Abstract
Colorectal cancer screening rates are below optimal. As part of a pilot clinic-based pragmatic study aiming to raise rates of colorectal-cancer screening, we explored patients’ reasons for not responding to a direct-mailed screening invitation. We conducted telephone interviews with patients who were mailed a fecal immunochemical test (FIT) but who did not return it to the lab. Interviews were audio-recorded, transcribed, and coded for thematic analysis. We met our goal of 20 interviews (10 in English and 10 Spanish; 75 % female). Reasons for not completing tests were fear of results or cost of follow-up colonoscopy (n = 9); not having received the test in the mail (n = 7); concerns about mailing fecal matter or that test results could be mixed up (n = 6); and being busy or forgetful (n = 4). Efforts to improve uptake of colorectal cancer screening in a direct-mailed program ought to address concerns identified in our study.
Electronic supplementary material
The online version of this article (doi:10.1007/s13142-014-0276-x) contains supplementary material, which is available to authorized users.
Keywords: Colorectal cancer screening, Direct-mailed fecal testing, Federally qualified health centers, Implementation, Pragmatic research, Qualitative interviews
INTRODUCTION
Colorectal cancer (CRC) is the second-leading cause of cancer death in the USA [1]. Nevertheless, screening rates are low, particularly among socioeconomically disadvantaged population subgroups. Data from the Behavioral Risk Factor Surveillance System (BRFSS) survey show that, in 2012, 35 % of adults aged 50 to 75 years—nearly 30 million people—were not up to date on CRC screening [2]. BRFSS data show lower rates of screening among those who lack healthcare coverage (37 vs. 69 % with health care insurance), those who lack a regular provider (31 vs. 69 % among those with a regular provider), and Hispanics (53 vs. 66 % for non-Hispanics) [2].
Clinic-based programs involving direct mailing of fecal tests to patients’ home have consistently improved colorectal screening participation, with effect sizes ranging from 6 to 24 % [3–6]. Such programs reduce structural barriers to screening by offering free testing and screening at home without a clinic visit. Despite the success of these efforts, a substantial proportion of participants still do not respond to these mailed attempts.
Previous research on factors that deter patients from getting screened, even when they are mailed a home-based test, is scarce. Limited available data from qualitative reports have identified hygienic concerns with collecting, storing, and sending fecal matter through the mail [6, 7] as well as misunderstanding instructions for completing the test [7].
We have previously reported on the methods and feasibility of our pilot pragmatic study, Strategies and Opportunities to STOP Colon Cancer in Priority Populations (STOP CRC), an EHR-enabled CRC screening intervention that could be scaled up to multiple safety net clinics [8]. We reported the proportion of patients successfully contacted (reach) and the proportion completing testing (effectiveness). Our current report adds to these findings by providing patients’ perspectives, obtained from in-depth patient interviews, of factors hindering their completion of CRC screening in general, and specifically of factors hindering receipt of screening following our direct-mail program. Our goal is ultimately to identify strategies for improving the program’s reach and effectiveness. Because our partnering organization was a Latino-serving federally qualified health center (FQHC), we explored differences in factors impeding completion of a FIT in both Spanish- and English-speaking groups.
METHODS
Study site and background
Our partnering pilot organization was Virginia Garcia Memorial Health Center (VGMHC), a Portland-based FQHC serving more than 32,000 unique patients at four primary care sites. Two of these primary-care sites participated in the pilot study. In 2012, VGMHC served 4,902 patients aged 50–74 years, of whom 41 % were Latino and 52 % were uninsured.
Briefly, patients who had a clinic visit in the past year were age-eligible for CRC screening (aged 50–74 years), were not up-to-date with screening, and met other study eligibility criteria (e.g., no history of CRC or inflammatory bowel disease) were eligible for participation in the pilot study. After stratification on key variables (insurance status and timing of last clinic visit), we randomly selected 213 patients for the screening program. The direct-mailed CRC screening program consisted of an introductory letter, a FIT kit with a postage-paid return envelope and instructions for completing the kit, and a reminder postcard (all materials were in English and Spanish). From the original list of 213 patients, 111 did not return the FIT kit for processing, had not opted out of the program, and were assumed to have a valid address. Interviews with these “non-responder” patients are the focus of this qualitative report.
Participants and recruitment
Recruitment letters were sent to all 111 patients (51 English and 60 Spanish speakers), and patients were called by phone to schedule an interview. Our goal was to interview 20 patients, half in English and half in Spanish. We also tried to balance participants in terms of ethnicity (Hispanic and non-Hispanic), age, and gender. Interviews were conducted by phone, audio-recorded, and lasted about 30–45 min. Participants were verbally consented and mailed a $20 gift card as compensation for their time. All interview procedures and materials were reviewed by the Institutional Review Board at Kaiser Permanente Northwest and qualified for a waiver of signed consent.
Data collection and analysis
The interview questions were developed by our lead qualitative researcher and were based on the previous qualitative interviews conducted with patients and providers at FQHCs [9, 10] and input from the research team. A draft set of questions was developed and reviewed with team members who had a range of expertise with colon cancer screening best practices, clinical expertise, and experience understanding the daily workings of FQHCs. The guide was reviewed until consensus was reached on both topics covered and their related questions/probes. The semi-structured, open-ended interview guide explored awareness of CRC and CRC screening, prior screening history, general barriers and facilitators to CRC screening, and reasons for an individual’s not completing or returning the test (i.e., mailing the kit to the laboratory).
All interviews were audio-recorded and transcribed verbatim. Interviews conducted in Spanish (by JJS, a trained bilingual interviewer) were also transcribed in Spanish to ensure integrity of meaning. We used a qualitative content analysis approach [11–16] with grounded theory coding techniques [17–20] to develop and apply a coding dictionary to the transcripts [17, 18]. Some codes denoted questions posed to the interview participants (e.g., reason for not returning FIT kit), while others represented issues that emerged during discussions (e.g., how to get a new FIT kit). All transcripts were coded by trained coders (JLS, JJS, and GC); Spanish-language transcripts were coded and reviewed by bilingual coders (JJS and GC). All coded transcripts were entered into Atlas.ti 5.0 (Scientific Software Development, 1997), a qualitative analysis software program used to electronically code and manage data, and to generate reports of coded text for ongoing thematic analysis [16, 21]. The team (JLS, JJS, and GC) subsequently reviewed reports generated from coded text and engaged in an iterative process of discussion and review that resulted in agreed upon and refined themes.
Data reliability and validity
We employed several strategies to improve the credibility and trustworthiness of our data [16, 22], including using trained interviewers; using a semi-structured interview guide; using a formal, team-based approach to analysis; conducting, transcribing, and analyzing Spanish interviews in Spanish to maintain integrity of meaning; and reviewing our findings with clinic staff against the feedback they were hearing from patients in the pilot program. We did not calculate intercoder reliability.
RESULTS
We sought to conduct 20 one-on-one interviews, a number consistent with the previous qualitative research [23–25]. A total of 137 call attempts were made to reach our goal of 20 completed participant interviews. Eight (7 %) individuals refused to do the interview (too busy or not interested), and 22 (20 %) were unreachable due to an undeliverable mailing address (10), discontinued or incorrect phone number (11), or were deceased (1). Participants were generally aged 50–64 years (85 %), female (75 %), and Hispanic (55 %); the majority (90 %) was currently receiving care at Virginia Garcia, with half for 6 years or more (Table 1).
Table 1.
Characteristics of interview participants (n = 20)
| Clinic site | Age group | Gender | Ethnicity | Primary language | Currently receives care at clinic | Years receiving care at clinic | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50–64 | 65–74 | Female | Male | Hispanic | Non-Hispanic | Spanish | English | Yes | No | <5 | 6+ | |
| Clinic A | 8 | 2 | 8 | 2 | 6 | 4 | 5 | 5 | 9 | 1 | 7 | 3 |
| Clinic B | 9 | 1 | 7 | 3 | 5 | 5 | 5 | 5 | 9 | 1 | 4 | 6 |
| Totals | 17 | 3 | 15 | 5 | 11 | 9 | 10 | 10 | 18 | 2 | 11 | 9 |
| 20 | 20 | 20 | 20 | 20 | 20 | |||||||
Interviews were conducted over the telephone in Portland, Oregon between June and August 2013
The results are organized into three categories: (1) patients’ awareness and understanding of CRC screening methods and prior CRC screening behaviors; (2) general barriers and facilitators to CRC screening; and (3) reasons for not completing screening.
General awareness of colorectal cancer screening methods (Table 2)
Table 2.
Awareness/understanding of colon cancer screening methods and prior history (n = 20) (participants may have expressed more than one belief or understanding about colon cancer or screening)
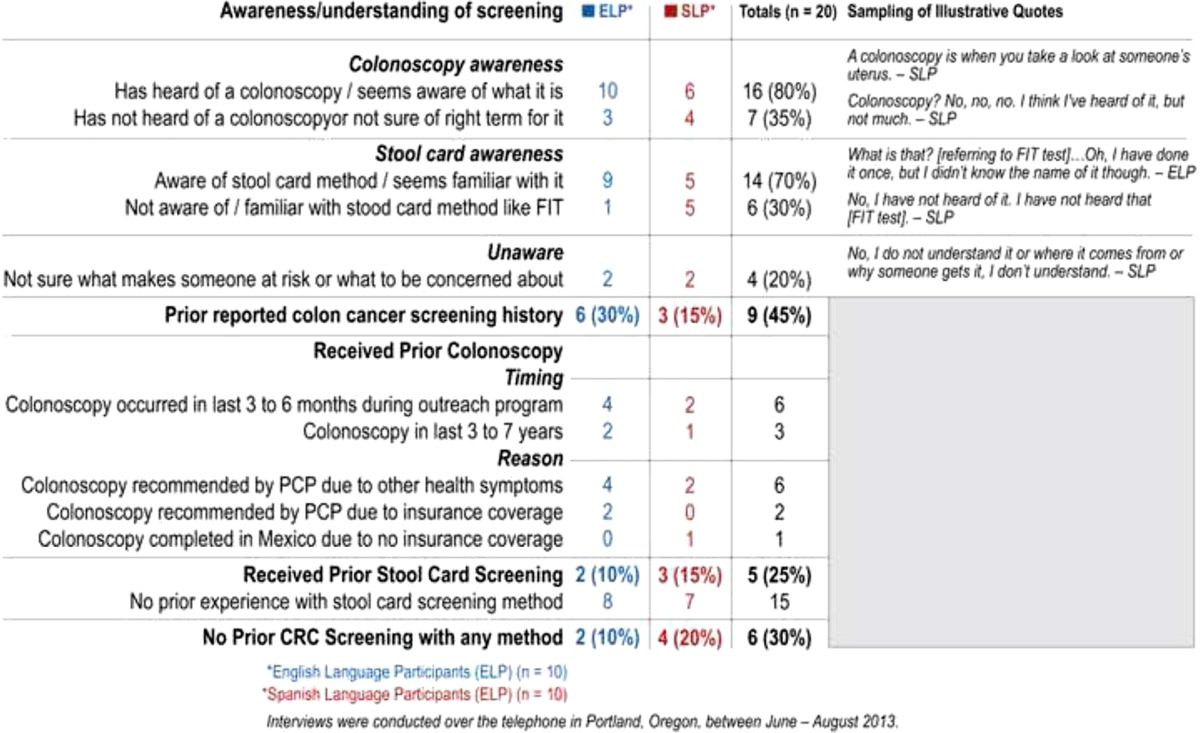
Awareness/understanding of screening methods
Most of the participants (70 %) were aware of the stool card method of screening, with half of the Spanish-speaking interviewees and 90 % of the English speakers familiar with it. Overall, 80 % (16) indicated knowing about colonoscopy for screening—100 % of English-speaking interviewees and 60 % of Spanish-speaking interviewees. Seven participants (four Spanish speakers) had either not heard of or were not aware of what a colonoscopy was, or they were not sure of the right terminology for a colonoscopy but could describe it (three English speakers). Forty-five percent of Spanish speaking participants (nine) expressed no awareness or familiarity with either colonoscopy or stool-card screening methods, as compared to only one (5 %) of the English-language participants. Furthermore, 20 % (two Spanish and two English) were uncertain about risk factors for colon cancer or what symptoms to look for. [See supplement for Table 1 summarizing additional information on patient belief and understanding about colon cancer risk and screening].
Prior history with CRC screening
Fourteen participants (70 %) described receiving prior CRC screening, with 45 % reporting screening by colonoscopy and 25 % by a stool-card method. Six participants (30 %) reported no prior CRC screening with any method. Twice as many English-speaking participants reported a colonoscopy (six) as did the Spanish language participants (three). Six of the participants (four English and two Spanish) said they had received a colonoscopy in the past 3 to 6 months, during the time of the outreach program. Eight of the nine people who reported having had a screening colonoscopy described doing so because of their PCP’s recommendation due to having health symptoms (six) or having the procedure covered by their health insurance (two). One Spanish-speaking participant described choosing to go to Mexico to complete a colonoscopy because of lack of insurance coverage. Three quarters (15) of participants indicated they had no prior experience with any type of stool-card method for CRC screening.
General barriers to CRC screening
Barriers to CRC screening in general (Table 3)
Table 3.
Barriers to colon cancer screening in general (n = 20) (participants may have expressed more than one belief or understanding about colon cancer screening)
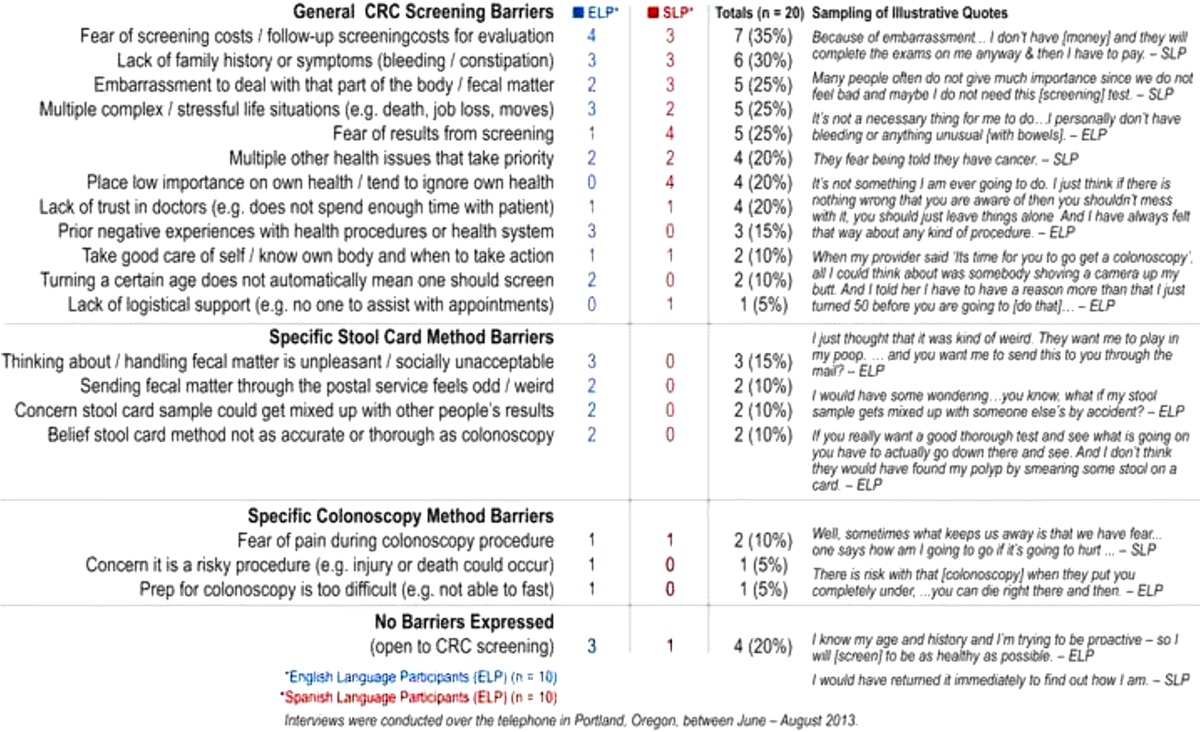
The most frequently mentioned barrier, cited by 35 % of participants (7), was concerns about the initial cost of screening, or subsequent follow up screening costs for evaluation, because of co-pays or lack of insurance. The second most frequently mentioned barrier, by 30 % of participants, (six) was lack of family history or symptoms. These barriers were equally cited by English- and Spanish-language participants. Experiencing embarrassment regarding “that part of the body” related to fecal matter, having a stressful life with multiple complexities, and having fear about the results of screening were all mentioned by a quarter of participants (five). However, Spanish-speaking participants cited fear of the results more frequently than English-language participants. Twenty percent of the participants (four) described having multiple other health issues that take priority over screening as a barrier. Placing low importance on their own health or ignoring it was cited solely by Spanish-language participants (four) as a barrier, while English speakers (three) solely cited having prior negative health procedures or health system interactions as a barrier to screening.
Ten percent of participants (one English and one Spanish) described fear of pain during a colonoscopy as a barrier to this method. Other colonoscopy screening barriers cited each once by English participants related to the preparation for the procedure being too complex to complete, and concern that it is a risky procedure that could lead to injury or death. For the stool-card method, only English speakers cited impediments. Three of these participants said that handling fecal matter is unpleasant, unsavory, and socially unacceptable. Additionally, English participants (two) also expressed concerns over the oddness of sending fecal matter through the mail, feared the results could become mixed up with others’ during processing, and said they believed it is a less accurate method than colonoscopy.
Facilitators to CRC screening in general
As with the barriers to completing specific CRC screening methods, Spanish-language participants also had less to say about particular facilitators to screening using either stool cards or colonoscopy. For the stool-card method, a quarter of participants (five) indicated that they were motivated by the belief that this method is a simple, affordable, and proactive way to initially screen for colon cancer. English speakers cited this facilitator more often than Spanish speakers. Preferring to avoid the preparation with colonoscopy (one) and fear of the invasiveness of the colonoscopy process (one) were expressed solely by English speakers; Spanish-language participants solely cited a preference for being provided a stool kit during a clinic encounter (one) and endorsed the clinic having a program to make the screening method affordable (one). None of the Spanish-language participants mentioned any factors that were facilitators for colonoscopy screening. Twenty percent of the English-language participants only (two) described three facilitators to colonoscopy screening: a preference to screen only once every 10 years (two), a belief colonoscopy is a more thorough and trustworthy option (two), and that colonoscopy was covered by insurance (two). [See supplement for Table 2 summarizing facilitators].
Specific reasons for not completing FIT kit outreach (Table 4)
Table 4.
Patient-reported reasons for FIT kit non-completion (n = 20)
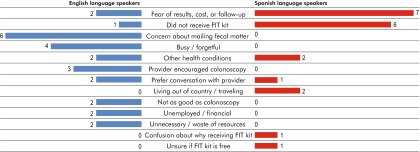
The most frequently cited reasons for not completing the FIT kit were fear of the results and the cost of follow-up screening. Almost half of the participants (nine) expressed these fears, describing concerns that the screening could lead to an invasive and costly follow-up colonoscopy. The second most frequently cited barrier, expressed by 35 % (seven) of participants, was not ever obtaining the FIT kit in the mail. These barriers were endorsed more often by Spanish-language participants than English-language participants. Concern about mailing fecal matter and mistrust that the samples could become mixed up when processed was solely endorsed as a barrier to completion by English-language participants (six). One fourth of the participants, both English and Spanish, described having ongoing health issues or symptoms that prevented them from completing or using the FIT kit method. Some of these participants expressed willingness to complete the FIT kit in the future if their health symptoms resolved. English speakers solely endorsed a genuine forgetfulness (four) due to having a busy life, as well as being unemployed or having financial challenges (two) as reasons for not completing the test. Two Spanish-language participants indicated they were either living or traveling out of the country (e.g., Mexico) during the time of the outreach program, and thus did not complete or return the kit. Other participants (three) disliked the impersonal nature of the outreach program (sent in the mail with no direct prior communication from their provider) for such a personal test. One Spanish speaker expressed a strong belief that this sort of screening should be conducted directly at the clinic or in their own doctor’s office since the screening involves a private part of the body. Additionally, two English speakers felt negatively about the mailed outreach, strongly expressing it as both unnecessary and a waste of multiple resources (e.g., paper, mailing costs, and people’s time). Three English-language participants, and no Spanish-language participants, explained they did not complete the FIT kit when received in the mail due to discussions with their providers that encouraged them to screen instead by colonoscopy since they had insurance that would cover the procedure. [See supplement for Table 3 illustrating patient quotes regarding reasons for FIT kit non-completion].
Overall, when asked about their willingness to screen in the future via a mailed stool-card approach, 70 % of participants (14) indicated they would be willing to use this method. One Spanish-speaking participant expressed being uncertain as to whether they would or not, and quarter of participants (five) clearly stated they would not consider screening using a mailed stool-card approach. Slightly, more Spanish speakers were either unwilling or uncertain about responding to and utilizing this approach in the future.
DISCUSSION AND CONCLUSION
Discussion
Overall, patients were aware of screening tests for colorectal cancer, though awareness was higher for English-speaking participants compared to Spanish-speaking participants. Commonly cited barriers to CRC screening as part of a direct-mail program were (1) fear of the results from screening, including concerns about the cost of follow-up colonoscopy; and (2) not having received the test kit in the mail. Other barriers included experiencing multiple life stressors, acute health concerns, and forgetfulness. Non-Hispanics also cited concerns about mailing fecal matter or that test results could easily be mixed up with others. Our findings can inform efforts to enhance colorectal cancer screening and reduce screening disparities in underserved populations.
The relatively low awareness of colorectal cancer screening among Spanish speakers, compared to English speakers, may reflect a lower level of attention given to this topic in Spanish-language media, compared to English-language media. Alternatively, it may reflect differences in the frequency of provider recommendations for testing based on patient characteristics, such as insurance status. Our team previously gathered qualitative data from clinic personnel at Virginia Garcia and other safety net clinics to assess clinic-level barriers and facilitators to CRC screening; a commonly cited reason for not recommending CRC screening was the limited capacity for low-cost colonoscopy among uninsured patients (both for initial screening and for diagnostic follow-up) [9]. Patients’ fears of the cost of screening and needed follow-up care for positive results were noted in the present study. Other studies have also documented this phenomenon [26, 27].
Our finding that 15 % of patients we interviewed reported having received a colonoscopy in the past 3–7 years underscores challenges in capturing colonoscopy information in electronic medical records. This issue has been reported previously and may reflect inconsistent updating of primary-care sites with colonoscopy records or the lack of a discrete field in the electronic health record to consistently extract this information [28, 29]. Further efforts are needed to improve the accuracy of previous colonoscopy receipt to ensure that the program is delivered to patients who need it.
We observed that a relatively high percentage of participants had received prior testing, but the proportions differed by test, with more having had a prior colonoscopy than a fecal test, perhaps reflecting the general trend in the USA for more age-eligible adults using this method. Most patients reporting having had a colonoscopy had completed testing in the prior 3–6 months, possibly as a result of receiving our program materials, even though our program focused solely on fecal testing. The cause of this phenomenon is likely that patients who received our introductory letter or FIT kit then called their doctors to discuss screening and were encouraged to obtain a colonoscopy instead. One patient described this scenario precisely. Provider bias toward colonoscopy has been documented in national data. Using data from the 2006–2007 National Survey or Primary Care Physicians (NSPCP), Klabunde and colleagues reported that 95 % of respondents expressed the belief that colonoscopy is very effective in reducing CRC mortality, while only a minority perceived guaiac-based FOBT (12 %); sigmoidoscopy (16 %); double-contrast barium enema (DCBE; 17 %); CT colonography (23 %); or fecal DNA testing (7 %) as effective [30].
Our observation that having no symptoms could be a barrier to screening is consistent with one previous qualitative report [31]. A limited number of previous reports have cited forgetfulness or procrastination as barriers to completing fecal testing [32, 33], and this was reported by a minority of our participants. Embarrassment was cited by our participants and reported in the previous literature [34, 35]. Our interviews also identified acute health concerns and life stressors that impeded completion of the test.
The fecal test-specific barriers we identified—that fecal matter is perceived as unpleasant, unsavory, and socially unacceptable—were identified in a qualitative report by Reynolds et al. and Chapple et al. [7, 36]. A small number of our participants considered it ‘odd’ to send fecal matter through the mail; similarly, O’Sullivan and others reported patients’ concerns about the public health risks of mailing fecal matter [7, 37]. Our study uniquely identified patients’ fears about the results being mixed up with others during processing. Nearly half of the participants expressed fear of the results and cost of screening or needed follow-up care, and more than a third reported not having received the program materials, including the FIT kit; this proportion was much higher among Spanish-speaking participants than English-speaking participants. Notably, a handful of Spanish-speaking participants reported having traveled to Mexico during the time period of the program mailings.
This study had some limitations which should be noted. Our small sample size may limit the generalizability of our findings to other settings. Our selection of participants by convenience may have led to selection bias, and social desirability bias may limit the accuracy of responses. Nevertheless, we employed several strategies to improve the credibility and trustworthiness of our data [16, 22], including transcribing and analyzing Spanish interviews in Spanish to maintain integrity of meaning and reviewing our findings with clinic staff against the feedback they were hearing from patients in the pilot program. Our analytic approach to stratify across language groups (English vs. Spanish) revealed important difference in levels of awareness and specific barriers to obtain colorectal cancer screening among English- and Spanish-speakers; these findings can support efforts to address colorectal cancer screening disparities.
Conclusion
Our findings underscore unique challenges with direct-mail interventions in FQHCs and might inform other efforts that rely on a mailed approach to improve rates of fecal testing in this setting. Further efforts to raise the rates of CRC screening using a direct-mail program might benefit from addressing patient barriers, by increasing CRC screening awareness, particularly for non-English speakers, identifying and promoting community resources for free or low-cost colonoscopy services (to allay concerns about the cost of screening and possible follow-up care), and promoting the social acceptability and safety of sending fecal matter in the mail.
Practice implications
Efforts to further target the program to those who need it may benefit from more complete capture of colonoscopy information in the electronic health record. Further exploration is needed to understand why a surprisingly high number of patients reported never having received the mailed components, though their mailings were not returned by the Post Office. Our study revealed that some participants traveled out of the country during the winter months, when our program kits were mailed, though other explanations are possible.
Electronic supplementary material
(JPEG 1084 kb)
(JPEG 1070 kb)
(JPEG 1735 kb)
Acknowledgments
The authors would like to acknowledge technical assistance provided by Leslie Bienen, DVM, MFA, and Christine Wilkins.
Human subjects statement
I confirm all patient/personal identifiers have been removed or disguised so the patient/person(s) described are not identifiable and cannot be identified through the details of the story.
Sources of funding
Research reported in this publication was supported by the National Center for Complementary and Alternative Medicine of the National Institutes of Health under Award Number UH2AT007782. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest
The authors have declared that they have no conflicts of interest.
Clinical Trials Registration Number
Footnotes
Implications
Practice: Health care providers ought to be aware of the barriers faced by patients in returning direct-mailed FIT tests.
Policy: Efforts to assure affordable low-cost colonoscopy are needed to allay patient fears about FIT testing.
Research: Further research is warranted to inform effective patient-centered strategies to encourage participation in colorectal cancer screening programs.
References
- 1.American Cancer Society . Colorectal Cancer Facts & Figures 2011–2013. Atlanta: American Cancer Society; 2012. [Google Scholar]
- 2.Centers for Disease Control Cancer screening—United States 2010. MMWR Morb Mortal Wkly Rep. 2012;61(03):41–45. [PubMed] [Google Scholar]
- 3.Green BB, Wang CY, Anderson ML, et al. An automated intervention with stepped increases in support to increase uptake of colorectal cancer screening: a randomized trial. Ann Intern Med. 2013;158:301–311. doi: 10.7326/0003-4819-158-5-201303050-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coronado GD, Golovaty I, Longton G, Levy L, Jimenez R. Effectiveness of a clinic-based colorectal cancer screening promotion program for underserved Hispanics. Cancer. 2011;117:1745–1754. doi: 10.1002/cncr.25730. [DOI] [PubMed] [Google Scholar]
- 5.Walsh JM, Salazar R, Kaplan C, Nguyen L, Hwang J, Pasick RJ. Healthy colon, healthy life (colon sano, vida sana): Colorectal cancer screening among Latinos in Santa Clara, California. J Cancer Educ. 2010;25:36–42. doi: 10.1007/s13187-009-0007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sequist TD, Zaslavsky AM, Marshall R, Fletcher RH, Ayanian JZ. Patient and physician reminders to promote colorectal cancer screening: a randomized controlled trial. Arch Intern Med. 2009;169(4):364–371. doi: 10.1001/archinternmed.2008.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapple A, Ziebland S, Hewitson P, McPherson A. What affects the uptake of screening for bowel cancer using a faecal occult blood test (FOBt): a qualitative study. Soc Sci Med (1982) 2008;66:2425–2435. doi: 10.1016/j.socscimed.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Coronado GD, Vollmer WM, Petrik AF, et al. Strategies and opportunities to STOP colon cancer in priority populations: pragmatic pilot study design and outcomes. BMC Cancer. 2014; 14. [DOI] [PMC free article] [PubMed]
- 9.Coronado GD, Petrik AF, Spofford M, Talbot J, Do HH, Taylor VM. Clinical perspectives on colorectal cancer screening at Latino-serving federally qualified health centers. Health Educ Behav. 2014. [DOI] [PMC free article] [PubMed]
- 10.Coronado GD, Farias A, Thompson B, Godina R, Oderkirk W. Attitudes and beliefs about colorectal cancer among Mexican Americans in communities along the USA–Mexico border. Ethn Dis. 2006;16:421–427. [PubMed] [Google Scholar]
- 11.Lofland L, Lofland J. Analyzing social settings: a guide to qualitative observation and analysis. 3. San Francisco CA: Wadsworth Publishing Inc; 1995. [Google Scholar]
- 12.Wolcott H. Transforming qualitative data: Description, analysis and interpretation. Thousand Oaks: Sage Publications; 1994. [Google Scholar]
- 13.Coffey A, Atkinson P. Making sense of qualitative data: Complementary research strategies. Thousand Oaks: Sage Publications; 1996. [Google Scholar]
- 14.Riessman C. Narrative analysis: Qualitative Research Methods Series 30. Newbury Park: Sage Publications; 1993. [Google Scholar]
- 15.Bernard HR. Research methods in anthropology: Qualitative and quantitative approaches. 2. Thousand Oaks: Sage; 1994. [Google Scholar]
- 16.Patton M. Qualitative evaluation and research methods. Thousand Oaks: Sage; 2002. [Google Scholar]
- 17.Elo S, Kyngas H. The qualitative content analysis process. J Adv Nurs. 2008;62:107–115. doi: 10.1111/j.1365-2648.2007.04569.x. [DOI] [PubMed] [Google Scholar]
- 18.Graneheim UH, Lundman B. Qualitative content analysis in nursing research: Concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today. 2004;24:105–112. doi: 10.1016/j.nedt.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Denzin N, Lincoln Y. The Sage Handbook of Qualitative Research. Thousand Oaks: Sage; 2011. [Google Scholar]
- 20.Strauss A, Corbin J. Basics of qualitative research: Techniques and procedures for developing grounded theory. Thousand Oaks: Sage; 2008. [Google Scholar]
- 21.Bernard H, Ryan G. Analyzing qualitative data: Systematic approaches. Los Angeles: Sage; 2010. [Google Scholar]
- 22.Lincoln YS, Guba EG. Establishing trustworthiness. Naturalistic inquiry. Newbury Park: Sage; 1985. pp. 289–331. [Google Scholar]
- 23.McMullen CK, Schneider J, Firemark A, Davis J, Spofford M. Cultivating engaged leadership through a learning collaborative: Lessons from primary care renewal in Oregon safety net clinics. Ann Fam Med. 2013;11(Suppl 1):S34–S40. doi: 10.1370/afm.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldstein AC, Schneider JL, Unitan R, et al. Health care worker perspectives inform optimization of patient panel-support tools: a qualitative study. Popul Health Manag. 2013;16:107–119. doi: 10.1089/pop.2012.0065. [DOI] [PubMed] [Google Scholar]
- 25.Feldstein AC, Perrin N, Liles EG, et al. Primary care colorectal cancer screening recommendation patterns: associated factors and screening outcomes. Med Decis Making. 2011. [DOI] [PMC free article] [PubMed]
- 26.Goodman MJ, Ogdie A, Kanamori MJ, Canar J, O’Malley AS. Barriers and facilitators of colorectal cancer screening among Mid-Atlantic Latinos: Focus group findings. Ethn Dis. 2006;16:255–261. [PubMed] [Google Scholar]
- 27.Harden E, Moore A, Melvin C. Exploring perceptions of colorectal cancer and fecal immunochemical testing among African Americans in a North Carolina community. Prev Chronic Dis. 2011;8:A134. [PMC free article] [PubMed] [Google Scholar]
- 28.Greiver M, Barnsley J, Glazier RH, Harvey BJ, Moineddin R. Measuring data reliability for preventive services in electronic medical records. BMC Health Serv Res. 2012;12:116. doi: 10.1186/1472-6963-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kern LM, Malhotra S, Barron Y, et al. Accuracy of electronically reported “meaningful use” clinical quality measures: a cross-sectional study. Ann Intern Med. 2013;158:77–83. doi: 10.7326/0003-4819-158-2-201301150-00001. [DOI] [PubMed] [Google Scholar]
- 30.Klabunde CN, Lanier D, Nadel MR, McLeod C, Yuan G, Vernon SW. Colorectal cancer screening by primary care physicians: Recommendations and practices, 2006–2007. Am J Prev Med. 2009;37:8–16. doi: 10.1016/j.amepre.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffman RM, Rhyne RL, Helitzer DL, et al. Barriers to colorectal cancer screening: Physician and general population perspectives, New Mexico, 2006. Prev Chronic Dis. 2011;8:A35. [PMC free article] [PubMed] [Google Scholar]
- 32.Janz NK, Lakhani I, Vijan S, Hawley ST, Chung LK, Katz SJ. Determinants of colorectal cancer screening use, attempts, and non-use. Prev Med. 2007;44:452–458. doi: 10.1016/j.ypmed.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Worthley DL, Cole SR, Esterman A, et al. Screening for colorectal cancer by faecal occult blood test: Why people choose to refuse. Intern Med J. 2006;36:607–610. doi: 10.1111/j.1445-5994.2006.01155.x. [DOI] [PubMed] [Google Scholar]
- 34.Goldman RE, Diaz JA, Kim I. Perspectives of colorectal cancer risk and screening among Dominicans and Puerto Ricans: stigma and misperceptions. Qual Health Res. 2009;19:1559–1568. doi: 10.1177/1049732309349359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly KM, Phillips CM, Jenkins C, et al. Physician and staff perceptions of barriers to colorectal cancer screening in Appalachian Kentucky. Cancer Control. 2007;14:167–175. doi: 10.1177/107327480701400210. [DOI] [PubMed] [Google Scholar]
- 36.Reynolds LM, Consedine NS, Pizarro DA, Bissett IP. Disgust and behavioral avoidance in colorectal cancer screening and treatment: a systematic review and research agenda. Cancer Nurs. 2013;36:122–130. doi: 10.1097/NCC.0b013e31826a4b1b. [DOI] [PubMed] [Google Scholar]
- 37.O’Sullivan I, Orbell S. Self-sampling in screening to reduce mortality from colorectal cancer: a qualitative exploration of the decision to complete a faecal occult blood test (FOBT) J Med Screen. 2004;11:16–22. doi: 10.1258/096914104772950709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(JPEG 1084 kb)
(JPEG 1070 kb)
(JPEG 1735 kb)


