Abstract
Fear avoidance model of chronic pain-based interventions are effective, but have not been successfully implemented into primary care. It was hypothesized that speed walking times and key measures of the fear avoidance model would improve following the brief intervention delivered in primary care. A brief primary care-based intervention (PCB) that included a single educational session, speed walking (an in vivo desensitization exposure task), and visual performance feedback was designed to reduce fear avoidance beliefs and improve function in 4 patients with chronic low back pain. A multiple baseline across subjects with a changing criterion design indicated that speed walking times improved from baseline only after the PCB intervention was delivered. Six fear avoidance model outcome measures improved from baseline to end of study and five of six outcome measures improved from end of study to follow-up. This study provides evidence for the efficacy of a brief PCB fear avoidance intervention that was successfully implemented into a busy clinic for the treatment of chronic pain.
Keywords: Fear avoidance model, Chronic pain, Primary care, Cognitive behavioral therapy, Speed walking
INTRODUCTION
Primary care physicians (PCP) report increased frustration with current treatment options for their patients with chronic noncancer pain (CNP) [1]. Historically, PCPs referred CNP patients to interdisciplinary pain center programs. Despite their proven efficacy, political factors and reimbursement practices have led to a decline in interdisciplinary pain programs [2]. Instead, for more than a decade, the treatment option for CNP is often a single modality pain intervention. Unfortunately, no evidence-based support has been found for the reversion to a single modality medical conceptualization and treatment of chronic pain [3]. As a result, primary care providers are left with limited options for their patients with CNP. PCPs options often are single modality treatment that is not effective or interdisciplinary treatment that is effective, but has limited availability or despite long-term cost effectiveness is perceived to be too expensive in the short term [2].
Redesign movements in primary care and health psychology may provide PCPs and health teams viable treatment options for their patients with CNP. Health psychologists advocate for behavioral interventions that go beyond strictly mental health services to include disease prevention, health risk behavior modification, disability reduction, enhanced effectiveness of medical treatments, and improved health-related quality of life [4]. Pain management professionals are also calling for a primary care-based interdisciplinary team approach to treatment [5]. Integration of behavioral health including pain treatment is now included within the larger Patient-Centered Medical Home (PCMH) movement. The PCMH is best described as a model or philosophy of primary care that is patient-centered, comprehensive, team-based, coordinated, accessible, and focused on quality and safety [6].
The PCMH model calls for a biopsychosocial rather than purely biomedical approach to patient care [7] and is the overall philosophy that guided development of the brief primary care-based (PCB) pain intervention employed in this study. The PCB intervention included a single education session [8], an in vivo rather than imagined exposure to the fearful stimuli of movement [9], and visual performance feedback (VPF). Single session pain education sessions have been found to change pain cognitions and physical performance [10] especially if it addresses the conviction that pain means tissue damage and catastrophizing [11].
The biopsychosocial-based fear avoidance (FA) model of chronic pain [12] is the foundation for the second component of the PCB intervention. The model proposes that cognitive misinterpretations result in fears related to pain, (re)injury, or movement. Pain-related fear of movement (kinesiophobia) leads to hypervigilance, muscular reactivity, and avoidance. In this context, fear of pain and the subsequent avoidance or escape behavior is similar to that seen in patients with a specific phobia [13]. Systematic desensitization [14] is a method commonly used to treat fear or anxiety through exposure to increasingly more anxiety-provoking encounters with the phobic stimuli while performing a behavior incompatible with avoidance or escape. Research over the past decade strongly supports the validity of the FA model for chronic pain [15], and thus suggests the effectiveness of exposure-based pain treatment. Various tasks involving movement or lifting have been used in exposure-based treatment [16]. Speed walking was chosen as the in vivo exposure task because of its demonstrated efficacy in the original behavioral work of Fordyce [9], and the task could be executed in the space available in a busy primary care clinic. Speed walking provides exposure to the feared task and an opportunity to change dysfunctional beliefs that maintain avoidance of walking faster. The final component of the PCB intervention was VPF [17]. VPF involves providing results of a targeted behavior that is graphed in lieu of verbal or written explanation. The time efficiency gained with VPF over other methods of feedback fits well in the primary care environment [18].
Early primary care-based efforts to translate interventions designed explicitly to change fear avoidance behavior and disability were no better than control/comparison interventions [8]. Interventions were found ineffective because of insufficient training for practitioners, and failure to select patients high on psychosocial risk factors such as fear avoidance [8]. We addressed these issues by designing a treatment approach based on previously successful interventions that could be delivered within the time and space constraints of a busy primary care setting by staff well trained in the delivery of biopsychosocial-based interventions for CNP [19]. Patients selected for this study scored high on a measure of fear avoidance.
The purpose of this study was to examine the effectiveness of this brief PCB intervention designed to reduce fear avoidance beliefs and improve function in patients with chronic low back pain. It was hypothesized that speed walking times and self-report measures of key components of the fear avoidance model would improve following the brief PCB intervention.
METHOD
Patients
Nine patients were identified by their physicians for participation in this study. Of the nine patients, three could not attend because of work obligations, a fourth was unable to attend due to unreliable transportation, and a fifth patient was disqualified because of a heart condition. Four patients met all criteria and agreed to participate in the study. Inclusion criteria for participation were (1) low back pain (LBP) of at least 6 month’s duration, (2) chronic noncancer LBP interfering significantly with activities of daily living, (3) reading and writing of English, (4) age of at least 19 years, and (5) substantial fear of movement with a raw score greater than 26 on the Tampa Scale for Kinesiophobia (TSK-11) [19]. Exclusion criteria were the presence of (1) a surgically correctable condition, (2) a coexisting medical condition that precluded an ability to do the required physical exercises, or (3) organic brain syndrome or psychosis. Ben (all patient names are pseudonyms) was a 64-year-old, married, Caucasian male with LBP for 240 months. Cathy was a 42-year-old, divorced, Caucasian female with LBP for 7 months. Debra was a 64-year-old, divorced, Caucasian female with LBP for 180 months. Ed was a 49-year-old, single, Caucasian male with LBP for 24 months.
The study was approved by the institutional review board and informed consent was obtained from each patient. Because of the multiple baseline with changing criteria design, patients were asked to come to the clinic for an hour each day for up to 20 days, as part of the consent process.
Research design
A multiple-baseline with changing criterion design was used to evaluate the efficacy of the brief PCB intervention on speed walking time. Delivery of the PCB intervention to each patient after varying lengths of time in the baseline phase was used to demonstrate a functional relationship between the intervention and speed walking times. This functional relationship can be most efficiently demonstrated through a within-subject design, rather than the use of a control or comparison group, providing confidence that the application of the intervention directly resulted in changes in walking times. The changing criterion aspect of the design was used because the ultimate speed walking time could not be emitted initially and required incremental improvement. Efficacy of the intervention was demonstrated with repeated improvement in speed walking time as the criterion was changed [20]. A pre-post-follow-up design was used to evaluate the intervention’s efficacy to change key elements of the fear avoidance model as assessed by six outcome measures.
Measures
Visual analogue scale
The visual analogue scale (VAS) is a single-item, patient-completed assessment of pain intensity. Patients use a 100-mm horizontal line with end points marked with labels “pain as bad as it can be” and “no pain” to record current pain intensity. The VAS has good psychometric properties and is a commonly used measure of pain level [21].
Tampa kinesiophobia scale
The TSK-11 is an 11-item measure that assesses fear of movement/(re)injury in back pain patients. Each item is rated on a 4-point Likert scale with scoring anchors labeled “Strongly Disagree,” “Disagree,” “Agree,” and “Strongly Agree.” A total score is calculated by summing across all items, with a possible range from 11 to 44; higher scores indicate greater fear of movement/(re)injury. The TSK-11 has demonstrated good internal consistency, test-retest reliability, responsiveness, concurrent validity, and predictive validity [22]. The TSK-11 possesses similar psychometric properties to the original TSK, but offers the advantage of brevity [22].
Pain and impairment relationship scale
The pain and impairment relationship scale (PAIRS) measures patients’ beliefs about the relationship between their pain and perceived impairment due to pain [23] and has been conceptualized as a measure of fear of pain [13]. The PAIRS consists of 15 items in the form of statements explicitly or implicitly attributing impairment to pain, with each item rated on a 7-point Likert scale anchored with degrees of agreement or disagreement. Higher scores on the PAIRS indicate a stronger belief that pain and impairment are related, and that one should restrict function in the presence of pain. The PAIRS has demonstrated good reliability and validity [24] and is sensitive to change following a cognitive-behavioral treatment program [25].
Catastrophizing subscale of the coping strategies questionnaire
The Coping Strategies Questionnaire (CSQ) is a 50-item inventory that assesses six cognitive and one behavioral pain coping strategies [26]. The catastrophizing subscale (CAT) of the CSQ consists of six items indicating the frequency with which each catastrophizing strategy is used (0 = “Never”, 6 = “Always”). Higher scores indicate a more exaggerated negative orientation toward pain stimuli and experience. The reliability and validity of the CAT has been well documented [26].
Beck depression inventory
The Beck Depression Inventory (BDI) is a 13-item self-report instrument that provides a quantitative measure of the cognitive and somatic aspects of depression. Each symptom is rated on a scale from 0 to 3. Lower scores indicate less depression. Considerable evidence is available on the reliability and validity of this commonly used measure [27].
Multidimensional pain inventory–pain interference scale
The MPI is composed of three sections with a total of 12 empirically derived subscales. The 9-item interference subscale used in this study produces a score that ranges from 0 to 6 with higher scores indicative of more perceived interference with daily activities. The MPI subscales including the Multidimensional Pain Inventory–Pain Interference Scale (MPI-IS) have demonstrated good reliability and validity when used together or as stand-alone scales [28].
Procedures
Orientation and baseline
The four patients participated in all aspects of the study individually, with no group involvement. The patients first completed consent procedures, and the TSK-11. The five remaining fear avoidance model measures were administered at baseline immediately prior to the collection of initial baseline walking time. Patients were informed regarding the purpose, execution, and safety issues of the walking track and program. The walking track was a 13.7 m (i.e., 45 ft) length of hallway in the patient use area of a primary care clinic. Patients executed a “semicircular” path at the turn-around points with no interruption of the gait rhythm. The turning maneuver at the ends of the track was demonstrated for safety reasons. During baseline, patients were asked to walk two laps, 54.9 m (i.e., 180 ft), of the walking track starting from a stationary standing position at the “start-finish” line. Patients were aware that they were timed, but no instructions were given regarding walking pace, as outlined by Fordyce [9]. The baseline walking time was recorded in seconds on a stopwatch by the study investigators. Patients completed three walking trials daily with a 5-min rest period between trials. Ben and Kathy reached a stable baseline in 6 trials over 2 days, Debra in 12 trials over 4 days, and Ed in 18 trials over 6 days.
Intervention
As each patient obtained a stable baseline, he or she was provided a 45-min education session that articulated the difference between acute and CNP. The patients were informed that their chronic LBP was not an indication of harm; consequently, plan-directed rather than pain-directed movement was an appropriate treatment. They were encouraged to take a management rather than curative approach to pain focusing more on outcomes related to function rather than total pain relief. The FA model of chronic pain along with a rationale for the speed walking task as an exposure strategy to overcome fear of movement was also presented. Patients then were given VPF regarding baseline data and asked to walk, not run, two laps on the hallway track, with the first goal set at 3 s faster than the average baseline time. Speed walking was timed in seconds by the study investigators. During each rest period, the patients were given an additional VPF regarding the last trial and the current goal. After three successful trials at the first goal, a second goal was set at 3 s faster than the average first goal performance. The intervention phase ended with three successful trials at the second goal and completion of the six outcome measures.
Follow-up
Two weeks following the completion of the intervention phase, each patient returned to the clinic and completed three trials of speed walking with VPF and 5 min of rest between trials. Each patient completed the six fear avoidance outcome measures to complete the study.
Treatment integrity and speed walking reliability
Two independent observers simultaneously recorded the occurrence or nonoccurrence of the delivery of 24 critical markers covering all aspects of the education session, speed walking exposure task, and VPF. Interobserver agreement was calculated using the point-by-point agreement ratio method [29], with interobserver agreement at 96 % for one patient and 100 % for the other three patients.
One of the three daily trials per patient included a second timer to check for timer agreement. All interobserver timings were within 0.05 s of the primary timer whose results were used in the study.
Data analysis
Speed walking data were analyzed using four methods: visual inspection of speed walking times, a comparison of speed walking mean times across conditions, an examination of percent of nonoverlapping data across conditions (PND) [30], and statistical analysis using hierarchical linear modeling (HLM).
The use of PND and HLM complements the visual analysis of data, where both methods of analysis provide information about the observed effects [31]. However, statistical analyses of single-subject or small n designs present a series of challenges, one of which is autocorrelation. This occurs because repeated observations within a subject tend to be more similar than observations coming from different subjects. Statisticians have developed a variety of strategies to accurately analyze single-subject or small n data, but there is no agreed-upon method applicable to all small n analyses [32]. One way to decide which statistical method to use is to conduct preliminary visual and statistical analyses on each dependent variable to assess the extent to which autocorrelation exists and the extent to which differences are observed in the data. In the present study, the preliminary analyses indicated significant autocorrelation, and the interest in both changes in level and slope during intervention and follow-up suggested that hierarchical linear modeling (using HLM 7.0) would be most appropriate. Primary research questions for this statistical model included the following: (1) Is there a stable baseline before intervention begins, (2) does the introduction of treatment result in a change in the slope observed, (3) does the introduction of treatment result in a change in the level observed, and (4 and 5) do follow-up data points also demonstrate a change in slope and/or level from baseline. Of note, both treatment conditions were combined for the purposes of this analysis, which appeared appropriate given the visual inspection of the data. The analysis was conducted with restricted maximum-likelihood estimation, which is less biased in small samples, but alters the interpretability of deviance as a model comparison statistic [36]. It should also be noted that while HLM can be used in small samples, this reduces generalizability of results, and in particular the magnitude of specific parameters (e.g., time-related changes in outcome) may be different in a separate or larger sample.
Participant questionnaire results for fear avoidance and outcome measures were analyzed using visual inspection and supplemented with statistical analysis of pre-post-follow-up data. The use of paired t tests allows each individual to serve as their own baseline, and directly compares the changes across participants. While this increases the power of this analysis, four subjects are too few to have confidence in a negative finding. Positive findings should be taken tenuously, but when observed, statistical significance may lend some degree of confidence to the difference between baseline and posttreatment or follow-up.
RESULTS
Results indicated that the PCB intervention had a positive influence on participants’ speed walking times as assessed by visual inspection of the data, percentage of nonoverlapping data, and statistical analysis. Visual inspection of the data and paired sample t tests provided evidence of the PCB intervention’s efficacy with patients’ perceived pain level, fear of movement, perceived relationship between pain and impairment, pain catastrophizing, depressive feelings, and interference of pain with daily activities.
Speed walking data
Visual inspection and comparison of means
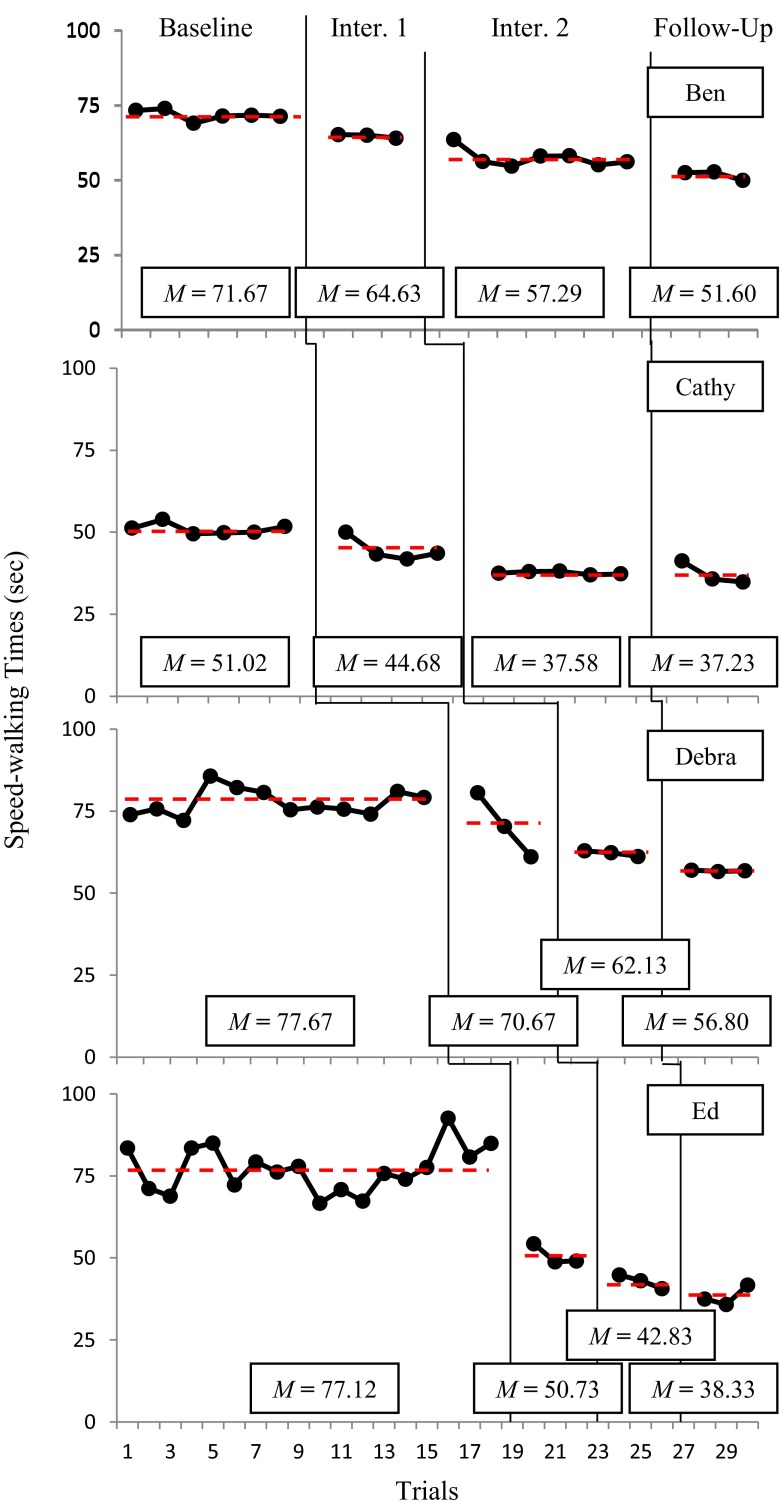
Visual inspection of the data presented in Fig. 1 suggests that the PCB intervention resulted in consistent and sustained reductions in participants’ speed walking times. Figure 1 also shows that despite persistent LBP which typically results in slower speed walking times, three of the four participants had 27–28 % faster times at Follow-up than at Baseline. The fourth participant demonstrated a 50 % reduction in speed walking time from Baseline to Follow-up.
Fig. 1.
Speed walking times across baseline, intervention (lecture, goal setting, visual performance feedback), and follow-up
Percentage of nonoverlapping data
Another way of assessing the efficacy of PCB intervention is to compute the percentage of nonoverlapping data (PND) between Baseline and Intervention [33]. The PND was computed by dividing the number of Intervention and Follow-up data points that exceeded the lowest initial Baseline data points by the total number of Intervention and Follow-up data points, respectively, and multiplying each result by 100 [30]. Results indicated that two of the four participants, Ben and Ed, had PND scores of 100 %, suggesting that the PCB intervention was “very effective” for these participants. PND scores for participants Cathy and Debra were 89 and 83 %, respectively, suggesting that the PCB intervention was “effective” for these patients [30] (Table 1).
Table 1.
Percentage of nonoverlapping data (PND) across intervention and follow-up conditions
| Patient | Baseline and intervention 1 and 2 PND (n) | Baseline and follow-up PND (n) |
|---|---|---|
| Ben | 100 (10) | 100 (3) |
| Cathy | 89 (9) | 100 (3) |
| Debra | 83 (6) | 100 (3) |
| Ed | 100 (6) | 100 (3) |
Statistical analysis of speed walking trials
A fourth way of assessing the efficacy of the speed walking aspect of the PCB intervention is HLM because it can account for autocorrelation, was able to model both changes in slope and level of the dependent variable, and allow individuals to have different numbers of observations [31, 34, 35].
HLM analysis sought a relatively parsimonious model with lower deviance, statistically significant predictors, and good interpretability. After starting with a basic (intercept only) model, we introduced each predictor sequentially, and removed predictors as they lost interpretability. This process is presented in Table 2. Initial models include few predictors, and the final model (model H) presents a parsimonious and statistically supportable model of the observed data.
Table 2.
Comparison of alternative HLM models of walking speed
| Model | Interceptb | Trials | TXa_level | TX_slope | FUa_level | FU_slope | Reliability | Deviance |
|---|---|---|---|---|---|---|---|---|
| A | 60.846** | 0.954 | 667.0 | |||||
| B | 75.092** | −1.410*** | 0.986 | 591.6 | ||||
| C | 75.430** | −1.301*** | −3.757* | 0.986 | 586.4 | |||
| D | 74.947** | −1.224*** | −0.361** | 0.987 | 588.1 | |||
| E | 74.156** | −1.194*** | 6.800 | −0.802* | 0.988 | 579.2 | ||
| F | 70.236*** | 0.027 | −1.273*** | −24.769*** | 0.988 | 541.8 | ||
| G | 69.023*** | 0.268 | −1.432*** | −1.490*** | 0.991 | 525.5 | ||
| H | 70.375*** | −1.260*** | −1.295*** | 0.992 | 527.9 |
*p < 0.05; **p < 0.01; ***p < 0.001
a TX treatment, FU follow-up
bIntercept represents the estimated average walking time at time zero, set at the first week of baseline. The variable trials represent each trial of the study, including baseline, treatment, and follow-up trials; statistically, trials represent the change in walking speed over time, not accounting for treatment. TX_slope is set at 0 during baseline and follow-up, and equal to trials during intervention phases; statistically, this variable represents the change in slope during intervention phases. TX_level is a dichotomous variable which represents the change in level of walking speed across intervention phases. FU_slope represents the change in slope during follow-up. FU_level represents the change in level of walking speed across follow-up observations
The interpretation of model H allows a statistical answer to the five research questions listed in the “METHOD” section (see table comments for additional information). In this case, the inclusion of a significant intercept simply indicates that at time 0 (i.e., the beginning of baseline), participants have a non-zero amount of time required to walk the established course (predicted at about 70.4 s). More importantly, by dropping the predictor variable trials, model H demonstrates that after accounting for changes in walking speed related to intervention and follow-up, there is no longer any slope. This specifically supports the presence of a stable baseline prior to intervention. Second, the inclusion of TX_slope and the noninclusion of TX_level indicate that the best way to interpret the effects of the intervention is that walking speed decreases steadily during intervention, as opposed to an immediate drop in time at the introduction of the intervention. According to this model, participants decrease about 1.26 s per trial during intervention. Finally, the inclusion of FU_slope and noninclusion of FU_level suggest that at follow-up, the participants continued to demonstrate ongoing reductions in walking speed of about 1.30 s per trial, at least while engaging in the follow-up trials. This is consistent with the observation that mean times decreased from baseline to intervention, and were lower yet in the follow-up trials.
Fear avoidance measures
Visual inspection of the mean scores across patients on measures of the key components of the fear avoidance model suggested that patients decreased their perceived pain level, fear of movement, belief that pain is related to impairment, catastrophizing in response to pain, depressive feelings, and interference of pain with daily activities. Closer inspection reveals some variation, as the first patient did not show improvement in fear of movement or depression, and the second did not report a decrease in pain interference (Table 3).
Table 3.
Mean scores across participants on measures relevant to fear avoidance
| Measure | Patients | Mean | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Visual analogue scale | |||||
| Baseline | 63 | 47 | 37 | 20 | 41.75 |
| Treatment | 69 | 51 | 4 | 3 | 31.75 |
| Follow-up | 31 | 12 | 7 | 15 | 16.25* |
| Tampa scale for kinesiophobia | |||||
| Baseline | 30 | 28 | 37 | 36 | 32.75 |
| Treatment | 34 | 20 | 28 | 18 | 25.00 |
| Follow-up | 31 | 19 | 29 | 17 | 24.00 |
| Pain and impairment relationship scale | |||||
| Baseline | 78 | 48 | 76 | 61 | 65.75 |
| Treatment | 57 | 38 | 45 | 38 | 44.50* |
| Follow-up | 58 | 26 | 51 | 33 | 42.00*** |
| Coping strategies questionnaire–catastrophizing scale | |||||
| Baseline | 3.16 | 1.33 | 3.33 | 1.33 | 2.29 |
| Treatment | 3.66 | 0.67 | 0.67 | 1.17 | 1.54 |
| Follow-up | 2.67 | 0.33 | 0.5 | 0.17 | 0.92** |
| Beck depression inventory | |||||
| Baseline | 14 | 1 | 17 | 5 | 9.25 |
| Treatment | 16 | 1 | 13 | 2 | 8.00 |
| Follow-up | 15 | 0 | 13 | 2 | 7.50 |
| Multidimensional pain inventory–interference scale | |||||
| Baseline | 4.10 | 2.18 | 5.22 | 3.70 | 3.80 |
| Treatment | 4.30 | 1.45 | 3.18 | 2.30 | 2.81 |
| Follow-up | 3.73 | 2.72 | 3.90 | 1.60 | 2.99 |
*p < 0.05; **p < 0.10; ***p = 0.001
Given the very small sample, inferential statistical tests for this are underpowered. This limits confidence in negative findings and generalizability. Positive findings with paired t tests, which use each subject as their own control, do suggest a greater statistical separation between baseline scores and those reported during intervention and follow-up. However, it should be noted that conducting 12 comparisons increases the risk of a type I error, and we have elected not to correct this, as it would further increase the odds of an already likely type II error. In short, these results should be taken with caution. With this in mind, it is notable that statistically significant effects were found for the following comparisons: The belief that pain is related to impairment (PAIRS) decreased after intervention (t(3) = 4.91, p = 0.016) and at follow-up (t(3) = 13.57, p = 0.001); pain scores (VAS) decreased at follow-up (t(3) = 3.69, p = 0.035), but not immediately after the intervention. There was also a nonsignificant trend for decreased catastrophizing at follow-up (t(3) = 2.71, p = 0.074), but not immediately after intervention.
DISCUSSION
This study provides evidence that a brief PCB intervention consisting of a single education session, speed walking as a graded in vivo exposure task, and VPF can produce measureable positive changes in fear avoidance beliefs and function for patients with chronic LBP. Multiple methods of examination consistently indicate that speed walking times improved from baseline only after the PCB intervention was delivered. In addition, visual inspection indicated that all six fear avoidance model outcome measures improved from baseline to end of study and five of six outcome measures improved from end of study to follow-up. Statistical results indicated a significant reduction in pain level from baseline to follow-up. Also, significant baseline to end of treatment and baseline to follow-up improvement were found for the PAIRS. The other measures of the FA model, the TSK, CAT, and BDI, exhibited visual differences while the PAIRS demonstrated more robust findings with both visual and statistical significance. These findings regarding the PAIRS have been found consistently in our work [25]. We have begun to conceptualize the PAIRS as a measure of one’s belief that one can function despite pain, a cornerstone to most cognitive-behavioral interventions for CNP.
This PCB intervention could be helpful for patients with LBP or other CNP with fear of movement as a prominent feature. PCP screening for fear of movement earlier in treatment, perhaps in the acute or subchronic phase, could prevent disability, reduce cost, and improve the quality of life with a return to activities consistent with patients’ values [15]. Information gathered from this intervention may help PCPs and primary care teams with treatment planning. For example, progress toward treatment goals could be reinforced. Lack of progress would provide immediate information beyond self-reported pain level to guide treatment planning. The team could quickly develop hypotheses about the causes for lack of progress, such as continued maintenance of inaccurate pain beliefs; undiscovered anatomical issues; positive reinforcement, such as compensation or attention for inactivity; or negative reinforcement, such as avoidance of unpleasant tasks or activities [9]. Future research might examine brief PCB interventions designed specifically to address factors that may be maintaining inactivity in patients with CNP for reasons other than fear of movement. Regardless of the reason for inactivity, clinic-based observation of activity provides the PCP with better data than patient self-report on which to address further treatment.
Speed walking was selected as the in vivo exposure task in this study for several reasons. First, speed walking is well-documented as an effective intervention and outcome measure in the behavioral work by Fordyce [9]. Second, speed walking fits well in our clinic space. Finally, all of the present patients expressed concern about walking speed, and it was speculated that improved walking speed might generalize to other valued functional activities. Anecdotally, generalization occurred as Ben reported that he started walking with his wife, Cathy hung pictures that had been sitting for a long time, Debra spent quality time shopping with her teenage daughter, and Ed walked around a lake near his house, something he had been fearful of doing. Assessment of patient-specific fear avoidance on tasks other than speed walking that can be implemented in a primary care clinic could be beneficial to patients and providers [16].
Our patients’ expressed willingness to participate, satisfaction with treatment, and had a very low no-show rate. They received direct handoff to study staff from their PCP, came to the same location, and interacted with the same office personal as their PCP, all advantages of integrated care [6]. Patients with fears related to movements other than walking, or schedules that do not allow daily sessions may require interventions tailored to their needs. Implementation to clinics with other characteristics may require alterations to the intervention. Although the implementation to other settings may require patient or clinic accommodations in the specific details, the overall design of this study may provide a template that can be used to structure and evaluate integrated PCB interventions. The present findings demonstrate the need for larger trials designed to examine the effectiveness of practical interventions delivered in real-life routine practice conditions.
While the intervention was successfully implemented in a busy primary care clinic, it was delivered by a health psychologist, not a primary care physician. Also, a key component of the intervention was a 45-min education session about pain and patient misperceptions. Delivery of a 45-min education session by a professional not typically present in medical clinics represents a major limitation of this study. The PCB intervention would likely be perceived as impractical and too time consuming by most busy primary care physicians [15]. Future studies might examine ways PCMH staff such as nurses or medical assistants could provide the intervention and gather activity data [6]. Additionally, advances in technology may provide viable alternatives for efficient delivery of the educational session and VPF. Nevertheless, the results provide support for greater integration of behavioral health providers into patient-centered primary care settings. The findings are consistent with the call for integrated interdisciplinary care for the treatment of chronic pain and illness conditions seen in primary care [37, 38].
In summary, this study provided preliminary evidence that a brief cognitive-behavioral intervention designed to reduce fear avoidance beliefs and improve function can be successfully delivered in a primary care clinic.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
Adherence to ethical standards
All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee on human experimentation at Creighton University and with the Helsinki Declaration of 1975, as revised in 2000.
Footnotes
Implications
Practice: A brief fear avoidance model based on cognitive-behavioral intervention can be effectively implemented into primary care.
Policy: Health care reform models and reimbursement policies should include access to integrated biopsychosocial treatments designed to improve function in chronic pain patients treated in primary care settings.
Research: Further research on design and delivery of cognitive behavioral interventions in the primary care setting is urgently needed.
References
- 1.Leverence RR, Williams RL, Potter M, et al. Chronic non-cancer pain: a siren for primary care–a report from the primary care multiethnic network (PRIME Net) J Am Board Fam Med. 2011;24:551–561. doi: 10.3122/jabfm.2011.05.110030. [DOI] [PubMed] [Google Scholar]
- 2.Loeser JD. Multidimensional pain management. In: Merskey H, Loeser JD, Dubner D, editors. The path of pain 1975–2005. Seattle: International Association for the Study for Pain; 2003. pp. 503–511. [Google Scholar]
- 3.Roth RS, Geisser ME, Williams DA. Interventional pain medicine: retreat from the biopsychosocial model of pain. Transl Behav Med. 2012;2:106–116. doi: 10.1007/s13142-011-0090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gatchel RJ, Oordt MS. Clinical health psychology and primary care. Washington D.C: American Psychological Association; 2003. [Google Scholar]
- 5.DeBar LL, Kindler L, Keefe FJ, et al. A primary care-based interdisciplinary team approach to the treatment of chronic pain utilizing a pragmatic clinical trials framework. Transl Behav Med. 2012;2:523–530. doi: 10.1007/s13142-012-0163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auxier AM, Miller BF, Rogers J. Integrated behavioral health and the patient-centered medical home. In: Talen MR, Burke Valeras A, editors. Integrated behavioral health in primary care. New York: Springer; 2013. pp. 33–52. [Google Scholar]
- 7.McDaniel SH. An introduction to primary care and psychology. Am Psychol. 2014;69:325–331. doi: 10.1037/a0036222. [DOI] [PubMed] [Google Scholar]
- 8.Pincus T, Smeets RJEM, Simmonds MJ, Sullivan MJL. The fear avoidance model disentangled: improving the clinical utility of the fear avoidance model. Clin J Pain. 2010;26:739–746. doi: 10.1097/AJP.0b013e3181f15d45. [DOI] [PubMed] [Google Scholar]
- 9.Fordyce WE. Behavioral methods for chronic pain and illness. St Louis: Mosby; 1976. [Google Scholar]
- 10.Louw A, Diener I, Butler DS, Puentedura EJ. The effect of neuroscience education on pain, disability, anxiety, and stress in chronic musculoskeletal pain. Arch Phys Med Rehabil. 2011;92:2041–2056. doi: 10.1016/j.apmr.2011.07.198. [DOI] [PubMed] [Google Scholar]
- 11.Moseley GL. Evidence for a direct relationship between cognitive and physical change during an education intervention in people with chronic pain. Eur J Pain. 2004;8:39–45. doi: 10.1016/S1090-3801(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 12.Vlaeyen JWS, Linton SJ. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain. 2012; 153: 1144-1147. [DOI] [PubMed]
- 13.Vlaeyen JWS, de Jong J, Sieben J, Crombez G. Graded exposure in vivo for pain-related fear. In: Turk DC, Gatchel RJ, editors. Psychological approaches to pain management: a practitioner’s handbook. 2. New York: Guilford Press; 2002. pp. 210–233. [Google Scholar]
- 14.Wolpe J. Psychotherapy by reciprocal inhibition. Palo Alto: Stanford University Press; 1958. [Google Scholar]
- 15.Crombez G, Eccleston C, Van Damme S, Valaeyen, JWS, Karoly P. Fear-avoidance model of chronic pain: the next generation. Clin J Pain. 2012; 475–483. [DOI] [PubMed]
- 16.Trost Z, France CR, Thomas JS. Examination of the photograph series of daily activities (PHODA) scale in chronic low back pain patients with high and low kinesiophobia. Pain. 2009;141:276–282. doi: 10.1016/j.pain.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Codding RS, Livanis A, Pace GM, Vaca L. Using performance feedback to improve treatment integrity of classwide behavior plans: an investigation of observer reactivity. J Appl Behav Anal. 2008;41:417–422. doi: 10.1901/jaba.2008.41-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burke RV, Howard MR, Peterson JL, Peterson RW, Allen KD. Visual performance feedback: effects on targeted and non-targeted staff. Behav Modif. 2012;36:687–704. doi: 10.1177/0145445511436007. [DOI] [PubMed] [Google Scholar]
- 19.de Jong JR, Vlaeyen JWS, Onghena P, Goossens MEJB, Geilen M, Mulder H. Fear of movement / (re)injury in chronic low back pain: education or exposure in vivo as mediator to fear reduction? Clin J Pain. 2005; 9–17 [DOI] [PubMed]
- 20.Hersen M, Barlow DH. Single case experimental designs: strategies for studying behavior change. New York: Pergamon Press; 1976. [Google Scholar]
- 21.Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976;2:175–84. doi: 10.1016/0304-3959(76)90113-5. [DOI] [PubMed] [Google Scholar]
- 22.Woby SR, Roach NK, Urmston M, Watson PJ. Psychometric properties of the TSK-11: a shortened version of the Tampa scale for Kinesiophobia. Pain. 2005;117:137–144. doi: 10.1016/j.pain.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 23.Riley JF, Ahern DK, Follick MJ. Chronic pain and functional impairment: assessing beliefs about their relationship. Arch Phys Med Rehabil. 1988;69:579–582. [PubMed] [Google Scholar]
- 24.Slater MA, Hall HF, Hampton Atkinson J, Garfin SR. Pain and impairment beliefs in chronic low back pain: validation of the Pain and Impairment Relationship Scale (PAIRS) Pain. 1991;41:51–56. doi: 10.1016/0304-3959(91)90146-O. [DOI] [PubMed] [Google Scholar]
- 25.Guck TP, Fleischer TD, Willcockson JC, Criscuolo CM, Leibrock LG. Predictive validity of the pain and impairment relationship scale in a chronic nonmalignant pain population. Arch Phys Med Rehabil. 1999;80:91–95. doi: 10.1016/S0003-9993(99)90313-1. [DOI] [PubMed] [Google Scholar]
- 26.Rosenstiel A, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. 1983;17:33–44. doi: 10.1016/0304-3959(83)90125-2. [DOI] [PubMed] [Google Scholar]
- 27.Gould J. A psychometric investigation of the standard and short form beck depression inventory. Psychiatr Rep. 1982;51:1167–1170. doi: 10.2466/pr0.1982.51.3f.1167. [DOI] [PubMed] [Google Scholar]
- 28.Jacob MC, Kerns RD. Assessment of the psychosocial context of the experience of chronic pain. In: Turk DC, Melzack R, editors. Handbook of pain assessment. 2. New York: Guilford Press; 2001. pp. 362–384. [Google Scholar]
- 29.Kazdin AE. Single-case research design: methods for clinical and applied settings. New York: Oxford University Press; 1982. [Google Scholar]
- 30.Scruggs TE, Mastropieri MA. Summarizing single-subject research: issues and applications. Behav Modif. 1998;22:221–242. doi: 10.1177/01454455980223001. [DOI] [PubMed] [Google Scholar]
- 31.Davis DH, Gagne P, Fredrick LD, Alberto PA, Waugh RE, Haardorfer R. Augmenting visual analysis in single-case research with hierarchical linear modeling. Behav Modif. 2013;37:62–89. doi: 10.1177/0145445512453734. [DOI] [PubMed] [Google Scholar]
- 32.Campbell JM, Herzinger CV. Statistics and single subject methodology. In: Gast DL, editor. Single subject methodology in behavioral sciences. New York: Routledge; 2010. [Google Scholar]
- 33.Kazdin AE. Methodological and interpretive problems in single-case experimental designs. J Consult Clin Psychol. 1978;46:629–642. doi: 10.1037/0022-006X.46.4.629. [DOI] [PubMed] [Google Scholar]
- 34.DeLucia C, Pitts SC. Applications of individual growth curve modeling for pediatric psychology research. J Pediatr Psychol. 2006;31:1002–1023. doi: 10.1093/jpepsy/jsj074. [DOI] [PubMed] [Google Scholar]
- 35.Raudenbush SW, Bryk AS. Hierarchical linear models: applications and data analysis methods. 2. Thousand Oaks: Sage Publications; 2002. [Google Scholar]
- 36.Singer JD, Willett JB. Applied longitudinal data analysis: modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 37.Hunter CL, Goodie JL, Oordt MS, Dobmeyer AC. Integrated behavioral health in primary care: step-by-step guidance for assessment and intervention. Washington, DC: American Psychological Association; 2009. [Google Scholar]
- 38.McDaniel SH, Fogarty CT. What primary care psychology has to offer the patient-centered medical home. Prof Psychol Res Pract. 2009;40:483–492. doi: 10.1037/a0016751. [DOI] [Google Scholar]