Abstract
The cognitive impairments associated with schizophrenia have long been known to involve deficits in working memory (WM) capacity. To date, however, the causes of WM capacity deficits remain unknown. The present study examined selective attention impairments as a putative contributor to observed capacity deficits in this population. To test this hypothesis, we used an experimental paradigm that assesses the role of selective attention in WM encoding and has been shown to involve the prefrontal cortex and the basal ganglia. In experiment 1, participants were required to remember the locations of 3 or 5 target items (red circles). In another condition, 3-target items were accompanied by 2 distractor items (yellow circles), which participants were instructed to ignore. People with schizophrenia (PSZ) exhibited significant impairment in memory for the locations of target items, consistent with reduced WM capacity, but PSZ and healthy control subjects did not differ in their ability to filter the distractors. This pattern was replicated in experiment 2 for distractors that were more salient. Taken together, these results demonstrate that reduced WM capacity in PSZ is not attributable to a failure of filtering irrelevant distractors.
Key words: schizophrenia, working memory, selective attention
Introduction
People with schizophrenia (PSZ) have large reductions in working memory (WM) capacity that are strongly correlated with broader measures of cognitive function.1,2 However, the origins of this impairment remain unknown. Among healthy young adults, individual differences in WM capacity have been linked to variations in selective attention: individuals with lower capacity indices tend to have difficulty storing only relevant items and excluding irrelevant items (for a review, see Cowan and Morey3). This has also been demonstrated in electrophysiological studies, which provide direct evidence that low-capacity participants store irrelevant items in WM.4 In contrast, the increase in capacity that is observed across child development does not appear to involve increased attentional selectivity, as even very young children show biased storage of relevant items and exclusion of irrelevant items.5,6 Furthermore, electrophysiological data indicate that the factors that explain individual differences in WM among healthy people differ from the factors that explained differences among PSZ.7 These data suggest that there are multiple mechanisms implicated in capacity limitations across development, across individuals, and perhaps, across disease. The aim of the present work was to determine the degree to which failures in selective attention are responsible for the WM capacity deficits in schizophrenia.
We addressed this issue previously in a set of 4 experiments in which subjects were instructed to store items of a particular color or shape in WM.8 Although PSZ had reduced WM capacity in these experiments, we found that they were able to selectively store the relevant information in WM just as well as healthy control subjects (HCS). Smith and colleagues9 also found that PSZ were able to selectively encode and store target words in WM at the exclusion of nontarget words, which were distinguished by word color. Taken together, these data suggest that the capacity impairment in PSZ arises from mechanisms that are distinct from those that account for individual differences in healthy adults.
This evidence for intact selection has been challenged by 2 recent studies. Hahn and colleagues10 reported substantial deficits in selection in PSZ when distracting stimuli were more salient than the to-be-remembered items in a spatial memory task. Similarly, Mayer and colleagues11 reported WM capacity reductions in PSZ were associated with the degree of vulnerability to attentional capture by a salient distractor during a visual search task. These 2 experiments suggest that selection may break down in the face of highly salient distractors where the irrelevant information may have a bottom-up advantage relative to task-relevant stimuli. In addition, the study by Hahn and colleagues10 used a spatial memory paradigm, raising the possibility that selection mechanisms may fail when locations, rather than simple visual features, are to be attended. The 2 experiments described below were designed to evaluate these possibilities.
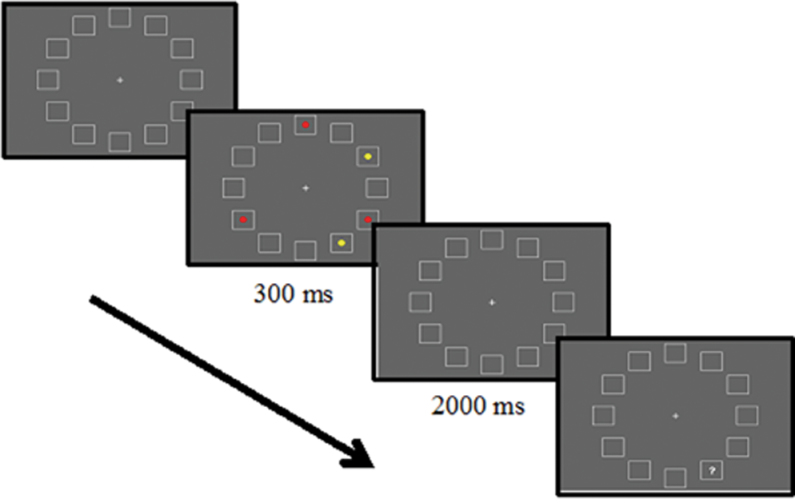
In the first experiment, we used a spatial WM paradigm that was designed to assess the role of filtering distractors during WM encoding and that has been validated in both lesion and functional magnetic resonance imaging (fMRI) studies.12,13 Participants were shown a display of 12 placeholders arranged in a circular array (see figure 1) and were asked to remember the locations of either 3 or 5 red target circles that appeared in randomly selected placeholders. On half of the 3-target trials, 2 yellow distractor circles were presented and participants were instructed to ignore these yellow distractors. Following a brief delay period, 1 of the 12 placeholders was probed, and participants were asked to indicate whether a target had been presented at that location.
Fig. 1.
Task sequence depicting a 3:2 trial from experiment 1. Following a fixation period with 12 empty placeholders, a memory array appears for 300ms. After a 2000-ms delay period, one of the placeholders is probed and participants indicate whether or not the space was occupied by a target.
If PSZ are more susceptible to spatial distractors,10,11 it may be predicted that (1) the presence of yellow distractors will have a dramatic effect on performance in PSZ, and (2) the magnitude of this effect will be directly associated with WM capacity. This prediction is bolstered by reports that accuracy in this task is dependent upon prefrontal and basal ganglia function12,13; ie, adequate filtering of the irrelevant distractors appears to be supported by structures that are known to be impaired in PSZ (see Luck and Gold14 and Perez-Costas et al15 for a review).
Alternately, if selective attention is relatively preserved in PSZ, decrements in spatial WM capacity should be observed in the absence of increased filtering costs. In experiment 2, the salience of the distractors was manipulated in order to examine the competition between top-down and bottom-up factors in the control of WM encoding. As in experiment 1, the comparison of performance in the presence vs the absence of distractors highlights the role of filtering deficits in WM capacity.
Experiment 1
Experiment 1 (figure 1) closely replicated the well-validated paradigm used in previous fMRI and neuropsychological studies.12,13 In 1 trial type, 3 red targets appeared with no distractors (the 3:0 condition); in a second trial type, 3 red targets appeared with 2 yellow distractors (the 3:2 condition); in a third trial type, 5 red targets appeared with no distractors (the 5:0 condition). Following presentation of the memory array, there was a delay period, after which one of the spatial locations was probed and participants were asked to indicate whether or not a target had been present at that location. If selective attention is impaired in schizophrenia, performance of PSZ would be expected to decline more dramatically between the 3:0 and 3:2 conditions than performance in the healthy control group. Alternatively, if PSZ have intact selection but limited storage capacity, group difference should be observed mostly in the performance decline between the 3:0 and the 5:0 condition, which maximally stresses WM capacity. Finally, it was expected that WM capacity estimates derived from this paradigm would be correlated with standardized measures of cognitive ability as shown previously.1,2
Methods
Participants
Forty-one individuals with a Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) diagnosis of schizophrenia or schizoaffective disorder and 32 psychiatrically healthy individuals participated in the experiment. Demographic information is provided in table 1. The groups were statistically similar on gender (χ 2 = 0.02; P = .89), age (t = 0.09; P = .93), race (χ 2 = 0.04; P = .98), and parental education, a proxy measure of socioeconomic status (t = 0.39; P = .70). However, PSZ had significantly fewer years of education (t = 4.60; P < .001) and lower IQ than did HCS (t = 6.54; P < .001). Diagnosis was confirmed using the Structured Clinical Interview for the DSM-IV (SCID-I/P, First et al16), as well as review of medical records and informant reports when appropriate. All PSZ were reported to be clinically stable by their mental health providers and had not received any changes in medication dosage for at least 4 weeks prior to testing. Haloperidol dose equivalents were calculated according to the formula recommended by Andreasen and colleagues17 (table 1). All HCS were free from any current Axis I diagnosis and Schizotypal Personality Disorder and were not taking any psychiatric medications. Participants in both groups were between the ages of 18 and 55 and reported no history of neurological injury. PSZ were recruited from the Maryland Psychiatric Research Center and other community clinics, whereas HCS were recruited by way of random digit dialing, web advertising, and word of mouth.
Table 1.
Demographic Information From Experiment 1 (mean ± SD)
| Healthy Controls | Schizophrenia Patients | |
|---|---|---|
| Gender (M:F) | 19:13 | 25:16 |
| Age | 40.34±10.27 | 40.56±10.90 |
| Race (AA:C:other) | 12:19:1 | 15:25:1 |
| Education (years) | 14.88±1.95 | 12.61±2.19*** |
| Parental education | 13.23±1.89 | 13.45±2.62 |
| Haloperidol dose equivalent (mg/day) | — | 12.46±12.40 |
| BPRS | — | 34.95±7.61 |
| WASI | 116.44±10.64 | 96.63±15.20*** |
| MATRICS total | 55.84±9.00 | 30.36±14.37*** |
| WRAT-4 | 107.56±13.25 | 93.71±12.51*** |
| WTAR | 110.59±11.99 | 95.85±15.56*** |
Note: BPRS = Brief Psychiatric Rating Scale;
MCCB = MATRICS Consensus Cognitive Battery; WASI = Wechsler Abbreviated Scale of Intelligence; WRAT = Wide Range Achievement Test; WTAR = Wechsler Test of Adult Reading.
***P < .001.
Neuropsychological and Symptom Measures
Several standardized neuropsychological measures were administered to examine current and premorbid cognitive functioning in PSZ and HCS: (1) the MATRICS Consensus Cognitive Battery (MCCB, Nuechterlein et al18); (2) the Wide Range Achievement Test 4 (WRAT-4, Wilkinson and Robertson19); (3) the Wechsler Test of Adult Reading (WTAR, Wechsler20); and (4) the Wechsler Abbreviated Scale of Intelligence (WASI, Wechsler21). Finally, the Brief Psychiatric Rating Scale (BPRS, Overall and Gorham22) was used to measure symptom severity in the present sample.
Stimuli and Procedure
The experimental task included 3 trial types: one in which 3 red circles, or “targets,” were presented with no distractors (3:0), one in which 3 red circle targets were presented along with 2 yellow circle distractors (3:2), and one in which 5 red circle targets were presented (5:0).
Stimuli were presented on a cathode ray tube monitor with a dark gray background (x = 0.242, y = 0.237, 1.03 cd/m2) at a viewing distance of 75cm. Targets were 1° red circles (x = 0.650, y = 0.315, 22.48 cd/m2) and distractors were 1° yellow circles (x = 0.437, y = 0.457, 77.69 cd/m2). On each trial, 12 square placeholders were arranged in an equidistant circle around central fixation with a visual angle of 10°. A memory array, consisting of either 3 red targets (3:0), 3 red targets and 2 yellow distractors (3:2), or 5 red targets (5:0) was presented for 300ms, followed by a 2-s delay. A probe then appeared at 1 of the 12 locations, prompting participants to indicate whether or not that location had been occupied by a target. On 50% of the trials, the probe was presented at a target location. In the 3:2 condition, the probe appeared at a distractor location 30% of the time. The probe remained on the screen until a response was made. Participants completed 6 blocks of 60 trials, for a total of 120 trials for each of the 3 trial types. The number of items stored in WM, or K, was estimated for each trial type using the formula K = (hit rate − false alarm rate) × set size.23
Results
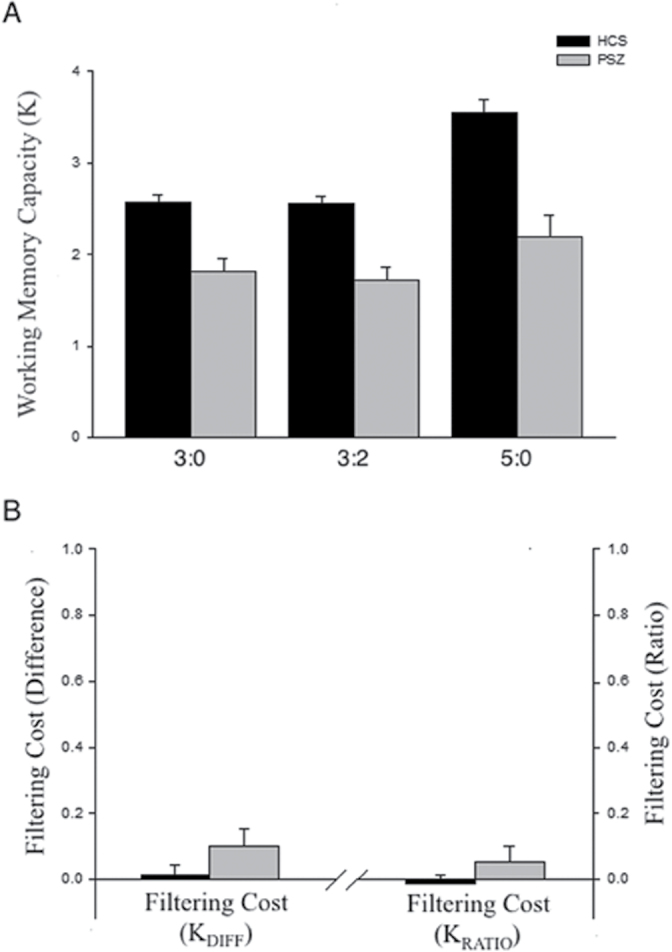
K for each group and trial type is presented in figure 2. A 2-way ANOVA revealed a main effect of group (F 1,71 = 22.67; P < .001), indicating that the estimated number of stored items was significantly lower in PSZ than in HCS for all 3 conditions. This difference between groups, collapsed across trial types, was large (Cohen’s d = 1.13) (d = 2 * √partial eta squared/(1- partial eta squared)).24
Fig. 2.
Number of items stored in WM (K ± standard error of the mean) from experiment 1. (A) The mean number of items stored for each condition, (B) the filtering cost. 3:0 = 3 targets, 0 distractors; 3:2 = 3 targets, 2 distractors; 5:0 = 5 targets, 0 distractors. K was lower in PSZ compared with HCS for all 3 trial types (P < .001) and did not decline significantly between the 3:0 and the 3:2 conditions for either group (P’s > .13). PSZ and HCS did not differ in K DIFF (P = .12) or K RATIO (P = .21). HCS = healthy control subject; PSZ = people with schizophrenia; WM = working memory.
There was also a main effect of trial type (F 2,142 = 77.07; P < .001), and pairwise comparison analyses (Bonferroni corrected) revealed that K was significantly larger in the 5:0 condition than in the 3:0 and 3:2 conditions for both PSZ (P’s < .05) and HCS (P’s < .001). K measures the number of items held in WM, and it underestimates storage capacity when the set size is less than a given participant’s storage capacity. Thus, the finding of higher K values for the 5:0 condition than for the 3:0 and 3:2 conditions indicates that capacity was greater than 3 for a number of participants of both groups. K did not differ significantly between the 3:0 and 3:2 conditions for either group (P’s > .13; Cohen’s d = 0.08 for HCS, 0.32 for PSZ), demonstrating that neither patients’ nor controls’ WM storage was affected by the presence of distracting stimuli.
The ANOVA also indicated a group × condition interaction (F 2,142 = 12.46; P < .001). HCS and PSZ diverged regarding the increase in K from the 3:0 to the 5:0 condition, with HCS exhibiting a significantly larger increase compared with PSZ (t = 4.09; P < .001; Cohen’s d = 0.94), indicative of larger storage capacity in HCS. To explore the interaction effect further, filtering cost was calculated in 2 ways: first, as the difference in K between the 3:0 and 3:2 conditions (K DIFF), and second as the proportion of this difference in K over 3:0 K (K RATIO; ie, the proportion of capacity that is lost due to distraction). That is, K DIFF was computed by subtracting 3:2 K from 3:0 K, whereas K RATIO was computed as K DIFF divided by 3:0 K.
As shown in figure 2, the filtering cost as measured by K DIFF and K RATIO was near zero in both groups. An independent samples t test revealed no significant group difference in K DIFF (t = 1.56; P = .12; Cohen’s d = 0.36) or K RATIO (t = 1.27; P = .21; Cohen’s d = 0.29). In fact, the difference in filtering cost between HCS and PSZ was only 0.09 K units (less than 1/10th of one item). This difference in K between groups is substantially smaller than the between-group difference in K for the 3 trial types, which ranged from 0.75 to 1.36 items in WM.
A Bayes factor analysis25 was conducted to determine the likelihood that no true difference in filtering cost exists between the groups. The OR for group differences in K DIFF indicated that the null hypothesis was 1.73 times more probable than the hypothesis that the groups differ in filtering cost (OR rose to 2.66 in the case of K RATIO). Thus, although PSZ exhibited substantially lower WM capacity, no evidence of impaired filtering was observed. By contrast, HCS and PSZ diverged significantly regarding the relative increase in K from the 3:0 to the 5:0 condition. HCS exhibited a significantly larger increase in K between the 3:0 and 5:0 conditions compared with PSZ (t = 4.09; P < .001; Cohen’s d = 0.94). Taken together, these results indicate that reductions in WM capacity can be observed in PSZ in the absence of impaired selective attention.
Finally, the relationship between WM capacity (K in the 5:0 condition) and several measures of cognitive ability were examined (the 5:0 condition was used to avoid restrictions of range in the 3:0 and 3:2 conditions). In both groups, K was strongly associated with general cognitive ability as measured by the MCCB total score, the MCCB WM subscale score, and the WASI total score (r’s = .50–.64; P’s < .01), and moderately correlated with cognitive ability as measured by the WTAR and WRAT-4 (r’s = .34–.41). Importantly, K was not associated with the magnitude of K DIFF for either group (r’s = −.03–.10; P’s > .60), indicating that participants’ ability to filter out irrelevant stimuli was unrelated to WM capacity in this task. K RATIO was also not significantly correlated with overall K for HCS (r = −.06, P = .73), but exhibited a trend-level association with K for PSZ (r = −0.29, P =0.07; see supplementary table 1). Medication dose was not significantly associated with K or filtering cost in PSZ (r’s < .23; P’s > .15).
Subsample Analysis
To rule out the possibility that the lower mean K in PSZ artificially masked a filtering deficit, we performed a subsample analysis in which we identified pairs of HCS and PSZ with similar K values in the 3:0 condition (N = 17 in each group; see supplementary table 2 for sample characteristics). The subsample exhibited the same pattern of results as the whole sample. There was a main effect of trial type (F 2,64 = 55.06; P < .001), with the largest K values in the 5:0 condition (P < .001) and no significant difference in K between the 3:0 and 3:2 conditions for either group (P’s > .12; Cohen’s d = 0.02 for HCS, 0.54 for PSZ). Importantly, there was no group × condition interaction (F 2,64 = 0.26; P = .77). Independent samples t tests conducted to explicitly examine filtering cost differences between groups revealed that PSZ and HCS were similar on K DIFF (t = 1.33; P = .19; Cohen’s d = 0.46) and K RATIO (t = 1.48; P = .15; Cohen’s d = 0.51). Finally, the subsample exhibited a similar pattern of correlations with neuropsychological and symptom variables as the full sample (see supplementary table 3).
Discussion
We found that PSZ exhibited a substantial reduction in WM capacity but did not exhibit an exaggerated filtering cost compared with healthy individuals, a finding that is underscored by the absence of a significant correlation between filtering cost and WM capacity. Given this pattern of results, it is difficult to attribute the reduced capacity observed in PSZ to impaired filtering. Moreover, an examination of the differences in filtering costs between groups revealed that the distractor stimuli decreased WM capacity in PSZ by only an additional 0.09 items compared with HCS. It is unlikely that the robust group differences in WM capacity found in all 3 trial types, which ranged from 0.75 to 1.36 items, can be explained by a filtering cost that only interfered with the storage of 9% of one item.
The logic of conventional statistical tests does not ordinarily allow strong conclusions to be drawn from null findings. However, several factors allow us to draw conclusions from the present results. First, previous studies have shown that neural activity is significantly modulated by the presence of distractors in the 3:2 condition in this paradigm.12,13 This paradigm therefore provides a sensitive means of assessing filtering ability. Second, overall WM capacity was strongly correlated with broader measures of cognitive performance, indicating that our WM measures were valid. Third, we found large differences in capacity between PSZ and HCS, demonstrating that we had substantial power to detect group differences and that our samples of PSZ and HCS were not unusual. Finally, the Bayes factor analysis provided positive evidence that the hypothesis of no difference in filtering between groups was more likely to be true than the alternative.
The present findings are consistent with those reported by Gold and colleagues8 suggesting that the reduction in WM capacity observed in PSZ is independent of deficits in selective attention. However, the possibility remains that PSZ may have an impairment in filtering stimuli that are highly salient, as found by Hahn and colleagues.10 Furthermore, while the subsample did not exhibit significant between-group differences in filtering cost, effect sizes were nonetheless larger than in the full sample. Such findings hint at the possibility of a distraction effect in PSZ that might not have been detectable using the present sample. We therefore replicated and expanded upon the paradigm of experiment 1 by augmenting the salience of the distractor items.
Experiment 2
Experiment 2 was conducted to determine whether PSZ would exhibit greater impairment than HCS in filtering out stimuli that are highly salient. Two additional distractor conditions were therefore added to the experimental design from experiment 1, and the 5-target (5:0) condition was eliminated. In the new distractor conditions, targets were red squares whereas distractors were either rotating red, rotating yellow, or stationary-yellow squares. Given that motion is a highly salient stimulus feature that strongly captures attention,26 it was expected that the rotating distractors would capture attention more readily than the stationary-yellow distractors. Furthermore, it was expected that performance would be most dramatically impacted in the condition in which targets and distractors share the same color (rotating red), because on these trials only the motion signal can indicate whether a given item is relevant. Given that rotating-red distractors are expected to capture attention more readily due to their overlap with the task-relevant target color, we refer to these stimuli as behaviorally relevant distractors. This terminology is used henceforth to distinguish task-relevant attentional capture from bottom-up salience that characterizes the rotating-yellow distractors (see Theeuwes27 for an elaboration upon this distinction). If PSZ are uniquely impaired in filtering out stimuli that are perceptually salient (ie, Leonard et al28) or behaviorally relevant, the filtering cost should be significantly greater for PSZ than for HCS in the rotating-red and rotating-yellow distractor conditions.
Methods
Participants
Forty-two individuals with a DSM-IV diagnosis of schizophrenia or schizoaffective disorder and 36 psychiatrically healthy individuals participated in the present experiment (see table 2 ). Of these participants, 16 PSZ and 11 HCS also participated in experiment 1. Groups were statistically similar on gender (χ 2 = 0.26; P = .61), age (t = 0.68; P = .50), race (χ 2 = 0.14; P = .93), and parental education (t = 1.21; P = .23). However, HCS had more years of education than PSZ (t = 4.25; P < .001), and a significantly higher IQ (t = 7.96; P < .001). Diagnostic status was confirmed using the same criteria as in experiment 1, and all inclusion criteria remained the same.
Table 2.
Demographics Information From Experiment 2
| Healthy Controlsa | Schizophrenia Patientsb | |
|---|---|---|
| Gender (M:F) | 22:14 | 28:14 |
| Age | 38.28±11.94 | 39.98±10.10 |
| Race (AA:C:other) | 13:21:2 | 16:23:3 |
| Education (years) | 15.11±1.91 | 13.05±2.32*** |
| Parental education | 14.21±2.08 | 13.55±2.65 |
| Haloperidol dose equivalent (mg/day) | — | 10.53±7.85 |
| BPRS | — | 37.32±7.09 |
| WASI | 118.00±8.63 | 97.83±13.51*** |
| MATRICS total | 54.35±8.38 | 30.18±14.13*** |
| WRAT-4 | 111.59±13.77 | 96.05±11.39*** |
| WTAR | 113.50±10.46 | 98.85±14.26*** |
Note: Abbreviations are explained in the first footnote to table 1.
aCognitive testing is not available for 4 healthy control participants.
bCognitive testing is not available for 2 schizophrenia patients.
***P < .001.
Neuropsychological measures
Same as in experiment 1.
Stimuli and Procedures
The experimental task included 4 trial types: one in which 3 red squares, or “targets,” were presented with no distractors; one in which 3 red targets were presented along with 2 stationary-yellow distractors; one in which the 3 red targets were presented with 2 rotating yellow distractors; and one in which the 3 red targets were presented with 2 rotating red distractors. All other features of the experiment were identical between experiments 1 and 2, with the exception that probe stimuli never cued a distractor stimulus location. Participants completed 6 blocks of trials that were intermixed with respect to trial type, for a total of 180 trials of the no-distractor condition, and 60 trials of each distractor condition. Statistical tests remained the same as those conducted in experiment 1.
Results
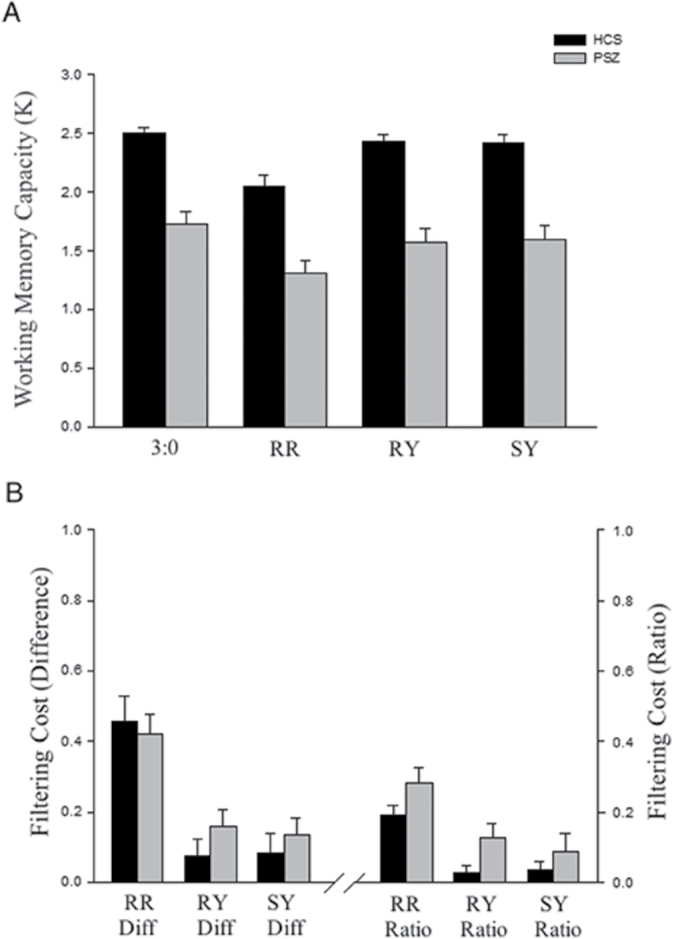
K for each group and trial type is presented in figure 3. A two-way ANOVA revealed a main effect of group (F 1,76 = 39.34; P < .001), indicating that WM capacity was significantly lower for PSZ in all 4 conditions, with a large effect size (Cohen’s d = 1.44).
Fig. 3.
Number of items stored in WM (K ± standard error of the mean) from experiment 2. (A) The mean number of items stored for each condition, (B) the filtering cost for each of the 3 distractor conditions. 3:0 = 3 Targets, 0 Distractors; RR = 3 Targets, 2 Rotating-Red Distractors; RY = 3 Targets, 2 Rotating-Yellow Distractors; SY = 3 Targets, 2 Stationary-Yellow Distractors. K was lower in PSZ compared with HCS for all 4 conditions (P < .001), yet PSZ and HCS did not differ in K DIFF for any of the 3 distractor types (P’s > .23). PSZ exhibited larger K RATIO in the RY condition only (P = .03). HCS = healthy control subject; PSZ = people with schizophrenia; WM = working memory.
There was also a main effect of trial type (F 3,228 = 35.93; P < .001), mainly due to reductions in K that were observed in both groups in the rotating-red distractor condition. However, the group × condition interaction was not significant (F 3,228 = 0.67; P = .57). Thus, PSZ were not impacted by distractor stimuli to a greater degree than HCS. Given the lack of an interaction, the main effect of condition was examined by collapsing across PSZ and HCS. Pairwise comparisons (Bonferroni corrected) revealed that accuracy in the rotating-red distractor condition was significantly reduced relative to the no-distractor condition (P < .001). There was also a small but significant decline in K in the rotating-yellow (P < .001) and stationary-yellow distractor conditions (P < .05) compared with the no-distractor condition. Medication dose was not significantly associated with K or filtering cost in PSZ (r’s < .29; P’s > .07).
To visualize these effects, filtering cost was again calculated in 2 ways: (1) subtracting the K of each of the distractor conditions from the K of the no-distractor condition (K DIFF; figure 3B), and (2) as the proportion of this difference in K over 3:0 K (K RATIO). As in experiment 1, the filtering cost associated with stationary-yellow (SYDIFF; SYRATIO) and rotating-yellow (RYDIFF; RYRATIO) distractors was small for both groups. The filtering cost associated with the rotating-red distractors (RRDIFF; RRRATIO) was substantially larger than the filtering cost associated with the rotating- and stationary-yellow distractors for both groups (t’s = 3.62–4.77; P’s < .001), but was nearly equivalent between PSZ and HCS. Independent samples t tests revealed that the 2 groups did not differ in RRDIFF (t = 0.41; P = .69; Cohen’s d = 0.09), RYDIFF (t = 1.22; P = .23; Cohen’s d = 0.28), or SYDIFF (t = 0.66; P = .51; Cohen’s d = 0.15). In fact, the group difference in filtering cost combined across all 3 distractor conditions was only 0.03 K units.
PSZ and HCS began to diverge somewhat when filtering cost was measured as a proportion of overall K (K RATIO). Independent samples t tests revealed that the proportion of K lost to distraction was significantly larger in PSZ for RYRATIO (t = 2.25; P < .05; Cohen’s d = 0.50), and larger than HCS at the level of a trend for RRRATIO (t = 1.87; P = .07; Cohen’s d = 0.42). The source of the contrasting findings between the difference score and ratio score is easily understood: while the difference scores were identical between groups, the ratio scores reflect the impact of lower baseline performance in the 3:0 condition for PSZ. Replicating the results from experiment 1, SYRATIO did not differ between groups (t = 0.98; P = .33; Cohen’s d = 0.22). Thus, PSZ may exhibit more interference than HCS for highly salient distractors (rotating red and yellow distractors), but not for moderately salient distractors (static yellow distractors).
Finally, a Bayes factor analysis revealed that the null hypothesis (no group differences in filtering costs) was substantially more likely to be true than the alternative hypothesis in the case of RRDIFF (OR = 5.31), RYDIFF (OR = 3.40), and SYDIFF (OR = 5.11) distractor conditions. In the case of ratio indices of filtering cost, the ORs were 1.18 for RRRATIO, 0.59 for RYRATIO, and 3.71 for SYRATIO. That is, the null hypothesis was more likely to be true in the case of rotating-red and stationary-yellow distractors, but less likely to be true in the case of rotating-yellow distractors.
Subsample Analysis
As in experiment 1, a subsample of participants was selected for additional analysis by identifying pairs of HCS and PSZ participants with similar K in the 3:0 condition (N = 18 in each group; see supplementary table 4 for sample characteristics). This subsample again exhibited a similar pattern of results to that of the whole sample. There was a main effect of trial type (F 3,102 = 16.95; P < .001), with no group × condition interaction (F 3,102 = 0.37; P = .77). Independent samples t tests conducted to explicitly examine filtering cost differences between groups revealed that PSZ and HCS were similar on filtering cost when measured as the difference between conditions (RRDIFF: t = 0.32, P = .75, Cohen’s d = 0.11; RYDIFF: t = 0.91, P = .37, Cohen’s d = 0.30; SYDIFF: t = 0.35, P = .73; Cohen’s d = 0.11) and as the proportion of K lost due to distraction (RRRATIO: t = 0.31, P = .76, Cohen’s d = 0.11; RYRATIO: t = 0.69, P = .50, Cohen’s d = 0.23; SYRATIO: t = 0.35, P = .73, Cohen’s d = 0.12).
Discussion
We found that PSZ exhibited a robust decrease in WM storage compared with HCS, whereas the 2 groups showed grossly similar effects of distraction. As in experiment 1, controlling for overall K did not change this pattern of results. The rotating-red distractors strongly captured attention, as indicated by substantial decreases in K compared with no-distractor trials. Despite the increased demands on selective attention, PSZ did not exhibit exaggerated filtering deficits compared with HCS for these highly potent distractors. The average difference in filtering cost between groups was, again, minute (0.03 K units)—too small to account for the large between-groups difference in K for each of the 4 trial types (0.74–0.85 K units). Furthermore, the Bayes factor analysis revealed that the null hypothesis was 3–5 times more likely to be true than the hypothesis of group differences in filtering cost.
Some evidence of impaired filtering was observed in PSZ for the highly salient rotating distractors when the filtering cost was measured as a proportion of K, which takes into account baseline differences between groups in WM capacity. PSZ may therefore lose a greater percentage of WM storage to distraction than do HCS, particularly when distracting stimuli are characterized by very high bottom-up salience. However, given that greater distraction was observed only after reduced capacity was taken into account, greater distraction cannot serve as an explanation for reduced capacity. Rather, some other factor must be driving the overall reduction in WM capacity.
General Discussion
The present study was designed to test the hypothesis that the reduced WM capacity estimates observed in PSZ are attributable to failures in selective attention. Despite the fact that patients’ K scores were substantially decreased compared with HCS in all conditions, no substantial difference in filtering was observed between the patient and control groups (experiment 1), even when distractor items exerted strong attentional capture (experiment 2). Taken together, these findings provide strong evidence against the hypothesis that impaired WM capacity in PSZ is largely a consequence of impaired filtering.
Although the reduced WM capacity in PSZ cannot be entirely explained by increased distractibility, it is possible that PSZ exhibit increased distractibility in addition to reduced capacity. Some evidence of this was observed, but the evidence was mixed. When distractibility was quantified as a proportional decrease in K rather than an absolute decrease in K, PSZ exhibited significantly greater distractibility than HCS for the rotating yellow distractors in experiment 2 (but not for the static distractors in either experiment). This is consistent with prior evidence that PSZ have difficulty suppressing highly salient distractors that activate the magnocellular visual pathway.28–30 Nevertheless, the present results clearly demonstrate that capacity reduction can be observed in the absence of impaired selective attention, making this cognitive function an unlikely candidate as the principal underlying cause of WM capacity reduction in PSZ.
Perhaps the most striking observation from the present study is the lack of group differences in filtering mechanisms that are mediated by the basal ganglia and prefrontal cortex. Functional imaging and focal lesion data have indicated that these structures play a critical role in the filtering and storage mechanisms that support WM in this experimental paradigm.12,13 Perhaps the degree of impairment in these areas is less dramatic in PSZ than in the lesion groups, thereby decreasing the likelihood that substantial filtering costs would be observed. Another possible explanation for these results is that PSZ may be employing different strategies such as a general tendency to limit the scope of attentional resources to a subset of available target items.7,31 Future studies using fMRI and other imaging techniques will be particularly important for distinguishing between these intriguing hypotheses.
The National Institute for Mental Health Research Domain Criteria (NIMH RDoC) initiative proposes that WM is a central construct for understanding psychopathology. An implicit assumption of this initiative is that variation in behavior along a single dimension can be attributed to variation in a single underlying neural mechanism. The present results suggest that this assumption is not valid in the domain of WM. Specifically, there have been multiple demonstrations that individual differences in WM capacity in the healthy population are related to individual differences in filtering,4,32 but the present results indicate that the reduction in WM capacity observed in PSZ is not caused by impaired filtering (see also Leonard et al7). Moreover, increases in WM over child development and declines in aging are also not well explained by changes in filtering.6,33 Thus, it appears that individual variation in WM is not continuous along a single dimension, which suggests that there may be different routes to capacity reduction in health and disease.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
National Institute of Mental Health (R01 MH065034 and R01 MH080066 to J.G.).
Supplementary Material
References
- 1. Johnson MK, McMahon RP, Robinson BM, et al. The relationship between working memory capacity and broad measures of cognitive ability in healthy adults and people with schizophrenia. Neuropsychology. 2013;27:220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol. 2005;114:599–611. [DOI] [PubMed] [Google Scholar]
- 3. Cowan N, Morey CC. Visual working memory depends on attentional filtering. Trends Cogn Sci. 2006;10:139–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428:748–751. [DOI] [PubMed] [Google Scholar]
- 5. Cowan N, AuBuchon AM, Gilchrist AL, Ricker TJ, Saults JS. Age differences in visual working memory capacity: not based on encoding limitations. Dev Sci. 2011;14:1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cowan N, Morey CC, AuBuchon AM, Zwilling CE, Gilchrist AL. Seven-year-olds allocate attention like adults unless working memory is overloaded. Dev Sci. 2010;13:120–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leonard CJ, Kaiser ST, Robinson BM, et al. Toward the neural mechanisms of reduced working memory capacity in schizophrenia. Cereb Cortex. 2013;23:1582–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gold JM, Fuller RL, Robinson BM, McMahon RP, Braun EL, Luck SJ. Intact attentional control of working memory encoding in schizophrenia. J Abnorm Psychol. 2006;115:658–673. [DOI] [PubMed] [Google Scholar]
- 9. Smith EE, Eich TS, Cebenoyan D, Malapani C. Intact and impaired cognitive-control processes in schizophrenia. Schizophr Res. 2011;126:132–137. [DOI] [PubMed] [Google Scholar]
- 10. Hahn B, Robinson BM, Kaiser ST, et al. Failure of schizophrenia patients to overcome salient distractors during working memory encoding. Biol Psychiatry. 2010;68:603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mayer JS, Fukuda K, Vogel EK, Park S. Impaired contingent attentional capture predicts reduced working memory capacity in schizophrenia. PLoS One. 2012;7:e48586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baier B, Karnath HO, Dieterich M, Birklein F, Heinze C, Müller NG. Keeping memory clear and stable–the contribution of human basal ganglia and prefrontal cortex to working memory. J Neurosci. 2010;30:9788–9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2008;11:103–107. [DOI] [PubMed] [Google Scholar]
- 14. Luck SJ, Gold JM. The construct of attention in schizophrenia. Biol Psychiatry. 2008;64:34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perez-Costas E, Melendez-Ferro M, Roberts RC. Basal ganglia pathology in schizophrenia: dopamine connections and anomalies. J Neurochem. 2010;113:287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 17. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. [DOI] [PubMed] [Google Scholar]
- 19. Wilkinson GS, Robertson GJ. Wide Range Achievement Test 4 Professional Manual. Lutz, FL: Psychological Assessment Resources; 2006. [Google Scholar]
- 20. Wechsler D. Wechsler Test of Adult Reading. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 21. Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 22. Overall J, Gorham D. The Brief Psychiatric Rating-Scale. Psychol Reports. 1962;10:799–812. [Google Scholar]
- 23. Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav Brain Sci. 2001;24:87–114; discussion 114. [DOI] [PubMed] [Google Scholar]
- 24. Cohen J Statistical Power Analysis for the Behavioral Sciences, 2nd ed. New Jersey: Lawrence Erlbaum Associates, Inc.; 1988. [Google Scholar]
- 25. Rouder JN, Speckman PL, Sun D, Morey RD, Iverson G. Bayesian t tests for accepting and rejecting the null hypothesis. Psychon Bull Rev. 2009;16:225–237. [DOI] [PubMed] [Google Scholar]
- 26. Girelli M, Luck SJ. Are the same attentional mechanisms used to detect visual search targets defined by color, orientation, and motion? J Cogn Neurosci. 1997;9:238–253. [DOI] [PubMed] [Google Scholar]
- 27. Theeuwes J. Top-down and bottom-up control of visual selection. Acta Psychol (Amst). 2010;135:77–99. [DOI] [PubMed] [Google Scholar]
- 28. Leonard CJ, Robinson BM, Hahn B, Gold JM, Luck SJ. Enhanced distraction by magnocellular salience signals in schizophrenia. Neuropsychologia. 2014;56:359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martínez A, Hillyard SA, Dias EC, et al. Magnocellular pathway impairment in schizophrenia: evidence from functional magnetic resonance imaging. J Neurosci. 2008;28:7492–7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Butler PD, Martinez A, Foxe JJ, et al. Subcortical visual dysfunction in schizophrenia drives secondary cortical impairments. Brain. 2007;130:417–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hahn B, Robinson BM, Harvey AN, et al. Visuospatial attention in schizophrenia: deficits in broad monitoring. J Abnorm Psychol. 2012;121:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fukuda K, Vogel EK. Individual differences in recovery time from attentional capture. Psychol Sci. 2011;22:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jost K, Bryck RL, Vogel EK, Mayr U. Are old adults just like low working memory young adults? Filtering efficiency and age differences in visual working memory. Cereb Cortex. 2011;21:1147–1154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.