Abstract
Concanavalin A and wheat germ agglutinin are as effective as insulin in enhancing the rate of glucose transport and in inhibiting epinephrine-stimulated lipolysis in isolated adipocytes. These lectins, also like insulin, inhibit basal as well as epinephrine-stimulated adenylate cyclase activity of membranes obtained from homogenates of fat cells. Low concentrations of wheat germ agglutinin enhance the specific binding of insulin to receptors of fat cells and liver membranes. Higher concentrations of this plant lectin, as well as of concanavalin A, competitively displace the binding of insulin to receptors in these tissues. These effects are equally apparent in insulin-binding proteins solubilized from membranes, indicating that the plant lectins interact directly with insulin receptors. All of the effects observed with the plant lectins are reversed by simple sugars that bind specifically to these plant proteins. Agarose derivatives of the plant lectins effectively adsorb solubilized insulin-binding proteins, and these can be eluted with buffers containing specific simple sugars. The possible implications of these findings to certain biological properties (mitogenicity) of these lectins and to the mechanism of action of other growth-promoting substances are considered.
Keywords: glucose transport, lipolysis, adenylate cyclase, affinity chromatography, lymphocyte transformation, growth factors
Full text
PDF
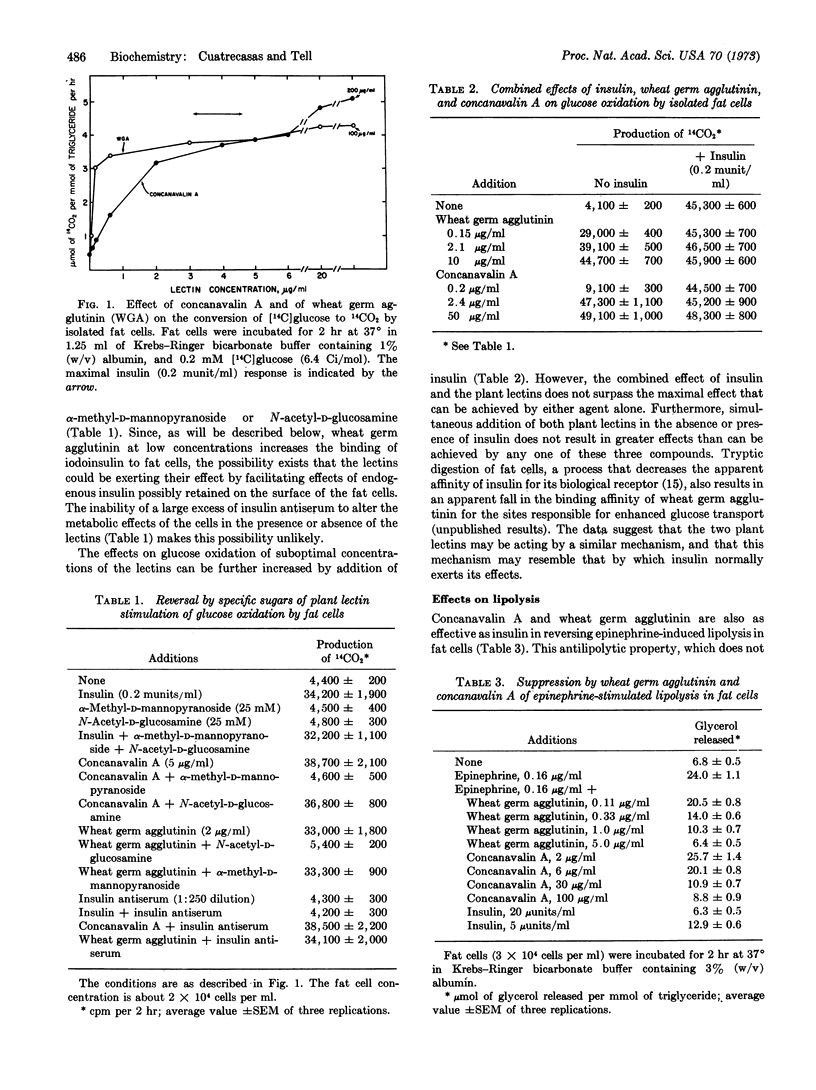

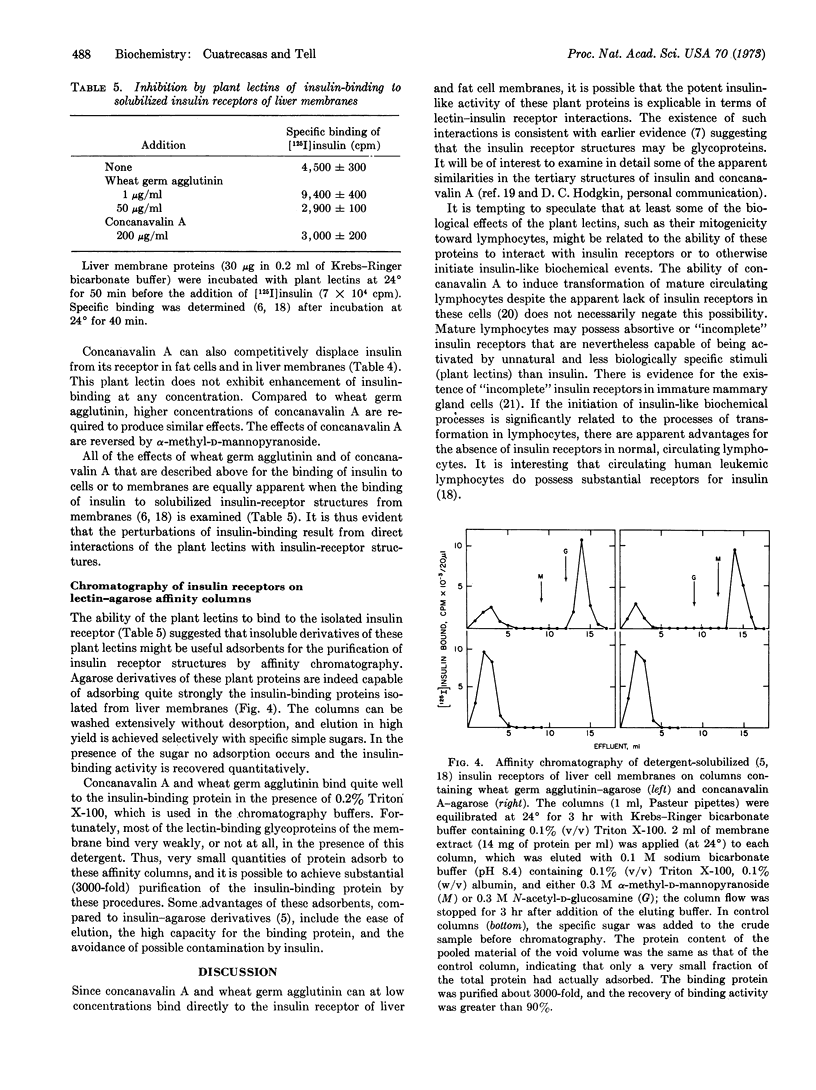

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berlin R. D. Effect of concanavalin A on phagocytosis. Nat New Biol. 1972 Jan 12;235(54):44–45. doi: 10.1038/newbio235044a0. [DOI] [PubMed] [Google Scholar]
- Blecher M. Effects of insulin and phospholipase A on glucose transport across the plasma membrane of free adipose cells. Biochim Biophys Acta. 1967 Jun 6;137(3):557–571. doi: 10.1016/0005-2760(67)90137-3. [DOI] [PubMed] [Google Scholar]
- Butcher R. W., Sneyd J. G., Park C. R., Sutherland E. W., Jr Effect of insulin on adenosine 3',5'-monophosphate in the rat epididymal fat pad. J Biol Chem. 1966 Apr 10;241(7):1651–1653. [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography and purification of the insulin receptor of liver cell membranes. Proc Natl Acad Sci U S A. 1972 May;69(5):1277–1281. doi: 10.1073/pnas.69.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Insulin--receptor interactions in adipose tissue cells: direct measurement and properties. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1264–1268. doi: 10.1073/pnas.68.6.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Isolation of the insulin receptor of liver and fat-cell membranes (detergent-solubilized-( 125 I)insulin-polyethylene glycol precipitation-sephadex). Proc Natl Acad Sci U S A. 1972 Feb;69(2):318–322. doi: 10.1073/pnas.69.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P., Parikh I. Adsorbents for affinity chromatography. Use of N-hydroxysuccinimide esters of agarose. Biochemistry. 1972 Jun 6;11(12):2291–2299. doi: 10.1021/bi00762a013. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Properties of the insulin receptor isolated from liver and fat cell membranes. J Biol Chem. 1972 Apr 10;247(7):1980–1991. [PubMed] [Google Scholar]
- Cuatrecasas P. Properties of the insulin receptor of isolated fat cell membranes. J Biol Chem. 1971 Dec 10;246(23):7265–7274. [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Cutrecasas P. Perturbation of the insulin receptor of isolated fat cells with proteolytic enzymes. Direct measurement of insulin-receptor interactions. J Biol Chem. 1971 Nov;246(21):6522–6531. [PubMed] [Google Scholar]
- Edelman G. M., Cunningham B. A., Reeke G. N., Jr, Becker J. W., Waxdal M. J., Wang J. L. The covalent and three-dimensional structure of concanavalin A. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2580–2584. doi: 10.1073/pnas.69.9.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepp K. D., Renner R. Insulin action on the adenyl cyclase system: Antagonism to activation by lipolytic hormones. FEBS Lett. 1972 Feb 1;20(2):191–194. doi: 10.1016/0014-5793(72)80791-9. [DOI] [PubMed] [Google Scholar]
- Hershko A., Mamont P., Shields R., Tomkins G. M. "Pleiotypic response". Nat New Biol. 1971 Aug;232(33):206–211. [PubMed] [Google Scholar]
- Hintz R. L., Clemmons D. R., Underwood L. E., Van Wyk J. J. Competitive binding of somatomedin to the insulin receptors of adipocytes, chondrocytes, and liver membranes. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2351–2353. doi: 10.1073/pnas.69.8.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho R. J., Jeanrenaud B. Insulin-like action of ouabain. I. Effect on carbohydrate metabolism. Biochim Biophys Acta. 1967 Aug 8;144(1):61–73. doi: 10.1016/0005-2760(67)90077-x. [DOI] [PubMed] [Google Scholar]
- Hsie A. W., Jones C., Puck T. T. Further changes in differentiation state accompanying the conversion of Chinese hamster cells of fibroblastic form by dibutyryl adenosine cyclic 3':5'-monophosphate and hormones. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1648–1652. doi: 10.1073/pnas.68.7.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illiano G., Cuatrecasas P. Modulation of adenylate cyclase activity in liver and fat cell membranes by insulin. Science. 1972 Feb 25;175(4024):906–908. doi: 10.1126/science.175.4024.906. [DOI] [PubMed] [Google Scholar]
- Krug U., Krug F., Cuatrecasas P. Emergence of insulin receptors on human lymphocytes during in vitro transformation. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2604–2608. doi: 10.1073/pnas.69.9.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J. F., Dill I. K., Holmlund C. E. Comparison of the effects of Bacillus subtilis protease, type 8 (subtilopeptidase A), and insulin on isolated adipose cells. J Biol Chem. 1967 Aug 25;242(16):3659–3664. [PubMed] [Google Scholar]
- Kuo J. F. Stimulation of glucose utilization and inhibition of lipolysis by polyene antibiotics in isolated adipose cells. Arch Biochem Biophys. 1968 Sep 20;127(1):406–412. doi: 10.1016/0003-9861(68)90243-9. [DOI] [PubMed] [Google Scholar]
- Lockwood D. H., Lipsky J. J., Meronk F., Jr, East L. E. Actions of polyamines on lipid and glucose metabolism of fat cells. Biochem Biophys Res Commun. 1971 Aug 6;44(3):601–607. doi: 10.1016/s0006-291x(71)80125-0. [DOI] [PubMed] [Google Scholar]
- Majerus P. W., Brodie G. N. The binding of phytohemagglutinins to human platelet plasma membranes. J Biol Chem. 1972 Jul 10;247(13):4253–4257. [PubMed] [Google Scholar]
- Makman M. H. Conditions leading to enhanced response to glucagon, epinephrine, or prostaglandins by adenylate cyclase of normal and malignant cultured cells. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2127–2130. doi: 10.1073/pnas.68.9.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minemura T., Crofford O. B. Insulin-receptor interaction in isolated fat cells. I. The insulin-like properties of p-chloromercuribenzene sulfonic acid. J Biol Chem. 1969 Oct 10;244(19):5181–5188. [PubMed] [Google Scholar]
- Novogrodsky A., Katchalski E. Lymphocyte transformation induced by concanavalin A and its reversion by methyl-alpha-D-mannopyranoside. Biochim Biophys Acta. 1971 Jan 28;228(2):579–583. doi: 10.1016/0005-2787(71)90064-5. [DOI] [PubMed] [Google Scholar]
- Oka T., Topper Y. J. Insulin-sepharose and the dynamics of insulin action. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2066–2068. doi: 10.1073/pnas.68.9.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl S. L., Birnbaumer L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. I. Properties. J Biol Chem. 1971 Mar 25;246(6):1849–1856. [PubMed] [Google Scholar]
- Powell A. E., Leon M. A. Reversible interaction of human lymphocytes with the mitogen concanavalin A. Exp Cell Res. 1970 Oct;62(2):315–325. doi: 10.1016/0014-4827(70)90560-4. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- RYLEY J. F. Studies on the metabolism of the protozoa. 4. Metabolism of the parasitic flagellate Strigomonas oncopelti. Biochem J. 1955 Mar;59(3):353–361. doi: 10.1042/bj0590353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran J. A new simple method for separation of adenosine 3',5'-cyclic monophosphate from other nucleotides and its use in the assay of adenyl cyclase. Anal Biochem. 1971 Sep;43(1):227–239. doi: 10.1016/0003-2697(71)90128-x. [DOI] [PubMed] [Google Scholar]
- Rodbell M. Metabolism of isolated fat cells. II. The similar effects of phospholipase C (Clostridium perfringens alpha toxin) and of insulin on glucose and amino acid metabolism. J Biol Chem. 1966 Jan 10;241(1):130–139. [PubMed] [Google Scholar]
- Smith J. W., Steiner A. L., Parker C. W. Human lymphocytic metabolism. Effects of cyclic and noncyclic nucleotides on stimulation by phytohemagglutinin. J Clin Invest. 1971 Feb;50(2):442–448. doi: 10.1172/JCI106511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A. A., Zenser T. V. Separation of cyclic 3',5'-nucleoside monophosphates from other nucleotides on aluminum oxide columns. Application to the assay of adenyl cyclase and guanyl cyclase. Anal Biochem. 1971 Jun;41(2):372–396. doi: 10.1016/0003-2697(71)90156-4. [DOI] [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. Restriction of the mobility of lymphocyte immunoglobulin receptors by concanavalin A. Proc Natl Acad Sci U S A. 1972 Mar;69(3):608–612. doi: 10.1073/pnas.69.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]



