Abstract
Neurexin1 (Nrxn1) and Neuroligin3 (Nlgn3) are cell adhesion proteins, which play an important role in synaptic plasticity that declines with advancing age. However, the expression of these proteins during aging has not been analyzed. In the present study, we have examined the age-related changes in the expression of these proteins in cerebral cortex and hippocampus of 10-, 30-, 50-, and 80-week-old male mice. Reverse transcriptase polymerase chain reaction (RT-PCR) analysis indicated that messenger RNA (mRNA) level of Nrxn1 and Nlgn3 significantly increased from 10 to 30 weeks and then decreased at 50 weeks in both the regions. However, in 80-week-old mice, Nrxn1 and Nlgn3 were further downregulated in cerebral cortex while Nrxn1 was downregulated and Nlgn3 was upregulated in hippocampus. These findings were corroborated by immunoblotting and immunofluorescence results. When the expression of Nrxn1 and Nlgn3 was correlated with presynaptic density marker synaptophysin, it was found that synaptophysin protein expression in cerebral cortex was high at 10 weeks and decreased gradually up to 80 weeks, whereas in hippocampus, it decreased until 50 weeks and then increased remarkably at 80 weeks. Furthermore, Pearson’s correlation analysis showed that synaptophysin had a strong relation with Nrxn1 and Nlgn3 in cerebral cortex and with Nlgn3 in hippocampus. Thus, these findings showed that Nrxn1 and Nlgn3 are differentially expressed in cerebral cortex and hippocampus which might be responsible for alterations in synaptic plasticity during aging.
Keywords: Neurexin, Neuroligin, Aging, Synaptophysin, Presynaptic density marker, Synaptic plasticity
Introduction
A common characteristic of advancing age is a gradual decline in cognitive functions associated with the progressive reduction of structural and functional plasticity in brain regions such as cerebral cortex and hippocampus that play a key role in cognition (Benice et al. 2006; Driscoll et al. 2006; Hara et al. 2012; Van der Jeugd et al. 2013). Studies in rats and non-human primates have demonstrated that aging impairs the functional integrity of prefrontal cortex neurons (Morrison and Baxter 2012) which is associated with decline in structural plasticity and cognitive performance (Dumitriu et al. 2010; Bloss et al. 2011, 2013). Moreover, studies in human and animal models suggest that age-associated deficits of brain functions are probably more related to alterations in synaptic connectivity and plasticity than with neuronal loss (Gleichmann et al. 2012; Sibille 2013; Labarrière et al. 2014). Thus, it is likely that a change of the integrity of the synapse mediating learning and memory contributes to the decline in cognitive function. The prefrontal cortex and hippocampus are mostly associated with age-related cognitive decline in human and primates (Paulesu et al. 1993; Levy and Goldman-Rakic 1999). Moreover, the hippocampus is the brain structure known to change first during normal aging (Geinisman et al. 1995; Winocur and Gagnon 1998), which has been observed behaviorally as a change in hippocampus-dependent cognitive performance in rodents and humans (Rapp et al. 1997; Maguire and Cipolotti 1998; Rosenbaum et al. 2001).
During memory formation, structural and functional modifications of both presynaptic and postsynaptic components have been widely reported. These changes can occur both at previously existing synapses and at synapses that are newly formed in response to learning-induced stimuli. The functional and structural alterations in both presynaptic and postsynaptic elements are dynamically coupled during the induction and maintenance of synaptic plasticity. The synaptic proteins neurexins (Nrxns) and neuroligins (Nlgns) have emerged as a pair of fascinating candidates for underlying synaptic plasticity. Presynaptic Nrxns form trans-synaptic complexes with postsynaptic Nlgns (Ushkaryov et al. 1992; Yamagata et al. 2003) and play an important role in differentiation, maturation, and stabilization of both excitatory and inhibitory synapses (Dean et al. 2003; Graf et al. 2004; Chubykin et al. 2007; Budreck and Scheiffele 2007; Krueger et al. 2012). These proteins not only facilitate the assembly of functional units on their own side of the synapses but also regulate synaptic specialization on the opposite side of a nascent synapse through their trans-synaptic interactions (Dean and Dresbach 2006). By simultaneous binding to postsynaptic density 95 (PSD95), Nlgns link the postsynaptic density and control ionotropic neurotransmitter receptors to the exocytotic machinery of the presynaptic terminals (El-Husseini et al. 2000; Wittenmayer et al. 2009). Remarkably, mutations in Nlgn3/4 are involved in autism spectrum disorders (ASDs) which have been shown to destabilize the Nrxns-Nlgns complexes (Arac et al. 2007; Chen et al. 2008). In addition, a retention of Nlgns in the endoplasmic reticulum as well as a decrease in its affinity for Nrxns have been associated with some of those mutations (Comoletti et al. 2004; Zhang et al. 2009), suggesting that impaired trafficking and protein binding properties of Nrxns-Nlgns might be related to the disease.
Synaptic plasticity is an essential cellular mechanism underlying learning and memory (Martin et al. 2000). Various proteins like synaptophysin, N-methyl-D-aspartate receptor subunit 1, PSD95, and calcium/calmodulin-dependent protein kinase II alpha, which are involved in neurotransmission at the synapses, are associated with synaptic plasticity (Yang et al. 2014). Synaptophysin is a synaptic vesicle glycoprotein essential for synapse formation in the culture hippocampal neurons (Tarsa and Goda 2002) and is also associated well with differences in cognitive performance in mice (Mulder et al. 2004). The loss of pre-synaptic vesicle protein in hippocampus correlates with the cognitive decline in Alzheimer’s disease (Reddy et al. 2005).
Although age-related changes in the expression of Nrxn1 and NLgn3 have not been studied, there are some reports on the expression of these proteins during development, mentioning that these proteins increase with maturation of nervous system in rodents (Püschel and Betz 1995; Song et al. 1999). Therefore, we have analyzed the age-related changes in the expression of Nrxn1 and Nlgn3 messenger RNA (mRNA) and protein in cerebral cortex and hippocampus during normal aging. In the present study, reverse transcriptase polymerase chain reaction (RT-PCR), immunoblotting, and immunofluorescence were performed to analyze the age-related changes in expression of Nrxn1, Nlgn3, and synaptophysin (immunoblotting) in the cerebral cortex and hippocampus of young, adult, middle age, and old male mice. The different age groups were assigned based on the average life span of mice. In addition, Nrxn1 and nlgn3 expression was correlated with presynaptic density marker synaptophysin by Pearson’s r correlation.
Materials and methods
Animals
The inbred Swiss albino mice were maintained under a 12-h light-dark cycle (light period 7:00 a.m. to 7:00 p.m.) at 23–24 °C in the animal house of Department of Zoology, Banaras Hindu University, Varanasi, India. The mice were handled and used according to the guidelines of the Institutional Animal Ethical Committee, Banaras Hindu University, Varanasi, India. They were provided with food and water ad libitum. A total of 28 mice were used. These mice were divided into four groups (n = 7): young (10 weeks), adult (30 weeks), middle age (50 weeks), and old (80 weeks). The four groups were assigned based on the average lifespan (100 weeks) of the mice. The mice that completed 50 % of the average lifespan were taken as middle age. The cerebral cortex and hippocampus were dissected out on ice after killing the mice, and tissues were stored at −80 °C for subsequent mRNA and protein analyses (n = 4 mice per group). For histological studies, the whole brain of young, adult, middle age, and old mice were processed for sectioning (n = 3 mice per group).
Semiquantitative reverse transcriptase polymerase chain reaction
Total RNA was isolated from the cerebral cortex and hippocampus of 10-, 30-, 50-, and 80-week-old male mice using the TRI Reagent kit (Sigma-Aldrich, USA) according to the manufacturer’s instruction. It was estimated by taking absorbance at 260 nm, and RNA sample with A260/280 of 1.8 was used. RNA from all ages was resolved on 1.2 % agarose formaldehyde gel to check its integrity by ethidium bromide staining of 28S and 18S rRNA. For complementary DNA (cDNA) synthesis, 3 μg of total RNA and 200 ng random hexamer (Fermentas International Inc, Canada) were mixed in 11-μl reaction volume and denatured at 70 °C for 5 min. Further, 2 μl of 10× reaction buffer, 2 μl of 10 mM dNTP mix, and 20 U of RNase inhibitor (New England Biolabs, USA) were added; the volume was made up to 19 μl and incubated at 25 °C for 5 min. Thereafter, 200 U of M-MuLv reverse transcriptase (New England Biolabs, USA) was added, and the tube was incubated first at 25 °C for 10 min and then at 42 °C for 1 h in a thermal cycler (Applied Biosystem, USA). The reaction was terminated by heating at 70 °C for 10 min and the resulting cDNA was stored at −80 °C.
For expression analysis, the cDNA was amplified using the following specific primers: Nrxn1, sense TTACTCTGGCTGCTGCAATG and anti-sense GTCTGAAATCCAGGCCACAT (Kolozsi et al. 2009); Nlgn3, sense CGTGTATAGCTCTATCCCGGAG and anti-sense ATTGCTAGCCTCTTCC-TCCTCT (Kolozsi et al. 2009); and internal control glyceraldehyde 3-phosphate dehydrogenase (GAPDH), sense GTCTCCTGCGACTTCAGC and anti-sense TCATTGTCATACCAGGAAATGA-GC (Xu et al. 2008). The annealing temperature was optimized using gradient RT-PCR amplification from 48 to 64 °C, and the number of cycles was standardized at optimum temperature from 20 to 36 cycles. For further experiments, the following optimized conditions were used for PCR amplification of Nrxn1 (94 °C for 30 s, 54 °C for 30 s, 72 °C for 30 s for 28 cycles), Nlgn3 (94 °C for 30 s, 62 °C for 45 s, 72 °C for 30 s for 28 cycles), and GAPDH (94 °C for 30 s, 52 °C for 30 s, 72 °C for 30 s for 26 cycles). The RT-PCR amplicons were run on 2 % agarose gel.
Immunoblotting
The cerebral cortex and hippocampus of 10-, 30-, 50-, and 80-week mice were used to prepare 10 % homogenate in RIPA buffer. The homogenate was centrifuged at 10,000 g at 4 °C for 10 min and supernatant was stored at −80 °C until use. The amount of protein in homogenate was estimated (Bradford 1976) and 30 μg protein was denatured, resolved by 10 % Tris-glycine SDS-PAGE, and transferred onto polyvinylidene fluoride (PVDF) membrane (Millipore, USA). The membrane was blocked in 5 % (w/v) non-fat milk prepared in 1× phosphate-buffered saline (PBS) for 3 h at room temperature and incubated overnight with primary antibodies (goat anti-Nrxn1, 1:500; goat anti-Nlgn3, 1:500; mouse anti-synaptophysin, 1:5000; and mouse anti-β-actin horseradish peroxidase conjugated, 1:20,000) at 4 °C. Goat polyclonal Nrxn1 (SC-14093) and Nlgn3 (SC-14334) antibodies were purchased from Santa Cruz Biotechnology (USA), mouse anti-synaptophysin (611880) from BD Biosciences, and mouse anti-β-actin antibody (A3854) from Sigma Aldrich (USA). After washing three times (5 min each) in 0.1 % PBST (0.1 % Tween 20 in 1× PBS), the membrane was incubated with HRP-conjugated secondary antibodies obtained from Bangalore Genei (India) (rabbit anti-goat for both Nrxn1 and Nlgn3, 1:2000, and rabbit anti-mouse for synaptophysin, 1:2000, for 2 h). Finally, the membrane was washed three times (5 min each) in 0.1 % PBST and detected by enhanced chemiluminescence (ECL) (Western bright, Advansta, USA) method.
Immunofluorescence
The brain of 10-, 30-, 50-, and 80-week male mice was processed to prepare 7-μm-thick transverse sections as described earlier (Kumar and Thakur 2014). The sections were fixed in acetone at −20 °C for 15 min and rinsed three times in PBS for 5 min each and incubated with 10 % goat serum in 0.1 % PBST at room temperature for 2 h to block the non-specific sites. Then, anti-Nrxn1 antibody (1:50) and anti-Nlgn3 (1:50) primary antibody were added and incubated overnight at 4 °C. Primary antibody was not added in negative control slides. Further, the sections were incubated at room temperature for 2 h in fluorescein isothiocyanate (FITC) conjugated anti-goat IgG (1:500) for both Nrxn1 and Nlgn3. Finally, it was rinsed three times in 1× PBS, mounted in vectashield mountant containing 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Vector Laboratories Inc., USA) and detected under a fluorescence microscope using FITC filter.
Statistical analysis
Molecular data were analyzed by performing one-way ANOVA followed by Tukey test on SPSS statistics for windows (version 16) for all parameters. The RT-PCR signal intensity was normalized against GAPDH and immunoblotting signal intensity against β-actin. The signal of immunofluorescence was analyzed by spot densitometry tool of Alpha EaseFC software (Alpha Innotech Corp., USA). Integrated density value (IDV)/unit area of cerebral cortex and dentate gyrus for different ages were calculated for Nrxn1 and Nlgn3 after deducting the value of negative control. Pearson’s r statistics method was used for correlation analysis. Values were reported as mean ± SEM (n = 4 mice per group for RT-PCR and immunoblotting, n = 3 mice per group for immunofluorescence), and p values <0.05 were considered as statistically significant.
Results
Effect of age on Nrxn1 expression in mouse cortex and hippocampus
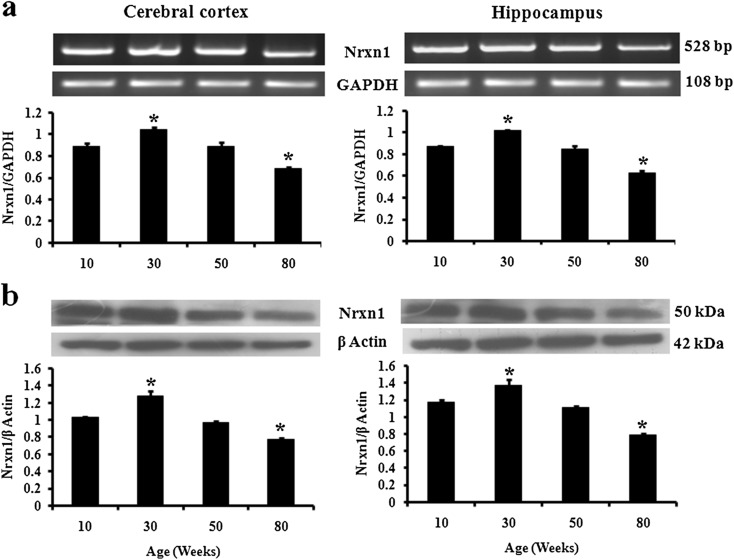
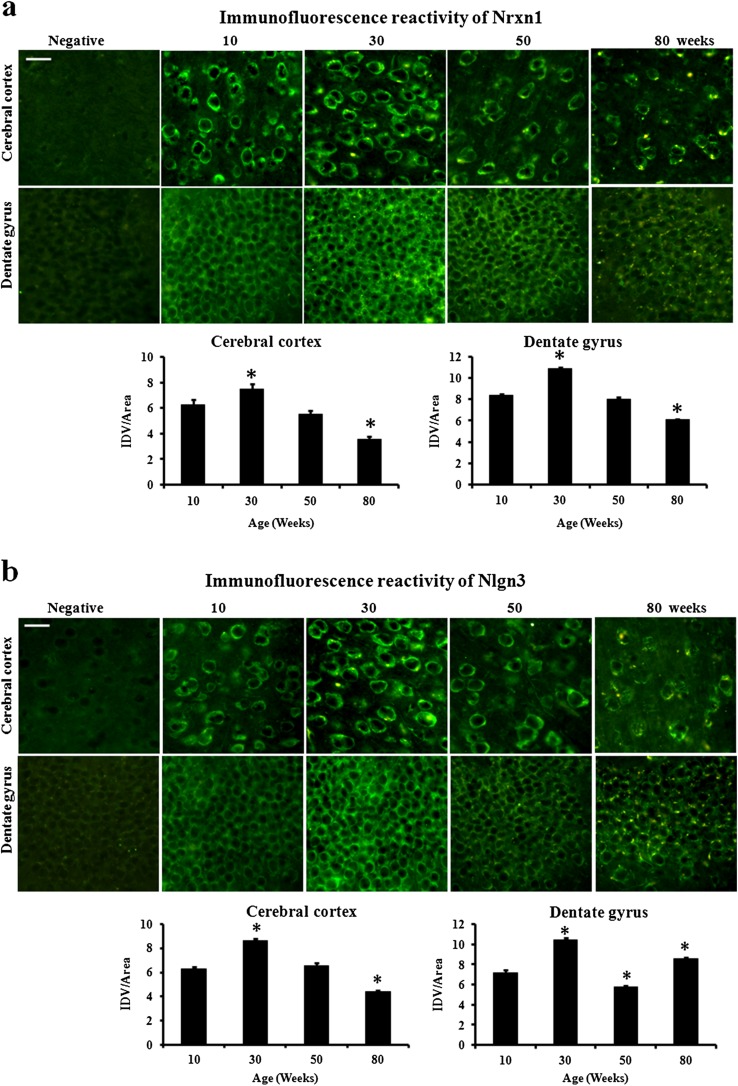
For exploring the age-related changes in the expression of Nrxn1, we performed RT-PCR, immunoblotting, and immunofluorescence in cerebral cortex and hippocampus of 10-, 30-, 50-, and 80-week male mice. As compared to 10-week mice, the level of mRNA significantly increased in both cerebral cortex (17 %) and hippocampus (16 %) of 30-week mice (Fig. 1a). Similarly, protein level also increased by 19 % in cerebral cortex and 15 % in hippocampus of 30-week mice (Fig. 1b). Thereafter, the level decreased in 50-week mice in both the regions. Further, mRNA level decreased significantly in 80-week cerebral cortex (23 %) and hippocampus (28 %) as compared to that of 10-week mice. Similarly, the protein level was significantly lower in 80-week cerebral cortex (24 %) and hippocampus (25 %). Thereafter, immunofluorescence result was analyzed in cerebral cortex and dentate gyrus of 10-, 30-, 50-, and 80-week-old mouse brain sections (Fig. 3a). As compared to 10-week mice, the immunofluorescence reactivity significantly increased in 30-week mice in both cerebral cortex (19 %) and dentate gyrus (30 %). Further, the immunofluorescence reactivity decreased significantly in 80-week cerebral cortex (44 %) and dentate gyrus (27 %). Thus, immunofluorescence results further confirmed the immunoblotting analysis. In the immunofluorescence signals of old mice, we observed yellow lipofuscin spots in FITC filter in brain sections of both cerebral cortex and dentate gyrus which are a marker of aging (Sohal and Brunk 1989).
Fig. 1.
Nrxn1 mRNA and protein are differentially affected by aging in mouse cerebral cortex and hippocampus. Expression analysis of Nrxn1 a mRNA and b protein in cerebral cortex and hippocampus of 10-, 30-, 50-, and 80-week male mice. GAPDH and β-actin served as endogenous control for RT-PCR and immunoblotting, respectively. The data are expressed as mean ± SEM (n = 4). The statistical significance is indicated by asterisk (p < 0.05) when compared with 10-week mice
Effect of age on Nlgn3 expression in mouse cortex and hippocampus
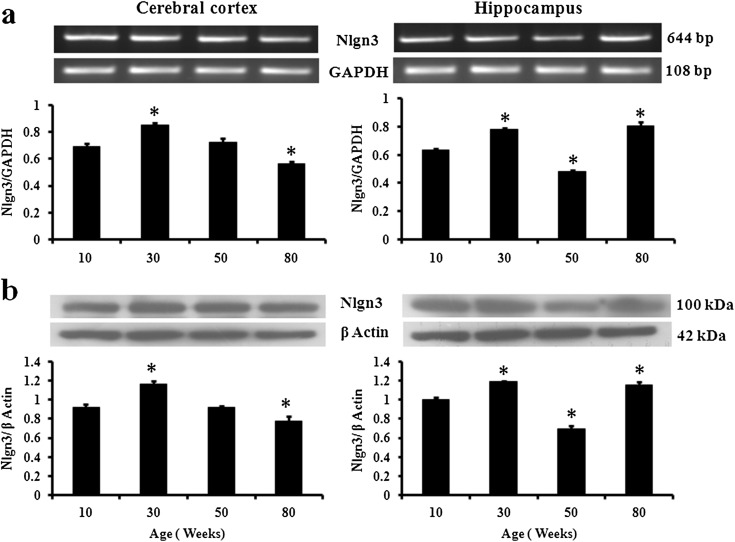
The level of Nlgn3 mRNA (Fig. 2a) significantly increased in 30-week mice as compared to that in 10-week mice in both cerebral cortex (22 %) and hippocampus (22 %) and thereafter decreased significantly in 80-week cerebral cortex (23 %). However, in hippocampus, the mRNA level decreased significantly in 50-week mice (25 %) but increased in 80-week mice (26 %). The immunoblotting results showed similar pattern of expression (Fig. 2b). Protein level significantly increased in 10- to 30-week mice in both cerebral cortex and hippocampus. Thereafter, the protein level decreased significantly in hippocampus (31 %) of 50-week mice. In 80-week mice, the protein level decreased in cerebral cortex (15 %) but increased in hippocampus (15 %). The immunofluorescence reactivity of Nlgn3 in both cerebral cortex and dentate gyrus was similar to expression pattern observed in RT-PCR and immunoblotting (Fig. 3b).
Fig. 2.
Nlgn3 mRNA and protein are differentially affected by aging in mouse cerebral cortex and hippocampus. Expression analysis of Nlgn3 a mRNA and b protein in cerebral cortex and hippocampus of 10-, 30-, 50-, and 80-week male mice. GAPDH and β-actin served as endogenous control for RT-PCR and immunoblotting, respectively. The data are expressed as mean ± SEM (n = 4). The statistical significance is indicated as by asterisk (p < 0.05) when compared with 10-week mice
Mouse cortical and hippocampal Nrxn1 and Nlgn3 expression is correlated with presynaptic density marker synaptophysin during aging
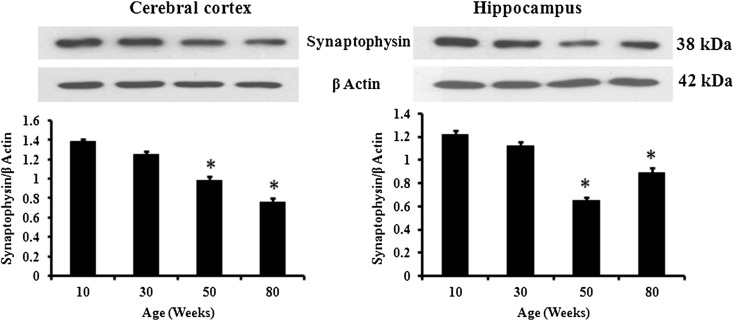
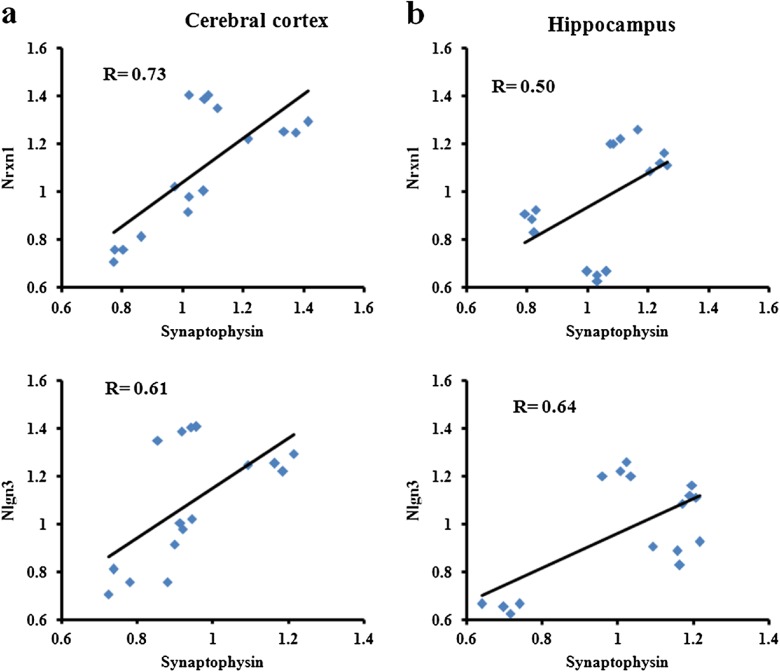
Presynaptic density marker protein synaptophysin expression was analyzed by immunoblotting in both cerebral cortex and hippocampus of 10-, 30-, 50-, and 80-week male mice (Fig. 4). The protein level was found highest in both cerebral cortex and hippocampus of 10-week mice and decreased gradually in cerebral cortex of 30-week (10 %), 50-week (30 %), and 80-week (46 %) mice. Similarly, the expression decreased in hippocampus of 30- and 50-week (47 %) mice but increased in 80-week mice. Further, Pearson’s r correlation statistics was performed to analyze the correlation of Nrxn1 and Nlgn3 with synaptophysin (Fig. 5a, b) and showed positive correlation in cerebral cortex (R = 0.73 for Nrxn1 and 0.61 for Nlgn3) and hippocampus (R = 0.50 for Nrxn1 and 0.64 for Nlgn3) of 10-, 30-, 50-, and 80-week male mice.
Fig. 4.
Presynaptic density marker protein synaptophysin is differentially affected by aging in mouse cerebral cortex and hippocampus. Immunoblotting analysis of synaptophysin in cerebral cortex and hippocampus of 10-, 30-, 50-, and 80-week male mice. The data are expressed as mean ± SEM (n = 4). The statistical significance is indicated by asterisk (p < 0.05) when compared with 10-week mice
Fig. 5.
Nrxn1 and Nlgn3 are positively correlated with synaptophysin in cerebral cortex and hippocampus of male mice. Pearson’s r correlation plot between relative density values (RDVs) of synaptophysin protein with Nrxn1 and Nlgn3 in a cerebral cortex and b hippocampus of 10-, 30-, 50-, and 80-week male mice. Nrxn1 and Nlgn3 proteins show positive correlation with synaptophysin during aging. Points in the form of bricks indicate the age groups of the mice
Discussion
The present study was conducted to investigate the age-related changes in the expression of synaptic proteins Nrxn1 and Nlgn3 in young, adult, middle age, and old male mice. Our results showed increased Nrxn1 and Nlgn3 level in cerebral cortex and hippocampus of adult mice as compared to young. Neurexins function as heterophilic cell adhesion molecules (Nguyen and Sudhof 1997) and affect the fusion of neurotransmitter vesicles with the plasma membrane (Puschel and Betz 1995). The regulation of vesicle fusion as well as cell adhesion may affect the progression of growth cone toward their target (axonogenesis) and transition from neurite extension to synaptogenesis. The postsynaptic Nlgns form heterophilic connections with Nrxns and are thus important for synapse formation and cognitive functions (Dean and Dresbach 2006). These proteins even induce the differentiation of presynaptic and postsynaptic specialization respectively in adjoining non-neuronal cells (Scheiffele et al. 2000; Graf et al. 2004). Moreover, their interaction is involved in neuronal plasticity mechanism and neuronal disorders such as autism (Südhof 2008). It has also been reported that the synaptic plasticity reduces during aging (Burke and Barnes 2006) and impairs executive functions such as working memory and attention (Tisserand and Jolles 2003). Therefore, it is likely that Nrxn1 and Nlgn3 are involved in age-related decline in cognitive function. Thus, the increased level of these proteins might be involved, particularly in synaptic plasticity or in adult neurogenesis. The hippocampus is the important site for neurogenesis in the adult mammalian brain, which includes the formation of neurons from neural precursor cells involving neuronal proliferation, differentiation, neurite growth, and synaptic integration (Zhao et al. 2008). In old cerebral cortex, the level of both Nrxn1 and Nlgn3 decreased, which might be due to loss of neurons during aging (Michael 2006).
Further, to understand the significance of age-related differential expression of Nrxn1 and Nlgn3 in cerebral cortex and hippocampus, we analyzed the expression of presynaptic density marker synaptophysin. Pearson’s correlation analysis showed a positive correlation of synaptophysin expression with Nrxn1 and Nlgn3 in cerebral cortex and hippocampus. Similar to Nlgn3, the level of synaptophysin also increased in old hippocampus as also reported earlier (Benice et al. 2006). In this study, it has been suggested that increased synaptophysin may be involved in a mechanism by which hippocampus might compensate age-related functional deficits (Benice et al. 2006). In another study, increased synaptophysin expression has been reported to be involved in a compensatory response to amyloid precursor protein-derived cognitive deficit of mouse model of Alzheimer’s disease (King and Arendash 2002). However, in rhesus macaque, synaptophysin expression decreased in both prefrontal cortex and hippocampus with aging (Gwendolen et al. 2010). Therefore, we hypothesized that the increased expression of Nlgn3 might support the compensatory functions in old hippocampus. In addition, difference in the synaptophysin expression can reflect variation in the number of synapses and synaptic vesicles per synapse, or the amount of synaptophysin in the vesicle (Frick and Fernandez 2003). The increased synaptophysin might represent an increased synaptic vesicle pool in response to an age-related reduction in neuron and synapse number. Thus, differential expression of Nrxn1 and Nlgn3 might be involved in impairment of synaptic plasticity during aging.
Conclusion
In conclusion, Nrxn1 and Nlgn3 show age-related variation in expression at both mRNA and protein levels in cerebral cortex and hippocampus of male mice. This shows a positive Pearson’s correlation with presynaptic density marker synaptophysin. The increased expression of Nlgn3 and synaptophysin in aged hippocampus might be involved in the compensatory function in age-related cognitive decline.
Acknowledgments
The present study was supported by grants from the Department of Biotechnology, Government of India, to MKT [BT/PR3996/MED/97/57/2011], and research fellowship to DK from the Council of Scientific Industrial Research, India [09/013(0253)/2009-EMR-I].
Conflict of interest
The authors do not have any potential conflict of interest including any financial, personal, or academic aspect.
References
- Arac D, Boucard AA, Ozkan E, Strop P, Newell E, Südhof TC, Brunger AT. Structures of neuroligin-1 and the neuroligin-1/neurexin-1 beta complex reveal specific protein-protein and protein-Ca2+ interactions. Neuron. 2007;56:992–1003. doi: 10.1016/j.neuron.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Benice TS, Rizk A, Kohama S, Pfankuch T, Raber J. Sex-differences in age-related cognitive decline in C57BL/6 J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience. 2006;137:413–423. doi: 10.1016/j.neuroscience.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Bloss EB, Janssen WG, Ohm DT, Yuk FJ, Wadsworth S, Saardi KM, McEwen BS, Morrison JH. Evidence for reduced experience-dependent dendritic spine plasticity in the aging prefrontal cortex. J Neurosci. 2011;31:7831–7839. doi: 10.1523/JNEUROSCI.0839-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloss EB, Puri R, Yuk F, Punsoni M, Hara Y, Janssen WG, McEwen BS, Morrison JH. Morphological and molecular changes in aging rat prelimbic prefrontal cortical synapses. Neurobiol Aging. 2013;34:200–210. doi: 10.1016/j.neurobiolaging.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Budreck EC, Scheiffele P. Neuroligin-3 is a neuronal adhesion protein at GABAergic and glutamatergic synapses. Eur J Neurosci. 2007;26:1738–1748. doi: 10.1111/j.1460-9568.2007.05842.x. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Chen X, Liu H, Shim AHR, Focia PJ, He X. Structural basis for synaptic adhesion mediated by neuroligin–neurexin interactions. Nat Struct Mol Biol. 2008;15:50–56. doi: 10.1038/nsmb1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, Sudhof TC. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54:919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoletti D, De Jaco A, Jennings LL, Flynn RE, Gaietta G, Tsigelny I, Ellisman MH, Taylor P. The Arg451Cys neuroligin-3 mutation associated with autism reveals a defect in protein processing. J Neurosci. 2004;24:4889–4893. doi: 10.1523/JNEUROSCI.0468-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C, Dresbach T. Neuroligins and neurexins: linking cell adhesion, synapse formation and cognitive function. Trends Neurosci. 2006;29:21–29. doi: 10.1016/j.tins.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, Scheiffele P. Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci. 2003;6:708–716. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Howard SR, Stone JC, Monfils MH, Tomanek B, Brooks WM, Sutherland RJ. The aging hippocampus: a multi-level analysis in the rat. Neuroscience. 2006;139:1173–1185. doi: 10.1016/j.neuroscience.2006.01.040. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Hao JD, Hara Y, Kaufmann J, Janssen WGM, Lou W, Rapp PR, Morrison JH. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with age-related cognitive impairment. J Neurosci. 2010;30:7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini A, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- Frick KM, Fernandez SM. Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiol Aging. 2003;24:615–626. doi: 10.1016/S0197-4580(02)00138-0. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Detoledo-Morrell L, Morrell F, Heller RE. Hippocampal markers of age-related memory dysfunction: behavioral, electrophysiological and morphological perspectives. Prog Neurobiol. 1995;45:223–252. doi: 10.1016/0301-0082(94)00047-L. [DOI] [PubMed] [Google Scholar]
- Gleichmann M, Zhang Y, Wood WH, III, Becker KG, Mughal MR, Pazin MJ, Praaga H, Kobiloa T, Zonderman AB, Troncoso JC, Markesbery WR, Mattson MP. Molecular changes in brain aging and Alzheimer’s disease are mirrored in experimentally silenced cortical neuron networks. Neurobiol Aging. 2012;33:205e1–205e18. doi: 10.1016/j.neurobiolaging.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwendolen EH, Steven GK, Henryk FU, Jacob R. Age-related decreases in SYN levels associated with increases in MAP-2, apoE, and GFAP levels in the rhesus macaque prefrontal cortex and hippocampus. Age. 2010;32:283–296. doi: 10.1007/s11357-010-9137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Rapp PR, Morrison JH. Neuronal and morphological base of cognitive decline in aged rhesus monkeys. Age. 2012;34:1051–1073. doi: 10.1007/s11357-011-9278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DL, Arendash GW. Maintained synaptophysin immunoreactivity in Tg2576 transgenic mice during aging: correlations with cognitive impairment. Brain Res. 2002;926:58–68. doi: 10.1016/S0006-8993(01)03294-2. [DOI] [PubMed] [Google Scholar]
- Kolozsi E, Mackenzie RN, Roullet FI, deCatanzaro D, Foster JA. Prenatal exposure to valproic acid leads to reduced expression of synaptic adhesion molecule neuroligin 3 in mice. Neuroscience. 2009;163:1201–1210. doi: 10.1016/j.neuroscience.2009.07.021. [DOI] [PubMed] [Google Scholar]
- Krueger DD, Tuffy LP, Papadopoulos T, Brose N. The role of neurexins and neuroligins in the formation, maturation, and function of vertebrate synapses. Curr Opin Neurobiol. 2012;22:412–422. doi: 10.1016/j.conb.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Kumar D, Thakur MK. Perinatal exposure to bisphenol-A impairs spatial memory through upregulation of neurexin1 and neuroligin3 expression in male mouse brain. PLoS ONE. 2014;9:e110482. doi: 10.1371/journal.pone.0110482. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Labarrière M, Thomas F, Dutar P, Pollegioni L, Wolosker H, Billard JM. Circuit-specific changes in D-serine-dependent activation of the N-methyl-D-aspartate receptor in the aging hippocampus. Age. 2014;36:9698. doi: 10.1007/s11357-014-9698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic P. Association of storage and processing functions in the dorsolateral prefrontal cortex of the non-human primate. J Neurosci. 1999;19:5149–5158. doi: 10.1523/JNEUROSCI.19-12-05149.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Cipolotti L. Selective sparing of topographical memory. J Neurol Neurosurg Psychiatry. 1998;65:903–909. doi: 10.1136/jnnp.65.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Michael JF. Rodent models of brain aging and neurodegeneration. Age. 2006;28:219–220. doi: 10.1007/s11357-006-9028-2. [DOI] [Google Scholar]
- Morrison JH, Baxter MG. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci. 2012;13:240–250. doi: 10.1038/nrn3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder M, Jansen PJ, Janssen BJ, van de Berg WD, van der Boom H, Havekes LM, de Kloet RE, Ramaekers FC, Blokland A. Low-density lipoprotein receptor-knockout mice display impaired spatial memory associated with a decreased synaptic density in the hippocampus. Neurobiol Dis. 2004;16:212–219. doi: 10.1016/j.nbd.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Sudhof TC. Binding properties of neuroligin 1 and neurexin 1beta reveal function as heterophilic cell adhesion molecules. J Biol Chem. 1997;272:26032–26039. doi: 10.1074/jbc.272.41.26032. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith C, Frackowiak R. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Puschel AW, Betz H. Neurexins are differentially expressed in the embryonic nervous system of mice. J Neurosci. 1995;15:2849–2856. doi: 10.1523/JNEUROSCI.15-04-02849.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Kansky MT, Roberts JA. Impaired spatial information processing in aged monkeys with preserved recognition memory. NeuroReport. 1997;8:1923–1928. doi: 10.1097/00001756-199705260-00026. [DOI] [PubMed] [Google Scholar]
- Reddy PH, Mani G, Park BS, Jacques J, Murdoch G, Whetsell W, Jr, Kaye J, Manczak M. Differential loss of synaptic proteins in Alzheimer’s disease: implications for synaptic dysfunction. J Alzheimers Dis. 2005;7:103–117. doi: 10.3233/jad-2005-7203. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Winocur G, Moscovitch M (2001) New views on old memories: re-evaluating the role of the hippocampal complex. Behav Brain Res 127:183–97. [DOI] [PubMed]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/S0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Sibille E. Molecular aging of the brain, neuroplasticity and vulnerability to depression and other brain-related disorders. Dialogues Clin Neurosci. 2013;15:53–56. doi: 10.31887/DCNS.2013.15.1/esibille. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Brunk UT. Lipofuscin as an indicator of oxidative stress and aging. Adv Exp Med Biol. 1989;266:17–26. doi: 10.1007/978-1-4899-5339-1_2. [DOI] [PubMed] [Google Scholar]
- Song JY, Ichtchenko K, Südhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci U S A. 1999;96:1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarsa L, Goda Y. Synaptophysin regulates activity-dependent synapse formation in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 2002;99:1012–1016. doi: 10.1073/pnas.022575999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisserand DJ, Jolles J. On the involvement of prefrontal networks in cognitive ageing. Cortex. 2003;39:1107–1128. doi: 10.1016/S0010-9452(08)70880-3. [DOI] [PubMed] [Google Scholar]
- Ushkaryov YA, Petrenko AG, Geppert M, Sudhof TC. Neurexins: synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science. 1992;257:50–56. doi: 10.1126/science.1621094. [DOI] [PubMed] [Google Scholar]
- Van der Jeugd A, Vermaercke B, Derisbourg M, Lo AC, Hamdane M, Blum D, Buée L, D'Hooge R. Progressive age-related cognitive decline in tau mice. J Alzheimers Dis. 2013;37:777–788. doi: 10.3233/JAD-130110. [DOI] [PubMed] [Google Scholar]
- Winocur G, Gagnon S. Glucose treatment attenuates spatial learning and memory deficits of aged rats on tests of hippocampal function. Neurobiol Aging. 1998;19:233–241. doi: 10.1016/S0197-4580(98)00057-8. [DOI] [PubMed] [Google Scholar]
- Wittenmayer N, Körber C, Liu H, Kremer T, Varoqueaux F, Chapman ER, Brose N, Kuner T, Dresbach T. Postsynaptic neuroligin1 regulates presynaptic maturation. PNAS. 2009;106:13564–13569. doi: 10.1073/pnas.0905819106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XH, Ouyang J, Xie PH, Chen JH. Changes of activity and expression of protein phosphatase type 2A during the apoptosis of NB4 and MR2 cells induced by arsenic trioxide. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2008;16:1021–1025. [PubMed] [Google Scholar]
- Yamagata M, Sanes JR, Weiner JA. Synaptic adhesion molecules. Curr Opin Cell Biol. 2003;155:621–632. doi: 10.1016/S0955-0674(03)00107-8. [DOI] [PubMed] [Google Scholar]
- Yang L, Zhang J, Zheng K, Shen H, Chen X. Long-term ginsenoside Rg1 supplementation improves age-related cognitive decline by promoting synaptic plasticity associated protein expression in C57BL/6 J mice. J Gerontol A Biol Sci Med Sci. 2014;69:282–294. doi: 10.1093/gerona/glt091. [DOI] [PubMed] [Google Scholar]
- Zhang C, Milunsky JM, Newton S, Ko J, Zhao G, Maher TA, Tager-Flusberg H, Bolliger MF, Carter AS, Boucard AA, Powell CM, Südhof TC. A neuroligin-4 missense mutation associated with autism impairs neuroligin-4 folding and endoplasmic reticulum export. J Neurosci. 2009;29:10843–10854. doi: 10.1523/JNEUROSCI.1248-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]