Abstract
Myofascial trigger points (MTrPs) are palpable, tender nodules in taut bands of skeletal muscle that are painful on compression. MTrPs are characteristic findings in myofascial pain syndrome (MPS). The role of MTrPs in the pathophysiology of MPS is unknown. Localization, diagnosis, and clinical outcome measures of painful MTrPs can be improved by objectively characterizing and quantitatively measuring their properties. The goal of this study was to evaluate whether ultrasound imaging and elastography can differentiate symptomatic (active) MTrPs from normal muscle. Patients with chronic (>3 months) neck pain with spontaneously painful, palpable (i.e., active) MTrPs and healthy volunteers without spontaneous pain (having palpably normal muscle tissue) were recruited for this study. The upper trapezius muscles in all subjects were imaged, and the echotexture was analyzed using entropy filtering of B-mode images. Vibration elastography was performed by vibrating the muscle externally at 100 Hz. Color Doppler variance imaging was used to quantify the regions of color deficit exhibiting low vibration amplitude. The imaging measures were compared against the clinical findings of a standardized physical exam. We found that sites with active MTrPs (n = 14) have significantly lower entropy (p < 0.05) and significantly larger nonvibrating regions (p < 0.05) during vibration elastography compared with normal, uninvolved muscle (n = 15). A combination of both entropy analysis and vibration elastography yielded 69% sensitivity and 81% specificity in discriminating active MTrPs from normal muscle. These results suggest that active MTrPs have more homogeneous texture and heterogeneous stiffness when compared with normal, unaffected muscle. Our methods enabled us to improve the imaging contrast between suspected MTrPs and surrounding muscle. Our results indicate that in subjects with chronic neck pain and active MTrPs, the abnormalities are not confined to discrete isolated nodules but instead affect the milieu of the muscle surrounding palpable MTrPs. With further refinement, ultrasound imaging can be a promising objective method for characterizing soft tissue abnormalities associated with active MTrPs and elucidating the role of MTrPs in the pathophysiology of MPS.
Keywords: ultrasound, superficial muscle, texture image analysis, myofascial trigger point, tissue differentiation, tissue characterization, ultrasonic imaging, entropy filtering
Introduction
Chronic soft tissue pain syndromes and myofascial pain, in particular, are widely prevalent in the population and have a significant impact on function and disability.1 It is reported that approximately 15% of routine medical clinic visits are the result of soft tissue pain concerns.2 The prevalence is considerably higher in pain clinics3 where soft tissue pain may account for 85% of these visits. Myofascial pain syndrome (MPS)4 most commonly involves the neck and low back. Characteristic findings in MPS are myofascial trigger points (MTrPs). MTrPs are palpable, discrete, and hyperirritable nodules in a taut band of skeletal muscle. Active MTrPs are associated with spontaneous pain in surrounding tissue and/or distant sites.5
The etiology and pathophysiology of MTrPs are currently unknown. The current diagnostic criteria for active MTrPs are based on palpation and compression of the nodule, which reproduces the individual’s spontaneous pain complaint. Until recently, there were no pathophysiological findings in the muscle associated with MTrPs. However, microanalytical studies of the local biochemical milieu of active MTrPs have demonstrated elevated local levels of inflammatory mediators, neuropeptides, catecholamines, and proinflammatory cytokines compared with asymptomatic muscle.6 Our research group has previously shown that MTrPs can be identified as hypoechoic regions on ultrasound images, and their mechanical properties, that is, stiffness and viscosity, can be objectively quantified7–9; however, the soft tissue neighborhood of the MTrP and the nature of the MTrP have not been previously explored. Characterization and evaluation of the soft tissue neighborhood of MTrPs can provide clues regarding the underlying pathophysiology and their relevance in MPS.10,11 The objective of this study was to develop an image-based technique for detecting differences between MTrPs and surrounding muscle as well as between muscle of healthy control subjects and subjects with active MTrPs through analysis of B-mode and color Doppler variance images.
Method
Subject Selection
Subjects recruited for this study were divided into two groups: volunteers experiencing symptoms of neck pain consistently over the past three months (active subjects) and volunteers who were pain-free (healthy control subjects). Those in the active group had at least one active MTrP in one or both upper trapezii and were evaluated, as described below, to rule out any potential causes of their symptoms other than MTrPs. The control group was pain-free and had no palpable MTrPs at standardized locations in the upper trapezii. Each participant provided informed consent prior to involvement in the study. The study procedures were approved by the Chesapeake Review Board.
All subjects underwent a thorough physical examination. A physiatrist palpated the central region of the upper trapezius muscle, approximately midway between the cervical vertebrae and the acromion process. Following Travell and Simon’s diagnostic criteria,1 this examiner determined the presence or absence of MTrPs in the muscle. Four sites (two sites per side) were marked on each subject, in the central region of the upper trapezius muscle within 6 cm of the muscle’s midline, approximately midway between the cervical vertebrae and the acromion process, as described in Ballyns et al.9 A site was classified as “active” if it was both spontaneously painful (pain present without provocation) and had at least one palpable nodule whose palpation reproduced the characteristic pain complaint, indicative of an active MTrP. Sites in symptomatic subjects were considered “normal in symptomatic” if no palpable nodule was found in that specified region of the upper trapezius muscle. Palpably normal sites in asymptomatic control subjects were classified as “normal.”
MTrP Localization
Ultrasound imaging was performed bilaterally on the upper trapezius using the SonixRP US system (Ultrasonix Medical Corporation, Vancouver, BC) and a 5~14-MHz ultrasonic linear array transducer (L14-5, central frequency 9.5 MHz). Imaging settings, that is, time gain compensation (TGC), depth, and sector size, were kept consistent for all subjects. The sonographer was blinded to the clinical status of each subject. In this study, MTrPs were identified on ultrasound images using the methods we have previously described.6 Briefly, hypoechoic areas were first detected using B-mode imaging; typically palpable MTrPs appear as focal darker areas.7,8 An external vibration source (Mini Vibrator; North Coast Medical, Inc., Morgan Hill, California), around 100 Hz was then applied to the surrounding muscle, and a color Doppler variance image was acquired (Figure 1). MTrPs have been previously found to be stiffer9 and thus vibrate with lower amplitude, and appear as focal areas of color deficit in the color variance image (Figure 1b).
Figure 1.
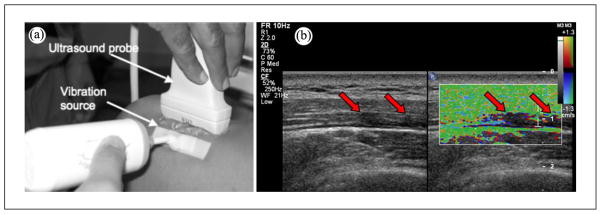
Localization of MTrPs using vibration elastography. (a) An external massager generates shear waves that propagate through the muscle. (b) Hypoechoic areas (MTrPs) in B-mode image are observed to be vibrating less than the surrounding muscle in color variance image.
MTrPs = myofascial trigger points.
Note: Figure is available in full color in the online version at uix.sagepub.com
Image Processing: Entropy Filtering Analysis
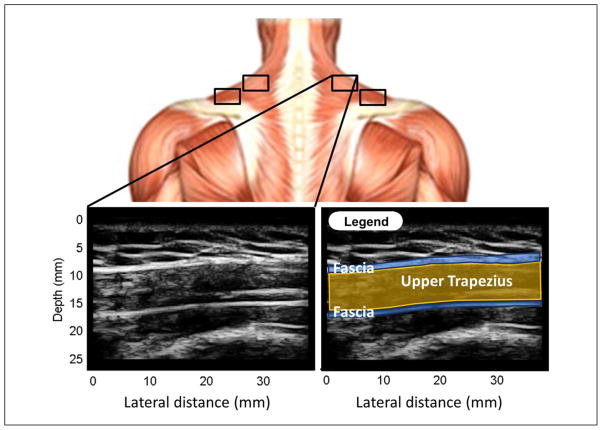
The B-mode images for upper trapezii of each subject included 256 scan lines per frame, full sector size, and imaging depth of 2.5 cm. The B-mode images were 8-bit envelope data after dynamic range compression and image processing. Four sites (two sites per side) in the upper trapezius muscle were investigated per subject (Figure 2), and two B-mode and color variance images were acquired at each site (Figure 1). Entropy filtering was performed on a total of 232 images (29 subjects, 4 sites per subject and 2 images per site) using the MATLAB (Mathworks, Natick, Massachusetts) image processing toolbox. MTrP size was determined from the color variance images using ImageJ12 following procedures previously described.8
Figure 2.
Upper trapezius sites and a representative B-mode image of it. Top image shows the approximate location of four sites of the upper trapezius investigated in this study. Bottom left is a representative B-mode image of an upper trapezius site. The legend, at the bottom right, helps identify the fascia (blue lines) and the upper trapezius (yellow).
Note: Figure is available in full color in the online version at uix.sagepub.com
Parameters estimated from two images acquired at each site were averaged. The two acquisitions were performed sequentially by removing the ultrasound probe and repositioning. The echotexture of the B-mode images were analyzed using entropy, a statistical measure of the probability distribution of the gray levels.
The entropy filter returns a scalar value for each pixel of the input image so that each output pixel contains the entropy value of the neighborhood around the corresponding pixel in the input image.
The entropy, E, assigned to each pixel, is evaluated with the following formula:
where pn is the probability density of the nth gray level (of 256 levels) within the neighborhood of the input pixel. For a uniform gray scale distribution with a 9 × 9 pixel neighborhood, entropy is maximum and equal to 6.32 – pn is equal to 1/n for uniformly distributed gray levels with n = 80 (number of gray levels in a 9 × 9 pixel cell except the input pixel). Summation in (1) is therefore performed on 80 elements only and entropy is equal to log2(80). A minimum entropy value of 0 is obtained when all the pixels, within a neighborhood, have the same intensity.
The neighborhood is a circle in pixel (with radius equal to 5 pixels), but it is an ellipse in spatial dimensions because of the different axial and lateral resolutions. Its area is given by π(5 × 0.078) × (5 × 0.07) = 0.43 mm2 (0.078 mm and 0.07 mm are lateral and axial resolutions, respectively). The wavelength used for imaging is around 0.162 mm, which is about 23% of the minor axis of the neighborhood (alternatively, the minor axis of the neighborhood is about 4.3 times larger than the wavelength used for imaging).
The entropy measure can be used to characterize the texture of an input image.13 However, because the texture of a tissue image reflects its scatterers’ properties, mean entropy values of selected regions from images of the upper trapezius of symptomatic and asymptomatic patients were used as a measure of heterogeneity of the tissue itself. This analysis has been used for differentiating normal from abnormal myocardial structure14–16 as well as characterizing human brain tissues17 and liver cancer.18
A B-mode image of the upper trapezius collected from a symptomatic subject (Figure 2) demonstrates that it is easy to discriminate among subcutaneous tissue (layer above the fascia), the fascia that surrounds the upper trapezius (two blue thin lines), and the underlying muscle (below the fascia). The region of interest (ROI), that is, upper trapezius, was manually marked in each image and analysis was performed on those regions only. Size of the ROI was therefore dictated by the size of the muscle itself. In the entropy-filtered image, regions with low entropy are regions that are homogeneously bright or dark in the B-mode image (Figure 3a) and appear dark in the filtered image (Figure 3b).
Figure 3.
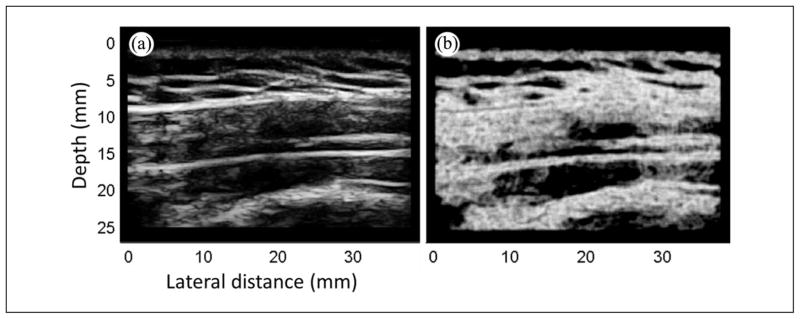
Entropy filtering. (a) B-mode image and (b) its entropy image.
To understand whether there were any differences in echotexture between the belly of the muscle and the fascial border, the ROI was divided into two parts (Figures 4a–c). The muscle belly was defined to be the central part of the upper trapezius (60% of the whole ROI) and obtained by cropping the ROI by 20% on both the upper and lower boundaries (Figure 4b). This cropped part identifies the fascial border, defined to be the part of the upper trapezius close to the fascia (40% of the whole ROI; Figure 4c). This set of entropy analyses was designed to quantify and distinguish partial contributions to entropy of nodular MTrPs (Figure 4b) from those of regions near the fascial border (Figure 4c).
Figure 4.
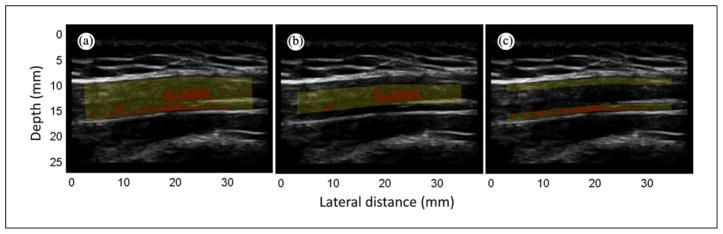
Region of interest (ROI). B-mode image is of an “active” subject. Yellow mask highlights the ROI, red regions have entropy < 4. (a) Whole ROI, (b) muscle belly (60% of the ROI), (c) fascial border (40% of the ROI).
Note: Figure is available in full color in the online version at uix.sagepub.com
In previous studies, our group has shown that MTrPs appear as hypoechoic regions in B-mode images.7–9 In this study, we investigated whether entropy filtering can help to localize those regions and to find whether the measure of their mean entropy can be used to discriminate among “active,” “normal in symptomatic,” and “normal” sites in the upper trapezius muscle. Therefore, an entropy threshold equal to four was chosen based on receiver operating characteristic (ROC) curves analysis. This entropy threshold was used throughout all the analyses presented in this article.
Statistical Analysis
Statistical analysis was performed using PASW 18 (IBM SPSS Inc., Chicago, Illinois). Mann–Whitney U test was performed to examine the effect of the tissue type (“active” sites vs. both “normal” and “normal in symptomatic”) on the tissue texture (mean entropy) and on the mean area of the regions with entropy less than 4. The dependent variables, mean entropy, and mean area of regions with entropy less than 4 were normally distributed as assessed by the Shapiro–Wilk test (p < 0.05); however, variances among independent groups (“normal,” “normal in symptomatic,” and “active”) were unequal as assessed by Levene’s test for equality of variances (p < 0.05).
The subject’s age was normally distributed among both “normal” and “active” groups. T test between those groups was performed to evaluate whether subjects in one group were statistically significantly older than the other. Statistical significance was determined when p < 0.05.
Results
A total of 29 subjects were studied: 15 asymptomatic, healthy (i.e., with no pain and no palpable MTrPs) control subjects (9 men and 6 women, age range 28 ± 8 years, M ± SD) and 14 active (i.e., with chronic neck pain and with palpable active MTrPs) subjects (4 men and 10 women, age range 36 ± 12).
ROC curves were generated for entropy levels of 3, 4, and 5. The area under the curve (AUC) for all ROC was equal to 0.68 (Figure 5a), demonstrating that all three thresholds were equally effective in discriminating “normal” from “active” sites. A qualitative visual analysis indicated that a threshold of entropy equal to 4 yielded regions that had the closest correspondence between hypoechoic regions on B-mode images, and therefore a threshold of 4 was selected to localize MTrPs. Regions with entropy of less than 4 (red regions in Figure 4a–c) were further characterized as homogeneous from the speckle (image texture) point of view and as possible sites of MTrPs or a taut band from the clinical point of view. In most of the entropy-filtered images, we observed a tiny elongated region of low entropy (entropy < 4) at the border of the fascia (Figure 4a–c). Anisotropy of muscle fiber alignment at the fascial border or the presence of a taut band may explain this phenomenon.
Figure 5.
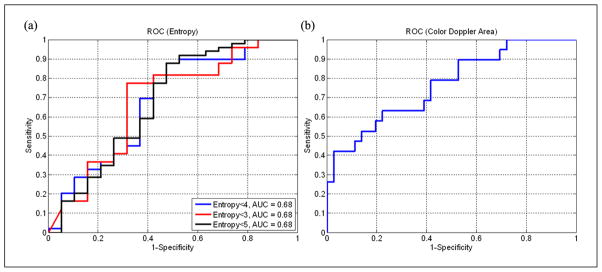
ROC curves. (a) ROC curves evaluated for entropy levels equal to 3 (red line), 4 (blue line) and 5 (black line). AUC is for all cases equal to 0.68. (b) ROC curve evaluated for MTrPs area measured using color Doppler imaging. AUC of color Doppler area is 0.77.
ROC = receiver operating characteristic; AUC = area under the curve; MTrPs = myofascial trigger points.
Note: Figure is available in full color in the online version at uix.sagepub.com
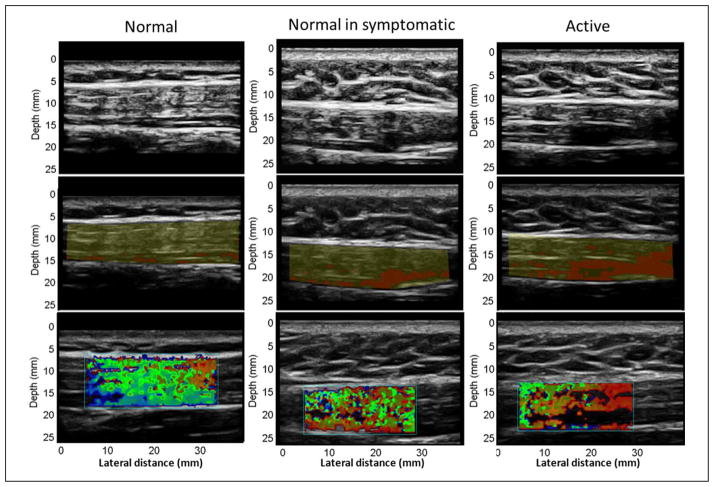
A first analysis was performed considering the whole upper trapezius (Figure 4a). In this case, “active” sites had significantly (p < 0.05) lower mean entropy compared with the “normal” sites, whereas the texture of the images of “normal in symptomatic” subjects was not significantly different from the “active” sites (Table 1, pFigure 6). Sizes of those regions with entropy less than 4 are significantly larger ( < 0.05) in “active” sites than in “normal” sites (Table 2, Figure 7). Based on the physical examination of subjects with MPS and active MTrPs, we have observed that the findings on ultrasound are often not localized to a single isolated nodule, but instead are more widespread and affect the soft tissue milieu of the affected muscle. Therefore, while a discrete nodule that reproduces the characteristic spontaneous pain on palpation may be identified in one location of the muscle, the surrounding muscle tissue often appears heterogeneous to palpation. We define “normal” in terms of no palpable nodule and no pain; however, the “normal” sites in symptomatic patients may indeed have different physical properties from muscle in healthy subjects without pain. We sought to capture this in our analysis and therefore differentiated between “normal in symptomatic” sites and normal tissue in healthy controls. A set of B-mode, entropy, and color Doppler–matched images of “normal,” “normal in symptomatic,” and “active” sites summarize our observations (Figure 8).
Table 1.
Mean Entropy Results.
| p Value “Normal” vs. “Active” | p Value “Normal” vs. “Normal in Symptomatic” | p Value “Normal in Symptomatic” vs. “Active” | |
|---|---|---|---|
| Whole ROI | 0.049* | 0.246 | 0.276 |
| Muscle belly | 0.083 | 0.038* | 0.381 |
| Fascial border | 0.016* | 0.275 | 0.098 |
ROI = region of interest. A statistical test (Mann–Whitney U test) was performed between mean entropy measured from healthy control subjects and active subjects. “Normal” sites have statistically significant (p < 0.05) lower mean entropy than “active” sites when the whole ROI or only the fascial border are analyzed. Mean entropy evaluated in the muscle belly is significantly higher in “normal” sites than in “normal in symptomatic” sites.
Figure 6.
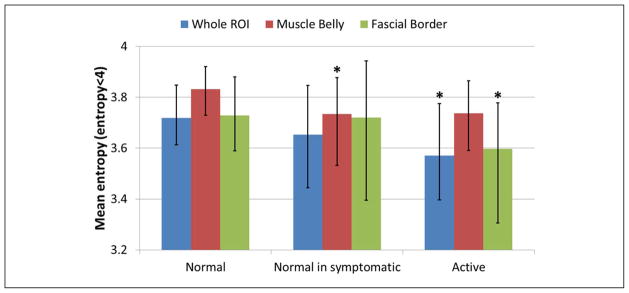
Mean entropy evaluated in whole ROI (blue bars), muscle belly (red bars), and fascial border (green bars). Bars are median, and error bars are first and third quartiles. Groups significantly different from “normal” (p < 0.05) are marked with asterisks.
ROI = region of interest.
Note: Figure is available in full color in the online version at uix.sagepub.com
Table 2.
Mean Area Results.
| p Value “Normal” vs. “Active” | p Value “Normal” vs. “Normal in Symptomatic” | p Value “Normal in Symptomatic” vs. “Active” | |
|---|---|---|---|
| Whole ROI | 0.028* | 0.097 | 0.357 |
| Muscle belly | 0.123 | 0.030* | 0.302 |
| Fascial border | 0.009* | 0.155 | 0.138 |
ROI = region of interest. A statistical test (Mann–Whitney U test) was performed between mean areas (with entropy < 4) measured from healthy control subjects and active subjects. “Normal” sites have statistically significant (p < 0.05) smaller regions with entropy < 4 than “active” sites when the whole ROI or only the fascial border are analyzed. Mean area evaluated in the muscle belly is significantly smaller in “normal” sites than in “normal in symptomatic” sites.
Figure 7.
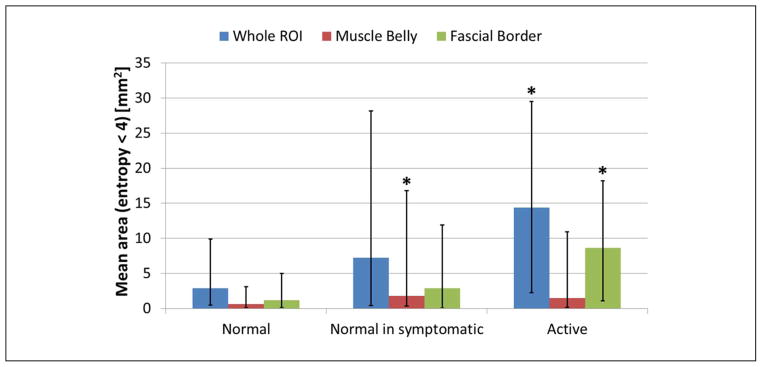
Mean area of regions with entropy < 4 evaluated in whole ROI (blue bars), muscle belly (red bars), and fascial border (green bars). Bars are median, and error bars are first and third quartiles. Groups significantly different from “normal” (p < 0.05) are marked with asterisks.
ROI = region of interest.
Note: Figure is available in full color in the online version at uix.sagepub.com
Figure 8.
B-mode, entropy mask, and sonoelasticity matched images. From top to bottom: first row are B-mode images, second row are entropy masks where in red are regions with entropy < 4, third row are sonoelasticity images. From left to right: first column are images of a “normal” site, second column are images of a “normal site in symptomatic,” third column are images of an “active” site.
Note: Figure is available in full color in the online version at uix.sagepub.com
In a second set of analyses, the ROI was divided into muscle belly (60% of the ROI) and fascial border (40% of the ROI). Results obtained on the muscle belly showed that a statistical difference between healthy control subjects and active subjects can only be found between “normal” and “normal in symptomatic” sites whereas no statistical difference was found between the echotexture of the muscle belly between “normal” and “active” sites (Table 1). Entropy level in fascial border is significantly lower (p < 0.05) in “active” compared with “normal” sites (Table 1), and regions with entropy lower than 4 are significantly larger (p < 0.05) in “active” than in “normal” sites (Table 2).
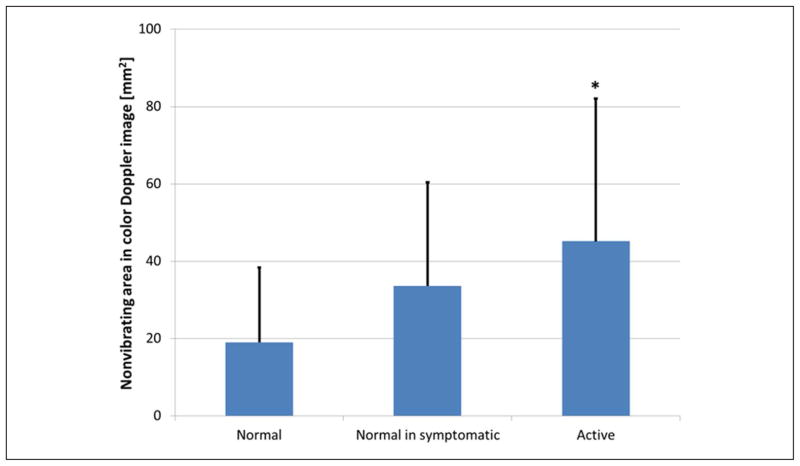
Color Doppler variance images were analyzed, as previously described,9 and showed that nonvibrating regions are significantly larger (p < 0.05) in “active” compared with “normal” sites (Figure 9). This finding is consistent with our previously published results. However, when sizes of nonvibrating regions are compared with low entropy (entropy < 4) regions, we found that the latter are significantly (p < 0.05) smaller than the former.
Figure 9.
Nonvibrating region size measurements from color Doppler images. Blue bars are means, error bars are standard deviations, asterisks are groups statistically different (p < 0.05) than “normal.”
Note: Figure is available in full color in the online version at uix.sagepub.com
The size of nonvibrating regions in color Doppler images was also found to be a good discriminator for tissue at “normal” and “active” sites (AUC of the ROC curve shown in Figure 5b is 0.77). Since both mean entropy and color Doppler areas were found to be able to successfully discriminate between tissue in healthy control subjects and active subjects (Figure 5), a linear discriminant analysis was performed between those quantities and between mean entropy and area of the regions with entropy lower than 4. In Figure 10, both sets of parameters separate “normal” sites from those that are “active.” However, a test accounting for mean entropy and color Doppler area (Figure 10a) offers higher sensitivity and specificity than the other that uses mean entropy and mean area of regions with entropy < 4.
Figure 10.
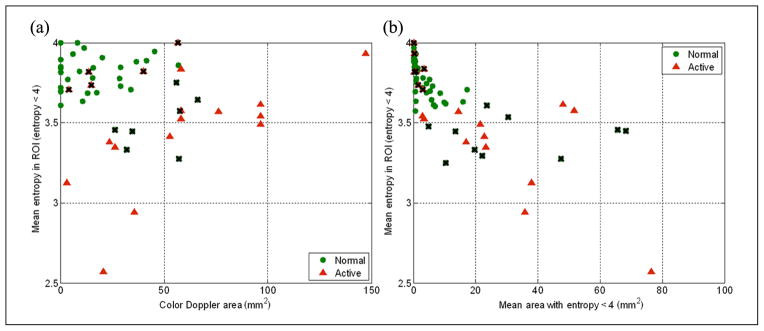
Linear discriminant analysis. (a) Mean entropy versus color Doppler area has sensitivity equal to 0.74 and specificity equal to 0.81. (b) Mean entropy versus mean area with entropy < 4 has sensitivity equal to 0.63 and specificity equal to 0.80. Green dots are related to “normal” sites, red triangles to “active” sites, and black crosses highlight false positives and false negatives.
Note: Figure is available in full color in the online version at uix.sagepub.com
A study of the size distributions of regions with entropy less than 4 within a ROI showed that, especially at “normal” sites, most of the regions with low entropy were small (less than 8 mm2). This finding can also be observed in Figure 10b. This indicates that the echotexture of the tissue at “normal” sites is locally heterogeneous compared with tissue at “active” sites. Results reported in Figure 10b, Figure 6, Figure 7, Table 1, and Table 2 show that large regions of homogeneous echotexture are likely found at “active” sites and that they are mainly located close to the fascial border.
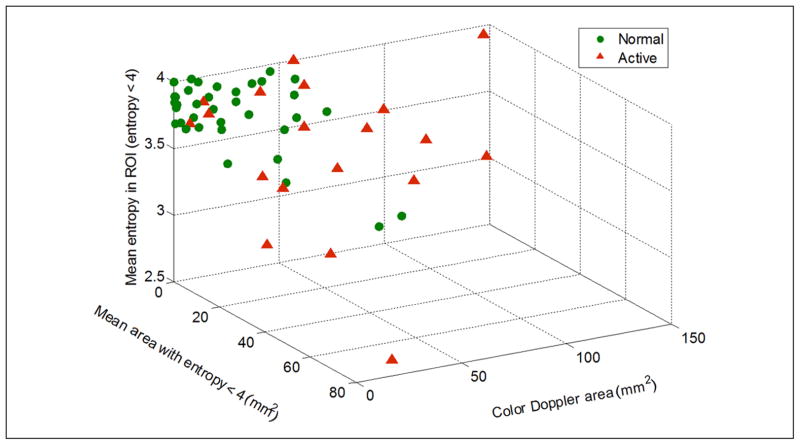
The test for discriminating between “normal” and “active” sites might be improved by combining all three quantities: mean entropy, mean area of regions with entropy < 4, and color Doppler area. A 3D scatter plot in Figure 11 shows how those parameters can successfully separate “normal” from “active” sites. Discriminant analysis, performed using the data from the whole ROI, shows that sensitivity and specificity of this test are equal to 69% and 81%, respectively. “Active” sites are characterized by larger regions with lower entropy that do not vibrate (or vibrate much less than their surrounding tissue) under external vibration.
Figure 11.
3D scatter plot of mean entropy, color Doppler area, and mean area with entropy < 4, evaluated using the whole ROI, shows clear separation between “normal” and “active” sites. “Active” sites are characterized by larger regions with low entropy (entropy < 4) and large color deficit in color Doppler imaging. Sensitivity is equal to 69%, and specificity is equal to 81%.
ROI = region of interest.
Note: Figure is available in full color in the online version at uix.sagepub.com
Active subjects were significantly older (p < 0.05) than control subjects, and this difference is important in biological tissue characterization. We investigated whether age was a confounding factor in the entropy analysis and found that low entropy values are almost equally distributed between older and younger subjects as shown in Figure 12. Therefore, the statistical difference observed in this preliminary study between “normal” and “active” sites is not explained by age.
Figure 12.
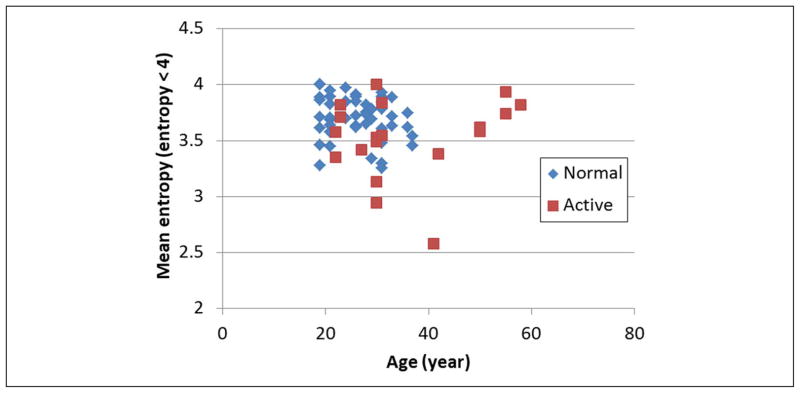
Mean entropy versus subject age. Absence of separation between “normal” and “active” shows that statistical difference observed in this preliminary study between those groups is not explained by age. This test was performed using the whole ROI.
ROI = region of interest.
Note: Figure is available in full color in the online version at uix.sagepub.com
To evaluate the repeatability of the entropy and color Doppler analysis, we acquired data from a single active subject by removing and repositioning the transducer six times. The mean entropy value measured from the whole ROI was equal to 3.43 with standard deviation equal to 0.075 (which is 2.18% of the mean value). Mean area of the region with entropy < 4 was equal to 48.38 mm2 with standard deviation equal to 3.18 mm2 (which is 6.57% of the mean value). Size of nonvibrating regions in color Doppler image was equal to 41.3 mm2 with standard deviation equal to 3.09 mm2 (which is 7.49% of the mean value). Therefore, we believe that the entropy and color Doppler area measurements are repeatable.
Discussion
Entropy analysis was performed on B-mode images of the upper trapezius of active subjects and healthy control subjects. The entropy is a statistical measure of the speckle pattern distribution and can be used to characterize the echotexture of the image. Echotexture of tissue images of “normal” sites is significantly more heterogeneous than “active” sites, revealing that this measure can be used as a tool to objectively discriminate between muscle tissue of active subjects and healthy control subjects. Echotexture of a tissue image reflects acoustic properties of the tissue itself, that is, acoustic impedance, scatterer distribution, size, and shape.16 A tissue region appears heterogeneous (high entropy) if scatterers are uniformly distributed within the ROI. Homogeneous echotexture (low entropy), instead, could indicate a sparse scatterer distribution. A localized region of sparse scatterer distribution and hypoechogenicity can be caused by fluid accumulation (edema) or an increase in blood volume, whereas hyperechogenicity of the tissue can be caused by fatty infiltration.12,19,20 In the absence of any infiltrations, hypoechogenicity and low entropy can be explained by a local change of scatterer concentration or shape. Scatterer concentration and shape changes can occur when fibers contract16; thus, the low entropy could be indicative of local contractures in the muscle. Scatterer shape can also change when fiber alignment and spatial direction change.16
The presence of localized regions of low entropy, that is, localized texture homogeneity, in symptomatic muscle makes the tissue macroscopically more heterogeneous than “normal” muscle that has a relatively uniform echotexture (high entropy). This finding agrees with our group’s previous observations7–9: subjects with active MTrPs showed spherical or band-like hypoechoic (darker) regions with an increase in fiber alignment heterogeneity.
It is important to note that age can have an effect on the appearance of the muscle. One limitation of our study sample is that the healthy control subjects were not age matched with the active subjects. However, in our preliminary analysis, age did not appear to significantly correlate with entropy.
In this study, the control group (“normal”) comprised subjects with no spontaneous pain. However, the muscle tissue in these subjects could be heterogeneous, possibly including latent MTrPs that may be too small to palpate. These would be manifested in our imaging measures contributing to the variance observed in this group. Results from this study reveal that regions within the muscle with low entropy are significantly larger in active subjects than healthy control subjects. Nonvibrating regions identified through vibration elastography are also significantly larger in active subjects than healthy control subjects. These findings indicate there might be a relationship between acoustical and mechanical properties of the tissue. Large regions with low entropy and large stiffer regions may possibly be two manifestations of the same phenomena (i.e., muscle contracture). This study makes clear that real-time visual identification of a large hypoechoic region in a muscle belly can be misleading in the diagnosis of an active MTrP. However, the presence of elongated hypoechoic regions close to the fascial border (in B-mode images) in conjunction with vibration elastography offers real-time data useful to qualitatively discriminate local tissue properties.
The proposed entropy analysis enables echotexture characterization. Our results show that this method can highlight regions of the muscle that correspond to palpable MTrPs. However, the entropy analysis is not sufficiently sensitive to uncover subtle differences in texture between MTrPs and the surrounding soft tissue. A more sophisticated texture analysis technique needs to be developed in the future to make echotexture-based muscle tissue characterization more sensitive. However, our preliminary findings demonstrate that the soft tissue abnormalities in subjects with chronic neck pain and active MTrPs are not restricted to isolated discrete nodules, but extend into the surrounding muscle milieu.
It is important to note that a B-mode image only shows a 2D slice of the milieu of the muscle. The macroscopic appearance of muscle is better appreciated in three dimensions. Figure 13 shows a volume rendering of a stack of entropy-filtered images acquired using a mechanically-scanned linear array probe (4D L14-5; Ultrasonix Medical Corporation, Vancouver, BC). The complex 3D appearance of the regions of low entropy is apparent. In future studies, we will quantify the echotexture in 3D to better characterize the muscle milieu.
Figure 13.
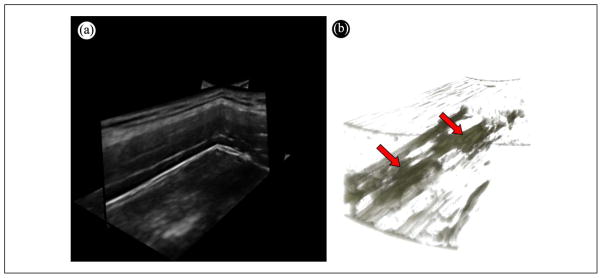
3D rendering of a MTrP. (a) Multiplanar view of a 3D volume of the upper trapezius acquired using a mechanically-scanned linear probe. (b) 3D volume rendering of the entropy-filtered stack of images in (a) showing the 3D structure of the regions with low entropy.
MTrPs = myofascial trigger points.
Note: Figure is available in full color in the online version at uix.sagepub.com
Conclusion
The data presented demonstrate that echotexture analysis using local entropy within the whole ROI or fascial border can distinguish between subjects with painful trigger points and those without pain, that is, between “active” and “normal” groups. No significant differences were found from the echotexture point of view between palpably normal tissue in both “normal in symptomatic” and “normal” sites except when the analysis was performed at the muscle belly. However, significant differences were found among all three categories of sites with vibration elastography. A combination of both entropy analysis performed using the whole ROI and entropy threshold equal to 4, and vibration elastography imaging yielded 69% sensitivity and 81% specificity. Characterizing the echotexture and mechanical properties of muscle tissue enhanced contrast between MTrPs and surrounding tissue, enabling improved delineation of suspected MTrPs in the milieu of the muscle. In the future, measurements of attenuation and backscattering properties of the tissue should be addressed to fully characterize MTrPs.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported in part by Grant 1R01-AR057348 from the National Institutes of Arthritis and Musculoskeletal and Skin Diseases at the National Institutes of Health.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Martin BI, Deyo RA, Mirza SK, Turner JA, Comstock BA, Hollingworth W, et al. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299(6):656–64. doi: 10.1001/jama.299.6.656. [DOI] [PubMed] [Google Scholar]
- 2.Skootsky SA, Jaeger B, Oye RK. Prevalence of myofascial pain in general internal medicine practice. West J Med. 1989;51(2):157–60. [PMC free article] [PubMed] [Google Scholar]
- 3.Gerwin RD. Classification, epidemiology, and natural history of myofascial pain syndrome. Curr Pain Headache Rep. 2001;5(5):412–20. doi: 10.1007/s11916-001-0052-8. [DOI] [PubMed] [Google Scholar]
- 4.Fishbain DA, Goldberg M, Meagher BR, Steele R, Rosomoff H. Male and female chronic pain patients categorized by DSM-III psychiatric diagnostic criteria. Pain. 1986;26:181–97. doi: 10.1016/0304-3959(86)90074-6. [DOI] [PubMed] [Google Scholar]
- 5.Simons DG, Travell JG, Simons PT. Travell and Simons’ Myofascial pain and dysfunction: the trigger point manual. Vol. I. Upper half of body. 2. Baltimore, MD: Williams and Wilkins; 1999. [Google Scholar]
- 6.Shah JP, Danoff JV, Desai MJ, Parikh S, Nakamura LY, Phillips TM, et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008;89:16–23. doi: 10.1016/j.apmr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Sikdar S, Shah JP, Gebreab T, Yen RH, Gilliams E, Danoff J, et al. Novel applications of ultrasound technology to visualize and characterize myofascial trigger points and surrounding soft tissue. Arch Phys Med Rehabil. 2009;90:1829–38. doi: 10.1016/j.apmr.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballyns JJ, Shah JP, Hammond J, Gebreab T, Gerber LH, Sikdar S. Objective sonographic measures for characterizing myofascial trigger points associated with cervical pain. J Ultrasound Med. 2011;30:1331–40. doi: 10.7863/jum.2011.30.10.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballyns JJ, Turo D, Otto P, Shah J, Hammond J, Gebreab T, et al. Office-based elastography technique for quantifying mechanical properties of skeletal muscle. J Ultrasound Med. 2012;31:1209–19. doi: 10.7863/jum.2012.31.8.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mense S, Simons DG. Muscle pain: understanding its nature, diagnosis and treatment. Philadelphia, PA: Lippincott Williams and Wilkins; 2001. [Google Scholar]
- 11.Simons DG, Mense S. Understanding and measurement of muscle tone as related to clinical muscle pain. Pain. 1998;75:1–17. doi: 10.1016/S0304-3959(97)00102-4. [DOI] [PubMed] [Google Scholar]
- 12.Rasband WS. ImageJ. U.S. National Institutes of Health; Bethesda, Maryland, USA: 1997–2012. Available from: URL: http://imagej.nih.gov/ij/ [Google Scholar]
- 13.Gonzalez RC, Woods RE, Eddins SL. Digital image processing using MATLAB. Vol. 11. Upper Saddle River, NJ: Prentice Hall; 2003. [Google Scholar]
- 14.Skorton DJ, Collins SM, Nicholas J, Pandian NB, Bean JA, Kerber RE. Quantitative texture analysis in two-dimensional echocardiography: application to the diagnosis of experimental myocardial contusion. Circulation. 1983;68:217–23. doi: 10.1161/01.cir.68.1.217. [DOI] [PubMed] [Google Scholar]
- 15.Skorton DJ, Collins SM, Woskoff SD, Bean JA, Melton HE. Range- and azimuth- dependent variability of image texture in two dimensional echocardiogram. Circulation. 1983;68:834–40. doi: 10.1161/01.cir.68.4.834. [DOI] [PubMed] [Google Scholar]
- 16.Shung KK, Thieme GA. Ultrasonic scattering in biological tissue. ch 10. Boca Raton, FL: CRC Press Inc; 1993. [Google Scholar]
- 17.Lerski RA, Straughan K, Schad LR, Boyce D, Blüml S, Zuna I., VIII MR image texture analysis—an approach to tissue characterization. Magn Reson Imaging. 1993;11:873–87. doi: 10.1016/0730-725x(93)90205-r. [DOI] [PubMed] [Google Scholar]
- 18.Raeth U, Schlaps D, Limberg B, Zuna I, Lorenz A, van Kaick G, et al. Diagnostic accuracy of computerized B-scan texture analysis and conventional ultrasonography in diffuse parenchymal and malignant liver disease. J Clin Ultras. 1985;13:87–99. doi: 10.1002/jcu.1870130203. [DOI] [PubMed] [Google Scholar]
- 19.Khoury V, Cardinal E, Brassard P. Atrophy and fatty infiltration of the supraspinatus muscle: sonography versus MRI. AJR Am J Roentgenol. 2008;190:1105–11. doi: 10.2214/AJR.07.2835. [DOI] [PubMed] [Google Scholar]
- 20.Shung KK, Thieme GA. Ultrasonic scattering in biological tissue. ch 11. Boca Raton, FL: CRC Press Inc; 1993. [Google Scholar]