Abstract
There is a growing use of psychostimulants such as methylphenidate (Ritalin; dopamine reuptake inhibitor) for medical treatments and as cognitive enhancers in the healthy. Methylphenidate is known to produce some addiction-related gene regulation. Recent findings in animal models show that selective serotonin reuptake inhibitors (SSRIs) including fluoxetine can potentiate acute induction of gene expression by methylphenidate, thus indicating an acute facilitatory role for serotonin in dopamine-induced gene regulation. We investigated whether repeated exposure to fluoxetine in conjunction with methylphenidate in adolescent rats facilitated a gene regulation effect well-established for repeated exposure to illicit psychostimulants such as cocaine - blunting (repression) of gene inducibility. We measured, by in situ hybridization histochemistry, the effects of a 5-day repeated treatment with methylphenidate (5 mg/kg), fluoxetine (5 mg/kg) or a combination on the inducibility (by cocaine) of neuroplasticity-related genes (Zif268, Homer1a) in the striatum. Repeated methylphenidate treatment alone produced minimal gene blunting, while fluoxetine alone had no effect. In contrast, fluoxetine added to methylphenidate robustly potentiated methylphenidate-induced blunting for both genes. This potentiation was widespread throughout the striatum, but was most robust in the lateral, sensorimotor striatum, thus mimicking cocaine effects. For illicit psychostimulants, blunting of gene expression is considered part of the molecular basis of addiction. Our results thus suggest that SSRIs such as fluoxetine may increase the addiction liability of methylphenidate.
Keywords: cocaine, cognitive enhancer, dopamine, gene expression, psychostimulant, SSRI antidepressant
Introduction
Scores of people are being exposed to psychotropic medications (e.g., antidepressants, psychostimulants) on a regular basis. For example, it was estimated that in 2008 approximately 3 million children between 4 and 17 years of age in the US alone were treated with psychostimulant medications such as amphetamine and methylphenidate for attention-deficit hyperactivity disorder (ADHD) (Swanson et al. 2011). Furthermore, there is increasing use of these psychostimulants as “cognitive enhancers” by healthy children and adults (Greely et al. 2008; Kollins 2008; Wilens et al. 2008). It is difficult to determine the exact magnitude of such medication abuse (Kollins 2008; Wilens et al. 2008), but one estimate indicates that, in 2008 in the US, about 8.5% of the population over 12 had a history of nonmedical use of prescription psychostimulants, and as many as 11 million prescriptions out of 38 million may have been diverted for nonmedical use (Swanson et al. 2011).
Exposure to psychostimulants, especially during the sensitive period of brain development, is of concern because studies in animal models show that these drugs can induce maladaptive neurobehavioral changes suggestive of an increased risk for drug addiction and other neuropsychiatric disorders later in life (for reviews, see Carlezon & Konradi 2004; Andersen 2005; Carrey & Wilkinson 2011). Moreover, increasing spread of psychostimulant use also enhances the likelihood of accidental coexposure with other psychotropic medications such as antidepressants, and almost nothing is known on the neurobiological consequences of such drug coexposure.
There is consensus that changes in gene regulation are critical for psychostimulant addiction and other long-lasting behavioral pathologies (Renthal & Nestler 2008). Studies over the last decade have described in some detail the effects of acute and repeated treatment with medical psychostimulants such as methylphenidate on gene regulation in addiction-related neuronal systems, including the striatum and cortex (Steiner & Van Waes 2013). Comparisons with the molecular effects of illicit psychostimulants, such as cocaine, show that these medications have the potential to impact many genes in a similar way, but other genes appear less affected than by cocaine (Steiner & Van Waes 2013). For example, similar to other psychostimulants, methylphenidate induces the expression of immediate-early genes, including c-Fos, Zif268, deltaFosB and Homer1a in striatal neurons (e.g., Brandon & Steiner 2003; Chase et al. 2005; Yano & Steiner 2005a; Chase et al. 2007; Cotterly et al. 2007). Differences between methylphenidate and cocaine/amphetamine were noted, for instance, in the effects on neuropeptides in the striatum. Thus, in one study, acute methylphenidate robustly induced substance P, while having minimal or no effects on dynorphin and enkephalin expression (Yano & Steiner 2005b). This is in contrast to cocaine and amphetamine, which reliably induce all three neuropeptides (see Steiner & Van Waes 2013, for review). Moreover, repeated treatment with methylphenidate alone produced blunting (repression) of c-Fos and Zif268 inducibility, whereas Homer1a was minimally or not affected (Brandon & Steiner 2003; Cotterly et al. 2007), also in contrast to cocaine treatment (Unal et al. 2009).
The lesser impact of methylphenidate may be related to the differential neurochemical effects of methylphenidate as compared to cocaine; methylphenidate blocks the reuptake of dopamine and norepinephrine (among other actions), while cocaine also inhibits reuptake of serotonin, in addition to dopamine and norepinephrine (see Yano & Steiner 2007). This hypothesis is supported by our recent findings showing that serotonin-enhancing drugs - selective serotonin reuptake inhibitor (SSRI) antidepressants - potentiate acute gene regulation by methylphenidate in the striatum (Steiner & Van Waes 2013). Thus, administering an SSRI (fluoxetine, citalopram) together with methylphenidate potentiated the acute induction of immediate-early genes (c-Fos, Zif268) and neuropeptides (substance P, dynorphin) by methylphenidate in striatal neurons (Steiner et al. 2010; Van Waes et al. 2010; Van Waes et al. 2012).
Will SSRIs thus modify the addiction liability of methylphenidate? Our above studies demonstrated SSRI-potentiated gene regulation for acute methylphenidate treatments. However, effects of repeated drug exposure are likely more relevant for the addiction liability. In animal models, repeated pretreatment with cocaine or amphetamine facilitates subsequent psychostimulant seeking and self-administration, suggesting an increased abuse/addiction liability (c.f. Steiner & Van Waes 2013). Similarly, previous studies found that repeated methylphenidate pretreatment facilitated the subsequent acquisition of cocaine self-administration in rats (Brandon et al. 2001; Schenk & Izenwasser 2002; Crawford et al. 2011). In these studies, animals treated before or during adolescence were more sensitive for this facilitation than those treated as adults. Further work demonstrated that increased cocaine taking after repeated methylphenidate pretreatment in adolescent rats (Brandon et al. 2001) was accompanied by altered gene regulation by cocaine in the striatum (Brandon & Steiner 2003). These molecular changes included blunting of immediate-early gene induction by a subsequent cocaine challenge.
In the present study, we investigated, again in adolescent rats, whether fluoxetine given in conjunction with repeated methylphenidate treatment would modify the gene blunting by methylphenidate. For comparison with our previous findings, we assessed the molecular response to a subsequent cocaine challenge (Brandon & Steiner 2003) and a repeated pretreatment regimen and molecular markers similar to our previous studies (Brandon & Steiner 2003; Cotterly et al. 2007). The two immediate-early gene markers investigated, Zif268 and Homer1a, are relevant for neuronal plasticity; Zif268 is a transcription factor (Knapska & Kaczmarek 2004), and Homer1a is a synaptic plasticity modulator (Thomas 2002) that is implicated in drug-induced neuroplasticity related to addiction (for review, see Szumlinski et al. 2008). Gene expression was mapped throughout the striatum in order to identify the functional domains affected by these treatments. Our previous studies showed that repeated cocaine treatment reliably blunts the induction of both Zif268 and Homer1a (Unal et al. 2009), while repeated treatment with methylphenidate alone produced blunting of Zif268 induction (Brandon & Steiner 2003; Cotterly et al. 2007), but had minimal effects on Homer1a expression (Cotterly et al. 2007). Our present findings demonstrate that adding fluoxetine to methylphenidate strongly potentiates blunting of both gene markers.
Materials and Methods
Subjects
Male Sprague–Dawley rats (five weeks old at the beginning of the drug treatment; Harlan, Madison, WI, USA) were housed 2 per cage under standard laboratory conditions (12:12h light/dark cycle; lights on at 07:00h) with food and water available ad libitum. Experiments were performed between 13:00 and 17:00h. Prior to the drug treatment, the rats were allowed one week of acclimation during which they were repeatedly handled. All procedures met the NIH guidelines for the care and use of laboratory animals and were approved by the Rosalind Franklin University Animal Care and Use Committee.
Drug treatments
Rats received 5 daily injections of vehicle (i.p., V), methylphenidate HCl (5 mg/kg, MP; in 0.02% ascorbic acid, 1 ml/kg; Sigma, St. Louis, MO, USA), fluoxetine HCl (5 mg/kg, FLX; Sigma), or methylphenidate plus fluoxetine. On day 6, they received a cocaine challenge (25 mg/kg, C; cocaine HCl, Sigma) or vehicle (basal expression) (groups V/C, MP/C, FLX/C, MP+FLX/C, V/V, MP/V, FLX/V, MP+FLX/V, n=6-15).
Tissue preparation and in situ hybridization histochemistry
The rats were killed with CO2 40 min after the vehicle or cocaine challenge injection. The brain was rapidly removed, frozen in isopentane cooled on dry ice and then stored at -30 °C until cryostat sectioning. Coronal sections (12 μm) were thaw-mounted onto glass slides (Superfrost/Plus, Daigger, Wheeling, IL, USA), dried on a slide warmer and stored at -30 °C. In preparation for the in situ hybridization histochemistry, the sections were fixed in 4% paraformaldehyde/0.9% saline for 10 min at room temperature, incubated in a fresh solution of 0.25% acetic anhydride in 0.1 M triethanolamine/0.9% saline (pH 8.0) for 10 min, dehydrated, defatted for 2 × 5 min in chloroform, rehydrated, and air-dried. The slides were then stored at -30 °C until hybridization.
Oligonucleotide probes (48-mers; Invitrogen, Rockville, MD, USA) were labeled with [33P]-dATP as described earlier (Willuhn et al. 2003). The probes had the following sequence: Zif268 (Egr1), complementary to bases 352-399, GenBank accession number M18416; Homer1a, bases 1493-1540, AB003726. One hundred μl of hybridization buffer containing labeled probe (∼3 × 106 cpm) was added to each slide. The sections were coverslipped and incubated at 37 °C overnight. After incubation, the slides were first rinsed in four washes of 1× saline citrate (150 mM sodium chloride, 15 mM sodium citrate), and then washed 3 times 20 min each in 2× saline citrate/50% formamide at 40 °C, followed by 2 washes of 30 min each in 1× saline citrate at room temperature. After a brief water rinse, the sections were air-dried and then apposed to X-ray film (BioMax MR-2, Kodak) for 5-9 days.
Analysis of autoradiograms
Gene expression in the striatum was assessed in sections from 3 rostrocaudal levels [rostral, approximately at +1.6 mm relative to bregma (Paxinos & Watson 1998); middle, +0.4 (Fig. 1); caudal, -0.8] in a total of 23 sectors mostly defined by their predominant cortical inputs (see Willuhn et al. 2003; Yano & Steiner 2005a). Eighteen of these sectors represent the caudate-putamen, and 5 the nucleus accumbens.
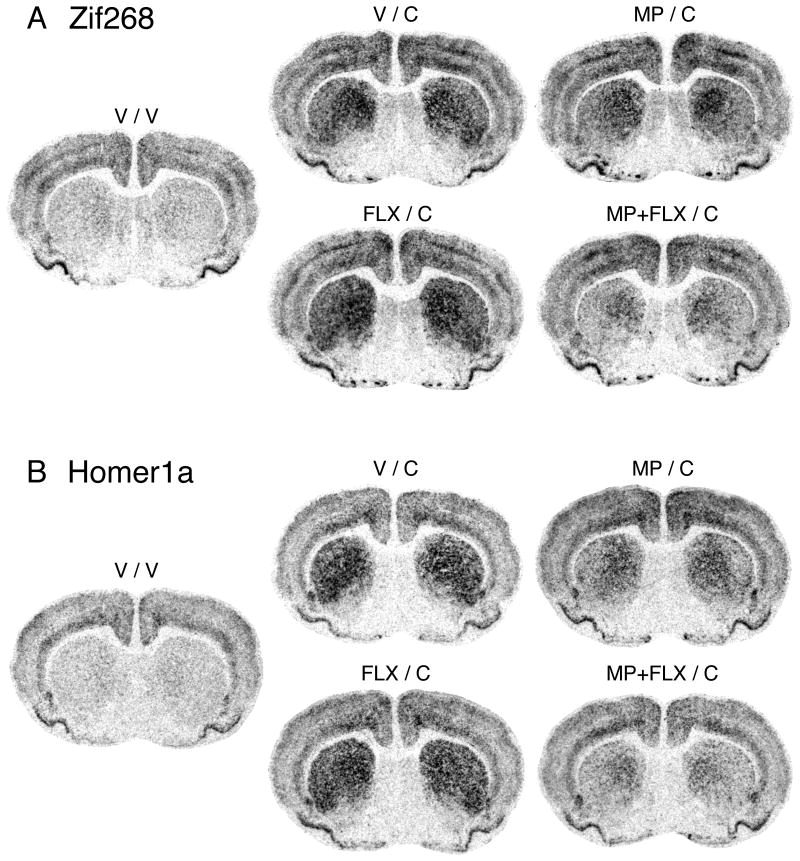
Figure 1.
Fluoxetine potentiates repeated methylphenidate-induced gene blunting in the striatum. Illustrations of film autoradiograms depict Zif268 (A) and Homer1a expression (B) in coronal sections from the middle striatum in rats that received 5 daily injections of vehicle (V), methylphenidate (5 mg/kg; MP), fluoxetine (5 mg/kg; FLX), or methylphenidate + fluoxetine (MP+FLX), followed on day 6 by vehicle (V) or a cocaine challenge (25 mg/kg; C). The maximal hybridization signal is in black.
Hybridization signals on film autoradiograms were measured by densitometry (NIH Image; Wayne Rasband, NIMH, Bethesda, MD, USA). The films were captured using a light table (Northern Light, Imaging Research, St. Catharines, Ontario, Canada) and a Sony CCD camera (Imaging Research). The “mean density” value of a region of interest was measured by placing a template over the captured image. Mean densities were corrected for background by subtracting mean density values measured over white matter (corpus callosum). Values from corresponding regions in the two hemispheres were then averaged. The illustrations of film autoradiograms displayed in Figure 1 are computer-generated images, and are contrast-enhanced where necessary. Maximal hybridization signal is black.
Statistics
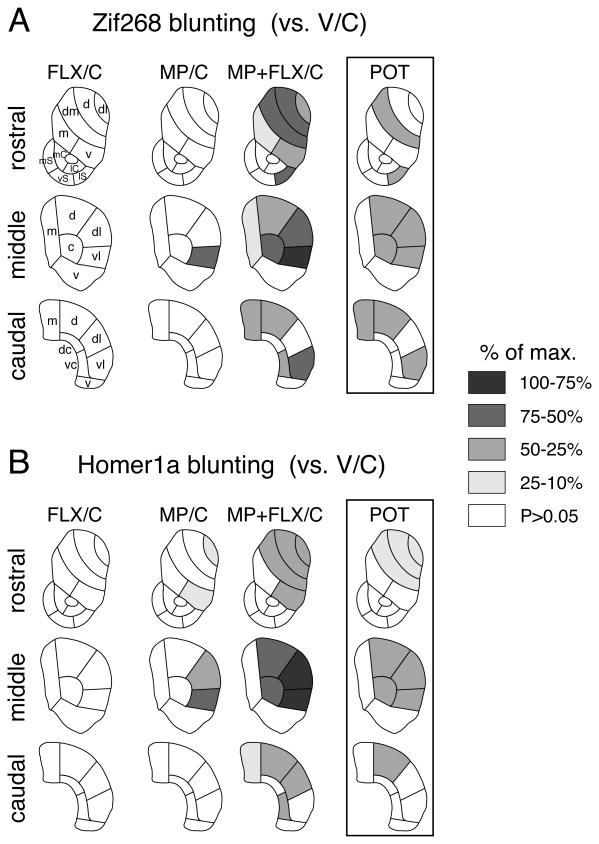
Treatment effects were determined by two-factor ANOVA with repeated treatment (V, MP, FLX, MP+FLX) and challenge (V, C) as between-subject variables. Newman-Keuls post hoc tests were used to describe differences between individual groups (Statistica, StatSoft, Tulsa, OK, USA). For illustrations of topographies (maps, Fig. 3), the decrease in gene induction (blunting) (vs. V/C) in a given region was expressed as the percentage of the maximal decrease observed for either probe (% max.). The regional distributions of the fluoxetine potentiation of Zif268 versus Homer1a blunting, and of Zif268 blunting (present study) versus acute Zif268 expression (Van Waes et al. 2010) were compared by Pearson correlations.
Figure 3.
Topography of fluoxetine-potentiated gene blunting in the striatum. Maps depict the distribution of blunting [i.e., the difference (decrease) vs. acute cocaine, V/C] for Zif268 (A) and Homer1a induction (B) in the rostral, middle and caudal striatum in rats treated with 5 daily injections of fluoxetine (5 mg/kg; FLX/C), methylphenidate (5 mg/kg; MP/C), or methylphenidate + fluoxetine (MP+FLX/C), followed on day 6 by the cocaine challenge. The differences between MP+FLX/C and MP/C groups are shown on the right (POT). The data are normalized relative to the maximal decrease observed (% of max.) for either gene. Sectors with a significant decrease (P<0.05) vs. acute cocaine controls (V/C) are shaded as indicated. Sectors without significant difference are in white. Caudate-putamen: c, central; d, dorsal; dc, dorsal central; dl, dorsolateral; dm, dorsomedial; m, medial; v, ventral; vc, ventral central; vl, ventrolateral. Nucleus accumbens: mC, medial core; lC, lateral core; mS, medial shell; vS, ventral shell; lS, lateral shell.
Results
Cocaine induces Zif268 and Homer1a expression in the striatum
Consistent with previous findings (e.g., Willuhn et al. 2003; Unal et al. 2009), acute administration of cocaine (cocaine challenge in vehicle-pretreated animals, V/C) induced Zif268 and Homer1a expression on all three rostrocaudal levels of the striatum (middle: Figs. 1 and 2; rostral, caudal: data not shown). Thus, for Zif268, a statistically significant increase in expression was observed in eighteen of the 23 striatal sectors, and for Homer1a, in nineteen sectors (P<0.05, V/C vs. V/V). The most robust cocaine-induced gene expression occurred in sensorimotor sectors of the middle (Figs. 1 and 2) and caudal striatum (not shown), as reported previously (e.g., Willuhn et al. 2003; Unal et al. 2009; see Steiner & Van Waes 2013).
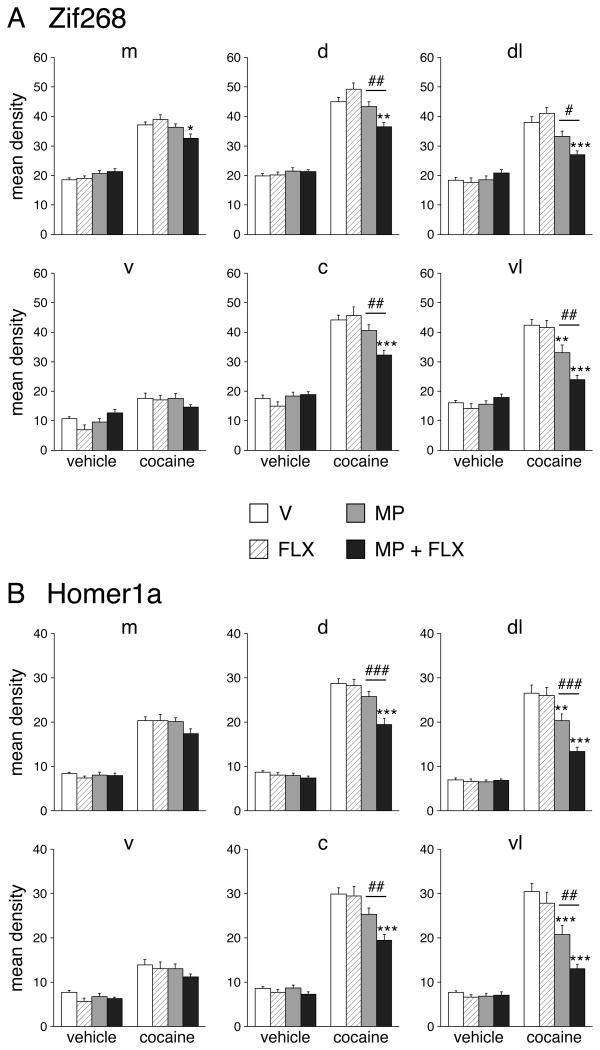
Figure 2.
Fluoxetine potentiation of repeated methylphenidate-induced Zif268 and Homer1a blunting in the middle striatum. Mean density values (mean ± SEM) for Zif268 (A) and Homer1a expression (B) in rats that received 5 injections of vehicle (V), methylphenidate (5 mg/kg; MP), fluoxetine (5 mg/kg; FLX), or methylphenidate + fluoxetine (MP+FLX), followed on day 6 by a vehicle or cocaine challenge (25 mg/kg; C), are given for the medial (m), ventral (v), dorsal (d), central (c), dorsolateral (dl), and ventrolateral (vl) sectors. The potentiation of blunting displayed a medial-lateral gradient, with the most robust effects laterally. *P<0.05, **P<0.01 and ***P<0.001 vs. V/C; #P<0.05, ##P<0.01 and ###P<0.001, MP+FLX/C vs. MP/C (potentiation).
Effects of repeated treatment with methylphenidate and/or fluoxetine on cocaine-induced Zif268 and Homer1a expression in the striatum
Repeated pretreatment with methylphenidate (5 mg/kg), fluoxetine (5 mg/kg) or methylphenidate+fluoxetine had no effect on basal expression of Zif268 or Homer1a 24 h after the last drug injection (i.e., 40 min after the vehicle challenge on day 6) (23 sectors: all P>0.05; middle: Fig. 2; rostral, caudal: not shown).
Repeated pretreatment with 5 mg/kg of methylphenidate alone (MP/C) produced statistically significant blunting of cocaine-induced Zif268 expression (P<0.05, vs. V/C) in only one of the 23 striatal sectors (middle: ventrolateral sector; Figs. 1A, 2A and 3A), although a similar tendency was seen in many sectors with a robust acute Zif268 response (V/C). Homer1a induction was somewhat more affected. Significant blunting in MP/C animals was observed in four sectors (rostral: dorsolateral, ventral; middle: dorsolateral, ventrolateral; Figs. 1B, 2B and 3B), with a similar tendency again present in more sectors.
In contrast to methylphenidate, repeated treatment with fluoxetine (5 mg/kg) alone had no impact on cocaine-induced gene regulation, neither in the caudate-putamen nor in the nucleus accumbens (Figs. 1, 2 and 3). None of the 23 sectors showed significant changes in Zif268 or Homer1a induction by cocaine in the fluoxetine-pretreated group (P>0.05, FLX/C vs. V/C; Figs. 2 and 3).
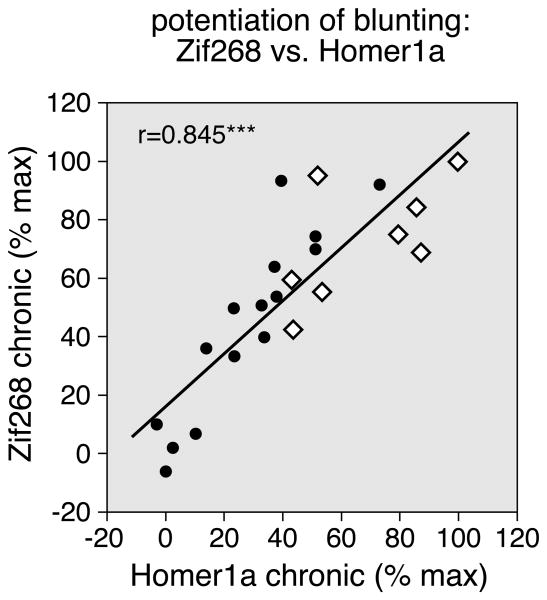
However, when given in conjunction with methylphenidate, fluoxetine potentiated repeated methylphenidate-induced blunting of gene inducibility by cocaine in the striatum (Figs. 1, 2 and 3). The fluoxetine potentiation of blunting was reflected by a higher proportion of the 23 striatal sectors displaying significantly decreased Zif268 or Homer1a induction after the methylphenidate+fluoxetine treatment (P<0.05, MP+FLX/C vs. V/C), compared with methylphenidate alone (MP/C vs. V/C) (Zif268: 15 sectors vs. 1 sector; Homer1a: 12 vs. 4; Figs. 2, 3). Direct statistical comparisons showed that Zif268 and Homer1a blunting were significantly more robust in the MP+FLX/C group than in the MP/C group in nine and eight of the 23 sectors, respectively (P<0.05, Fig. 3, POT). The correlation analysis showed that the regional distribution in the striatum of the potentiation of blunting by fluoxetine was similar for Zif268 and Homer1a (Zif268 × Homer1a: r=0.845, P<0.001; Fig. 4). Overall, the potentiation was most robust in sectors of the sensorimotor (lateral) striatum (Figs. 2, 3, and 4).
Figure 4.
Relationship between the potentiation of blunting for Zif268 vs. that for Homer1a induction after repeated drug treatment. The scatterplot shows that the difference in induction in MP+FLX/C vs. MP/C animals (potentiation) is correlated for Zif268 and Homer1a, in the 23 sectors (r=0.845). The values are expressed as the percentages of the maximal potentiation for each gene. The open diamonds indicate sensorimotor sectors. ***P<0.001.
Regional distribution of fluoxetine potentiation of methylphenidate-induced gene regulation: potentiation of blunting (repeated treatment) versus potentiation of acute induction
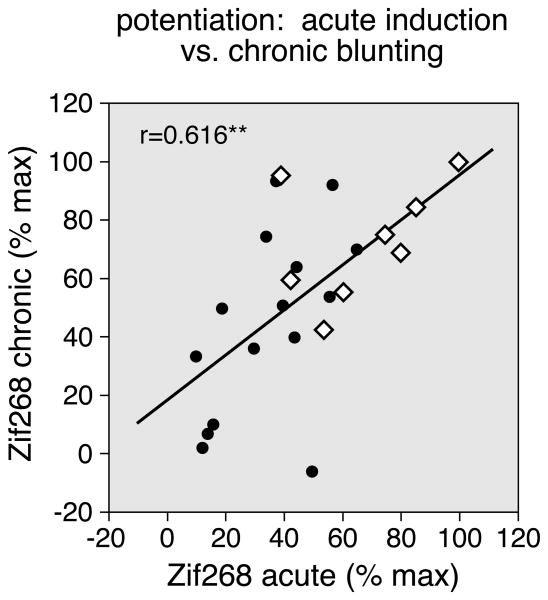
For repeated cocaine treatment, the degree of gene blunting was directly related to the magnitude of the initial (acute) gene induction in a given striatal region - the greater the induction after the first drug administration, the more blunted the induction after chronic treatment, consistent with a compensatory neuroadaptation (Willuhn et al. 2003; Unal et al. 2009). It was thus of interest to determine whether the fluoxetine potentiation of striatal gene regulation showed a similar relationship. We compared the fluoxetine potentiation of Zif268 blunting after the repeated treatment (present study) with the potentiation of acute Zif268 induction by methylphenidate, as reported before (Van Waes et al. 2010). Our results show that, across the 23 striatal sectors, the magnitude of the potentiation of Zif268 blunting (“chronic”, present study) was indeed positively correlated with the magnitude of the potentiation of acute Zif268 induction (Van Waes et al. 2010) (r=0.616, P<0.01; Fig. 5). Such acute gene regulation is thus a good indicator for chronic effects for this gene. Moreover, this correlation underscores our finding that the fluoxetine potentiation is greatest in sensorimotor sectors (Fig. 5, open diamonds).
Figure 5.
Relationship between the potentiation of acute Zif268 induction vs. that of Zif268 blunting after repeated drug treatment. The scatterplot depicts the correlation between the potentiation of acute Zif268 induction (difference between MP+FLX and MP groups; Van Waes et al. 2010) and the potentiation of Zif268 blunting (chronic; difference between MP+FLX/C and MP/C groups; present study), in the 23 sectors (r=0.616). The values are expressed as the percentages of the maximal potentiation for each gene. The open diamonds indicate sensorimotor sectors. **P<0.01.
Discussion
The present study is the first to show potentiated gene regulation in the striatum after repeated methylphenidate+fluoxetine treatment. We investigated whether fluoxetine given in conjunction with methylphenidate would modify the effects of repeated methylphenidate treatment on gene induction by a subsequent cocaine challenge. We previously showed that repeated treatment with a high dose of methylphenidate (10 mg/kg) in adolescent rats resulted in robust blunting of gene induction by cocaine (Brandon & Steiner 2003). The present methylphenidate threshold dose (5 mg/kg; Brandon & Steiner 2003; Yano & Steiner 2005b) alone produced only a modest blunting. However, adding fluoxetine (5 mg/kg), which by itself had no effect, strongly potentiated blunting of Zif268 and Homer1a induction by cocaine throughout most of the striatum, mimicking effects of repeated cocaine exposure (Unal et al. 2009). To the extent that such gene blunting is predictive of the abuse/addiction liability (see Steiner & Van Waes 2013), our findings suggest an enhanced abuse/addiction liability after this combination treatment.
SSRIs potentiate gene regulation by methylphenidate
Similar to other psychostimulants (Steiner & Van Waes 2013), methylphenidate-induced gene regulation in the striatum is mediated by dopamine receptor activation (Yano et al. 2006; Alburges et al. 2011). However, serotonin contributes to gene regulation by psychostimulants such as cocaine (e.g., Bhat & Baraban 1993; Lucas et al. 1997; Horner et al. 2005; Szucs et al. 2005). These findings led to the hypothesis that the reduced effects of methylphenidate, as compared with those of cocaine, might be related to the lack of methylphenidate effects on serotonin (Yano & Steiner 2007). Consistent with this hypothesis, acute treatment with the SSRIs fluoxetine or citalopram potentiated methylphenidate-induced expression of c-Fos, Zif268, substance P and dynorphin in the striatum (Steiner et al. 2010; Van Waes et al. 2010; Van Waes et al. 2012). This acute potentiation was most robust in the lateral, sensorimotor striatum.
The present study extends these findings to chronic gene regulation; fluoxetine also potentiates at least some molecular changes induced by repeated methylphenidate treatment. We show this effect for one of the best-established alterations in gene regulation after repeated psychostimulant treatments, blunting of gene inducibility (Maze & Nestler 2011). Repeated methylphenidate treatment (5 mg/kg, 5 days) alone produced minor blunting of Zif268 and Homer1a induction by the cocaine challenge (25 mg/kg) (statistically significant in 1 and 4 of the 23 sectors, respectively). Fluoxetine given in conjunction with methylphenidate enhanced blunting for both genes (significant in 15 and 12 sectors, greater in 9 and 8 sectors). While these effects were thus widespread, they were also most robust in the lateral, sensorimotor striatum, thus again mimicking effects of cocaine treatment (Unal et al. 2009).
To the best of our knowledge, only one other study has investigated the effects of repeated methylphenidate+SSRI treatment on gene regulation and behavior (Warren et al. 2011). In this study, rats received methylphenidate (2 mg/kg) and/or fluoxetine (2.5 mg/kg) twice daily between postnatal days 20 and 34, and gene expression in the midbrain (dopamine cell body area) and behavioral effects were assessed (without drug challenge) either one day after the treatment or 2 months later when the animals were adults. Results showed that the combination treatment produced various molecular changes involving ERK signaling and transcription factors (e.g., CREB, Zif268, mTOR) in the ventral tegmental area. Some of these effects were enhanced, some were reversed, compared with the effects of methylphenidate alone. Importantly, many of these changes endured into adulthood (Warren et al. 2011).
Gene blunting in our present study was measured 1 day after the repeated treatment. We previously showed that gene blunting induced by repeated cocaine treatment (25 mg/kg, 5 days) in adults is long-lasting; it persisted almost undiminished for at least 3 weeks after the 5-day treatment (Unal et al. 2009). With a similar endurance in adolescents (present study), these effects would thus also be expected to last well into the adulthood of the animals. Future studies will have to determine how long the striatal gene regulation effects may last.
Potential mechanisms underlying gene blunting and its potentiation by serotonin
Several mechanisms have been advanced to explain blunting of gene expression after repeated psychostimulant treatments (Steiner & Van Waes 2013). These include systems-level neuroadaptations as well as intracellular (epigenetic) modifications. The former include upregulated dynorphin signaling in the striatum, which has been demonstrated after repeated cocaine/amphetamine exposure in animal models as well as in human drug users (Steiner & Gerfen 1998; Shippenberg et al. 2007). Dynorphin expression is also increased by methylphenidate (Brandon & Steiner 2003; Yano & Steiner 2005b; Alburges et al. 2011), and this response is potentiated by fluoxetine (Van Waes et al. 2012). Dynorphin acts, at least in part, as a negative feedback mechanism (Steiner & Gerfen 1998) to limit dopamine and glutamate input to striatal neurons, and, given that both neurotransmitters are critical for psychostimulant-induced gene expression, dynorphin may thereby limit gene induction (see Steiner & Van Waes 2013, for discussion). On the other hand, the long-lasting endurance of gene blunting after psychostimulant treatment may require mechanisms involving epigenetic modifications (e.g., histone hypoacetylation or methylation) (Maze & Nestler 2011). A role for such epigenetic regulation has recently been shown for blunting of c-Fos induction after repeated amphetamine treatment (Renthal et al. 2008).
Which serotonin receptors mediate the SSRI potentiation of striatal gene regulation? Interactions between serotonin and dopamine are complex, involving several serotonin receptor subtypes and brain areas (Muller & Huston 2006; Bubar & Cunningham 2008). A number of serotonin receptor subtypes are expressed in striatal neurons (Barnes & Sharp 1999), and facilitatory effects on striatal gene expression have been shown for serotonin 5-HT1B, 5-HT2A, 5-HT3 and other receptor agonists (e.g., Lucas et al. 1997; Szucs et al. 2005). Interestingly, a recent study demonstrated potentiation of methylphenidate-induced locomotion by a 5-HT1B receptor agonist (Borycz et al. 2008). However, these drugs were administered systemically (as were the SSRIs in our studies) and may thus also have modified striatal inputs by actions at receptors in other brain areas (for example, in the midbrain; Warren et al. 2011). Future studies using local drug administration and receptor-selective agents will have to determine the receptor subtypes and sites that mediate the potentiation of methylphenidate-induced gene regulation in the striatum.
Clinical relevance
The functional consequences of blunted (transcription factor) gene inducibility remain unclear, but as the integrity of neurons depends on balanced regulation of gene expression to ensure that cellular components with limited half-lives are replenished and cellular plasticity is maintained, it can be assumed that drug-induced gene blunting disrupts cell homeostasis/function (see Steiner & Van Waes 2013). Such gene blunting thus serves as a convenient marker to identify the brain areas/neurons altered by a repeated drug treatment.
The lateral (sensorimotor) striatum that was preferentially affected in our studies is critical for stimulus-response (habit) learning, and drug-induced molecular changes in this part of the striatum are implicated in aberrant habit formation and compulsive behavior in drug addiction, and relapse to drug seeking after previous drug exposure (Everitt & Robbins 2005; see Steiner & Van Waes 2013, for discussion). Methylphenidate-induced molecular changes in these striatal circuits may thus contribute to the previously observed facilitation of cocaine seeking and taking (Brandon et al. 2001). Our findings suggest that fluoxetine may enhance such effects of methylphenidate. However, the behavioral consequences may not be restricted to drug seeking/taking. In the study by Warren et al. (2011) discussed above, the juvenile rats pretreated with methylphenidate+SSRI displayed, as adults, increased sensitivity to cocaine and sucrose reward (place preference conditioning), but also enhanced reactivity to stress- and anxiety-eliciting situations.
The above preclinical studies may indicate potential health risks for methylphenidate-SSRI coexposure in humans. How widespread is methylphenidate-SSRI coexposure resulting from pharmacological treatments and cognitive enhancer use? SSRIs such as fluoxetine are often the first-line treatment for several depressive and anxiety disorders (Petersen et al. 2002) and are given to millions of patients in the United States alone every year. As discussed, methylphenidate is used both in the treatment of conditions such as ADHD and as a recreational drug and cognitive enhancer (Greely et al. 2008; Kollins 2008; Wilens et al. 2008). Combination therapies of methylphenidate plus an SSRI are indicated for several conditions, including ADHD and anxiety/depression comorbidity (Safer et al. 2003; Bhatara et al. 2004; Kollins 2008). Methylphenidate is also combined with SSRIs as augmentation therapy and as acceleration treatment for SSRIs in major depressive disorder (e.g., Lavretsky et al. 2003; Nelson 2007). It is currently unknown how much accidental methylphenidate-SSRI coexposure occurs as a result of cognitive enhancer use by patients on SSRIs.
The addiction liability of methylphenidate alone is more limited compared with illicit psychostimulants (Svetlov et al. 2007), and it remains unclear whether proper medical use of drugs such as methylphenidate (low oral doses) has detrimental effects (Kollins 2008; Wilens et al. 2008). However, cognitive enhancer/recreational use can involve snorting and intravenous injection of the drug, which produce higher plasma levels and thus greater risks for neuronal and related behavioral changes (for review, see Steiner & Van Waes 2013). Future studies will have to determine whether coexposure with SSRIs increases the addiction liability of methylphenidate, as our gene regulation effects suggest (Steiner & Van Waes 2013).
Acknowledgments
This work was supported in part by USPHS grants DA031916 and DA011261 (H.S.).
Footnotes
Conflict of Interest: None.
Author Contributions: VVV and HS were responsible for the study concept and design. VVV, MV and JB performed the experiments, and acquired and analyzed the data. VVV and HS wrote the manuscript. All authors critically reviewed content and approved final version of the manuscript.
References
- Alburges ME, Hoonakker AJ, Horner KA, Fleckenstein AE, Hanson GR. Methylphenidate alters basal ganglia neurotensin systems through dopaminergic mechanisms: A comparison with cocaine treatment. J Neurochem. 2011;117:470–478. doi: 10.1111/j.1471-4159.2011.07215.x. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Stimulants and the developing brain. Trends Pharmacol Sci. 2005;26:237–243. doi: 10.1016/j.tips.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Bhat RV, Baraban JM. Activation of transcription factor genes in striatum by cocaine: role of both serotonin and dopamine systems. J Pharmacol Exp Ther. 1993;267:496–505. [PubMed] [Google Scholar]
- Bhatara V, Feil M, Hoagwood K, Vitiello B, Zima B. National trends in concomitant psychotropic medication with stimulants in pediatric visits: practice versus knowledge. J Atten Disord. 2004;7:217–226. doi: 10.1177/108705470400700404. [DOI] [PubMed] [Google Scholar]
- Borycz J, Zapata A, Quiroz C, Volkow ND, Ferré S. 5-HT(1B) receptor-mediated serotoninergic modulation of methylphenidate-induced locomotor activation in rats. Neuropsychopharmacology. 2008;33:619–626. doi: 10.1038/sj.npp.1301445. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, Baker LK, White FJ. Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology. 2001;25:651–661. doi: 10.1016/S0893-133X(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Steiner H. Repeated methylphenidate treatment in adolescent rats alters gene regulation in the striatum. Eur J Neurosci. 2003;18:1584–1592. doi: 10.1046/j.1460-9568.2003.02892.x. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Prospects for serotonin 5-HT2R pharmacotherapy in psychostimulant abuse. Prog Brain Res. 2008;172:319–346. doi: 10.1016/S0079-6123(08)00916-3. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Konradi C. Understanding the neurobiological consequences of early exposure to psychotropic drugs: linking behavior with molecules. Neuropharmacology. 2004;47:47–60. doi: 10.1016/j.neuropharm.2004.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrey N, Wilkinson M. A review of psychostimulant-induced neuroadaptation in developing animals. Neurosci Bull. 2011;27:197–214. doi: 10.1007/s12264-011-1004-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase T, Carrey N, Soo E, Wilkinson M. Methylphenidate regulates activity regulated cytoskeletal associated but not brain-derived neurotrophic factor gene expression in the developing rat striatum. Neuroscience. 2007;144:969–984. doi: 10.1016/j.neuroscience.2006.10.035. [DOI] [PubMed] [Google Scholar]
- Chase TD, Carrey N, Brown RE, Wilkinson M. Methylphenidate regulates c-fos and fosB expression in multiple regions of the immature rat brain. Dev Brain Res. 2005;156:1–12. doi: 10.1016/j.devbrainres.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Cotterly L, Beverley JA, Yano M, Steiner H. Dysregulation of gene induction in corticostriatal circuits after repeated methylphenidate treatment in adolescent rats: Differential effects on zif 268 and homer 1a. Eur J Neurosci. 2007;25:3617–3628. doi: 10.1111/j.1460-9568.2007.05570.x. [DOI] [PubMed] [Google Scholar]
- Crawford CA, Baella SA, Farley CM, Herbert MS, Horn LR, Campbell RH, Zavala AR. Early methylphenidate exposure enhances cocaine self-administration but not cocaine-induced conditioned place preference in young adult rats. Psychopharmacology. 2011;213:43–52. doi: 10.1007/s00213-010-2011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Greely H, Sahakian B, Harris J, Kessler RC, Gazzaniga M, Campbell P, Farah MJ. Towards responsible use of cognitive-enhancing drugs by the healthy. Nature. 2008;456:702–705. doi: 10.1038/456702a. [DOI] [PubMed] [Google Scholar]
- Horner KA, Adams DH, Hanson GR, Keefe KA. Blockade of stimulant-induced preprodynorphin mRNA expression in the striatal matrix by serotonin depletion. Neuroscience. 2005;131:67–77. doi: 10.1016/j.neuroscience.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Knapska E, Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog Neurobiol. 2004;74:183–211. doi: 10.1016/j.pneurobio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Kollins SH. ADHD, substance use disorders, and psychostimulant treatment: current literature and treatment guidelines. J Atten Disord. 2008;12:115–125. doi: 10.1177/1087054707311654. [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Kim MD, Kumar A, Reynolds CF. Combined treatment with methylphenidate and citalopram for accelerated response in the elderly: an open trial. J Clin Psychiatry. 2003;64:1410–1414. doi: 10.4088/jcp.v64n1202. [DOI] [PubMed] [Google Scholar]
- Lucas JJ, Segu L, Hen R. 5-Hydroxytryptamine1B receptors modulate the effect of cocaine on c-fos expression: converging evidence using 5-hydroxytryptamine1B knockout mice and the 5-hydroxytryptamine1B/1D antagonist GR127935. Mol Pharmacol. 1997;51:755–763. doi: 10.1124/mol.51.5.755. [DOI] [PubMed] [Google Scholar]
- Maze I, Nestler EJ. The epigenetic landscape of addiction. Ann N Y Acad Sci. 2011;1216:99–113. doi: 10.1111/j.1749-6632.2010.05893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller CP, Huston JP. Determining the region-specific contributions of 5-HT receptors to the psychostimulant effects of cocaine. Trends Pharmacol Sci. 2006;27:105–112. doi: 10.1016/j.tips.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Nelson JC. Augmentation strategies in the treatment of major depressive disorder. Recent findings and current status of augmentation strategies. CNS Spectr. 2007;12(Suppl 22):6–9. doi: 10.1017/s1092852900016011. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1998. [Google Scholar]
- Petersen T, Dording C, Neault NB, Kornbluh R, Alpert JE, Nierenberg AA, Rosenbaum JF, Fava M. A survey of prescribing practices in the treatment of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:177–187. doi: 10.1016/s0278-5846(01)00250-0. [DOI] [PubMed] [Google Scholar]
- Renthal W, Carle TL, Maze I, Covington HE, Truong HT, Alibhai I, Kumar A, Montgomery RL, Olson EN, Nestler EJ. Delta FosB mediates epigenetic desensitization of the c-fos gene after chronic amphetamine exposure. J Neurosci. 2008;28:7344–7349. doi: 10.1523/JNEUROSCI.1043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008;14:341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safer DJ, Zito JM, DosReis S. Concomitant psychotropic medication for youths. Am J Psychiatry. 2003;160:438–449. doi: 10.1176/appi.ajp.160.3.438. [DOI] [PubMed] [Google Scholar]
- Schenk S, Izenwasser S. Pretreatment with methylphenidate sensitizes rats to the reinforcing effects of cocaine. Pharmacol Biochem Behav. 2002;72:651–657. doi: 10.1016/s0091-3057(02)00735-9. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Exp Brain Res. 1998;123:60–76. doi: 10.1007/s002210050545. [DOI] [PubMed] [Google Scholar]
- Steiner H, Van Waes V. Addiction-related gene regulation: Risks of exposure to cognitive enhancers vs. other psychostimulants. Prog Neurobiol. 2013;100:60–80. doi: 10.1016/j.pneurobio.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Van Waes V, Marinelli M. Fluoxetine potentiates methylphenidate-induced gene regulation in addiction-related brain regions: Concerns for use of cognitive enhancers? Biol Psychiatry. 2010;67:592–594. doi: 10.1016/j.biopsych.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetlov SI, Kobeissy FH, Gold MS. Performance enhancing, non-prescription use of Ritalin: a comparison with amphetamines and cocaine. J Addict Dis. 2007;26:1–6. doi: 10.1300/J069v26n04_01. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Wigal TL, Volkow ND. Contrast of medical and nonmedical use of stimulant drugs, basis for the distinction, and risk of addiction: comment on Smith and Farah (2011) Psychol Bull. 2011;137:742–748. doi: 10.1037/a0024898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szucs RP, Frankel PS, McMahon LR, Cunningham KA. Relationship of cocaine-induced c-Fos expression to behaviors and the role of serotonin 5-HT2A receptors in cocaine-induced c-Fos expression. Behav Neurosci. 2005;119:1173–1183. doi: 10.1037/0735-7044.119.5.1173. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD. Homers regulate drug-induced neuroplasticity: implications for addiction. Biochem Pharmacol. 2008;75:112–133. doi: 10.1016/j.bcp.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas U. Modulation of synaptic signalling complexes by Homer proteins. J Neurochem. 2002;81:407–413. doi: 10.1046/j.1471-4159.2002.00869.x. [DOI] [PubMed] [Google Scholar]
- Unal CT, Beverley JA, Willuhn I, Steiner H. Long-lasting dysregulation of gene expression in corticostriatal circuits after repeated cocaine treatment in adult rats: Effects on zif 268 and homer 1a. Eur J Neurosci. 2009;29:1615–1626. doi: 10.1111/j.1460-9568.2009.06691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Waes V, Beverley J, Marinelli M, Steiner H. Selective serotonin reuptake inhibitor antidepressants potentiate methylphenidate (Ritalin)-induced gene regulation in the adolescent striatum. Eur J Neurosci. 2010;32:435–447. doi: 10.1111/j.1460-9568.2010.07294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Waes V, Carr B, Beverley JA, Steiner H. Fluoxetine potentiation of methylphenidate-induced neuropeptide expression in the striatum occurs selectively in direct pathway (striatonigral) neurons. J Neurochem. 2012;122:1054–1064. doi: 10.1111/j.1471-4159.2012.07852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren BL, Iñiguez SD, Alcantara LF, Wright KN, Parise EM, Weakley SK, Bolaños-Guzmán CA. Juvenile administration of concomitant methylphenidate and fluoxetine alters behavioral reactivity to reward- and mood-related stimuli and disrupts ventral tegmental area gene expression in adulthood. J Neurosci. 2011;31:10347–10358. doi: 10.1523/JNEUROSCI.1470-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Adler LA, Adams J, Sgambati S, Rotrosen J, Sawtelle R, Utzinger L, Fusillo S. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47:21–31. doi: 10.1097/chi.0b013e31815a56f1. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Sun W, Steiner H. Topography of cocaine-induced gene regulation in the rat striatum: Relationship to cortical inputs and role of behavioural context. Eur J Neurosci. 2003;17:1053–1066. doi: 10.1046/j.1460-9568.2003.02525.x. [DOI] [PubMed] [Google Scholar]
- Yano M, Beverley JA, Steiner H. Inhibition of methylphenidate-induced gene expression in the striatum by local blockade of D1 dopamine receptors: Interhemispheric effects. Neuroscience. 2006;140:699–709. doi: 10.1016/j.neuroscience.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Yano M, Steiner H. Methylphenidate (Ritalin) induces Homer 1a and zif 268 expression in specific corticostriatal circuits. Neuroscience. 2005a;132:855–865. doi: 10.1016/j.neuroscience.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Yano M, Steiner H. Topography of methylphenidate (Ritalin)-induced gene regulation in the striatum: differential effects on c-fos, substance P and opioid peptides. Neuropsychopharmacology. 2005b;30:901–915. doi: 10.1038/sj.npp.1300613. [DOI] [PubMed] [Google Scholar]
- Yano M, Steiner H. Methylphenidate and cocaine: the same effects on gene regulation? Trends Pharmacol Sci. 2007;28:588–596. doi: 10.1016/j.tips.2007.10.004. [DOI] [PubMed] [Google Scholar]