2 (6.9-27.8) | 15.0 (7.2-26.4) | |||
| Time 2, mean (SD) | 18.0 (5.3) | 17.4 (5.5) | t104 = 0.61 | .54 | |
| Median (range) | 17.7 (9.6-28.8) | 17.2 (8.4-28.0) | |||
| Scan interval, y | 2.3 (0.6) | 2.6 (0.7) | t104 = 2.45 | .02 | |
| Median (range) | 2.13 (0.98-3.70) | 2.42 (1.26-3.91) | |||
| Comorbid Axis I disorders, lifetimec | |||||
| Anxiety disorder | … | 9 | |||
| Alcohol/substance abuse | … | 2 | |||
| Behavioral disorder | … | 5 | |||
| Depressive mood | … | 15 | |||
| Bipolar disorder | … | 0 | |||
| Other Axis II disorders, lifetime | … | 7 |
Abbreviation: COS, childhood-onset schizophrenia.
None of the nonpsychotic siblings were receiving antipsychotic medication. Only 3 had taken either selective serotonin reuptake inhibitor or stimulant medication at some point in their lifetime.
Socioeconomic status was measured using the Hollingshead index.30 Higher scores reflect lower socioeconomic status.
Axis I disorders were not present in the control group.
A group of 57 unrelated healthy individuals serving as controls was selected from a sample of community volunteers recruited as part of a prospective study of normal brain development. They were matched for sex, age, and scan interval. Controls were free of lifetime medical or psychiatric disorders, as determined by clinical examination and standardized interview. Psychiatric illness in a first-degree relative was also exclusionary. The mean (SD) midinterval age for the control group was 16.9 (5.3) years.
The parental socioeconomic status score was obtained using the Hollingshead index.30 With the current cohort, it was not possible to obtain controls with matched socioeconomic status; however, for COS probands, we have not seen any significant influence of socioeconomic status on the structural findings and do not expect this in siblings.
The study was approved by the National Institute of Mental Health institutional review board. Written informed consent was obtained from parents and controls and patients older than 18 years, and written informed assent was obtained from minors.
Mri Acquisition
All images were acquired using a 1.5-T MRI scanner (Signa; General Electric) at the National Institutes of Health Clinical Center, Bethesda, Maryland. Three-dimensional (acquisition matrix, 256 × 256 × 124; voxel dimension, 0.9375 × 0.9375 × 1.5 mm), T1-weighted, fast-spoiled gradient-echo MRI volumes were acquired longitudinally. Imaging parameters were axial section thickness, 1.5 mm; echo time, 5 milliseconds; repetition time, 24 milliseconds; flip angle, 45°; and field of view, 24 cm. All participants were scanned identically at baseline and follow-up.
All structural scans collected on the 1.5-T MRI scanner were acquired with the standard single-channel quadrature coil (General Electric). The head coil was unchanged over the years. After each software and gradient upgrade, a set of participants was scanned before and after the change to reestablish reliability. To date, we have found no significant difference in measurements before and after these upgrades.
Image Processing
Scalp and other nonbrain tissues were removed to ensure that the tissue change maps were derived from brain tissues and not affected by the growing thickness of the skull or scalp. A whole-brain binary mask and brain tissue type classification (GM, WM, and cerebrospinal fluid) were generated for each participant's raw baseline scan using a skull-stripping meta-algorithm.31,32 This algorithm uses a skull-stripped reference image and identifies a consensus-based brain mask based on the results of several independent skull-stripping algorithms. The skull-stripped brain image data were then converted to binary masks and manually edited to eliminate any segmentation errors. A follow-up mask was then created by linearly registering the baseline to the follow-up image and applying the resulting transformation parameters to the baseline mask. Again, visual inspection and manual editing were used to ensure segmentation accuracy. A nonparametric, nonuniform, intensity normalization (N3) bias field correction algorithm was applied to reduce the intensity inhomogeneity in the MRIs caused by non-uniformities in the radio frequency receiver coils.33 Skull-stripped follow-up scans were globally aligned to the same individual's baseline scan using 12-parameter affine registration, and both were then aligned to the International Consortium for Brain Mapping single-subject, high-resolution brain template34 to adjust for intersubject differences in overall brain size and head alignment. The scaling factors for longitudinal registration were recorded and later used to rescale the Jacobian maps. All images were resampled with an isotropic matrix of 220 voxels in the x, y, and z dimensions; each interpolated voxel was 1 mm ×1 mm ×1 mm.
Minimal Deformation Template
A minimal deformation template (MDT) was created on the basis of data from 40 healthy controls to serve as an unbiased group-average template, as previously reported.27 Briefly, an affine average image was first created from globally aligned baseline scans. Each scan was then nonlinearly registered to the affine average template, using a nonlinear, inverse, consistent elastic registration algorithm.35 The deformation field was determined by maximizing a compound measure of the mutual information of the aligned image intensities and the elastic energy of the deformation. The nonlinear average brain image was created by voxelwise averaging of the intensities of the resampled images that were nonlinearly registered to the affine average template. Finally, the MDT was created by adjusting the nonlinear average with an inverse geometric centering of the displacement fields.36 The MDT was subsequently used as the target for intersubject registration.
Longitudinal Growth Maps
Individual 3-dimensional maps of brain tissue growth, also known as Jacobian maps, were derived from the deformation fields that spatially align the follow-up scan to the baseline scan, using elastic image registration.35,37 The Jacobian maps were then resampled with the displacement vector fields that spatially normalized the baseline scans to the MDT. Spatial normalizations among different brains enable cross-subject and group comparisons. To compute maps of growth rates (annual tissue change), the individual Jacobian maps were divided by their interscan intervals in years. Finally, a scaling factor, derived from the 12-parameter affine registration of baseline and follow-up scans, was applied to include any global growth modeled by the 12-parameter affine registration. All statistical analyses were based on the annualized Jacobian maps adjusted for the scaling of the brain at baseline.
Voxelwise Linear Regressions
At each point in the brain, a linear regression model was fitted to model the relationships between the growth rate and factors influencing the growth rate. Predictors included the midinterval age of the participant (B1), sex (B2), diagnosis (B3; nonpsychotic siblings of COS patients, 1; matched healthy controls, 0), age × diagnosis interactions (B4), and an age-squared term (B5) to model any evidence for a nonzero rate of acceleration or deceleration in the effect of age on growth rates.
In this model, the growth rate is directly modeled; therefore, if the age term fits, it implies a deceleration or acceleration of the growth rate. The age-squared term is included to assess even higher-order effects, ie, if the rate of acceleration or deceleration itself depends on age. As is typically the case, the age × diagnosis interaction term is designed to detect any group difference in the age effect on growth rates.
We applied these statistics using voxelwise (at each point in the brain) multiple regressions to assess whether the local rate of brain tissue change was significant and whether it depended on the covariates of interest. Statistical or “P value” maps were generated to visualize the pattern of voxelwise significance. To control for false-positives, we enforced a standard false discovery rate (FDR) correction for multiple statistical comparisons across voxels in the whole brain and inside each brain lobe, using the conventionally accepted false-positive rate of 5% (q = 0.05).38
Results
Average Growth Rates In Different Age Groups
We studied 3 age- and sex-matched groups (16 participants per group) representing the following age groups: preadolescents (age, 7 to <14 years), postpubertal adolescents (age, 14 to <18 years), and young adults (age, 18-28 years). The mean (SD) ages for the healthy control groups were 11.0 (1.5) (6 male/10 female), 16.0 (1.4) (9 male/7 female), and 22.3(2.8) years (8 male/8 female), respectively; the corresponding mean ages for the COS sibling groups were 10.0(1.5) (7 male/9 female; 3 individuals had depressive mode disorders), 16.1 (1.4) years (8 male/8 female; 5 individuals had depressive mode disorders), and 22.1(3.0) years (4 male/12 female; 6 subjects had depressive mode disorders), respectively. The average ages were not significantly different between siblings and controls within any of the age ranges (2-sample t tests; preadolescence, P=.06; adolescence, P=.82; young adult, P=.84). Demographic data for these subgroups are available in the eTable (http://www.archgenpsychiatry.com).
We compared growth rates in each age range, and only in the youngest age group (age, 7 to <14 years) did we find a significant difference in growth rates (Figure 1A and B). The COS siblings showed slower parietal WM growth rates than healthy controls at similar ages (FDR, q=0.05, critical P=.001) in the bilateral parietal WM; a post hoc analysis, split by hemisphere, detected a growth rate difference only on the left side (critical P=.004). The growth rate difference was no longer detectable at older ages (Figure 1C and D). From the 3-dimensional maps shown, the growth rates in the siblings appeared to surpass those of healthy controls at later ages, at least locally in the brain (eg, see the blue region in Figure 1D), but this effect did not survive the standard FDR-based correction for multiple comparisons.
Figure 1.
Contrast maps show growth rate differences between siblings of patients with childhood-onset schizophrenia and healthy controls. A, Red areas signify regions with faster growth rates in controls, observed in parietal white matter. B, Significant difference in growth rate was noted in the youngest age group. C and D, Later ages showed no significant differences.
Average Growth Rates
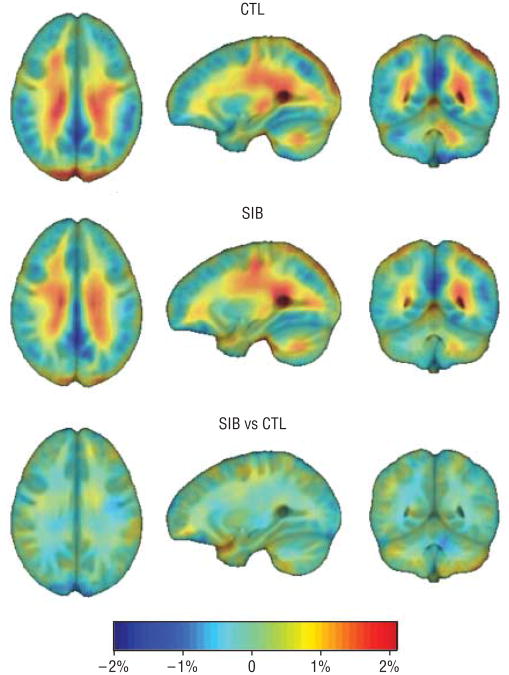
Figure 2 shows a 3-dimensional map of the mean growth rates (in percentage per year) for healthy children and nonpsychotic siblings of patients with COS and a comparison of growth rates between the 2 groups when all age groups were combined. Both groups showed active WM growth, as expected (1%-2% per year, bilaterally). When all age groups were combined, there was no significant difference overall between the 2 groups across the whole brain spatially.
Figure 2.
Mean growth rate maps for healthy controls (CTL), nonpsychotic siblings of patients with childhood-onset schizophrenia (SIB), and group difference maps. Both groups show stereotypical patterns of rapid growth in the white matter, and difference maps are near zero across the whole brain suggesting similar growth rates. Standard radiologic convention was used to display the images.
Age Effects On Growth Rates In Healthy Controls
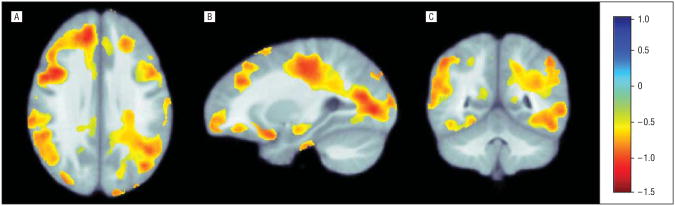
According to the correlation coefficient maps (β maps, Figure 3A) and P value maps (Figure 3B), the rate of WM growth slows as the healthy controls age, by as much as 0.5% to 1% per year in some regions. Critical P values for the age effects (B1) after correction for multiple comparisons were as follows: whole brain, P=.01; parietal lobe, P = .01; frontal lobe, P = .02; occipital lobe, P = .009; and temporal lobe, P = .02.
Figure 3.
Partial correlation coefficient maps of the age effects (B1). A, Transverse view. B, Sagittal view. C, Coronal view. The correlation coefficient maps show how age affects growth rates in healthy controls, corrected for multiple comparisons inside each brain lobe. In these regions, there is evidence that growth rates slow with age. The color bar shows the annual rate of change in the growth rate (as a percentage) per year. As such, the red colors show that the annual growth rate drops by approximately 0.5% to 1% per year. This is in line with common sense because the growth rate should slow as children age. Standard radiologic convention was used to display the images.
Age Effects On Growth Rates In Healthy Siblings Of Cos Patients
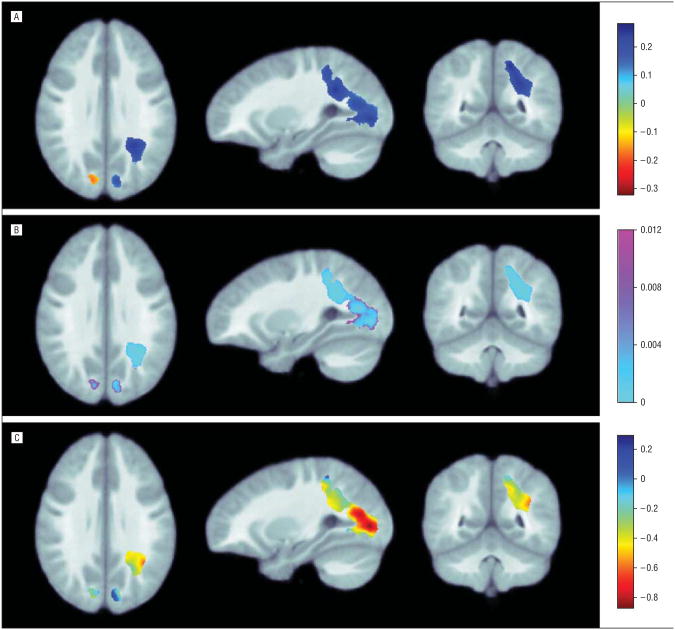
The B4 term in the linear regression model accounts for any group difference in age effects on growth rates between siblings of patients with COS and healthy controls (Figure 4A, B). Figure 4C shows the age effects on growth rates in the siblings. In the parietal and occipital lobes, the deceleration of WM growth rates was less (weaker) in the siblings compared with healthy controls. In other words, the siblings of patients with COS sustained a more constant growth rate compared with the controls (the sibling WM growth was not slowing as much as the growth for healthy controls). The growth rates in the siblings were slowing by approximately 0.2% to 0.8% per year (Figure 4C), while the rates in healthy controls were slowing by 0.5% to 1% per year (Figure 3A). Critical P values for the age × diagnosis interaction (B4) after correction for multiple comparisons were as follows: parietal lobe, P = .004; occipital lobe, P=.01. The age × diagnosis interactions for the whole brain and the frontal and temporal lobes were not significant.
Figure 4.
Maps of age effects on growth rates. A, Partial correlation coefficient maps for interaction between age effect and diagnosis in white matter (B4). B, P maps of significance for interaction between age effect and diagnosis in white matter (B4). C, Derived age effects in siblings of patients with childhood-onset schizophrenia (COS) corrected for multiple comparisons inside the white matter of each brain lobe (B1 + B4). White matter growth rates slow with age in nonpsychotic siblings of patients with COS. The color bar shows the annual rate of change in the growth rate (in percentage) per year. The red colors show that the annual growth rate drops by approximately 0.2% to 0.8% per year, less than those in healthy controls. Standard radiologic convention was used to display the images.
The Age-Squared Effect On Growth Rates
We identified a significant age-squared effect on growth rates, a term modifying the age effect on growth rates (note that this is an “age-cubed” effect on brain volumes). Signs of the correlation coefficients for B5 were positive, in contrast to the values of B1, the age effects on growth rates. The fit of the age-squared term showed that the rate of acceleration or deceleration was also changing; this is reasonable because all rates and their higher-order temporal derivative should reach equilibrium when the growth rate approaches zero. The growth rate plateaus before it declines in older age (at approximately 30 years), so it is reasonable that the β values of B5 were much smaller than B1 (B5 is typically approximately 25- to 50-fold smaller than B1). Critical P values for the age-squared term (B5) after correction for multiple comparisons were as follows: whole brain, P=.009; parietal lobe, P=.007; frontal lobe, P=.01; occipital lobe, P= .006; and temporal lobe, P=.02.
Baseline Differences
To assess whether any group differences were detectable at baseline, we performed a voxel × voxel analysis of brain volume differences, comparing siblings with controls, for each age group separately. We applied a standard FDR correction over the whole cerebral WM, as well as inside each brain lobe. In this baseline analysis, no group differences survived the standard FDR-based correction for multiple comparisons.
Comment
To our knowledge, this is the first study to explore WM growth rates from early childhood through adulthood in nonpsychotic siblings of patients with COS. Nonpsychotic siblings showed abnormally slow WM growth rates before age 14. These differences normalized over time and were no longer detectable after the typical age at onset for COS. This normalization effect has also been reported for the GM deficits in these siblings.12,13 As with healthy controls, the siblings showed nonlinear (slowing) WM growth with age; however, the initial brain growth rate in the siblings, which was slower than that of the controls, was maintained, whereas growth rate in the controls slowed more rapidly with age. This process of initial lag followed by normalization was detected significantly in parietal regions and qualitatively in prefrontal regions (Figure 1).
Nonpsychotic full siblings share approximately 50% of their genetic variations with the patients, manifest no schizophrenia spectrum symptoms, and have no antipsychotic medication exposure. Any shared brain abnormalities are likely to be markers of genetic liability for the illness.8 Prior studies of this population12 showed that nonpsychotic siblings shared GM deficits in prefrontal and temporal cortices at early ages and normalized as the siblings grew older, past the typical age at onset for schizophrenia. Gray matter deficits may therefore be age specific. Alternatively, the age-dependent normalization in growth rates may be a sign of “resilience” in siblings who do not become ill. It is important to determine whether the slowed growth seen here in siblings is mechanistically similar to that seen in individuals with schizophrenia. Such a study might examine both the siblings and patients and calculate relative risk of shared regions of abnormal growth. Supporting the notion that delayed growth is shared by siblings and patients, an earlier study,22 the only longitudinal study of patients with COS to date, compared 3-dimensional maps of local WM growth rates in patients with COS with those in healthy children (12 participants per group; age range, 12-16 years). We found slowed WM growth rates throughout the brain in the COS group. We did not include the COS patients in the current study to avoid duplication of data and difficulties in matching a 3-way sample on age, available scan numbers, scan intervals, and other parameters. We are in the process of collecting additional scans from COS patients and will expand the analysis to include controls, nonpsychotic siblings of patients with COS, and COS patients when we obtain a matched sample of COS patients.
Relatively few studies have addressed abnormalities of brain growth rates in schizophrenia, and still fewer have addressed them in patients' relatives. Volumetric studies show altered lobar WM volumes in schizophrenia15,20; however, some studies have reported larger39 and others smaller20,40,41 WM volumes in patients compared with controls, depending on the region examined. More recently, studies of WM integrity and connectivity using DTI have shown widespread reductions in the FA values in patients with schizophrenia compared with controls, as discussed in a review.20 However, the question whether these abnormalities are endophenotypes remains unaddressed. Volumetric studies, for the most part, have failed to show WM volume abnormalities in adult siblings of patients with schizophrenia.42-46 However, a recent study47 showed lower WM FA in the left prefrontal cortex and hippocampus in siblings of patients with schizophrenia. None of these studies included younger siblings or a longitudinal component covering the age range from children to young adults. As in the case of GM abnormalities, the WM alterations in siblings may normalize by late adolescence and hence are not seen in these samples. In a recent DTI study, Boos et al26 detected no WM abnormalities in adult siblings of patients with schizophrenia, but the arcuate fasciculus was described as thicker in siblings compared with probands. This raises the possibility of a compensatory thickening for possible functional normalization. Our findings suggest a similar compensatory increase in parietal (and perhaps prefrontal) WM, suggesting that a sustained growth rate with age (unlike that in healthy individuals) may compensate for an early lag in brain growth. This is supported to some extent by our regression analysis showing that growth trajectories between siblings and controls differ (Figure 4).
In siblings, the GM deficits normalize with age, and the parietal GM deficits are the earliest to appear as well as to normalize.12 The parietal GM deficits also normalize in patients with COS as they age.48 Thus, whether the growth rate alteration is primary or secondary to underlying GM changes is a difficult issue to determine with current MRI resolution. Slowed WM growth may therefore be an early-age endophenotype that normalizes with age. Studies of neurocircuitry development will examine this further. Siblings may also show more pronounced functional neurocircuitry abnormalities that improve with age.
In COS as well as adult schizophrenia, the GM and WM deficits are most commonly located in the frontal and temporal lobes.14 In this study, the growth abnormalities were localized mainly to occipital and parietal areas. Interestingly, occipital and parietal GM deficits are the ones to normalize earliest in the siblings of patients with COS. Taken together, these findings suggest that these regions may have a more resilient, early compensatory/corrective response in healthy siblings.
It may be useful to relate the WM growth to the profiles of cortical GM loss in patients and their siblings, for example, using GM thickness as a covariate to determine whether it accounts for the rates of WM growth or differences in those rates. As detailed by Gogtay et al in 2007,12 the cortical thickness measurements in our prior reports, defined as the distance between linked vertices of the GM and WM boundaries, were calculated at 40 962 cortical points. Even so, there is no natural point-to-point correspondence between each GM thickness measure (at a cortical point) and a WM volume measure (over a volume). Including cortical thickness as a covariate that might explain some of the variance in WM change would be difficult to achieve, since it would not be clear which part of the cortex to use for each internal WM point (such a mapping might be possible with DTI scans). Additionally, the GM trajectory follows a wavelike pattern, so average thickness might not be ideal. Future DTI studies may make it possible to relate GM to WM trajectories and determine how variance in one tracks or correlates to variance in the other.
The group aged 18 years or older was chosen because previous GM studies, now replicated in 2 independent samples,12,13 showed striking normalization of deficits by age 18 (the end of adolescence). We could have divided the sample as before and after age 18; however, we believed that it was scientifically interesting to see the changes at earlier ages. The division between 7 to less than 14 and 14 to less than 18 years was guided primarily by the available number of individuals with scans and an effort to balance the 3 groups. There is no consensus definition in the literature regarding prepubertal and post-pubertal age; however, motivated by these and prior GM findings, we are making strong efforts to recruit more siblings in early age groups and hope to map the nonlinear growth from early ages well into adulthood. In this study, we did not assess pubertal stage (eg, using Tanner scales), although we used pubertal stage as a convenient way to refer to the age groups (preadolescents and postpubertal adolescents). Healthy siblings do not undergo a physical examination (which is not permitted by the current institutional review board–approved design of the protocol); hence, staging according to the Tanner scales was not done.
To control for false-positives when conducting multiple statistical comparisons across the brain, we applied a standard FDR correction over the whole cerebral WM as well as inside each brain lobe. Generally, using a larger search region (whole-brain WM) will be more conservative than the smaller regions of interest (individual brain lobes). Group differences in age effects on growth rates were significant only in the parietal and occipital lobes, not at the whole-brain level, suggesting a local and relatively subtle effect. Our study is exploratory in that it consists of a unique group of siblings in a specific age range. As such, a less conservative approach can be justified to identify differences in unaffected individuals with an increased risk of psychosis.
Based on our past experiments,49 the MDT converges to a sharp and stable image once 20 to 40 brains are included, with a negligible difference obtained by adding more. Because of this, we chose 40 participants to make the MDT. In theory, one can use all participants; however, from experience and studies of how the mean template converges with increasing sample size,49,50 it is not vital and would increase computational demands (in terms of both time and memory). For smaller studies (<40 participants), one could use all available individuals. Participants were selected in an unbiased way (ie, randomly) but with certain criteria (age and sex) to ensure that the MDT group (n =40) was representative of the larger group (n =57). We balanced the subgroup demo-graphically to avoid any risk of bias in aligning new scans to the MDT.
The difference in interscan interval was significant but was relatively small. The interval used should not affect our analysis, since we divided the change maps by the scan interval. All final growth maps represent the average amount of change per year (or estimated growth rate). In other words, we normalized each tissue-change map to show the annual change rates. We might have estimated growth in the healthy children with slightly higher accuracy because longer intervals tend to involve greater changes, and the reproducibility error of serial scanning may be lower as a proportion of the overall change.
We did not collect IQ data on the siblings of patients with COS; only vocabulary scores were obtained. However, we are now collecting IQ scores on all newly recruited siblings, and these will be included in future analyses. Mismatch of IQ between study groups is always a limitation, since it is difficult to acquire IQ-matched controls. Also, lower IQ is part of the schizophrenia phenotype, and there is an argument to be made that findings should not be covaried for IQ.
Our study has several limitations. We examined the largest sample of nonpsychotic siblings of COS patients to date from a longitudinal perspective, but only 2 scans were included per participant. The use of each person's baseline scan as a reference to compute growth provides excellent statistical power to detect change, but cohort effects are possible because different individuals were present at each end of the broad age range examined. Although the average ages were not significantly different between the siblings and controls in any of the age ranges examined (all P>.05), there was a trend for an age difference in the youngest age group (7 to < 14 years; P=.06), in which a significant difference in growth rate was identified. Even so, we note that this slight difference, if regarded as a confounder, would have worked against the findings we reported here. The youngest group had siblings with a mean (SD) age of 10.0 (1.5) years and controls with a mean age of 11.0 (1.5) years. If anything, growth is expected to slow as a child ages, so the marginally older controls should have slightly slower growth than if we had scanned them a year younger. There is strong a fortiori evidence that the slower growth in siblings in this age range cannot be ascribed to the imperfect age matching because, if anything, the slight age difference would have worked against finding slower growth in the siblings, and we did find slower growth in the siblings. Matching with regard to sex was not ideal in the young adult group (18-28 years: healthy controls, 8 male/8 female; siblings of COS patients, 4 male/12 female). Even so, a strong sex difference in brain change rates is not expected at this age. Together with the possible cohort effect, we deem our findings preliminary. Also, limited neurocognitive data were available on siblings, precluding meaningful correlations with brain growth. Efforts are being made to acquire neurocognitive and functional imaging data to explore neurocircuitry development across this age range. Additionally, the youngest child in the study was 7 years old, so we cannot infer whether WM growth has slowed at earlier ages (birth to 7 years). We are actively recruiting younger individuals and hope to use a mixed-effects model (with ≥3 scans per sibling) in future studies to more fully model the trajectory of brain growth in the siblings of patients with COS. Despite these limitations, our study provides the first evidence of WM growth alterations in non-psychotic siblings of patients with COS as a time-limited trait marker. Ongoing studies with alternative imaging modalities will investigate the agreement across measures of brain maturation and examine connectivity patterns across development.
Supplementary Material
Acknowledgments
Funding/Support: This research was supported by grants EB01651, R01 EB007813, R01 EB008281, and R01 EB008432 from the National Institute for Biomedical Imaging and Bioengineering; RR019771 from the National Center for Research Resources; AG016570 and R01 AG020098 from the National Institute on Aging; and R01 HD050735 from the National Institute of Child Health and Human Development (Dr Thompson). Additional support was provided by grants P41 RR013642 (Dr Toga) from the National Center for Research Resources, a component of the National Institutes of Health, and National Institute of Mental Health intramural funding (Drs Gogtay, Clasen, Rapoport, and Giedd; Ms Stidd; and Messrs Weisinger and Chavez).
Footnotes
Financial Disclosure: None reported.
Online-Only Material: The eTable is available at http://www.archgenpsychiatry.com
Contributor Information
Dr Nitin Gogtay, Child Psychiatry Branch, National Institute of Mental Health, Bethesda, Maryland.
Dr Xue Hua, Imaging Genetics Center, Laboratory of Neuro Imaging, Department of Neurology, UCLA (University of California, Los Angeles) School of Medicine.
Ms Reva Stidd, Child Psychiatry Branch, National Institute of Mental Health, Bethesda, Maryland.
Mss Christina P. Boyle, Imaging Genetics Center, Laboratory of Neuro Imaging, Department of Neurology, UCLA (University of California, Los Angeles) School of Medicine.
Mss Suh Lee, Imaging Genetics Center, Laboratory of Neuro Imaging, Department of Neurology, UCLA (University of California, Los Angeles) School of Medicine.
Messrs Brian Weisinger, Child Psychiatry Branch, National Institute of Mental Health, Bethesda, Maryland.
Messrs Alex Chavez, Child Psychiatry Branch, National Institute of Mental Health, Bethesda, Maryland.
Dr Jay N. Giedd, Child Psychiatry Branch, National Institute of Mental Health, Bethesda, Maryland.
Dr Liv Clasen, Child Psychiatry Branch, National Institute of Mental Health, Bethesda, Maryland.
Dr Arthur W. Toga, Imaging Genetics Center, Laboratory of Neuro Imaging, Department of Neurology, UCLA (University of California, Los Angeles) School of Medicine.
Dr Judith L. Rapoport, Child Psychiatry Branch, National Institute of Mental Health, Bethesda, Maryland.
Dr Paul M. Thompson, Imaging Genetics Center, Laboratory of Neuro Imaging, Department of Neurology, UCLA (University of California, Los Angeles) School of Medicine.
References
- 1.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60(12):1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- 2.Gogtay N. Cortical brain development in schizophrenia: insights from neuroimaging studies in childhood-onset schizophrenia. Schizophr Bull. 2008;34(1):30–36. doi: 10.1093/schbul/sbm103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rapoport JL, Gogtay N. Childhood onset schizophrenia: support for a progressive neurodevelopmental disorder. Int J Dev Neurosci. 2011;29(3):251–258. doi: 10.1016/j.ijdevneu.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157(1):16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 5.Lawrie SM, Abukmeil SS. Brain abnormality in schizophrenia: a systematic and quantitative review of volumetric magnetic resonance imaging studies. Br J Psychiatry. 1998;172:110–120. doi: 10.1192/bjp.172.2.110. [DOI] [PubMed] [Google Scholar]
- 6.Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162(12):2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 7.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49(1-2):1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 9.Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7(10):818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- 10.Boos HB, Aleman A, Cahn W, Hulshoff Pol H, Kahn RS. Brain volumes in relatives of patients with schizophrenia: a meta-analysis. Arch Gen Psychiatry. 2007;64(3):297–304. doi: 10.1001/archpsyc.64.3.297. [DOI] [PubMed] [Google Scholar]
- 11.Gogtay N, Ordonez A, Herman DH, Hayashi KM, Greenstein D, Vaituzis C, Lenane M, Clasen L, Sharp W, Giedd JN, Jung D, Nugent TF, III, Toga AW, Leibenluft E, Thompson PM, Rapoport JL. Dynamic mapping of cortical development before and after the onset of pediatric bipolar illness. J Child Psychol Psychiatry. 2007;48(9):852–862. doi: 10.1111/j.1469-7610.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- 12.Gogtay N, Greenstein D, Lenane M, Clasen L, Sharp W, Gochman P, Butler P, Evans A, Rapoport J. Cortical brain development in nonpsychotic siblings of patients with childhood-onset schizophrenia. Arch Gen Psychiatry. 2007;64(7):772–780. doi: 10.1001/archpsyc.64.7.772. [DOI] [PubMed] [Google Scholar]
- 13.Mattai AA, Weisinger B, Greenstein D, Stidd R, Clasen L, Miller R, Tossell JW, Rapoport JL, Gogtay N. Normalization of cortical gray matter deficits in nonpsychotic siblings of patients with childhood-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 2011;50(7):697–704. doi: 10.1016/j.jaac.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gogtay N, Vyas NS, Testa R, Wood SJ, Pantelis C. Age of onset of schizophrenia: perspectives from structural neuroimaging studies. Schizophr Bull. 2011;37(3):504–513. doi: 10.1093/schbul/sbr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makris N, Seidman LJ, Ahern T, Kennedy DN, Caviness VS, Tsuang MT, Goldstein JM. White matter volume abnormalities and associations with symptomatology in schizophrenia. Psychiatry Res. 2010;183(1):21–29. doi: 10.1016/j.pscychresns.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skudlarski P, Jagannathan K, Anderson K, Stevens MC, Calhoun VD, Skudlarska BA, Pearlson G. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry. 2010;68(1):61–69. doi: 10.1016/j.biopsych.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters BD, Blaas J, de Haan L. Diffusion tensor imaging in the early phase of schizophrenia: what have we learned? J Psychiatr Res. 2010;44(15):993–1004. doi: 10.1016/j.jpsychires.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Pérez-Iglesias R, Tordesillas-Gutiérrez D, Barker GJ, McGuire PK, Roiz-Santiañez R, Mata I, de Lucas EM, Quintana F, Vazquez-Barquero JL, Crespo-Facorro B. White matter defects in first episode psychosis patients: a voxelwise analysis of diffusion tensor imaging. Neuroimage. 2010;49(1):199–204. doi: 10.1016/j.neuroimage.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Péez-Iglesias R, Tordesillas-Gutiérrez D, McGuire PK, Barker GJ, Roiz-Santiañez R, Mata I, de Lucas EM, Rodríguez-Sánchez JM, Ayesa-Arriola R, Vazquez-Barquero JL, Crespo-Facorro B. White matter integrity and cognitive impairment in first-episode psychosis. Am J Psychiatry. 2010;167(4):451–458. doi: 10.1176/appi.ajp.2009.09050716. [DOI] [PubMed] [Google Scholar]
- 20.Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, Jolesz FA, Shenton ME. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41(1-2):15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashtari M, Cottone J, Ardekani BA, Cervellione K, Szeszko PR, Wu J, Chen S, Kumra S. Disruption of white matter integrity in the inferior longitudinal fasciculus in adolescents with schizophrenia as revealed by fiber tractography. Arch Gen Psychiatry. 2007;64(11):1270–1280. doi: 10.1001/archpsyc.64.11.1270. [DOI] [PubMed] [Google Scholar]
- 22.Gogtay N, Lu A, Leow AD, Klunder AD, Lee AD, Chavez A, Greenstein D, Giedd JN, Toga AW, Rapoport JL, Thompson PM. Three-dimensional brain growth abnormalities in childhood-onset schizophrenia visualized by using tensor-based morphometry. Proc Natl Acad Sci U S A. 2008;105(41):15979–15984. doi: 10.1073/pnas.0806485105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hulshoff Pol HE, Schnack HG, Mandl RC, Brans RG, van Haren NE, Baaré WF, van Oel CJ, Collins DL, Evans AC, Kahn RS. Gray and white matter density changes in monozygotic and same-sex dizygotic twins discordant for schizophrenia using voxel-based morphometry. Neuroimage. 2006;31(2):482–488. doi: 10.1016/j.neuroimage.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 24.Brans RG, van Haren NE, van Baal GC, Schnack HG, Kahn RS, Hulshoff Pol HE. Heritability of changes in brain volume over time in twin pairs discordant for schizophrenia. Arch Gen Psychiatry. 2008;65(11):1259–1268. doi: 10.1001/archpsyc.65.11.1259. [DOI] [PubMed] [Google Scholar]
- 25.Brans RG, van Haren NE, van Baal GC, Staal WG, Schnack HG, Kahn RS, Hulshoff Pol HE. Longitudinal MRI study in schizophrenia patients and their healthy siblings. Br J Psychiatry. 2008;193(5):422–423. doi: 10.1192/bjp.bp.107.041467. [DOI] [PubMed] [Google Scholar]
- 26.Boos HB, Mandl RC, van Haren NE, Cahn W, van Baal GC, Kahn RS, Hulshoff Pol H. Tract-based diffusion tensor imaging in patients with schizophrenia and their non-psychotic siblings. Eur Neuropsychopharmacol. doi: 10.1016/j.euroneuro.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Hua X, Leow AD, Levitt JG, Caplan R, Thompson PM, Toga AW. Detecting brain growth patterns in normal children using tensor-based morphometry. Hum Brain Mapp. 2009;30(1):209–219. doi: 10.1002/hbm.20498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andreasen NC. Methods for assessing positive and negative symptoms. Mod Probl Pharmacopsychiatry. 1990;24:73–88. doi: 10.1159/000418013. [DOI] [PubMed] [Google Scholar]
- 29.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association; p. 2000. text revision. [Google Scholar]
- 30.Hollingshead AB. Four Factor Index for Social Status. New Haven, CT: Yale University; 1975. [Google Scholar]
- 31.Leung K, Parker DS, Cunha A, Dinov I, Toga AW. IRMA: An image registration meta-algorithm evaluating alternative algorithms with multiple metrices. Sci Stat Database Manag. 2008;5069(Lecture Notes in Computer Science):612–617. doi: 10.1007/978-3-540-69497-7_46. [DOI] [Google Scholar]
- 32.Dinov I, Lozev K, Petrosyan P, Liu Z, Eggert P, Pierce J, Zamanyan A, Chakrapani S, Van Horn J, Parker DS, Magsipoc R, Leung K, Gutman B, Woods R, Toga A. Neuroimaging study designs, computational analyses and data provenance using the LONI pipeline. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0013070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 34.Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998;22(2):324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- 35.Leow A, Huang SC, Geng A, Becker J, Davis S, Toga A, Thompson P. Inverse consistent mapping in 3D deformable image registration: its construction and statistical properties. Inf Process Med Imaging. 2005;19:493–503. doi: 10.1007/11505730_41. [DOI] [PubMed] [Google Scholar]
- 36.Kochunov P, Lancaster J, Thompson P, Toga AW, Brewer P, Hardies J, Fox P. An optimized individual target brain in the Talairach coordinate system. Neuroimage. 2002;17(2):922–927. [PubMed] [Google Scholar]
- 37.Leow AD, Klunder AD, Jack CR, Jr, Toga AW, Dale AM, Bernstein MA, Britson PJ, Gunter JL, Ward CP, Whitwell JL, Borowski BJ, Fleisher AS, Fox NC, Harvey D, Kornak J, Schuff N, Studholme C, Alexander GE, Weiner MW, Thompson PM. ADNI Preparatory Phase Study. Longitudinal stability of MRI for mapping brain change using tensor-based morphometry. Neuroimage. 2006;31(2):627–640. doi: 10.1016/j.neuroimage.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 39.Mitelman SA, Brickman AM, Shihabuddin L, Newmark RE, Hazlett EA, Haznedar MM, Buchsbaum MS. A comprehensive assessment of gray and white matter volumes and their relationship to outcome and severity in schizophrenia. Neuroimage. 2007;37(2):449–462. doi: 10.1016/j.neuroimage.2007.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanskanen P, Haapea M, Veijola J, Miettunen J, Järvelin MR, Pyhtinen J, Jones PB, Isohanni M. Volumes of brain, grey and white matter and cerebrospinal fluid in schizophrenia in the Northern Finland 1966 Birth Cohort: an epidemiological approach to analysis. Psychiatry Res. 2009;174(2):116–120. doi: 10.1016/j.pscychresns.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Bartzokis G, Nuechterlein KH, Lu PH, Gitlin M, Rogers S, Mintz J. Dysregulated brain development in adult men with schizophrenia: a magnetic resonance imaging study. Biol Psychiatry. 2003;53(5):412–421. doi: 10.1016/s0006-3223(02)01835-8. [DOI] [PubMed] [Google Scholar]
- 42.Boos HB, Cahn W, van Haren NE, Derks EM, Brouwer RM, Schnack HG, Hulshoff Pol HE, Kahn RS. Focal and global brain measurements in siblings of patients with schizophrenia [published online January 17, 2011] Schizophr Bull. doi: 10.1093/schbul/sbq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cannon TD, van Erp TG, Huttunen M, Lönnqvist J, Salonen O, Valanne L, Poutanen VP, Standertskjöld-Nordenstam CG, Gur RE, Yan M. Regional gray matter, white matter, and cerebrospinal fluid distributions in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry. 1998;55(12):1084–1091. doi: 10.1001/archpsyc.55.12.1084. [DOI] [PubMed] [Google Scholar]
- 44.Goldman AL, Pezawas L, Mattay VS, Fischl B, Verchinski BA, Zoltick B, Weinberger DR, Meyer-Lindenberg A. Heritability of brain morphology related to schizophrenia: a large-scale automated magnetic resonance imaging segmentation study. Biol Psychiatry. 2008;63(5):475–483. doi: 10.1016/j.biopsych.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 45.Staal WG, Hulshoff Pol HE, Schnack HG, Hoogendoorn ML, Jellema K, Kahn RS. Structural brain abnormalities in patients with schizophrenia and their healthy siblings. Am J Psychiatry. 2000;157(3):416–421. doi: 10.1176/appi.ajp.157.3.416. [DOI] [PubMed] [Google Scholar]
- 46.Calabrese DR, Wang L, Harms MP, Ratnanather JT, Barch DM, Cloninger CR, Thompson PA, Miller MI, Csernansky JG. Cingulate gyrus neuroanatomy in schizophrenia subjects and their non-psychotic siblings. Schizophr Res. 2008;104(1-3):61–70. doi: 10.1016/j.schres.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hao Y, Yan Q, Liu H, Xu L, Xue Z, Song X, Kaneko Y, Jiang T, Liu Z, Shan B. Schizophrenia patients and their healthy siblings share disruption of white matter integrity in the left prefrontal cortex and the hippocampus but not the anterior cingulate cortex. Schizophr Res. 2009;114(1-3):128–135. doi: 10.1016/j.schres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 48.Greenstein D, Lerch J, Shaw P, Clasen L, Giedd J, Gochman P, Rapoport J, Gog-tay N. Childhood onset schizophrenia: cortical brain abnormalities as young adults. J Child Psychol Psychiatry. 2006;47(10):1003–1012. doi: 10.1111/j.1469-7610.2006.01658.x. [DOI] [PubMed] [Google Scholar]
- 49.Gutman B, Svarer C, Leow AD, Yanovsky I, Toga AW, Thompson PM. Creating Unbiased Minimal Deformation Templates for Brain Volume Registration. Barcelona, Spain: Organization for Human Brain Mapping; 2010. [Google Scholar]
- 50.Kochunov P, Lancaster JL, Thompson P, Woods R, Mazziotta J, Hardies J, Fox P. Regional spatial normalization: toward an optimal target. J Comput Assist Tomogr. 2001;25(5):805–816. doi: 10.1097/00004728-200109000-00023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.