Abstract
Until relatively recently, long-acting injectable (LAI) formulations were only available for first-generation antipsychotics and their utilization decreased as use of oral second-generation antipsychotics (SGA) increased. Although registry-based naturalistic studies show LAIs reduce rehospitalization more than oral medications in clinical practice, this is not seen in recent randomized clinical trials. PROACTIVE (Preventing Relapse Oral Antipsychotics Compared to Injectables Evaluating Efficacy) relapse prevention study incorporated efficacy and effectiveness features. At 8 US academic centers, 305 patients with schizophrenia or schizoaffective disorder were randomly assigned to LAI risperidone (LAI-R) or physician’s choice oral SGAs. Patients were evaluated during the 30-month study by masked, centralized assessors using 2-way video, and monitored biweekly by on-site clinicians and assessors who knew treatment assignment. Relapse was evaluated by a masked Relapse Monitoring Board. Differences between LAI-R and oral SGA treatment in time to first relapse and hospitalization were not significant. Psychotic symptoms and Brief Psychiatric Rating Scale total score improved more in the LAI-R group. In contrast, the LAI group had higher Scale for Assessment of Negative Symptoms Alogia scale scores. There were no other between-group differences in symptoms or functional improvement. Despite the advantage for psychotic symptoms, LAI-R did not confer an advantage over oral SGAs for relapse or rehospitalization. Biweekly monitoring, not focusing specifically on patients with demonstrated nonadherence to treatment and greater flexibility in changing medication in the oral treatment arm, may contribute to the inability to detect differences between LAI and oral SGA treatment in clinical trials.
Key words: relapse prevention, schizophrenia, psychotic symptoms, negative symptoms, clinical trial design
Introduction
Relapse prevention remains a major public health challenge in schizophrenia treatment. Symptom exacerbations increase personal suffering, psychosocial deterioration, family/societal burden, risk of harm to self and others, and impair progress toward recovery.1–3 It has even been suggested that repeated relapses are neurotoxic, aggravating the long-term course of illness.1
There is ample evidence that antipsychotic medication substantially reduces relapse risk.1–3 However, even partial nonadherence to oral medications may erode this potential benefit.2,4 Delivery of continuous, assured medication through long-acting injectable (LAI) antipsychotic formulations has a long history of use to minimize nonadherence.2,3 This potential clinical effect is not pharmacodynamic although there may be pharmacokinetic advantages based on lower and less variable plasma concentrations with LAI administration.3 The primary role of LAIs in relapse reduction is pragmatic. Once administered, there is no risk of missed daily doses, and if a patient fails to appear for a scheduled injection, clinicians are immediately aware of nonadherence well before any possible consequences. In contrast, patients may fail to fill oral medication prescriptions or report that they are taking medication when they are either partially or entirely nonadherent.4 Unfortunately, clinicians have difficulty in detecting these behaviors and tend to overestimate adherence in their patients.3,4
Although many support the face validity of assumptions regarding LAI superiority, there are disparate findings in both early studies with FGAs and more recent studies that investigate SGAs. Leucht and colleagues5 reported a 30% reduction in relapse rate favoring LAI over oral medications; this meta-analysis drew on studies stretching back 40 years, and most compared first-generation antipsychotics (FGA) oral and LAIs. They also reported that studies that targeted relapsing patients with confirmed nonadherence showed the most robust differences. There was a 14-year hiatus between the last studies with FGA LAIs and the first studies with second-generation antipsychotics (SGA) LAIs. A recent meta-analysis in this journal by Kishimoto and colleagues6 failed to find advantages for LAIs in general, but fluphenazine decanoate stood out as more effective than oral FGA medications. The finding of fluphenazine decanoate superiority may be a function, the time that the studies were done (1970s) when the only medications available were FGAs and therefore all used oral FGA comparators. Another meta-analysis of 19 studies published between 2000 and 2011 found no difference overall between LAI and oral medications.7 Kishimoto and colleagues8 also reported an overall relapse rate of 37% among patients receiving either oral or LAI formulations of FGAs, while the relapse rate on SGAs (predominately studies of oral SGAs) was 29%. In a most recent evaluation of mirror-image studies, LAIs were superior over oral medications with respect to reducing hospitalization.9 Disparate findings across studies and over time might reflect real drug differences and/or be accounted for by patient selection biases and methodological variations. Such variances have historically hampered clinical interpretation as to the most apt positioning of LAIs in our clinical armamentarium.
PROACTIVE (Preventing Relapse Oral Antipsychotics Compared to Injectables Evaluating Efficacy) was initiated in 2006 to inform clinical decision making by conducting an up-to-date relapse prevention study that included both efficacy/explanatory and effectiveness/pragmatic features. Table 1 presents study characteristics that highlight these considerations. LAI-R was selected because it was the only SGA LAI available in 2006. Historically, while LAI-oral comparative studies with FGAs showed similar patterns of relapse in a first year of treatment,2 possible differences in relapse rates emerged in the second year.10 Treatment in PROACTIVE lasted for up to two and half years, included patients who were within a year of prior relapse, and was multisite. This article focuses on relapse, rehospitalization, and symptom change.
Table 1.
Comparison of Efficacy/Explanatory and Effectiveness/Pragmatic Clinical Trials
| Domain | Effectiveness/Pragmatic Trial | Efficacy/Explanatory Trial |
|---|---|---|
| Participant eligibility | “All comers” | Limited to select, well-defined study population |
| Experimental intervention | Flexibility in implementation | Clearly delineated and rigorously followed |
| Comparison intervention | Usual practice | Clearly defined, often placebo condition rather than clinically driven choice |
| Companion intervention clinician expertise | Accommodates clinical styles in implementation | Rigidly applied to minimize clinician impact |
| Outcome | Clinically meaningful | Direct consequence of intervention |
| Adherence | No explicit measurement | Measured; might be exclusionary criterion |
| Clinician adherence to protocol | Effectiveness not monitored | Efficacy closely monitored |
| Analysis of primary outcome | Inclusive of all patients | Intention-to-treat |
Source: Adapted from Thorpe et al. 25
Methods
Patients with a Diagnostic and Statistical Manual of Mental Disorders IV-TR diagnosis of schizophrenia or schizoaffective disorder11 were enrolled at 8 US academic centers and, after providing informed consent and completing baseline assessments, were randomly assigned to receive either LAI-R or physician’s choice of oral SGA medication. Inclusion criteria included age between 18–65 years, symptom exacerbation within 12 months of screening but community dwelling for at least 4 weeks, at least moderately ill (Clinical Global Impressions [CGI] severity score of four or greater), and able to provide written informed consent. Exclusion criteria included first episode of psychosis, allergy to risperidone, inadequate prior response to risperidone, treatment-refractoriness and/or lack of response to clozapine, or medical instability. Pregnant or lactating women were excluded. Receipt of an LAI was not an exclusion criterion; patients could be randomized to either LAI-R or oral medication. If randomized to LAI-R and receiving a FGA LAI, they were switched to LAI-R. If randomized to oral, the LAI was discontinued and an oral SGA was initiated. The study (clinicaltrials.gov NCT00330863) was approved by Institutional Review Boards at all sites. Study conduct and safety were monitored by a National Institute of Mental Health, Data and Safety Monitoring Board. It was conducted between August 2006 and January 2011. Study duration was up to 30 months for the 68% of subjects enrolled by July 2008; for those subsequently enrolled, duration was between 17 and 29 months.
Study Medications
Following randomization, medication was administered openly, guided by an evidence-based Medication Manual developed by the investigators (available upon request). Subjects were seen biweekly; LAI-R subjects received injections, and Oral SGA subjects received a 2-week medication supply. Antipsychotic medications, provided by the manufacturers, were cost-free. We assessed adherence at biweekly visits. Subjects who missed scheduled appointments received follow-up reminders, but study staff did provide treatment in the community. Antipsychotic polypharmacy was discouraged. Mood stabilizers and/or antidepressant medications prescribed prior to randomization were continued. Anticholinergic medication was permitted for extrapyramidal side effects (benztropine mesylate preferred), and propranolol was recommended for akathisia. Insomnia could be treated with hypnotic agents (including benzodiazepines) or low dose quetiapine up to 200mg/d). LAI-R was initiated with a 25-mg injection. Oral antipsychotic medication was continued for at least 3 weeks before tapering with the goal of completing cross-titration within 6 weeks. Injection dosage could be increased as needed to 37.5 or 50 mg or reduced to 12.5mg. Subjects receiving a FGA LAI were switched to LAI-R and did not receive oral supplementation. Most subjects received gluteal injections, alternating sides. If symptoms worsened, dose increases of LAI-R were recommended. Oral risperidone supplementation was allowed to manage symptom exacerbation.
Subjects randomized to oral SGAs continued the oral SGA they were receiving or, if they were not receiving an oral SGA, any marketed oral SGAs could be selected. These included aripiprazole, olanzapine, quetiapine, risperidone, and ziprasidone at study initiation. Paliperidone, asenapine, and iloperidone were added upon Food and Drug Administration (FDA) approval. If subjects were receiving an LAI, it was discontinued and an oral SGA initiated. Oral antipsychotics could be changed based on lack of efficacy, side effects, or patient preference.
Assessment
Chart diagnosis was confirmed by diagnostic case conferences, led by the site Principal Investigator. Subjects were assessed by on-site clinical raters and study psychiatrists biweekly for medication adherence, vital signs, symptoms, and side effects. Symptoms were assessed with an abbreviated version of the Brief Psychiatric Rating Scale (BPRS)12; items included grandiosity, suspiciousness, unusual thought content, hallucinatory behavior, conceptual disorganization, mannerisms and posturing, blunted affect and emotional withdrawal, and the CGI.13 Use of services including hospitalization, emergency room visits, and crisis intervention was recorded. Quarterly visits included the full BPRS, CGI, the Scale for Assessment of Negative Symptoms (SANS),14 the Scale of Functioning (SOF),15 the Abnormal Involuntary Movement Scale,16 the Barnes Akathisia Scale,17 the Simpson-Angus Scale,18 a modified sexual side effects questionnaire,19 and the New Antipsychotics Metabolic Evaluation Scale.20 Fasting blood chemistry, glucose, cholesterol, and lipid profiles were evaluated quarterly. The BPRS, SANS, and CGI were completed by 2 “Master Raters.” The first, at The Zucker Hillside Hospital, was an MSW with extensive experience in completing these rating scales and training others to do so. In addition to completing assessments, she trained all site raters. The second, at the University of Iowa, was a research coordinator with many years of researcher assessment experience. They interviewed subjects using a live, 2-way video connection and were masked to treatment assignment. These highly experienced clinical assessors provided the standard for training and reliability and completed all assessments during the 5-year study. Interrater reliability was excellent, with intraclass correlations (ICC) of 0.75 between the Master Raters, ICC of 0.79 between site and Master Raters, and ICC of 0.75 among site raters.
Primary Outcome
Relapse, adapted from criteria first used by Csernansky and colleagues,21 was defined by (1) psychiatric hospitalization for worsening symptoms but not for social reasons; (2) increase in level of psychiatric care (eg, significant crisis intervention to avert hospitalization, emergency room visit, increase in frequency of contact to maintain outpatient status); (3) substantial clinical deterioration as indicated by a score of 6 (much worse) or 7 (very much worse) on the CGI-I scale, or a sustained increase in psychotic symptoms as rated by either site or the Master Rater; and (4) deliberate self-injury, suicidal or homicidal ideation that was judged clinically significant as determined by the investigator, violent behavior resulting in clinically significant injury to another person or property damage. Time of first and subsequent relapses was independently evaluated by a Relapse Monitoring Board (RMB) of schizophrenia experts, masked to treatment assignment. The RMB also identified less severe episodes of symptom exacerbation defined by substantial and sustained increases in psychotic symptoms that lasted at least 4 weeks (3 biweekly visit ratings).
Secondary Outcomes
Relapse did not require discontinuation of treatment or study participation. Reasons for treatment discontinuation included discontinuation of LAI-R in the injectable arm, receipt of a LAI in the oral arm, or receipt of clozapine in both. Subjects who discontinued randomized treatment continued to be assessed quarterly. Reasons for study discontinuation included serious and/or life-threatening adverse event (AE); serious and/or life-threatening clinical circumstances (eg, uncontrollable violence or suicidal behavior and/or severe relapse); withdrawal of consent; loss to follow-up; serious protocol violation; or administrative reasons.
Symptom and functioning measures included BPRS, SANS, and CGI ratings by the Master Raters, and SOF ratings by on-site raters.
Statistical Analysis
Subjects randomly assigned to treatment who received at least one dose of medication (LAI-R injection or oral prescription) were included in intent-to-treat analyses. The primary outcome was time to first relapse compared between the 2 groups by Kaplan-Meier survival analysis.21 Time to first hospitalization was also compared between groups. Secondary outcome measures included psychotic symptoms, other psychiatric symptoms, social functioning, and adverse effects.
BPRS measures and SANS affective flattening and alogia were highly positively skewed and were analyzed using generalized linear mixed model regression analyses with lognormal error distribution (SAS GLIMMIX, Version 9.2). Supplemental analyses specifying gamma-distributed error were very similar. SANS avolition-apathy and asociality-anhedonia, the CGI severity and improvement, and the 2 SOF variables were reasonably symmetric-unimodal and were analyzed using general linear mixed effects regression models that assumed normally distributed error (SAS MIXED, Version 9.2).22 Both statistical procedures assume data are missing at random. Mixed effects regression models used data after baseline, with baseline values as a covariate (except for CGI improvement). Fixed design effects were treatment, visit, and their interaction. First-degree autoregressive structure (AR1) was specified for the covariance matrices (up to ten post-baseline measures), and a random subject effect was included to model between-subject variability.23 Statistical significance was set at unadjusted 2-tailed P = .05.
Results
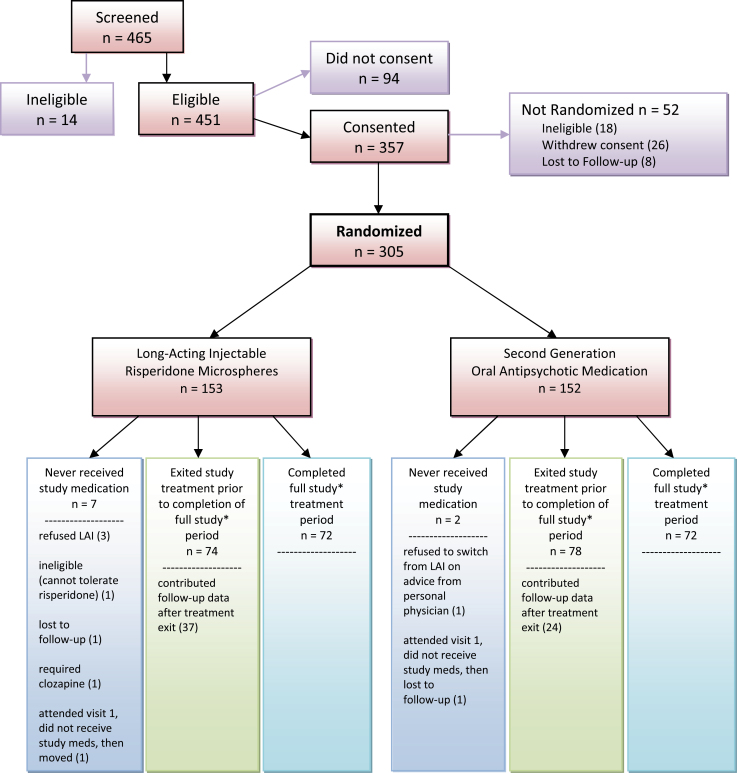
Figure 1 shows that 305 subjects were randomly assigned to treatment; 153 to LAI-R and 152 to Oral SGAs. Seven LAI-R subjects and 2 Oral SGA subjects received no medication after randomization and were not included in comparative analyses. At study entry, 276 patients were receiving antipsychotic medication; 29 were not. SGAs were received by 255 with risperidone most frequent (125). Seventy-one were receiving more than 1 antipsychotic; 32 were receiving an LAI antipsychotic; R-LAI 22, FGA LAI 10. Table 2 shows baseline subject characteristics by treatment group. Study subjects were on average 38 years old; 71% were male. Although age at first episode (23) and number of psychiatric hospitalizations (11) were comparable, LAI-R subjects had been hospitalized more recently (mean 22.4 months ago) than Oral SGA subjects (mean 40.4 months ago). Symptom ratings at baseline were comparable except for SANS Avolition/apathy, which was less severe in the Oral group and SOF scores that showed significantly better functioning in the Oral group.
Fig. 1.
Patient randomization, completion, and discontinuation rates in relapse prevention study of long-acting injectable (risperidone microspheres) and second-generation antipsychotic oral medications.
*Full study period ranged between 17 and 30 months, depending on enrollment date.
Thirty months of treatment was possible for 68% of the sample.
Table 2.
Baseline Characteristics of PROACTIVE Study Participants
| Variable | Total (n = 305) | LAI-R (n = 153) | Oral Second-Generation Antipsychotics (n = 152) | Test and Significance of Difference |
|---|---|---|---|---|
| Age (mean/SD) | 38.2±12.1 | 38.18±11.8 | 38.32±12.3 | t = 0.10, df = 303, P = .92 |
| Gender (%) | ||||
| Male | 218 (71%) | 108 (71%) | 110 (72%) | χ2 = 0.12, df = 1, P = .73 |
| Female | 87 (29%) | 45 (29%) | 42 (28%) | |
| Diagnosis (%) | ||||
| Schizophrenia NOS | 5 (2%) | 4 (3%) | 1 (1%) | χ2 = 1.3, df = 3, P = .74 (NOS, residual excluded) |
| SZ disorganized | 23 (8%) | 11 (7%) | 12 (8%) | |
| SZ paranoid | 123 (40%) | 66 (43%) | 57 (38%) | |
| SZ residual | 1 (0.3%) | 0 (0%) | 1 (1%) | |
| Schizoaffective | 99 (32%) | 46 (30%) | 53 (35%) | |
| SZ undifferentiated | 54 (18%) | 26 (17%) | 28 (18%) | |
| Age at first hospitalization (Mean/SD) | 22.8±8.8 (N = 283) | 23.0±9.2 (N = 143) | 22.6±8.4 (n = 140) | t = 0.41, df = 281, P = .68 |
| Number of hospitalizations (including current, mean/SD)a | 10.9±34.4 (N = 281) | 12.0±46.0 (N = 138) | 9.9±17.2 (N = 143) | t = 0.50, df = 279, P = .62 |
| Time (in months) since last hospitalization (mean/SD)b | 31.5±64.3 (N = 269) | 22.4±35.9 (N = 133) | 40.4±82.4 (N = 136) | t = 2.31, df = 267, P = .021 |
| Racial distribution (%) | ||||
| Caucasian | 159 (52%) | 81 (53%) | 78 (51%) | χ2 = 0.6, df = 2, P = .75 (Other excluded) |
| African American | 85 (28%) | 45 (29%) | 40 (26%) | |
| Hispanic | 58 (19%) | 27 (18%) | 31 (20%) | |
| Other | 3 (1%) | 0 (0%) | 3 (2%) | |
| Educational level (%) | ||||
| Some college or above | 130 (43%) | 66 (45%) | 64 (42%) | χ2 = 0.3, df = 2, P = .87 |
| High school graduate | 82 (27%) | 40 (27%) | 42 (28%) | |
| Below high school | 87 (29%) | 41 (28%) | 46 (30%) | |
| Employed at study entry (%) | 48 (16%) (N = 297) | 23 (16%) (N = 148) | 25(17%) (N = 149) | χ2 = 0.1, df = 1, P = .77 |
| Clinical Global Impressions | ||||
| Severity | 4.04±0.80 | 4.1±0.84 | 4.0±0.77 | t = 0.78, df = 303, P = .44 |
| Brief Psychiatric Rating Scale | ||||
| Total | 38.1±9.32 | 38.2±9.53 | 38.0±9.13 | t = 0.21, df = 302, P = .83 |
| Psychosis cluster | 2.7±1.09 | 2.7±1.12 | 2.7±1.08 | t = −0.03, df = 303, P = .97 |
| Anxiety/depression | 2.8±0.99 | 2.8±1.06 | 2.8±0.91 | t = 0.23, df = 303, P = .82 |
| Negative signs/symptoms | 1.6±0.66 | 1.6±0.67 | 1.6±0.65 | t = 0.66, df = 301, P = .51 |
| Excitement/activation | 1.4±0.56 | 1.4±0.56 | 1.4±0.56 | t = −0.09, df = 301, P = .93 |
| Scale for Assessment of Negative Symptoms | ||||
| Affective flattening | 1.9±0.97 | 1.9±0.97 | 1.9±0.97 | t = −0.29, df = 303, P = .77 |
| Alogia | 1.4±0.78 | 1.4±0.71 | 1.4±0.84 | t = −0.47, df = 303, P = .64 |
| Avolition/apathy | 3.2±1.10 | 3.4±1.03 | 3.0±1.14 | t = 2.68, df = 303, P = .008 |
| Asociality/anhedonia | 3.2±0.93 | 3.3±0.93 | 3.2±0.93 | t = 1.43, df = 301, P = .15 |
| Scale of Functioningb | ||||
| Sum of items 1–14 | 40.9±7.26 | 40.1±7.24 | 41.8±7.19 | t = −2.07, df = 300, P = .040 |
| Global rating (Item 15)b | 2.7±0.71 | 2.7±0.68 | 2.8±0.73 | t = −2.00, df = 300, P = .047 |
Note: LAI-R, long-acting injectable risperidone; NOS, not otherwise specified; SZ, schizophrenia.
aWilcoxson Rank-Sum test, P = .84; t test excluding outlier: t = 0.96, df = 278, P = .34; Poisson regression, P = .69.
bScale of Functioning was completed by on-site, unblinded raters. Higher score indicates better functioning.
Mean treatment duration for subjects was 551.2±341.8 days for LAI-R (N = 146) and 542.6±335.4 days for Oral SGA (N = 150; t = .22, df = 294, P = .83). In the LAI-R group, the modal dose received was 50mg (38%); 37.5mg (22.%); 25mg (22%); 12.5mg (6%); 62.5mg (5%); 75mg (5%). Seventy-four (30.4%) of LAI-R subjects received supplementation with oral antipsychotics after the initial transition period. For the Oral SGA group, risperidone was most frequently prescribed for 67 (44%); the mean (SD) of the modal prescribed dose was 5.1 (2.1). Olanzapine was prescribed for 30 (20%), mean (SD) dose of 23 (13.6); aripiprazole for 22 (14%), mean (SD) dose 23.4 (10.6); ziprasidone for 14 (9%), mean (SD) dose 142.8 (56.5); paliperidone for 9 (6%), mean (SD) dose 8.3 (2.9); quetiapine for 8 (5%), mean (SD) dose 525 (138.9); and iloperidone 1 (1%) dose 12. At study entry 26 (17.3%) changed from their prior antipsychotic medication. Further, during the course of study treatment, 43 (28%) had their oral antipsychotic changed. Concomitant psychotropic medications were used for 58% of LAI-R subjects and 61% of Oral SGA subjects. These included antidepressants—LAI-R 44% and Oral SGA 40%. mood stabilizers (18% and 20%), and anxiolytic/hypnotics (33% and 37%).
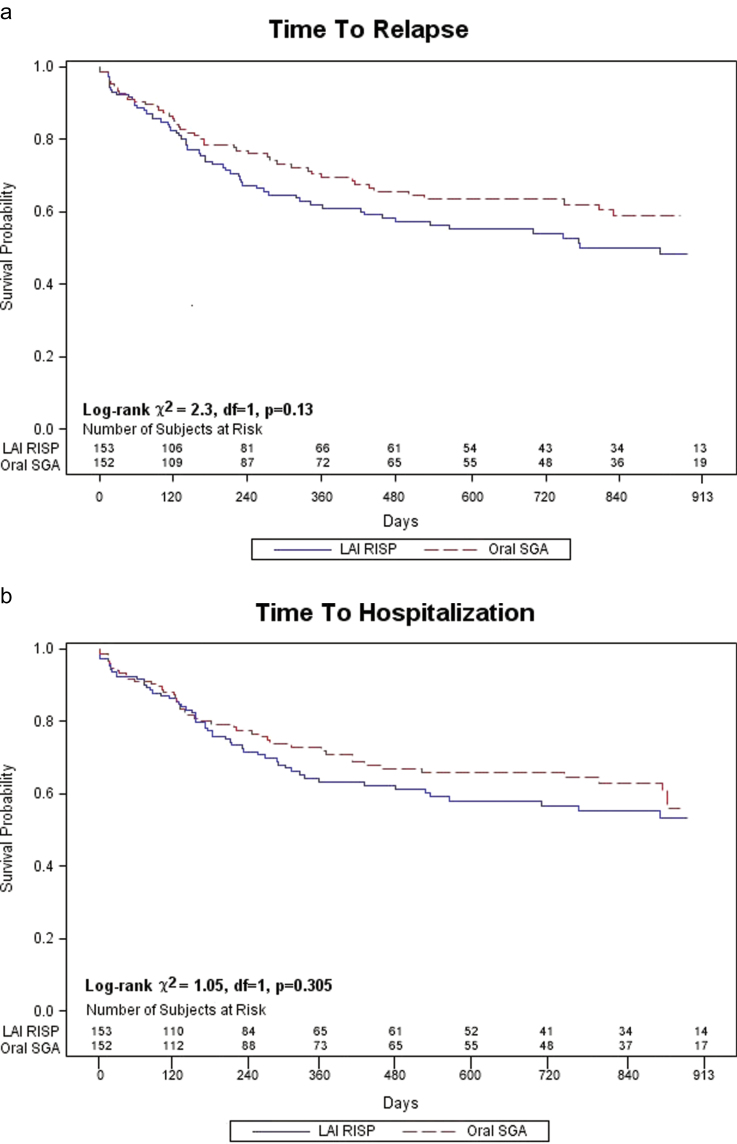
As shown in figure 1, 161 (53%) of subjects discontinued treatment before study end (81 in LAI-R and 80 in Oral SGA). Reasons for discontinuation were subject decision (LAI-R 21, Oral 12); clinical decision LAI-R 29, Oral 19); intervening illness or other administrative (LAI-R 17, Oral 28); lost to follow-up (LAI-R 12, Oral 18); and deceased (LAI-R 2, Oral 3). Overall 109 subjects (37%) experienced a relapse; 61/146 (42%) in the LAI-R group and 48/150 (32%) in the Oral SGA group (χ in the df = 1, P = .08). There was no significant difference between groups in time to first relapse (log rank χ, P = .08, df = 1, P = .13) or first hospitalization (log rank χ= 1, P = df = 1, P = .30; figure 2).
Fig. 2.
A comparison of time to first relapse (a) between patients receiving either long-acting injectable risperidone (LAI-R) or oral second-generation antipsychotics medications (b) and first hospitalization.
In between-group comparisons on the BPRS, SANS, CGI, and SOF (see supplementary tables), there were significant differences favoring LAI-R in the BPRS total score averaged over time (treatment F = 4.17, df = 1, 274, P = .042, Cohen’s d = 0.25; visit F = 8.25, df = 9, 1264, P < .0001; treatment × visit F = 0.70, df = 9, 1264, P = .71). Similar improvement over time, particularly in the second year, was observed favoring LAI-R for BPRS psychosis cluster (treatment F = 5.24, df = 1, 285, P = .023, Cohen’s d = 0.27; visit F = 5.36, df = 9, 1295, P < .0001; treatment × visit F = 2.35, df = 9, 1295, P = .013). There were no significant differences between treatments for the remaining BPRS symptom clusters of anxiety-depression, negative symptoms, and excitement. There was a significant difference favoring Oral SGA on SANS alogia (F = 4.46, df = 1, 279, P = .036, Cohen’s d = 0.25). Treatment differences on the other 3 SANS global ratings (affective flattening, avolition, asociality-anhedonia), CGI severity and improvement, and the 2 SOF measures (total score and global rating) were not significant.
Adverse Events
Six subjects died during the study, 2 in the LAI-R group (1 with apparently unrelated renal failure, 1 from suicide) and 4 in the Oral SGA group (1 from suicide, 1 due to unintentional illicit drug overdose, 1 unknown cause, and 1 of cardiac arrest after treatment exit). Table 3 presents treatment emergent AEs by treatment group. The table shows the most severe rating received for each AE. AE severity was low; the mean rating is less than 2 (mild) for all AEs in both groups. There was 1 statistically significant difference between groups; anorexia was lower in the Oral than the LAI-R arm. Two trend level differences (blurred vision and sedation/drowsiness) favored LAI-R.
Table 3.
Adverse Events Most Severe Level Recorded After Baseline by Treatment (Mean ± SD)
| Adverse Event | Total (n = 291)a | LAI-R (n = 143) | Oral Second-Generation Antipsychotics (n = 148) | Test and Significance of Difference |
|---|---|---|---|---|
| Bruising easily | 1.45±0.71 | 1.43±0.67 | 1.48±0.75 | t = −0.64, P = .52 |
| Rash | 1.48±0.74 | 1.53±0.78 | 1.44±0.71 | t = 1.06, P = .29 |
| Urticaria (hives, itching) | 1.66±0.77 | 1.60±0.69 | 1.71±0.84 | t = −1.20, P = .23 |
| Blurred vision | 1.84±0.79 | 1.76±0.73 | 1.91±0.83 | t = −1.63, P = .10 |
| Sedation/drowsiness | 2.43±0.83 | 2.34±0.87 | 2.53±0.78 | t = −1.90, P = .058 |
| Restlessness | 2.45±0.79 | 2.48±0.76 | 2.43±0.82 | t = 0.47, P = .64 |
| Insomnia | 2.37±0.92 | 2.38±0.89 | 2.36±0.94 | t = 0.18, P = .86 |
| Malaise (weakness, fatigue) | 2.18±0.86 | 2.22±0.85 | 2.14±0.87 | t = 0.75, P = .46 |
| Stiffness | 1.99±0.84 | 2.01±0.82 | 1.97±0.85 | t = 0.49, P = .63 |
| Tremor | 1.76±0.76 | 1.77±0.72 | 1.75±0.80 | t = 0.22, P = .83 |
| Dizziness | 1.80±0.81 | 1.82±0.79 | 1.78±0.82 | t = 0.43, P = .66 |
| Headache | 1.94±0.89 | 1.99±0.88 | 1.89±0.90 | t = 1.04, P = .30 |
| Fever | 1.25±0.47 | 1.27±0.49 | 1.24±0.46 | t = 0.53, P = .60 |
| Sore throat | 1.60±0.74 | 1.64±0.78 | 1.57±0.69 | t = 0.72, P = .47 |
| Dry mouth | 2.31±0.93 | 2.36±0.92 | 2.25±0.94 | t = 1.04, P = .30 |
| Hypersalivation | 1.80±0.87 | 1.76±0.80 | 1.84±0.94 | t = −0.74, P = .46 |
| Enuresis | 1.59±0.86 | 1.63±0.89 | 1.56±0.83 | t = −0.68, P = .50 |
| Constipation | 1.69±0.84 | 1.75±0.86 | 1.64±0.82 | t = 1.08, P = .28 |
| Diarrhea | 1.66±0.83 | 1.65±0.86 | 1.68±0.81 | t = −0.26, P = .80 |
| Anorexia (loss of appetite) | 1.77±0.85 | 1.89±0.86 | 1.69±0.84 | t = 2.00, P = .046 |
| Nausea | 1.75±0.81 | 1.78±0.82 | 1.72±0.80 | t = 0.70, P = .48 |
| Vomiting | 1.49±0.74 | 1.48±0.72 | 1.51±0.76 | t = −0.44, P = .66 |
| Menstrual irregularity (N = 122) | 1.58±0.91 | 1.62±0.96 | 1.55±0.86 | t = 0.41, df = 120, P = .68 |
| Breast tenderness/galactorrhea (N = 290) | 1.36±0.65 | 1.39±0.69 | 1.32±0.61 | t = 0.91, df = 288, P = .36 |
Note: LAI-R, long-acting injectable risperidone. Only subjects with postbaseline ratings are included.
a N = 291 (LAI-R: 143, Oral second-generation antipsychotics: 148), all t tests have df = 289 except as noted.
Discussion
We did not find a significant difference between schizophrenia subjects randomized to LAI-R or oral SGA medications in either time to relapse or hospitalization in a study that incorporated both explanatory and pragmatic design features. Controlling for time since last hospitalization, which was longer in the Oral SGA group, did not alter this finding. In contrast, increasing over the 30-month study course, we observed a more robust reduction in psychotic symptoms among patients who received the LAI formulation.
Following initial establishment of safety and efficacy in FDA-mandated registration trials, our field has shifted toward more pragmatic clinical trials in the belief that these may provide greater real-world generalizability than traditionally highly restrictive randomized, double-blind, placebo controlled studies.24,25 PROACTIVE had both efficacy/explanatory and effectiveness/pragmatic design features. We balanced the methodological rigor of explanatory trials (randomization to treatment, expert clinical care, and uniform, frequent monitoring), with pragmatic features (broad clinical representation, physician’s choice of treatment within the oral treatment arm, open clinical care following randomization, flexible dosing, and continued participation after experiencing a relapse). Consistent with an explanatory design, all subjects in the trial were seen every 2 weeks (as were those in many other studies), and both R-LAI and oral antipsychotic medications were provided. Frequent clinical contact might reduce relapse in both groups and reduce power to detect between-group differences. This effect could be a function of the supportive effects of clinical contacts, enhanced medication adherence monitoring, and the direct provision of oral medication, eliminating the need to fill prescriptions.2 Further, our patient population was not selected for documented nonadherence to medication, and requirements for informed consent to randomization to an LAI may have reduced the inclusion of these participants. Because the hypothesized advantage of LAIs is driven by enhanced medication adherence and detection of nonadherence, selection of patients who are more engaged in their care and therefore less likely to stop taking oral medication could make it more difficult to detect differences between LAI and oral treatment.2
PROACTIVE replicates and extends the results of a study with similar design in the VA population, largely confined to men. Rosenheck and colleagues26 reported hospitalization rates of 39% and 45%, respectively, among patients receiving LAI-R and oral SGA medications during a 2-year follow-up. This study, with a broader patient population, confirms the absence of a statistically significant difference between LAI and oral medications in preventing relapse and rehospitalization but does identify improvement over time in psychotic symptoms with LAI treatment.
Other recent studies using hospitalization or even broader criteria of relapse report similar outcomes: 46% relapse rate for LAI-R vs 43% for oral aripiprazole.27 A recent 2-year trial of LAI olanzapine and oral olanzapine reported similar relapse rates for both treatment groups (20% for LAI, 18% for oral) although patients receiving LAI olanzapine spent less time in hospital when they relapsed.28 A significant advantage for LAI-R (16%) was found compared with quetiapine (31%)29; and in the same study a numerical “descriptive” advantage compared with aripiprazole (27%).30 In a recent study of LAI-R risperidone vs oral quetiapine, Smeraldi and colleagues reported similar remission rates among patients in both treatment groups.31 In a post-hoc analysis of trials comparing LAI paliperidone to oral paliperidone, the risk of relapse was greater among patients receiving oral medications.32 Collectively, the results of modern-day LAI-oral comparisons are mixed. Moreover, as reviewed in the “Introduction” section, meta-analyses undertaken to clarify “the signal” from studies over time have added to the confusion and convey the overall impression of minimal (or perhaps, if any, only targeted) advantage for LAIs as a part of our clinical armamentarium.
One reason that overall 30-month relapse rate was relatively low (37%) may be the biweekly clinic visits, designed to match the timing of injections with LAI-R. This frequency is higher than current practice, and if it were an appropriate evidence-based standard of care, it could overwhelm an already burdened community mental health services system. However, there are ways to increase contact that do not require in-person clinic visits.33–35 A rigorous scientific evaluation of optimum frequency and model(s) of contact and interaction with follow-up and continuity of outpatient care for schizophrenia patients would be an important and timely contribution.
The timing of introduction of LAI treatment during the course of illness may also be important in understanding the role LAIs in the treatment of schizophrenia. Most studies that compared LAIs and oral medications have been in patients with chronic schizophrenia. A recent US study of LAI in first-episode schizophrenia patients36,37 and a European pharmacoepidemological study of LAI and oral antipsychotic medications used following initial hospitalization for schizophrenia38 raise the possibility that introduction of LAIs early in the course of illness might be advantageous. Other study models may also be useful in clarifying the role of LAIs. For example, a large, simple trial has never been conducted utilizing LAIs. This would entail randomization to LAI vs oral antipsychotic with no other “research” contacts or assessments and with a readily observed outcome such as hospitalization. Further possibilities include studies that focus on specific patient groups, such as those who are currently experiencing symptom exacerbation or have documented cognitive challenges in taking oral medications.
In conclusion, this was a relapse prevention trial with rigorous and innovative methods that incorporated a hybrid explanatory-pragmatic design. We found no significant differences in relapse or hospitalization between LAI-R and oral SGA medications. However, there was a symptomatic advantage in psychosis severity for patients who were treated with LAI-R, particularly over longer course of study treatment. Given the enormous personal suffering, family burden, disruption in functioning, and societal cost associated with preventable relapse and rehospitalization, this remains an important area of study.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Cooperative Agreement grants from the National Institute of Mental Health (1 U01MH070023) to N.R.S., J.M.K. (1 U01MH070011) to P.F.B. (1 U01MH070009) to D.C.G. (1 U01MH070012) to A.K. (1 U01MH070008) to J.L., J.B. (1 U01MH070017) to T.M. (1 U01MH070007) to A.J.M. (1 U01MH070010) to D.D.M. (1 U01MH070016) to D.R.W.
Supplementary Material
Acknowledgments
We acknowledge the contributions of coinvestigators and research teams at the 8 collaborating sites. They include Kim Carroll, Davin Dickerson, Sriram Ramaswamy, Lisa Raeke, Kelsey Shannahan, Adriana Foster, Simon Sebastian, Edna Stirewalt, Brian Cantley, Susan Ray, Richard Franco, Tim Holman, Jane Kerr, Tara Biehl, Nick Lemke. Rater training and supervision was provided by Joanne McCormack, MSW. NIMH collaborators and authors John Hsiao, MD, Joanne B. Severe, MS, and Stephen R. Marder, MD were instrumental in the development and design of the PROACTIVE study. Antipsychotic medications were provided by AstraZeneca, Bristol-Myers Squibb Company, Eli Lilly and Company, Janssen LP, and Pfizer Inc. All authors have specified their potential conflict of interests as part of the submission materials.
References
- 1. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 5. Treatment and prevention. Past, present, and future. Schizophr Res. 2010;122:1–23. [DOI] [PubMed] [Google Scholar]
- 2. Schooler NR. Relapse prevention and recovery in the treatment of schizophrenia. J Clin Psychiatry. 2006;67(suppl 5):19–23. [PubMed] [Google Scholar]
- 3. Kane JM, Correll CU. Past and present progress in the pharmacologic treatment of schizophrenia. J Clin Psychiatry. 2010;71:1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. 2013;12:216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leucht C, Heres S, Kane JM, Kissling W, Davis JM, Leucht S. Oral versus depot antipsychotic drugs for schizophrenia–a critical systematic review and meta-analysis of randomised long-term trials. Schizophr Res. 2011;127:83–92. [DOI] [PubMed] [Google Scholar]
- 6. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40:192–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kirson NY, Weiden PJ, Yermakov S, et al. Efficacy and effectiveness of depot versus oral antipsychotics in schizophrenia: synthesizing results across different research designs. J Clin Psychiatry. 2013;74:568–575. [DOI] [PubMed] [Google Scholar]
- 8. Kishimoto T, Agarwal V, Leucht S, Kane JM, Correll CU. Relapse prevention in schizophrenia: a systematic review and meta-analysis of second-generation antipsychotics versus first-generation antipsychotics. Mol Psychiatry. 2011;143:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kishimoto T, Nitta M, Borenstein M, Kane JM, Correll CU. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74:957–965. [DOI] [PubMed] [Google Scholar]
- 10. Hogarty GE, Schooler NR, Ulrich R, Mussare F, Ferro P, Herron E. Fluphenazine and social therapy in the aftercare of schizophrenic patients. Relapse analyses of a two-year controlled study of fluphenazine decanoate and fluphenazine hydrochloride. Arch Gen Psychiatry. 1979;36:1283–1294. [DOI] [PubMed] [Google Scholar]
- 11. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed: DSM-IV-TR. Washington, DC: American Psychiatric Press; 2000. [Google Scholar]
- 12. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962; 10:799–812. [Google Scholar]
- 13. Guy W. Clinical Global Impressions (CGI). In: Guy W, ed. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute of Mental Health; 1976:217–222. [Google Scholar]
- 14. Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Arch Gen Psychiatry. 1982;39:784–788. [DOI] [PubMed] [Google Scholar]
- 15. Rapaport MH, Bazzetta J, McAdams LA, Patterson T, Jeste DV. Validation of the scale of functioning in older outpatients with schizophrenia. Am J Geriat Psychiatry. 1996;4:218–28. [DOI] [PubMed] [Google Scholar]
- 16. Guy W. Abnormal Involuntary Movement Scale (AIMS). In: Guy W, ed. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute of Mental Health; 1976:534–537. [Google Scholar]
- 17. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. [DOI] [PubMed] [Google Scholar]
- 18. Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. [DOI] [PubMed] [Google Scholar]
- 19. Burke MA, McEvoy JP, Ritchie JC. A pilot study of a structured interview addressing sexual function in men with schizophrenia. Biol Psychiatry. 1994;35:32–35. [DOI] [PubMed] [Google Scholar]
- 20. Guzik LH, Erickson ZE, Mena SJ, Pierre JM, Ames D. Measuring patients’ subjective experiences taking antipsychotic medications using the Novel Antipsychotic Medication Experience Scale (NAMES): a preliminary Analysis. International Congress of Schizophrenia Research; March 28–April 1; San Diego, CA. Schizophr. Bull. 2009; 35 (supplement 1): 36, ID: 551878. [Google Scholar]
- 21. Csernansky JG, Mahmoud R, Brenner R; Risperidone-USA-79 Study Group. A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med. 2002;346:16–22. [DOI] [PubMed] [Google Scholar]
- 22. Liu C, Cao D, Chen P, Zagar T. RANDOM and REPEATED statements: how to use them to model the covariance structure in Proc Mixed. MidWestern SAS Users Group, Proceedings 2007. Paper S02-2007. www.mwsug.org/proceedings/2007/stats/MWSUG-2007-S02.pdf. Accessed May 7, 2014. [Google Scholar]
- 23. Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- 24. Lieberman JA, Stroup TS. The NIMH-CATIE Schizophrenia Study: what did we learn? Am J Psychiatry. 2011;168:770–775. [DOI] [PubMed] [Google Scholar]
- 25. Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol. 2009;62:464–475. [DOI] [PubMed] [Google Scholar]
- 26. Rosenheck RA, Krystal JH, Lew R, et al. ; CSP555 Research Group. Long-acting risperidone and oral antipsychotics in unstable schizophrenia. N Engl J Med. 2011;364:842–851. [DOI] [PubMed] [Google Scholar]
- 27. Macfadden W, Ma YW, Thomas Haskins J, Bossie CA, Alphs L. A prospective study comparing the long-term effectiveness of injectable risperidone long-acting therapy and oral aripiprazole in patients with schizophrenia. Psychiatry. 2010;7:23–31. [PMC free article] [PubMed] [Google Scholar]
- 28. Novice D, Ascher-Swanum H, Bertsch J, Detke H, McDonnell D, Hato JM. Risk of relapse and hospitalization in the 2-year open-label treatment of outpatients with schizophrenia randomized to olanzapine long acting injection or oral olanzapine. Presentation at the 3rd Biennial Conference of the Schizophrenia International Research Society; April 2012; Florence, Italy. [Google Scholar]
- 29. Gaebel W, Schreiner A, Bergmans P, et al. Relapse prevention in schizophrenia and schizoaffective disorder with risperidone long-acting injectable vs quetiapine: results of a long-term, open-label, randomized clinical trial. Neuropsychopharmacology. 2010;35:2367–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Arce Cordón R, Eding E, Marques-Teixeira J, Milanova V, Rancans E, Schreiner A. Descriptive analyses of the aripiprazole arm in the risperidone long-acting injectable versus quetiapine relapse prevention trial (ConstaTRE). Eur Arch Psychiatry Clin Neurosci. 2012;262:139–149. [DOI] [PubMed] [Google Scholar]
- 31. Smeraldi E, Cavallaro R, Folnegović-Šmalc V, Bidzan L, Emin Ceylan M, Schreiner A. Long-term remission in schizophrenia and schizoaffective disorder: results from the risperidone long-acting injectable versus quetiapine relapse prevention trial (ConstaTRE). Ther Adv Psychopharmacol. 2013;3:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Markowitz M, Fu DJ, Levitan B, Gopal S, Turkoz I, Alphs L. Long-acting injectable paliperidone palmitate versus oral paliperidone extended release: a comparative analysis from two placebo-controlled relapse prevention studies. Ann Gen Psychiatry. 2013;12:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McGorry PD, Purcell R, Goldstone S, Amminger GP. Age of onset and timing of treatment for mental and substance use disorders: implications for preventive intervention strategies and models of care. Curr Opin Psychiatry. 2011;24:301–306. [DOI] [PubMed] [Google Scholar]
- 34. Steinwachs DM, Roter DL, Skinner EA, et al. A web-based program to empower patients who have schizophrenia to discuss quality of care with mental health providers. Psychiatr Serv. 2011;62:1296–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spaniel F, Vohlídka P, Hrdlicka J, et al. ITAREPS: information technology aided relapse prevention programme in schizophrenia. Schizophr Res. 2008;98:312–317. [DOI] [PubMed] [Google Scholar]
- 36. Weiden PJ, Schooler NR, Weedon JC, Elmouchtari A, Sunakawa A, Goldfinger SM. A randomized controlled trial of long-acting injectable risperidone vs continuation on oral atypical antipsychotics for first-episode schizophrenia patients: initial adherence outcome. J Clin Psychiatry. 2009;70:1397–1406. [DOI] [PubMed] [Google Scholar]
- 37. Weiden PJ, Schooler NR, Weedon JC, Elmouchtari A, Sunakawa-McMillan A. Maintenance treatment with long-acting injectable risperidone in first-episode schizophrenia: a randomized effectiveness study. J Clin Psychiatry. 2012;73:1224–1233. [DOI] [PubMed] [Google Scholar]
- 38. Tiihonen J, Haukka J, Taylor M, Haddad PM, Patel MX, Korhonen P. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168:603–609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.