Abstract
BACKGROUND
The management of relapsed aggressive lymphomas remains problematic. Ixabepilone (BMS-247550, epothilone B analog), a potent inhibitor of tubulin disassembly, has promising preclinical and early-phase clinical activity in drug-resistant malignancies.
METHODS
This multicenter phase 2 clinical trial tested the activity and safety of ixabepilone in relapsed/refractory aggressive lymphoma patients with either chemosensitive (at least a partial response [PR] to most recent chemotherapy) or chemoresistant (less than PR to most recent chemotherapy) disease at 20 mg/m2 given intravenously weekly on days 1, 8, and 15 of a 28-day cycle.
RESULTS
Fifty-one enrolled patients with a median age of 66 years received at least 1 dose of ixabepilone. Diffuse large B-cell lymphoma (n = 25; 49%), mantle cell lymphoma (n = 16; 31%), and transformed follicular lymphoma (n = 5; 10%) were the most frequent histologies. Patients were heavily pretreated, with more than one-quarter having received 4 or more prior therapies. The overall response rate was 27% (14 of 51 patients) with 12% (6 patients) experiencing complete responses and 16% (8 patients) with PRs. All responses were in patients with chemosensitive disease. The median time to response was 2 cycles with a median duration of response of 9.7 months.
CONCLUSIONS
Ixabepilone was well-tolerated, with neutropenia, peripheral sensory neuropathy, fatigue, and nausea as the major toxicities. Ixabepilone has modest single-agent activity in patients with recurrent chemosensitive aggressive lymphomas.
Keywords: non-Hodgkin lymphoma, aggressive, refractory, relapsed, ixabepilone
INTRODUCTION
Major gains in front-line management of aggressive non-Hodgkin lymphomas (NHLs) have not yet translated to the setting of relapsed disease. Intensive salvage approaches with high-dose therapy and autologous stem cell transplant benefit an increasingly smaller subset of patients, and most patients with relapsed or primary refractory disease continue to have suboptimal outcomes and limited treatment choices with no established standard of care.1,2 Ixabepilone (BMS-247550; Bristol-Myers Squibb, Princeton, NJ) is a unique nontaxane tubulin inhibitor with preclinical activity in drug-resistant models that could have a role in treatment of lymphomas displaying progressive chemoresistance.
The epothilones are naturally occurring nontaxane tubulin polymerization stabilizers.3,4 Ixabepilone is a semisynthetic derivative of epothilone B that binds to a site on the tubulin protein in close proximity to taxane binding sites, and has greater potency than taxanes in multiple preclinical models. Ixabepilone has been studied in solid tumors, and is currently approved by the US Food and Drug Administration for use in locally advanced and metastatic breast cancer, either alone or in combination with capecitabine after failure of taxanes. Taxanes have previously been tested in NHL but have limited activity.5 Ixabepilone binding stabilizes α- and β-tubulin heterodimers and prevents depolymerization, leading to cell cycle arrest and apoptosis. In vitro ixabepilone is less susceptible to efflux by P-glycoprotein (ie, multidrug resistance-1 [MDR-1] efflux pump), a common mechanism of resistance for taxanes.6 In this regard, there is potential for activity in settings where other drugs lose their efficacy. Its unique mechanism of action relative to other agents commonly used in lymphomas makes ixabepilone an attractive agent to test in NHL.
MATERIALS AND METHODS
Patient Selection
Patients had histologically confirmed relapsed or refractory aggressive NHL. including grade 3 follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma (MCL), primary mediastinal B-cell lymphoma, Burkitt lymphoma, Burkitt-like B-cell lymphoma, and anaplastic large cell lymphoma (CD30+, T cell, null cell, or Hodgkin-like types). There were 2 cohorts: cohort 1 (“chemosensitive”) enrolled patients with a complete response (CR) or partial response (PR) lasting at least 4 weeks to their most recent therapy; cohort 2 (“chemoresistant”) enrolled patients with stable disease (SD) but less than a PR to their most recent therapy. Patients with progression on their most recent therapy were excluded. Notably, cohort 2 was initially defined as patients without a sustained response to front-line therapy of at least 4 weeks. However, accrual was severely limited and the above definition was instituted after discussions with the National Cancer Institute. There was no limit to the number of prior regimens.
Additional eligibility requirements included: 1) measurable disease (at least 1 lesion ≥ 10 mm in longest diameter); 2) at least 4 weeks since prior chemotherapy (6 weeks for nitrosoureas or mitomycin C), radiation, or surgery; 3) age ≥ 18 years; 4) life expectancy of greater than 3 months; and 5) Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2. Laboratory entry criteria included white blood cell count (WBC) > 3000/µL, an absolute neutrophil count (ANC) > 1200/µL, platelet count > 100,000/µL, total bilirubin ≤ 1.5 mg/dL, aspartate transaminase (AST) and alanine transaminase (ALT) ≤ 2.5 times the upper limit of normal, and a creatinine of ≤ 1.5 mg/dL, or a creatinine clearance ≥ 60 mL/minute.
Pertinent exclusion criteria were the presence of ≥ grade 2 peripheral neuropathy, pregnancy, known intracranial disease, history of allergic reaction to Cremophor, known human immunodeficiency virus disease, active second malignancy other than nonmelanoma skin cancer or carcinoma in situ of the cervix, or uncontrolled inter-current illness including but not limited to active infection, symptomatic congestive heart failure, cardiac arrhythmia, or unstable angina pectoris. Patients immediately eligible and willing to undergo hematopoietic stem cell transplantation were excluded. However, those requiring debulking of disease prior to transplant were allowed. Usage of St. John’s Wort, a potent inducer of cytochrome P450 3A4 (CYP3A4), or cimetidine, a potent inhibitor of CYP3A4, were prohibited during the study.
Informed consent was obtained from all enrolled participants. This study was approved by the institutional review boards of all participating centers and was conducted in accordance with the Declaration of Helsinki.
Drug Administration
Ixabepilone was administered intravenously (IV) at a dose of 20 mg/m2 over 1 hour weekly on days 1, 8, and 15 of a 28-day cycle following premedication with H1 and H2 antagonists. A single dose escalation to 25 mg/m2 was allowed for patients with < grade 4 leukopenia and neutropenia without other toxicities ≥ grade 2 in cycle 1. Treatment continued until progression, intolerance, or physician-patient discretion. Patients could be removed from the study if their response enabled their eligibility for stem cell transplantation. Supportive care, including the use of colony-stimulating factors, was allowed at the discretion of the treating physician.
Dose reduction to 15 mg/m2 was required for any patient with more than a 1-week treatment delay due to toxicity, grade 3 or 4 nausea and vomiting despite maximal medical intervention, grade 3 or 4 diarrhea, grade 2 neuropathy (sensory or motor) lasting 7 or more days, grade 3 neuropathy lasting < 7 days, and for those not meeting hematologic criteria for retreatment 2 weeks after the last dose. Those requiring further dose reductions or who developed ≥ grade 3 neuropathy lasting ≥ 7 days were removed from the study. No dose re-escalations were allowed after dose reductions.
Study Design
The primary endpoint of this multicenter phase 2 study was overall response rate in chemosensitive and chemoresistant relapsed aggressive lymphomas. Secondary endpoints included duration of response, progression-free survival (PFS), and overall survival (OS).
Separate 2-stage Simon designs were designed for the 2 cohorts. Cohort 1 (chemosensitive patients), required at least 7 responses in the first 22 patients (Ho = relative risk [RR] ≤ 30% versus H1 = RR ≥ 50%; alpha = 0.1; power = 90%) in order to proceed to the second stage of 24 additional patients. Ixabepilone would be deemed worthy of further study in this population if ≥ 18 responses were seen among the 46 total cohort 1 patients. Cohort 2 (chemoresistant patients) required at least 1 response in the first 15 enrolled patients (Ho = RR < 10% versus H1 = RR ≥ 30%; alpha = 0.1; power = 91%) in order to proceed to the second stage of 15 additional patients. The drug would be considered worthy of further study in this population if 7 or more responses were seen among the 30 total cohort 2 patients.
Response Evaluations
Any patient exposed to at least 1 dose of ixabepilone was in the intent-to-treat (ITT) population. Response assessment was done in both in the ITT population and in patients completing at least 2 cycles of therapy with computed tomography (CT) or magnetic resonance imaging imaging every 2 cycles or every 8 weeks while on therapy.7 Positron emission tomography (PET) scans were not required, but results were noted if performed.
Statistical Methods
Response rates are presented together with 95% confidence intervals (CIs) using the binomial distribution. Comparisons of responses between cohorts and across histologies were performed using Fisher’s exact test. The PFS (time to disease progression or death from any cause) and OS rates were calculated using the Kaplan-Meier8 estimator. For PFS, patients were censored at the time of last contact if alive and disease-free; for both PFS and OS, one patient was censored at the time of stem cell transplant. The effects of baseline covariates on PFS and OS were evaluated using the Cox proportional hazards model.9
RESULTS
Patient Characteristics
Fifty-two patients were enrolled at 9 centers from April 2003 to October 2009. One patient never received treatment and is excluded from analysis (Table 1). Accrual to the study was slow, and the study was ultimately halted for lack of accrual. There were 34 (67%) males and 17 (33%) females with a median age of 66 years (range, 44–90 years). Major histologies included: DLBCL (n = 25; 49%), MCL (n = 16; 31%), and transformed FL (n = 5; 10%). Patients had a median of 3 prior therapies; more than one-quarter of patients received 4 or more prior therapies. Forty-five (88%) patients had prior rituximab-containing regimens. Cohorts 1 and 2 were generally similar except for a slight trend toward a poorer performance status and increased bone marrow involvement in cohort 2 versus cohort 1. Eleven patients had prior autologous stem cell transplant, and 1 patient had prior allogeneic stem cell transplant.
TABLE 1.
Patient Characteristics
| Characteristic | All Patients n = 51 |
Cohort 1 n = 39 |
Cohort 2 n = 12 |
|---|---|---|---|
| Median age, y (range) | 66 (44–90) | 64 (44–90) | 73 (53–87) |
| Sex | |||
| Male | 34 (67%) | 27 (69%) | 7 (58%) |
| Female | 17 (33%) | 12 (31%) | 5 (42%) |
| Race | |||
| Caucasian | 46 (90%) | 34 (87%) | 12 (100%) |
| African American | 1 (2%) | 1 (3%) | 0 (0%) |
| Hispanic | 4 (8%) | 4 (10%) | 0 (0%) |
| Histology | |||
| DLBCL | 25 (49%) | 18 (46%) | 7 (58%) |
| MCL | 16 (31%) | 12 (31%) | 4 (33%) |
| FL | 3 (6%) | 3 (8%) | 0 (0%) |
| TFL | 5 (10%) | 4 (10%) | 1 (8%) |
| Othera | 2 (4%) | 2 (5%) | 0 (0%) |
| BM involvement | |||
| Yes | 9 (18%) | 6 (15%) | 3 (25%) |
| No | 41 (80%) | 32 (82%) | 9 (75%) |
| NA | 1 (2%) | 1 (3%) | |
| LDH elevation | |||
| Yes | 22 (43%) | 16 (41%) | 6 (50%) |
| No | 29 (57%) | 23 (59%) | 6 (50%) |
| ECOG PS | |||
| 0 | 21 (41%) | 17 (44%) | 4 (33%) |
| 1 | 24 (47%) | 19 (49%) | 5 (42%) |
| 2 | 3 (6%) | 1 (3%) | 2 (17%) |
| NA | 3 (6%) | 2 (5%) | 1 (8%) |
| Prior therapies: | |||
| 1 | 14 (27%) | 10 (26%) | 4 (33%) |
| 2–3 | 22 (43%) | 17 (44%) | 5 (42%) |
| 4 or more | 15 (29%) | 12 (31%) | 3 (25%) |
| Prior rituximab | |||
| Yes | 45 (88%) | 34 (87%) | 11 (92%) |
| No | 6 (12%) | 5 (13%) | 1 (8%) |
Other histologies: 1 patient with large cell lymphoma not otherwise classifiable and 1 patient with large cell lymphoma with Burkitt-like features.
Abbreviations: BM, bone marrow; DLBCL, diffuse large B-cell lymphoma; ECOG PS, Eastern Cooperative Oncology Group performance status; FL, follicular lymphoma; LDH, lactate dehydrogenase; MCL, mantle cell lymphoma; NA, not available; TFL, transformed follicular lymphoma.
Response
Among 51 patients exposed to at least 1 dose of study drug (ITT population), the overall response rate (ORR) was 27% (95% CI = 16%–42%) (Table 2) including 6 (12%) CR/CRu (unconfirmed complete response) and 8 (16%) PR. All responses occurred in cohort 1 (chemosensitive patients). Compared with cohort 2, cohort 1 had a significantly higher ORR (14 of 39 patients [36%] versus 0 of 12 patients [0%], P = .022). An additional 25% (n = 8 in cohort 1 and n = 5 in cohort 2) of patients had disease stabilization. Responses varied by histology: FL grade 3 (3 of 3 [100%]), transformed FL (2 of 5 [40%]), and DLBCL (7 of 25 [28%]), and MCL (2 of 16 [13%]). No responses were seen in the 2 patients with large cell lymphoma, not otherwise specified (NOS) or with Burkitt-like features. These differences were statistically significant (P = .021). Among 14 responders, 12 (86%) had received prior rituximab.
TABLE 2.
Patient Responses
| ITT Population n = 51 |
Cohort 1 n = 39 |
Cohort 2 n = 12 |
|
| CR/CRu | 6 (12%) | 6 (15%) | 0 (0%) |
| PR | 8 (16%) | 8 (21%) | 0 (0%) |
| SD | 13 (25%) | 8 (21%) | 5 (42%) |
| PD | 12 (24%) | 9 (23%) | 3 (25%) |
| NE | 12 (24%) | 8 (21%) | 4 (33%) |
| Patients Receiving ≥2 cyclesa n = 39 |
Cohort 1 n = 31 |
Cohort 2 n = 8 |
|
| CR/CRu | 6 (15%) | 6 (19%) | 0 (0%) |
| PR | 8 (21%) | 8 (26%) | 0 (0%) |
| SD | 13 (33%) | 8 (26%) | 5 (63%) |
| PD | 12 (31%) | 9 (29%) | 3 (38%) |
Although 41 patients received ≥2 cycles of therapy on protocol, only 39 of these had adequate response evaluations. One patient in cohort 1 was lost to follow-up after 2 cycles and 1 patient in cohort 2 was inadequately evaluated for response.
Abbreviations: CR, complete response; CRu, unconfirmed complete response; ITT, intention to treat; NE, not evaluated; PD, progressive disease; PR, partial response; SD, stable disease.
Among 41 patients completing at least 2 cycles of therapy, 2 patients were inevaluable for response: one was lost to follow-up and one had an inadequate response assessment. Among the 39 assessed patients, the ORR was 36% (95% CI = 21%–53%) including 6 CR/CRu and 8 PR. Significantly more responses were observed in cohort 1 versus cohort 2 (14 of 31 [45%] patients versus 0 of 8 [0%], P = .034 using Fisher’s exact test).
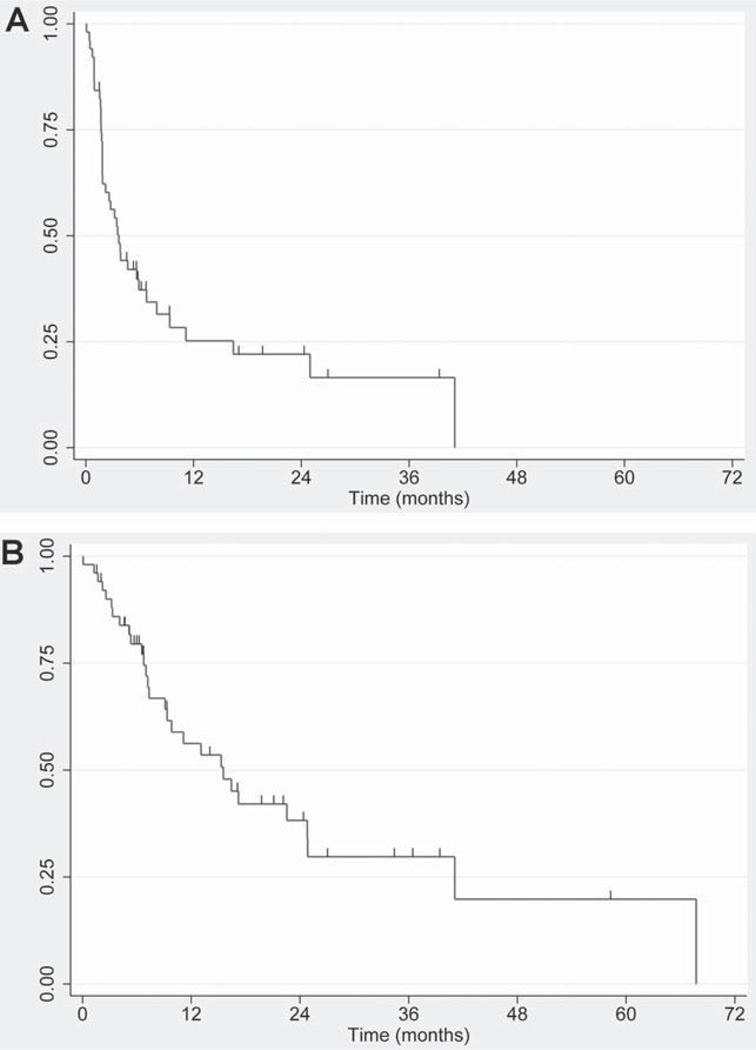
The median PFS and OS in the ITT population were 3.7 months and 15.5 months, respectively (Fig. 1). The median PFS and OS for the ITT population in cohort 1 (n = 39) were 3.7 and 16.7 months, respectively. The median duration of response among responders was 9.7 months, with 1 patient maintaining a response at >38.3 months. One patient achieving a CRu after 2 cycles proceeded to allogeneic stem cell transplant after 2 additional cycles. This patient was censored at the time of transplant and is alive and free of disease at 35 months follow-up.
Figure 1.
(A) Progression-free survival is shown. (B) Overall survival is shown. In both panels, tick marks indicate censored observations.
Univariate analysis identified lactate dehydrogenase (LDH) (hazard ratio [HR] = 1.9; P = .050) as the only significant predictor of PFS, whereas age (HR = 1.60 per decade increase in age; P = .003), ECOG performance status (HR = 2.4; P = .01), and LDH (HR = 3.6; P = .001) were statistically significant predictors of OS. In multivariate analysis, ECOG performance status (HR = 2.0; P = .04) and LDH (HR = 3.5; P = .004) remained significant predictors of OS.
Although not required by the study protocol, 18 of 51 patients had PET scans as part of their response evaluation. Among patients achieving a PR, SD, or PD, 4 of 15 (27%) had evidence of a significant metabolic response on PET scan, including 1 patient with SD and 2 with a PR achieving completely negative PET scans.
Study Drug Tolerance
A total of 41 of 51 patients (80%) completed at least 2 cycles of study drug and 17 (33%) received more than 2 cycles. The median cumulative dose administered was 246 mg delivered over a median of 2 cycles. The maximum cumulative dose received by any one patient was 570 mg. By cohort, 32 of 39 (82%) patients in cohort 1 and 9 of 12 (75%) patients in cohort 2 received at least 2 cycles of therapy. Ten patients did not complete 2 cycles of study drug due to: progressive disease (n = 8; 16%), refusal of further treatment (n = 1; 2%), and persistent peripheral neuropathy (n = 1; 2%).
Seven patients required a dose reduction due to grade 2 to 3 neutropenia (n = 3; 6%), grade 2 to 3 fatigue (n = 2; 4%), grade 2 thrombocytopenia (n = 1; 2%), and persistent grade 2 neuropathy (n = 1; 2%). Ten patients missed 1 or more doses of a cycle due to progressive disease (n = 4; 8%), persistent > grade 1 neuropathy (n = 4; 8%), grade 2 neutropenia (n = 1; 2%), and neutropenic fever with infection (n = 1; 2%). Thirteen patients required a dose delay due to neutropenia (n = 9; 18%), neutropenia with infection (n = 4; 8%), thrombocytopenia (n = 2; 4%), diarrhea (n = 1; 2%), peripheral neuropathy (n = 1; 2%), and delayed response evaluation (n = 1; 2%).
Toxicity
All patients were evaluable for toxicity (Table 3). Thirty-four (67%) patients experienced at least 1 episode of grade 3 or 4 toxicity including neutropenia (n = 17; 33%), leukopenia (n = 15; 29%), fatigue (n = 10; 20%), lymphopenia (n = 10; 20%), and sensory neuropathy (n = 6; 12%). Sensory neuropathy of any grade occurred in 31 (61%) patients with grade 1 or 2 in 25 (49%) patients and grade 3 in 6 (12%) patients, respectively. Motor neuropathy of grade 1 or 2 and grade 3 occurred in 4 (8%) and 2 (4%) patients, respectively. There were no grade 4 neuropathies observed. One patient with grade 3 sensory neuropathy opted for study discontinuation despite achieving a CR.
TABLE 3.
Toxicities (n = 51)
| Adverse Event (Attribution ≥3) |
Grade 1/2 n (%) |
Grade 3/4 n (%) |
|---|---|---|
| Alopecia | 11 (22%) | 0 (0%) |
| Dehydration | 0 (0%) | 4 (8%) |
| Dyspnea | 4 (8%) | 2 (4%) |
| Fatigue | 21 (41%) | 10 (20%) |
| GI toxicities | ||
| Anorexia | 14 (27%) | 1 (2%) |
| Constipation | 11 (22%) | 0 (0%) |
| Diarrhea | 15 (29%) | 2 (4%) |
| Nausea | 24 (47%) | 0 (0%) |
| Vomiting | 7 (14%) | 1 (2%) |
| Hematologic | ||
| Anemia | 23 (45%) | 3 (6%) |
| Leukopenia | 15 (29%) | 15 (29%) |
| Lymphopenia | 3 (6%) | 10 (20%) |
| Neutropenia | 4 (8%) | 17 (33%) |
| Thrombocytopenia | 17 (33%) | 2 (4%) |
| Hypersensitivity reaction | 1 (2%) | 0 (0%) |
| Liver function | ||
| Bilirubin increased | 2 (4%) | 0 (0%) |
| SGOT increased | 8 (16%) | 1 (2%) |
| SGPT increased | 5 (10%) | 1 (2%) |
| Neurologic | ||
| Ataxia | 1 (2%) | 0 (0%) |
| Motor neuropathy | 4 (8%) | 2 (4%)a |
| Muscle weakness | 2 (4%) | 1 (2%) |
| Myalgia | 9 (18%) | 4 (8%) |
| Sensory neuropathy | 25 (49%) | 6 (12%)a |
| Syncope | 0 (0%) | 2 (4%) |
None of these neuropathy adverse events were grade 4.
Abbreviations: GI, gastrointestinal; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvate transaminase.
DISCUSSION
The paucity of effective options for relapsed aggressive lymphomas makes the continued search for new agents mandatory. The vast majority of patients with relapsed or refractory aggressive lymphomas die of their disease, and the median survival following failure of autologous stem cell transplant is approximately 6 months. This phase 2 multicenter study sought to determine the overall and complete response rate to an antitubulin cytotoxic agent, ixabepilone, in a heavily pretreated group of patients with either “chemosensitive” or “chemoresistant” relapsed/refractory aggressive lymphomas as defined above. Given preclinical evidence that ixabepilone is less susceptible to common mechanisms of drug resistance, this was felt to be an important question to test in patients.
Fifty-one patients received at least 1 dose of ixabepilone and constitute the ITT population. This was a heavily pretreated group of patients with 22% of patients having previously undergone an autologous stem cell transplant, and more than one-quarter of patients having received at least 4 prior regimens; 88% of patients had relapsed after prior rituximab-containing regimens. Despite the promising preclinical data showing efficacy in chemorefractory models, no responses were observed among patients in the chemoresistant cohort as defined in this trial. On the other hand, among patients who had responded to and subsequently relapsed after their most recent chemotherapy, a response rate of 36% was observed. One patient with stable disease by CT criteria had a complete metabolic response by PET, and was successfully bridged to an allogeneic stem cell transplant; he remains alive and free of disease 3 years later. Of all histologies enrolled, those with either grade 3 FL or transformed FL had the highest response rate (63% ORR). Interestingly, the only complete responder in the study by O’Connor et al occurred in a FL patient, and overall responses were noted in 2 of 6 (33%) FL patients enrolled. Although it is difficult to make conclusions on the basis of these few patients, the pooled data from the study by O’Connor et al and our study suggest a signal in follicle center cell-derived lymphomas, which may be worth further investigation. Notably, at the time of enrollment of FL patients on our study, a distinction between grade 3a and 3b FL was not made; therefore, responses based on these categories are not available.
Others have evaluated ixabepilone in lymphoid malignancies. A phase 1 trial of ixabepilone in 61 advanced cancer patients included 15 patients with relapsed lymphomas.10 Six patients responded, including 2 with diffuse large B-cell lymphomas failing prior CHOP-based therapy; one of these patients achieved a complete remission and the other a partial remission.10 These results prompted a multicenter phase 2 study of ixabepilone in indolent lymphomas.11 O’Connor and colleagues tested ixabepilone on a weekly schedule (25 mg/m2 weekly for 3 of 4 consecutive weeks) in 28 patients (22 evaluable) with heavily pretreated indolent and mantle cell lymphomas. The response rate was approximately 30%, with activity observed across different histologies (FL, SLL/CLL, marginal zone lymphoma, MCL). The duration of response ranged from 2 to 8 months.
Even in our heavily pretreated population, ixabepilone was overall well tolerated, with the exception of neuropathic toxicity. The most common toxicities seen with this dose and schedule were hematologic, sensory neuropathy, fatigue, and nausea. Peripheral sensory neuropathy is a commonly reported toxicity with ixabepilone, with severity varying by dose and schedule. Phase 1/2 studies mostly done in patients with solid tumors have suggested that higher doses (ie, 40 mg/m2) given every 3 weeks are associated with a greater incidence of the characteristic length-dependent axonal polyneuropathy than with dosing schedules similar to those used in this trial.10 Here, we observed some degree of sensory neuropathy in 61% of patients, with grade 3 or 4 in 12% (6 of 51 patients). Overall, more patients on our trial experienced neuropathy than the 28% reported by O’Connor and colleagues when treating indolent NHL patients using the same dose and schedule.11 The reason for this is unclear, because patients on both studies received a median of 2 cycles of therapy with only 33% on our study and 21% on the study by O’Connor et al receiving more than 2 cycles. We also observed a motor neuropathy in 12% (6 of 51) of patients, which was not reported in other studies.
There are several limitations to the present study. First, this trial had a long accrual period, during which time several aspects of the care and treatment of patients with aggressive NHL have changed. In particular, rituximab became widely available soon after this study began and may have contributed to slow accrual. In addition, this trial used 1999 CT-based criteria,7 which have now been replaced with metabolic response criteria12 that incorporate PET scans into response assessments. As noted above, PET scans may have changed the response classification of several patients on our trial.
Part of the appeal to testing ixabepilone is that, outside of vinca alkaloids, few other agents commonly used in lymphomas target the microtubule apparatus. Paclitaxel and docetaxel have been tested in lymphomas, with limited single-agent activity.13–21 The largest study of 96 lymphoma patients found an overall response rate of 25%, but many other trials have reported response rates that were significantly lower. Although it is difficult to compare different phase 2 studies, it seems that the response rate to ixabepilone is at least similar and possibly superior to that of the taxanes. In addition, the single-agent response rate of ixabepilone is similar to those achieved by other agents currently in use. For example, single-agent gemcitabine obtains only a 20% overall response rate in aggressive lymphomas, but when combined with other agents, the response rate more than doubles, and gemcitabine-containing salvage regimens are now commonplace in relapsed aggressive lymphomas.22–24 Similarly, bendamustine has limited single-agent activity with an overall response rate of only 38% in relapsed aggressive lymphomas, and yet combinations with rituximab are promising with response rates of 55% even in elderly patients and those with refractory disease.25,26 Thus, ixabepilone may yet have a role in lymphomas, if the right combination can be developed.
Despite encouraging activity, the further development of ixabepilone for lymphoma faces considerable hurdles because of numerous other candidate drugs under development. Many of these have more immediate appeal because of their rational design, targeting B-cell receptor signaling or lymphoma-specific surface markers. In our opinion, though, it would be premature to abandon chemotherapy, because it remains the backbone of treatment, particularly in aggressive lymphomas. If multidrug combinations can be further improved by incorporating agents with a better therapeutic ratio, meaningful improvements in outcome may result. Ixabepilone, with its unique mechanism of action and limited myelosuppression, may be considered for incorporation in such multidrug regimens.
Acknowledgments
FUNDING SOURCES
This work was funded by National Institutes of Health grant N01CM62201 (Early Therapeutics Development with Phase II Emphasis).
Footnotes
CONFLICT OF INTEREST DISCLOSURE
The authors made no disclosure.
REFERENCES
- 1.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333:1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 3.Lee FY, Borzilleri R, Fairchild CR, et al. Preclinical discovery of ixabepilone, a highly active antineoplastic agent. Cancer Chemother Pharmacol. 2008;63:157–166. doi: 10.1007/s00280-008-0724-8. [DOI] [PubMed] [Google Scholar]
- 4.Lee FY, Borzilleri R, Fairchild CR, et al. BMS-247550: a novel epothilone analog with a mode of action similar to paclitaxel but possessing superior antitumor efficacy. Clin Cancer Res. 2001;7:1429–1437. [PubMed] [Google Scholar]
- 5.Younes A. Paclitaxel-based treatment of lymphoma. Semin Oncol. 1999;26(1) suppl 2:123–128. [PubMed] [Google Scholar]
- 6.Khrapunovich-Baine M, Menon V, Yang CP, et al. Hallmarks of molecular action of microtubule stabilizing agents: effects of epothilone B, ixabepilone, peloruside A, laulimalide on microtubule conformation. J Biol Chem. 2011;286:11765–11778. doi: 10.1074/jbc.M110.162214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;47:583–621. [Google Scholar]
- 9.Cox DR. Regression models and life tables (with discussion) J R Stat Soc Ser B. 1972;34:187–220. [Google Scholar]
- 10.Aghajanian C, Burris HA, 3rd, Jones S, et al. Phase I study of the novel epothilone analog ixabepilone (BMS-247550) in patients with advanced solid tumors and lymphomas. J Clin Oncol. 2007;25:1082–1088. doi: 10.1200/JCO.2006.08.7304. [DOI] [PubMed] [Google Scholar]
- 11.O’Connor OA, Portlock C, Moskowitz C, et al. A multicentre phase II clinical experience with the novel aza-epothilone Ixabepilone (BMS247550) in patients with relapsed or refractory indolent non-Hodgkin lymphoma and mantle cell lymphoma. Br J Haematol. 2008;143:201–209. doi: 10.1111/j.1365-2141.2008.07271.x. [DOI] [PubMed] [Google Scholar]
- 12.Cheson BD, Pfistner B, Juweid ME, et al. International Harmonization Project on Lymphoma. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 13.Budman DR, Petroni GR, Johnson JL, Cooper MR, Schlossman DM, Barcos M, et al. Phase II trial of docetaxel in non-Hodgkin’s lymphomas: a study of the Cancer and Leukemia Group B. J Clin Oncol. 1997;15:3275–3279. doi: 10.1200/JCO.1997.15.10.3275. [DOI] [PubMed] [Google Scholar]
- 14.Kahl BS, Bailey HH, Smith EP, Turman N, Smith J, Werndli J, et al. Phase II study of weekly low-dose paclitaxel for relapsed and refractory non-Hodgkin’s lymphoma: a Wisconsin Oncology Network Study. Cancer Invest. 2005;23:13–18. [PubMed] [Google Scholar]
- 15.Rizzieri DA, Sand GJ, McGaughey D, et al. Low-dose weekly paclitaxel for recurrent or refractory aggressive non-Hodgkin lymphoma. Cancer. 2004;100:2408–2414. doi: 10.1002/cncr.20245. [DOI] [PubMed] [Google Scholar]
- 16.Casasnovas RO, Haioun C, Dumontet C, et al. Phase II study of 3-hour infusion of high dose paclitaxel in refractory and relapsed aggressive non-Hodgkin’s lymphomas. Groupe d’Etude des Lymphomes de l’Adulte. Haematologica. 2000;85:502–507. [PubMed] [Google Scholar]
- 17.Press OW, LeBlanc M, O’Rourke TJ, et al. Phase II trial of paclitaxel by 24-hour continuous infusion for relapsed non-Hodgkin’s lymphomas: Southwest Oncology Group trial 9246. J Clin Oncol. 1998;16:574–578. doi: 10.1200/JCO.1998.16.2.574. [DOI] [PubMed] [Google Scholar]
- 18.Wilson WH, Chabner BA, Bryant G, et al. Phase II study of paclitaxel in relapsed non-Hodgkin’s lymphomas. J Clin Oncol. 1995;13:381–386. doi: 10.1200/JCO.1995.13.2.381. [DOI] [PubMed] [Google Scholar]
- 19.Younes A, McLaughlin P, Romaguera J, et al. Taxol plus topotecan plus rituximab (TTR) with G-CSF support: an effective salvage program for the treatment of patients with relapsed/refractory aggressive B-cell non-hodgkin lymphoma (NHL) who failed CHOP-like and platinum-based therapy. Session type: oral session [Abstract] Blood. 2003;102 abstract 489. [Google Scholar]
- 20.Younes A, Preti HA, Hagemeister FB, et al. Paclitaxel plus topotecan treatment for patients with relapsed or refractory aggressive non-Hodgkin’s lymphoma. Ann Oncol. 2001;12:923–927. doi: 10.1023/a:1011172215216. [DOI] [PubMed] [Google Scholar]
- 21.Younes A, Sarris A, Melnyk A, et al. Three-hour paclitaxel infusion in patients with refractory and relapsed non-Hodgkin’s lymphoma. J Clin Oncol. 1995;13:583–587. doi: 10.1200/JCO.1995.13.3.583. [DOI] [PubMed] [Google Scholar]
- 22.Crump M, Baetz T, Couban S, et al. Gemcitabine, dexamethasone, and cisplatin in patients with recurrent or refractory aggressive histology B-cell non-Hodgkin lymphoma: a Phase II study by the National Cancer Institute of Canada Clinical Trials Group (NCICCTG) Cancer. 2004;101:1835–1842. doi: 10.1002/cncr.20587. [DOI] [PubMed] [Google Scholar]
- 23.Fossa A, Santoro A, Hiddemann W, et al. Gemcitabine as a single agent in the treatment of relapsed or refractory aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 1999;17:3786–3792. doi: 10.1200/JCO.1999.17.12.3786. [DOI] [PubMed] [Google Scholar]
- 24.Wenger C, Stern M, Herrmann R, Rochlitz C, Pless M. Rituximab plus gemcitabine: a therapeutic option for elderly or frail patients with aggressive non Hodgkin’s lymphoma? Leuk Lymphoma. 2005;46:71–75. doi: 10.1080/10428190400007540. [DOI] [PubMed] [Google Scholar]
- 25.Vacirca J, Tabbara I, Acs P, Shumaker G. Bendamustine + rituximab as treatment for elderly patients with relapsed or refractory diffuse large B-cell lymphoma [Abstract] Blood. 2010;116 abstract 2806. [Google Scholar]
- 26.Weidmann E, Kim SZ, Rost A, et al. Bendamustine is effective in relapsed or refractory aggressive non-Hodgkin’s lymphoma. Ann Oncol. 2002;13:1285–1289. doi: 10.1093/annonc/mdf189. [DOI] [PubMed] [Google Scholar]