Abstract
Objective: To understand the underlying dynamic neurophysiological changes over the course of schizophrenia, it is important to study subjects longitudinally from the early stage of the illness. We previously reported that visual P300 was already impaired in patients with first-episode schizophrenia (FESZ). This study demonstrates how the visual P300, as well as earlier components P1, N1, and N200, changed at the 1-year follow-up after their initial measurement. Methods: Visual ERPs were recorded with the same experimental paradigm and acquisition protocol at both time points in FESZ (n = 18) and healthy comparison subjects (n = 24). Participants silently counted infrequent target stimuli (“x”) amid standard stimuli (“y”) presented on the screen while the 64-channel electroencephalogram was recorded. Results: FESZ showed smaller visual P300, N200, P1 (trend level) amplitude and delayed P300 and N1 latency at both time points; however, only P300 showed progressive amplitude reduction over the course of the illness at 1-year follow-up. P300 latency did not change over time in either group. FESZ showed significantly reduced Spatial Span total score at both time points, and there was a significant negative correlation between P300 peak amplitude and the Brief Psychiatric Rating Scale positive symptom score at baseline. Conclusion: These data show progressive P300 amplitude reduction in response to visual stimuli in the early stage of schizophrenia. These visual P300 findings support the concept of progression of schizophrenia, suggesting the usefulness of the visual P300 as a biological marker of progression.
Key words: EEG, first-episode schizophrenia, P300, longitudinal, progression of schizophrenia
Introduction
In recent years, schizophrenia has been often conceptualized as a developmental disorder.1,2 It usually manifests itself in late adolescence or early adulthood, which are times of robust maturational changes in several cognitive domains including selective attention, working memory, and inhibitory control.3 Studies examining volumetric abnormalities in first-episode schizophrenia identified significant progressive volume reductions of cortical gray matter involving frontal, temporal, and parietal lobes and left Heschl’s gyrus in first-episode schizophrenia patients, consistent with an accelerated trajectory of brain pathology postonset.4–6 These brain regions support cognitive functions that undergo maturation during late adolescence and early adulthood. Thus, the study of the trajectory of cognitive abnormalities at the early stage of the illness in a longitudinal design is important for the understanding of the underlying dynamics of neurophysiological changes over the course of schizophrenia.
P300 is one of the most useful indices of the underlying neurophysiological pathology in schizophrenia patients in the early stage of the disease because it is considered to reflect complex cognitive functions such as working memory and/or attention,7 both functions found to be abnormal in schizophrenia.8 Some cross-sectional studies suggested a possibility that P300 could reflect the progressive nature of a pathological process in schizophrenia patients.9,10 However, only longitudinal studies can provide data to clarify the nature of the disease progression. Furthermore, early studies suggested that visual P300 is more sensitive to schizophrenia state, while the auditory P300 was found more sensitive to trait.11,12
Visual N200 is also elicited by task-relevant stimuli, and it indexes stimulus classification.13,14 Visual N200 has been reported to be small in patients with schizophrenia mostly independently of the P300.15–19 Sensory-evoked components, P1 and N1, primarily indexing early stages of perceptual processing are also believed to be dissociable from P300 abnormality.20 These earlier components also need to be examined longitudinally in order to clarify the progression of schizophrenia pathophysiology and the contribution of early perceptual abnormalities to the development and progression of abnormalities at later cognitive stages. Deficits of the visual perceptual processing have been established in chronic schizophrenia.21 We previously reported that the visual P300 was already impaired in first-episode schizophrenia patients.22 In the current investigation, we examine how the impairment found in the first-episode individuals changes over time. In what follows, we report a longitudinal study of visual P300, as well as P1, N1, and N200, in first-episode schizophrenia. We measured P1, N1, N200, and P300 at baseline and approximately 1 year later (follow-up) in first-episode schizophrenia patients and in their healthy control subjects. We hypothesized that cognitive dysfunction indexed by the visual P300 would significantly progress over time.
Method
Subjects
The sample consisted of 18 patients with first-episode schizophrenia (FESZ), as well as 24 healthy comparison (HC) subjects. Subject recruitment was done as part of the Boston Longitudinal Assessment and Monitoring of Clinical Status and Brain Function in Adolescents and Adults (CIDAR) Center (www.bostoncidar.org). Fifteen of 24 HC and 14 of 18 FESZ had participated in the previous study,22 and the others were newly recruited into the study and underwent both the baseline and the follow-up assessments. The FESZ were recruited based on referrals from clinicians or through local hospitals and clinics, and HC were recruited through newspaper and web site advertisements. The study was approved by the local Institutional Review Board committees at Beth Israel Deaconess Medical Center and Harvard Medical School. All study participants (or legal guardians for those under 18 years) gave written informed consent prior to study participation and received payment for participation.
All the patients met DSM-IV-TR criteria for either schizophrenia, schizoaffective disorder, or schizophreniform disorder. DSM-IV diagnoses were based on interviews using the Structured Clinical Interview for DSM-IV-TR, Research Version23 and information from patient medical records. The HC were drawn from the same geographic bases as FESZ, with comparable age, gender, race and ethnicity, handedness, and parental socioeconomic status (see table 1). No HC met criteria for any current major DSM-IV-TR Axis I disorders or had a history of psychosis, major depression (recurrent), bipolar disorder, obsessive compulsive disorder, posttraumatic stress disorder, or developmental disorders. HC were also excluded if they had a history of psychiatric hospitalizations, prodromal symptoms, schizotypal or other Cluster A personality disorders, first-degree relatives with psychosis, or any current or past use of antipsychotics. Exclusion criteria for all subjects were sensory-motor handicaps; neurological disorders; medical illnesses that significantly impair neurocognitive function; diagnosis of mental retardation; education less than 5th grade if under 18 years of age or less than 9th grade if 18 years or above; not fluent in English; DSM-IV substance abuse in the past month; DSM-IV substance dependence, excluding nicotine, in the past 3 months; current suicidality; no history of electro convulsive therapy (ECT) within the past 5 years for FESZ and no history of ECT ever for HC; or study participation by another family member.
Table1.
Demographic, Neurocognitive, and Clinical Information
| HC (N = 24) | FESZ (N = 18) | Statistic | |||
|---|---|---|---|---|---|
| t or χ2 | df | P | |||
| Female sex, N (%) | 11 (45.8) | 5 (27.8) | 1.42 | 1 | .23 |
| Age at baseline | 20.0 (3.85) | 21.7 (4.55) | 3.85 | 40 | .18 |
| Age at follow-up | 21.0 (3.87) | 22.8 (4.54) | 3.87 | 40 | .17 |
| Inter-EEG interval (mo) | 12.7 (4.03) | 11.7 (3.33) | 4.03 | 40 | .38 |
| Handednessa | 0.7 (2.46) | 1.9 (3.25) | 2.46 | 40 | .17 |
| Education years | 13.4 (2.67) | 14.1 (2.69) | 2.67 | 40 | .45 |
| Parental SESb | 1.7 (0.91) | 2.2 (1.17) | 0.91 | 40 | .12 |
| Current IQc | 117.9 (23.66) | 105.5 (14.54) | 23.66 | 39 | .06 |
| Premorbid IQd | 118.4 (15.58) | 111.2 (16.41) | 15.58 | 40 | .15 |
| GAF | |||||
| Baseline | 84.1 (6.94) | 49.4 (13.40) | 10.91 | 40 | <.001* |
| Follow-up | 82.5 (9.51) | 62.1 (15.52) | 5.19 | 39 | <.001* |
| BPRS Total Score | |||||
| Baseline | 44.0 (13.7) | ||||
| Follow-up | 35.8 (10.6) | ||||
| BPRS Positive Symptom Score | |||||
| Baseline | 9.2 (3.9) | ||||
| Follow-up | 7.0 (2.5) | ||||
| BPRS Negative Symptom Score | |||||
| Baseline | 11.8 (5.2) | ||||
| Follow-up | 9.4 (2.6) | ||||
| Spatial Span subtest of the Wechsler Memory scale | |||||
| Baseline | 20.3 (3.6) | 16.9 (2.9) | 3.2 | 39 | .003** |
| Follow-up | 19.8 (3.1) | 16.4 (3.7) | 3.2 | 39 | .003** |
| Medication (CPZ equivalents) | |||||
| Baseline | 267.9 (206.8) | ||||
| Follow-up | 300.0 (300.4) | ||||
Note: Values are means (SDs) unless otherwise indicated. SES, socioeconomic status; CPZ, chlorpromazine.
aBased on the Annett Handedness Scale total score.
bBased on the Hollingshead method. Higher scores indicate lower socioeconomic status.
cEstimated from Vocab and Block Design T-scores of Wechsler Abbreviated Scale of Intelligence.
dEstimated from Reading scale of Wide Range Achievement Test.
*Patients with first-episode schizophrenia showed significantly lower Global Assessment of Functioning scale scores compared with healthy comparison subjects both at baseline and follow-up.
**Patients with first-episode schizophrenia showed significantly lower Spatial Span scores compared with healthy comparison subjects both at baseline and follow-up.
Mean duration of illness in FESZ at baseline was 13.8 ± 13.3 months. At baseline, 13 out of 18 FESZ were medicated with atypical antipsychotics. Daily dose chlorpromazine equivalent for the medicated subjects was 267.9±206.8.24,25 The following numbers of subjects received additional psychotropic medications: mood stabilizers: 1; antidepressants: 6; anxiolytics: 3.
At follow-up, 11 of 18 FESZ were medicated with atypical antipsychotics. Daily dose chlorpromazine equivalent for the medicated subjects was 300.0±300.4. The following numbers of the patients received additional psychotropic medications: mood stabilizers: 0; antidepressants: 5; anxiolytics: 2.
Handedness was assessed using the Annett Handedness Questionnaire.26 Premorbid intellectual abilities were estimated using the Reading subtest from the Wide Range Achievement Test-4,27 and current intellect was estimated from the Vocabulary and Block Design subtests of the Wechsler Abbreviated Scale of Intelligence.28 Parental socioeconomic status was evaluated using the Hollingshead two-factor index.29 All subjects were evaluated with the Global Assessment of Functioning (GAF) scale. In addition, clinical symptoms of FESZ were rated using the Brief Psychiatric Rating Scale (BPRS).30 Trained and experienced clinical researchers, all clinicians, with established reliability (prior Kappa scores ≥ 0.80) performed the BPRS ratings. Training was conducted by experienced senior staff members and involved viewing and practice with training tapes, as well as with clinical vignettes, followed by patient interviews with observation by a senior staff member until proper administration and the reliability of intraclass correlation coefficient greater than 0.80 was achieved. Given the fact that visual P300 indexes neurophysiological processes associated with visual working memory function, we have selected the Spatial Span subtest of the Wechsler Memory scale-III,31 a behavioral visual working memory test, in order to examine the relationship between 2 measures of the same function. We reasoned that because the electrophysiological and behavioral measures of visual working memory most likely tap into nonidentical but highly related processes, the correlation should be observed between the 2. All demographic and clinical data are summarized in table 1. BPRS positive symptom score included subscales of grandiosity, suspiciousness, hallucinations, unusual thought content, and conceptual disorganization, while BPRS negative symptom score included the subscales of disorientation, blunted affect, emotional withdrawal, motor retardation, self-neglect, uncooperativeness, mannerism, and posturing.32
The electroencephalograms (EEGs) were acquired with the same experimental paradigm and the acquisition protocol at baseline and follow-up.
Experimental Paradigm
Subjects were instructed to silently count infrequently (20%) presented target stimuli (letter “X,” 1.8 × 2.0cm total number: 36) among standard stimuli (letter “Y,” 1.8 × 2.0cm, total number: 144). The letters were white, presented centrally on a black computer screen placed approximately 100cm away from the subject’s eyes. The stimulus duration was 82 ms, and the interstimulus interval was 976ms (SuperLab 4.2, Cedrus Corporation).
EEG Data Acquisition and Processing
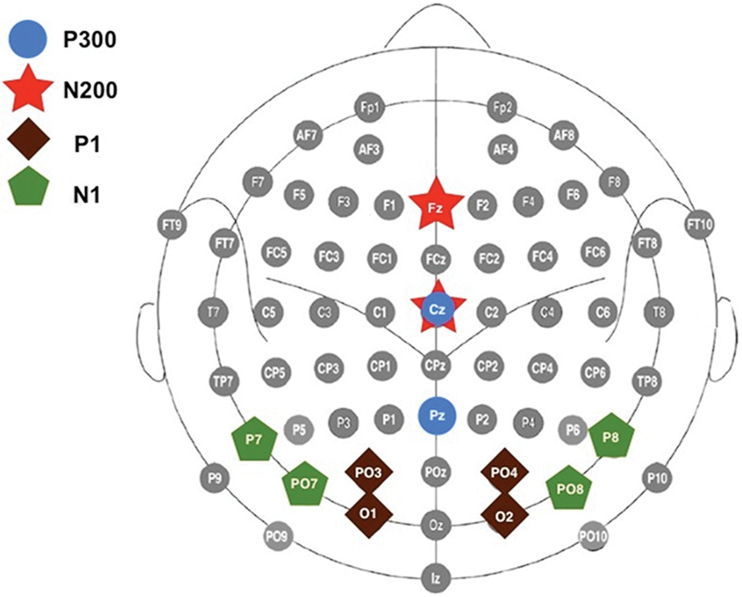
The EEG was recorded using custom-designed electrode caps with a 64-channel BioSemi Active-Two system (BioSemi B.V.). The EEG was acquired in a continuous mode at a digitization rate of 512 Hz, with a bandpass of DC–100 Hz, and stored for later analysis. Blinks and eye movements were monitored through electrodes placed on the outer canthi of the left and right eyes and above and below the left eye. The EEG data were processed off-line using Brain Vision Analyzer package (Brain Products, Germany) and referenced off-line to the algebraic mean of the right and left mastoids. Individual event-related potential (ERP) epochs, time-locked to the stimulus onset, were constructed starting 100ms prestimulus and ending 900ms after the stimulus onset. Eye blinks and movement artifacts were corrected by the Gratton, Coles, and Donchin method.33 Single-trial epochs containing muscle activity or amplifier blocking were rejected off-line before averaging (±100 μV criterion). Averages were calculated for target and standard conditions separately, after subtraction of the 100ms prestimulus baseline. P300 peak amplitude and latency were measured as the most positive data point between 350 and 650ms, and the N200 was identified as the most negative data point between 190 and 380ms poststimulus in the target stimulus averages. The P1 peak was defined as the most positive data point between 60 and 200ms, and the N1 was identified as the most negative data point between 75 and 250ms after stimulus onset in the standard stimulus averages. These peaks were measured at the electrode sites at which they were maximal (P300: Cz, Pz; N200: Fz, Cz; P1: PO3/PO4, O1/O2; N1: P7/P8, PO7/PO8). A schematic of the electrodes used in the analyses is provided in figure 1.
Fig. 1.
A schematic of the electrodes used in the analyses of P300 (Cz and Pz; blue circles), N200 (Fz and Cz: red stars), P1 (PO3, PO4, O1 and O2; brown squares), and N1 (P7, P8, PO7 and PO8; green pentagons).
The minimal number of trials for a subject to be included in the analyses was set at 75% (target: 27 trials, standard: 108 trials). All subjects fulfilled this criterion. The mean number of target and standard trials used to construct individual averages did not differ significantly between groups both at baseline and follow-up (t’s < 1.50, P > .15), with approximately 35 and 142 trials surviving artifact rejection.
Statistical Analyses
Independent t tests were used to assess group differences in age, handedness, time 1-time 2 EEG interval, education, parental socioeconomic status, estimated current/premorbid IQ, and the Global Assessment of Functioning scale. ERPs were analyzed with repeated measures ANOVAs with group (HC or FESZ) as a between factor and electrode and time (baseline or follow-up) as within factors.
Correlational Analyses
Because we have previously found correlations between visual P300 amplitude and BPRS positive symptom score,22 we examined correlations of the P300 amplitude at Pz with the BPRS positive symptom score in FESZ at baseline and at follow-up separately using Spearman’s rho to diminish the effect of any outliers. In addition, exploratory analyses of the correlations between percent change score of the P300 amplitude {calculated by the formula 100 × [(P300 amplitude at follow-up − P300 amplitude at baseline)/P300 amplitude at baseline]}, and baseline clinical scores (BPRS positive, BPRS negative, GAF scale, current IQ) were performed using the Spearman’s rho. In addition, P300 visual amplitude at both time 1 and time 2 was correlated with the Spatial Span subtest of the Wechsler Memory test results at both time points in FESZ.
Results
Demographic, Behavioral, Clinical, and Neuropsychological Variables
There were no significant differences in the age, handedness, time 1-time 2 EEG interval, education, parental socioeconomic status, estimated premorbid IQ, or current IQ between the 2 groups. FESZ showed a significantly lower GAF scale compared with HC both at baseline and follow-up (see table 1).
For target count, both groups at both time points were over 90% accurate and there were no group differences (t < 1.31, P > .20), so it is unlikely that the progression effects seen in FESZ were due to their worse performance, although we cannot dismiss the slim possibility that the patients remembered the number of the target stimuli from the first measurement.
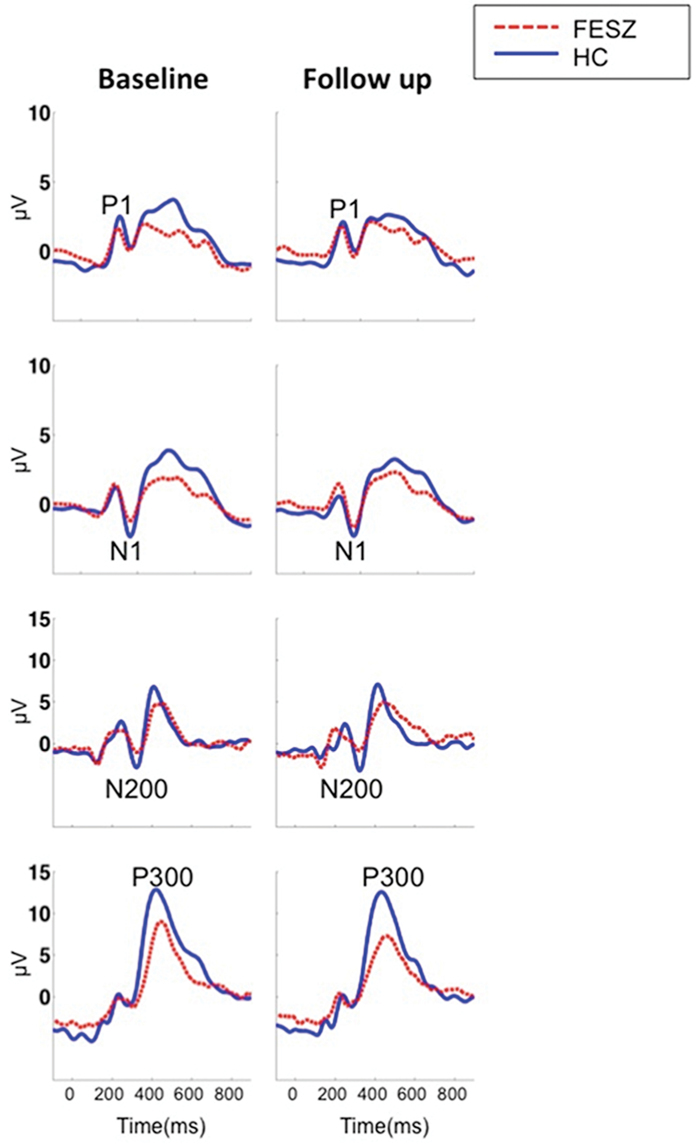
BPRS total and positive symptom score in FESZ improved over time (t = 3.4, P = .004 and t = 3.5, P = .003, respectively), but BPRS negative symptom score did not (t = 1.5, P = .14). FESZ showed significantly reduced Spatial Span total score at both time points compared with HC (t = 3.2, P = .003 and t = 3.2, P = .003). The Spatial Span total score did not change over time in both groups (t = 0.69, P = .49 and t = 1.02, P = .31, respectively). Figure 2 shows the visual ERPs grand averages in each group.
Fig. 2.
Visual ERPs grand average waveforms in FESZ and HC at baseline (left) and follow-up (right) measured at O1 for the P1, P8 for the N1, Fz for the N200, and Pz for the P300.
P300 Peak Amplitude and Latency
There was a main effect of group (F [1,40] = 12.74, P = 0.001), suggesting that P300 amplitudes were smaller in FESZ at both time points, which replicated our previous finding that the visual P300 was already impaired at the first measurement in FESZ.
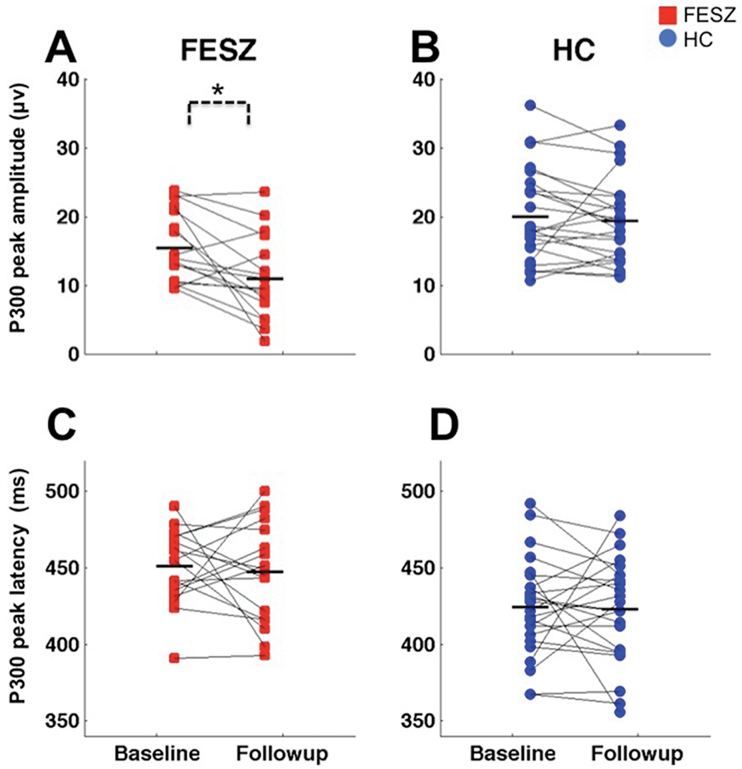
There was a main effect of time for P300 amplitude: F [1,40] = 8.30, P = .006, driven by the progressive P300 amplitude reduction in FESZ as evidenced by Time by Group interaction (F [1,40] = 3.79, P = .05): follow-up t tests found a significant P300 amplitude reduction in FESZ (t = 3.3, P = .004) but not in HC (t = 0.69, P = .49) (Figure 3, top).
Fig. 3.
Scattergrams of the P300 peak amplitudes at Pz at baseline and follow-up in FESZ (A), the P300 peak amplitudes at Pz at baseline and follow-up in HC (B), the P300 beak latencies at Pz at baseline and follow-up in FESZ (C), and the P300 peak latencies at Pz at baseline and follow-up in HC (D). The P300 peak amplitudes were significantly smaller and the P300 latencies were significantly delayed in FESZ compared with HC at baseline and follow-up. FESZ showed significant P300 amplitude reduction over time. Horizontal lines indicate group means.
P300 peak latency was delayed in FESZ at both time points (F [1,40] = 14.96, P < .001). There were no other significant main effects or interactions (F’s < 2.77, P’s > .10) (Figure 3, bottom).
N200 Peak Amplitude and Latency
There was a significant group by electrode interaction (F [1,40] = 4.22, P = .04). Follow up t tests for each electrode averaged over the 2 time points showed that the N200 amplitude was smaller in FESZ at Fz (t = −2.14, P = .03) but not at Cz (t = −1.26, P = .21). N200 amplitude did not show time effects (F’s < 0.77, P’s > .38).
N200 latency was significantly shorter centrally than frontally in both groups (F [1,40] = 36.26, P < .001) but did not differ between groups (F [1,40] = 1.63, P = .20). There were no other significant main effects or interactions (F’s < 1.35, P’s > .25).
P1 Peak Amplitude and Latency
P1 peak amplitude was smaller in FESZ at both time points, but this effect did not reach statistical significance (F [1,40] = 3.58, P = .06). There were no significant interactions (F’s < 2.45, P’s > .07).
P1 peak latency was shortest at O2 (F [3,38] = 4.16, P = .01) but showed no group differences (F [1,40] = 0.96, P = .33). No other significant effects were found (F’s < 1.26, P’s > .26).
N1 Peak Amplitude and Latency
N1 amplitude did not differ between groups (F [1,40] = 0.33, P = .56). There were no significant interactions (F’s < 1.55, P’s > .22).
N1 peak latency was delayed in FESZ (F [1,40] = 4.86, P = .03). There were no other significant main effects or interactions (F’s < 2.03, P’s > .16).
Correlations Between the P300 and Clinical Variables at Each Time Point
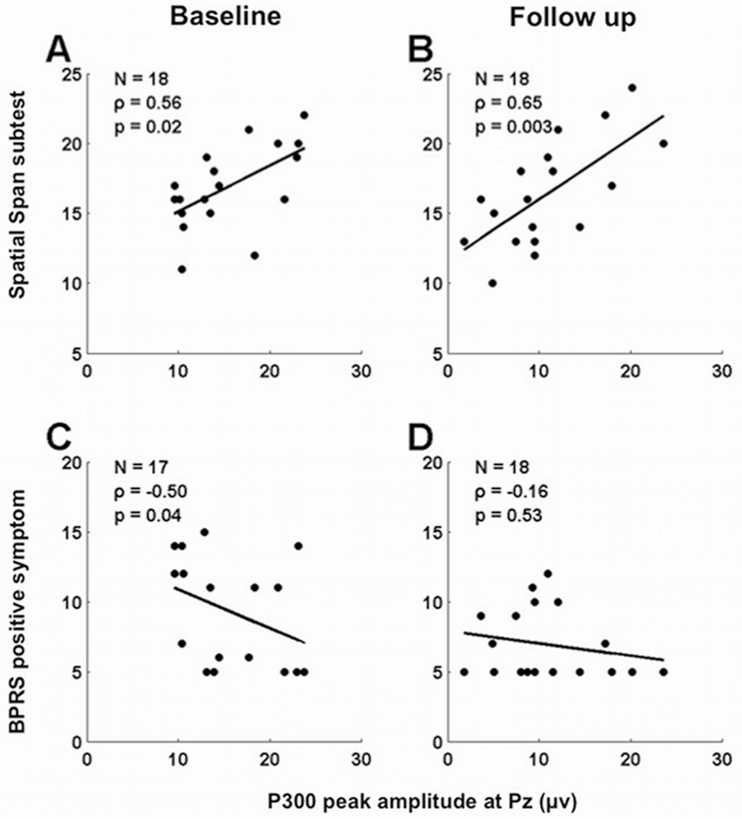
There was a significant negative correlation between P300 peak amplitude at Pz with the BPRS positive symptom score at baseline (rho = −0.50, P = .04), but this correlation lost significance at follow-up (rho = −0.16, P = .52). There were no significant correlations between P300 values and GAF scores (−0.13 < rho < 0.08, 0.60 < P < .89). There were significant positive correlations between the P300 peak amplitude at Pz with the Spatial Span total score at both time points in FESZ (rho = 0.56, P = .01 and rho = 0.65, P = .003; figure 4).
Fig. 4.
Scattergrams of the P300 peak amplitude and the Spatial Span subtest of the Wechsler Memory test at baseline (A); the P300 peak amplitude the Spatial Span subtest of the Wechsler Memory test at follow-up (B); the P300 peak amplitude and the BPRS positive symptoms at baseline (C); and the P300 peak amplitude and the BPRS positive symptoms at follow-up (D) in patients with FESZ. P300 peak amplitude at Pz was positively correlated with the Spatial Span total score at baseline and follow-up. Visual P300 peak amplitude at Pz was negatively correlated with the BPRS positive symptom score at baseline, but this correlation lost significance at follow-up.
No significant correlations were observed between P300 values and the chlorpromazine dose equivalent in FESZ (−0.14 < rho < 0.42, 0.07 < P < .63) at both time points.
Discussion
This study investigated the P300 ERP component in a visual oddball task longitudinally, as well as the correlations between P300 and the schizophrenia symptomatology and visual working memory performance. FESZ showed smaller visual P300 amplitude and prolonged P300 latency at both time points. Furthermore, FESZ showed significant progressive P300 amplitude reduction over time; in contrast, the latency did not change over time. FESZ also showed smaller N200 and P1 (trend level) amplitude and delayed N1 latency at both time points. However, only visual P300 amplitude showed significant time effects. The more severe positive symptoms were correlated with the smaller P300 amplitude at baseline, but this correlation was lost at follow-up. Although there is a possibility that visual P300 is unrelated to symptoms, we presume that the improvement in symptoms and greater clustering of the P300 amplitude around lower values (and thus smaller SD) at follow-up might be the main reasons of the lack of correlation. Thus, these data demonstrated that the neuropathology reflected in the visual P300 amplitude had progressed in spite of the fact that FESZ showed improvement in symptoms and the GAF score. As we hypothesized, the brain changes brought about by the pathological processes set in motion by the development of schizophrenia impacted the cognitive function of visual working memory, and these deficits persisted over time. Indeed, there was a specificity of the relationship between the visual working memory test results and visual P300 as an index of visual working memory function; FESZ showed impaired visual working memory as evaluated by the Spatial Span test, which correlated with the visual P300 amplitudes at both time points in this study. Note that despite the significant correlation at both the time points, the Spatial Span test did not change over time, while the visual P300 did. These results may suggest that there was another factor at work, which was associated with both the P300 generation and the Spatial Span test or that the Spatial Span test did not fully reflect the cognitive processes related to the visual P300. At the same time, in our subjects, the chlorpromazine equivalent scores did not correlate with P300 amplitudes (0.12 < rho < 0.22, 0.36 < P < .63) at either time point. Furthermore, the change in the chlorpromazine equivalent score {calculated by the formula 100 × [(chlorpromazine equivalent score at follow-up − chlorpromazine equivalent score at baseline)/chlorpromazine equivalent score at baseline]} did not correlate with the P300 change score (rho = 0.11, P = .68). Considering a finding from a meta-analysis on P300 in schizophrenia, which indicated that antipsychotic medications were unrelated to the P300 amplitude effect size,34 we conclude that the observed result was not confounded by antipsychotic medication.
In contrast to the P300 amplitude, the P300 latency did not change significantly over time, though it was prolonged significantly at both time points in FESZ. This may suggest that pathophysiological changes that could have impacted the P300 latency, such as white matter deficits, alterations in receptor function, or alterations in the components from different generators that contribute to the surface recorded waveform, are prominent at the onset of the disease but do not progress drastically at least in the early course of the illness. Although there have been a few cross-sectional diffusion tensor imaging (DTI) studies that suggested white matter pathology increased with age35 or duration of illness36,37 in schizophrenia patients, longitudinal DTI studies of first-episode patients are very limited.38 Further studies of longitudinal changes in white matter abnormalities are therefore also needed.
In this study, P300 amplitude in HC also showed a tendency to decrease over time, though the difference was not significant, and the P300 latency did not change over time. This likely reflects the normal development of visual P300 reported by Stige et al.,39 where visual P300 amplitude reduced with age but P300 latency did not change.
Cross-sectional studies in schizophrenia of auditory P300 have been more frequent than those of visual P300 and, in general, have reported a smaller visual P300 effect size than the auditory P300.34 Several studies that used the visual oddball task have reported smaller visual P300 amplitudes in patients with schizophrenia relative to healthy controls.40–43 Galderisi et al. also showed a reduction of the late positive complex to the target stimuli as examined by the Independent Principal Component analysis. Furthermore, time frequency analysis suggested the contribution of abnormal low frequency oscillations (theta or delta) to the visual P300 abnormality.41,42 Bestelmeyer et al.40 reported that visual P300 abnormality was specific to schizophrenia patients in the study where both schizophrenia and bipolar disorder patients were involved. Horan et al. reported normal visual P300 in the modified visual oddball paradigm, which contained emotional stimuli. 45 In the studies where both the auditory and visual oddball were used, Wood et al. demonstrated that P300 amplitude was reduced in both modalities,46 but other studies reported a discrepancy between the 2 modalities, namely impaired auditory P300 and intact visual P300.11,12,16,17,47,48 N200 amplitude was also reported to be small in the visual oddball paradigm,16,49 which we replicated in this study. P1 reduction in schizophrenia patients has been consistently reported in studies using magnocellular pathway–biased stimuli (low contrast/spatial frequency),50–52 while studies using parvocellular pathway–biased stimuli (high contrast/spatial frequency) or simple visual oddball tasks did not find the reduction.53,54 The finding that P1 group differences did not reach significant level in this study is probably due to a letter-discriminating oddball task, which would have tapped the parvocellular visual pathway rather than the magnocellular pathway. N1 amplitude to visual oddball tasks seems to be normal.16,17,40,46,54 One recent study suggested the usefulness of the N1 latency for diagnosis of schizophrenia.55 We also have shown the normal N1 amplitude but delayed N1 latency in the first-episode schizophrenia patients in this study. We did not replicate the N1 amplitude deficits in FESZ we found in our previous study,22 which also suggests the importance of selecting right tasks to see impairment in these early components.
In terms of longitudinal studies in schizophrenia patients, there have been several reports of longitudinal ERP changes in the context of the treatment effects over a relatively short time. For example, auditory gating deficits indexed by P50 have shown improvements after treatment,56,57 while P300 in a standard auditory oddball task tended to be stable through the course of treatment,58,59 although a study using a Go/No Go task and one that had a relatively long duration of follow-up have reported auditory P300 enhancement at the second measurement.56,60 Mathalon et al. investigated auditory and visual P300 longitudinally along with the clinical symptom fluctuations over time and concluded that both auditory and visual P300 had reflected symptom fluctuations but only auditory P300 could be used as a trait marker of schizophrenia,12 which may suggest that visual P300 is more sensitive to clinical state. Deficits of duration mismatch negativity (MMN), also considered to be a trait marker,61 have been reported to be stable over 1- to 2-year period in chronic schizophrenia patients. On the other hand, frequency MMN showed progressive reduction over time, which was highly correlated with the progressive volume reduction of the Heschl gyrus in patients with first-episode schizophrenia.63 Progressive reduction of the brain volume in schizophrenia has been frequently reported in the frontal64–72 and temporal regions.4,6,66,68,69,71,73 Furthermore, we have found progressive volume reduction of the parietal lobe in the patients with first-episode schizophrenia (Hosokawa et al., submitted). These regions have been also identified as including visual P300 generators.14,74 In this study, we have reported visual P1, N200 and P300 deficits; however, only visual P300 showed the progressive reduction at 1-year follow-up in the absence of the worsening of symptoms or cognitive function. Although it is possible that visual perceptual processing dysfunction especially in the magnocellular pathway, which manifested in the early course of the illness, might have contributed to abnormal working memory operations indexed by N200 and P300,25 further progressive P300 amplitude reduction, in the absence of such reductions in the P1 and N200, suggests, at the very least, that these processes are dissociable. While the current data cannot directly address the possibility of earlier sensory operations impacting the later memory-based operations, they speak against a simple model of a dysfunction “up the stream” dependent on the dysfunction “down the stream.” It is thus possible that the visual P300 progression observed in this study is the result of a progressive volume reduction of the P300 visual generators, or might be the consequence of degraded synaptic connectivity or both. Taken together, visual P300 might be more sensitive to the schizophrenia state than to trait and therefore can be used as a biological marker of progression in schizophrenia.
In summary, we found reduced visual P300 amplitude and delayed latency in FESZ at initial measurement and at the 1-year follow-up. Furthermore, the visual P300 amplitude showed progressive reduction over time in FESZ. Patients who had more positive symptoms showed more amplitude reduction at baseline but no significant correlation with symptoms at follow-up, perhaps because all patients experienced P300 amplitude reduction and the improvement in the positive symptoms at the follow-up, which contributed to reduced variance and hence a lack of correlation. These data are significant, and we believe novel, in showing a visual modality P300 progression in the early stage of schizophrenia, in contrast to less consistent or absent progression in the auditory P300. These visual P300 findings support the concept of progression of schizophrenia. They suggest the usefulness of the visual P300 as a biological marker of progression in schizophrenia, both at onset and follow-up, and potentially even in the prodromal period. The visual P300 would appear to be especially valuable as a biological marker because of its ease and economy of measurement.
Supplementary Material
Supplementary material is available at http://schizophre niabulletin.oxfordjournals.org.
Funding
Department of Veterans Affairs Medical Research Awards (Schizophrenia Center, Merit Awards to R.W.M. and M.E.S.); National Institute of Mental Health (K05MH070047 and R01MH50747 to M.E.S., R01MH40799 and R01MH052807 to R.W.M., CIDAR P50MH080272 to R.W.M. and M.E.S.); Strategic Young Researcher Overseas Visits Program for Accelerating Brain Circulation (S2208 to S.K.) and Grant-in-Aid for Young Scientists (B 22791129 to Y.H.) from Japan Society for the Promotion of Science; Fund for Pharmacopsychiatry Research (to Y.H.) from SENSHIN Medical Research Foundation.
Supplementary Material
Acknowledgments
We thank all participants who participated in the study, and T. Tasoff and K. D. Kim for their support as research assistants. This study was conducted as a part of the Boston CIDAR study, which refers to as “Longitudinal Assessment and Monitoring of Clinical Status and Brain Function in Adolescents and Adults.” All authors declare that they do not have conflicts of interest.
References
- 1. Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends Neurosci. 2009;32:485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lewis DA, González-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. [DOI] [PubMed] [Google Scholar]
- 3. Catts VS, Fung SJ, Long LE, et al. Rethinking schizophrenia in the context of normal neurodevelopment. Front Cell Neurosci. 2013;7:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kasai K, Shenton ME, Salisbury DF, et al. Progressive decrease of left superior temporal gyrus gray matter volume in patients with first-episode schizophrenia. Am J Psychiatry. 2003;160:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vita A, De Peri L, Deste G, Sacchetti E. Progressive loss of cortical gray matter in schizophrenia: a meta-analysis and meta-regression of longitudinal MRI studies. Transl Psychiatry. 2012;2:e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kasai K, Shenton ME, Salisbury DF, et al. Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2003;60:766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–336. [DOI] [PubMed] [Google Scholar]
- 9. O’Donnell BF, Faux SF, McCarley RW, et al. Increased rate of P300 latency prolongation with age in schizophrenia. Electrophysiological evidence for a neurodegenerative process. Arch Gen Psychiatry. 1995;52:544–549. [DOI] [PubMed] [Google Scholar]
- 10. Mathalon DH, Ford JM, Rosenbloom M, Pfefferbaum A. P300 reduction and prolongation with illness duration in schizophrenia. Biol Psychiatry. 2000;47:413–427. [DOI] [PubMed] [Google Scholar]
- 11. Duncan CC. Event-related brain potentials: a window on information processing in schizophrenia. Schizophr Bull. 1988;14:199–203. [DOI] [PubMed] [Google Scholar]
- 12. Mathalon DH, Ford JM, Pfefferbaum A. Trait and state aspects of P300 amplitude reduction in schizophrenia: a retrospective longitudinal study. Biol Psychiatry. 2000;47:434–449. [DOI] [PubMed] [Google Scholar]
- 13. Simson R, Vaughn HG, Jr, Ritter W. The scalp topography of potentials in auditory and visual discrimination tasks. Electroencephalogr Clin Neurophysiol. 1977;42:528–535. [DOI] [PubMed] [Google Scholar]
- 14. Soltani M, Knight RT. Neural origins of the P300. Crit Rev Neurobiol. 2000;14:199–224. [PubMed] [Google Scholar]
- 15. Bruder G, Kayser J, Tenke C, et al. The time course of visuospatial processing deficits in schizophrenia: an event-related brain potential study. J Abnorm Psychol. 1998;107:399–411. [DOI] [PubMed] [Google Scholar]
- 16. Egan MF, Duncan CC, Suddath RL, Kirch DG, Mirsky AF, Wyatt RJ. Event-related potential abnormalities correlate with structural brain alterations and clinical features in patients with chronic schizophrenia. Schizophr Res. 1994;11:259–271. [DOI] [PubMed] [Google Scholar]
- 17. Ford JM, White PM, Csernansky JG, Faustman WO, Roth WT, Pfefferbaum A. ERPs in schizophrenia: effects of antipsychotic medication. Biol Psychiatry. 1994;36:153–170. [DOI] [PubMed] [Google Scholar]
- 18. Potts GF, O’Donnell BF, Hirayasu Y, McCarley RW. Disruption of neural systems of visual attention in schizophrenia. Arch Gen Psychiatry. 2002;59:418–424. [DOI] [PubMed] [Google Scholar]
- 19. Vohs JL, Hetrick WP, Kieffaber PD, et al. Visual event-related potentials in schizotypal personality disorder and schizophrenia. J Abnorm Psychol. 2008;117:119–131. [DOI] [PubMed] [Google Scholar]
- 20. Sponheim SR, McGuire KA, Stanwyck JJ. Neural anomalies during sustained attention in first-degree biological relatives of schizophrenia patients. Biol Psychiatry. 2006;60:242–252. [DOI] [PubMed] [Google Scholar]
- 21. Dias EC, Butler PD, Hoptman MJ, Javitt DC. Early sensory contributions to contextual encoding deficits in schizophrenia. Arch Gen Psychiatry. 2011;68:654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oribe N, Hirano Y, Kanba S, et al. Early and late stages of visual processing in individuals in prodromal state and first episode schizophrenia: an ERP study. Schizophr Res. 2013;146:95–102. [DOI] [PubMed] [Google Scholar]
- 23. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York, NY: New York State Psychiatric Institute; 2002. [Google Scholar]
- 24. Stoll AL. The Psychopharmacology Reference Card. Belmont, MA: McLean Hospital; 2001. [Google Scholar]
- 25. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. [DOI] [PubMed] [Google Scholar]
- 26. Annett M. A classification of hand preference by association analysis. Br J Psychol. 1970;61:303–321. [DOI] [PubMed] [Google Scholar]
- 27. Wilkinson GS, Robertson GJ. Wide Range Achievement Test. 4th ed. Lutz, FL: Psychological Assessment Resources, Inc; 2006. [Google Scholar]
- 28. Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation, Harcourt Brace; 1999. [Google Scholar]
- 29. Hollingshead A. Two Factor Index of Social Position. New Haven, CT: Yale University Press; 1965. [Google Scholar]
- 30. Overall JGD. The brief psychiatric rating scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 31. Wechsler D. Wechsler Memory Scale - Third Edition Manual. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 32. Ruggeri M, Koeter M, Schene A, et al. ; EPSILON Study Group. Factor solution of the BPRS-expanded version in schizophrenic outpatients living in five European countries. Schizophr Res. 2005;75:107–117. [DOI] [PubMed] [Google Scholar]
- 33. Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55:468–484. [DOI] [PubMed] [Google Scholar]
- 34. Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology. 2003;40:684–701. [DOI] [PubMed] [Google Scholar]
- 35. Rosenberger G, Kubicki M, Nestor PG, et al. Age-related deficits in fronto-temporal connections in schizophrenia: a diffusion tensor imaging study. Schizophr Res. 2008;102:181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carpenter DM, Tang CY, Friedman JI, et al. Temporal characteristics of tract-specific anisotropy abnormalities in schizophrenia. Neuroreport. 2008;19:1369–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Friedman JI, Tang C, Carpenter D, et al. Diffusion tensor imaging findings in first-episode and chronic schizophrenia patients. Am J Psychiatry. 2008;165:1024–1032. [DOI] [PubMed] [Google Scholar]
- 38. Douaud G, Mackay C, Andersson J, et al. Schizophrenia delays and alters maturation of the brain in adolescence. Brain. 2009;132:2437–2448. [DOI] [PubMed] [Google Scholar]
- 39. Stige S, Fjell AM, Smith L, Lindgren M, Walhovd KB. The development of visual P3a and P3b. Dev Neuropsychol. 2007;32:563–584. [DOI] [PubMed] [Google Scholar]
- 40. Bestelmeyer PE. The visual P3a in schizophrenia and bipolar disorder: effects of target and distractor stimuli on the P300. Psychiatry Res. 2012;197:140–144. [DOI] [PubMed] [Google Scholar]
- 41. Donkers FC, Schwikert SR, Evans AM, Cleary KM, Perkins DO, Belger A. Impaired neural synchrony in the theta frequency range in adolescents at familial risk for schizophrenia. Front Psychiatry. 2011;2:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ergen M, Marbach S, Brand A, Başar-Eroğlu C, Demiralp T. P3 and delta band responses in visual oddball paradigm in schizophrenia. Neurosci Lett. 2008;440:304–308. [DOI] [PubMed] [Google Scholar]
- 43. Luck SJ, Kappenman ES, Fuller RL, Robinson B, Summerfelt A, Gold JM. Impaired response selection in schizophrenia: evidence from the P3 wave and the lateralized readiness potential. Psychophysiology. 2009;46:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Galderisi S, Maj M, Mucci A, Monteleone P, Kemali D. Lateralization patterns of verbal stimuli processing assessed by reaction time and event-related potentials in schizophrenic patients. Int J Psychophysiol. 1988;6:167–176. [DOI] [PubMed] [Google Scholar]
- 45. Horan WP, Foti D, Hajcak G, Wynn JK, Green MF. Intact motivated attention in schizophrenia: evidence from event-related potentials. Schizophr Res. 2012;135:95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wood SM, Potts GF, Hall JF, Ulanday JB, Netsiri C. Event-related potentials to auditory and visual selective attention in schizophrenia. Int J Psychophysiol. 2006;60:67–75. [DOI] [PubMed] [Google Scholar]
- 47. Pfefferbaum A, Ford JM, White PM, Roth WT. P3 in schizophrenia is affected by stimulus modality, response requirements, medication status, and negative symptoms. Arch Gen Psychiatry. 1989;46:1035–1044. [DOI] [PubMed] [Google Scholar]
- 48. Dichter GS, van der Stelt O, Boch JL, Belger A. Relations among intelligence, executive function, and P300 event related potentials in schizophrenia. J Nerv Ment Dis. 2006;194:179–187. [DOI] [PubMed] [Google Scholar]
- 49. Ford JM, White P, Lim KO, Pfefferbaum A. Schizophrenics have fewer and smaller P300s: a single-trial analysis. Biol Psychiatry. 1994;35:96–103. [DOI] [PubMed] [Google Scholar]
- 50. Foxe JJ, Doniger GM, Javitt DC. Early visual processing deficits in schizophrenia: impaired P1 generation revealed by high-density electrical mapping. Neuroreport. 2001;12:3815–3820. [DOI] [PubMed] [Google Scholar]
- 51. Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiatry. 2002;59:1011–1020. [DOI] [PubMed] [Google Scholar]
- 52. Schechter I, Butler PD, Zemon VM, et al. Impairments in generation of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia. Clin Neurophysiol. 2005;116:2204–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Butler PD, Martinez A, Foxe JJ, et al. Subcortical visual dysfunction in schizophrenia drives secondary cortical impairments. Brain. 2007;130:417–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. van der Stelt O, Frye J, Lieberman JA, Belger A. Impaired P3 generation reflects high-level and progressive neurocognitive dysfunction in schizophrenia. Arch Gen Psychiatry. 2004;61:237–248. [DOI] [PubMed] [Google Scholar]
- 55. Neuhaus AH, Popescu FC, Bates JA, Goldberg TE, Malhotra AK. Single-subject classification of schizophrenia using event-related potentials obtained during auditory and visual oddball paradigms. Eur Arch Psychiatry Clin Neurosci. 2013;263:241–247. [DOI] [PubMed] [Google Scholar]
- 56. Bender S, Schall U, Wolstein J, Grzella I, Zerbin D, Oades RD. A topographic event-related potential follow-up study on ‘prepulse inhibition’ in first and second episode patients with schizophrenia. Psychiatry Res. 1999;90:41–53. [DOI] [PubMed] [Google Scholar]
- 57. Oranje B, Aggernaes B, Rasmussen H, Ebdrup BH, Glenthøj BY. P50 suppression and its neural generators in antipsychotic-naive frst-episode schizophrenia before and after 6 months of quetiapine treatment. Schizophr Bull. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gallinat J, Riedel M, Juckel G, et al. P300 and symptom improvement in schizophrenia. Psychopharmacology (Berl). 2001;158:55–65. [DOI] [PubMed] [Google Scholar]
- 59. Molina V, Muñoz F, Martín-Loeches M, Casado P, Hinojosa JA, Iglesias A. Long-term olanzapine treatment and p300 parameters in schizophrenia. Neuropsychobiology. 2004;50:182–188. [DOI] [PubMed] [Google Scholar]
- 60. Higashima M, Nagasawa T, Kawasaki Y, et al. Auditory P300 amplitude as a state marker for positive symptoms in schizophrenia: cross-sectional and retrospective longitudinal studies. Schizophr Res. 2003;59:147–157. [DOI] [PubMed] [Google Scholar]
- 61. Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76:1–23. [DOI] [PubMed] [Google Scholar]
- 62. Light GA, Braff DL. Stability of mismatch negativity deficits and their relationship to functional impairments in chronic schizophrenia. Am J Psychiatry. 2005;162:1741–1743. [DOI] [PubMed] [Google Scholar]
- 63. Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gur RE, Cowell P, Turetsky BI, et al. A follow-up magnetic resonance imaging study of schizophrenia. Relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry. 1998;55:145–152. [DOI] [PubMed] [Google Scholar]
- 65. Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry. 2003;60:585–594. [DOI] [PubMed] [Google Scholar]
- 66. Lieberman JA, Tollefson GD, Charles C, et al. ; HGDH Study Group. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62:361–370. [DOI] [PubMed] [Google Scholar]
- 67. Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A. Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:148–157. [DOI] [PubMed] [Google Scholar]
- 68. Nakamura M, Salisbury DF, Hirayasu Y, et al. Neocortical gray matter volume in first-episode schizophrenia and first-episode affective psychosis: a cross-sectional and longitudinal MRI study. Biol Psychiatry. 2007;62:773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rapoport JL, Giedd JN, Blumenthal J, et al. Progressive cortical change during adolescence in childhood-onset schizophrenia. A longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56:649–654. [DOI] [PubMed] [Google Scholar]
- 70. Sun D, Stuart GW, Jenkinson M, et al. Brain surface contraction mapped in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Mol Psychiatry. 2009;14:976–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Thompson PM, Vidal C, Giedd JN, et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci U S A. 2001;98:11650–11655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. van Haren NE, Hulshoff Pol HE, Schnack HG, et al. Focal gray matter changes in schizophrenia across the course of the illness: a 5-year follow-up study. Neuropsychopharmacology. 2007;32:2057–2066. [DOI] [PubMed] [Google Scholar]
- 73. Keshavan MS, Haas GL, Kahn CE, et al. Superior temporal gyrus and the course of early schizophrenia: progressive, static, or reversible? J Psychiatr Res. 1998;32:161–167. [DOI] [PubMed] [Google Scholar]
- 74. Bledowski C, Prvulovic D, Hoechstetter K, et al. Localizing P300 generators in visual target and distractor processing: a combined event-related potential and functional magnetic resonance imaging study. J Neurosci. 2004;24:9353–9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.