Abstract
Negative symptoms in schizophrenia have been grouped into the 2 factors of apathy and diminished expression, which might be caused by separable pathophysiological mechanisms. Recently, it has been proposed that apathy could be due to dysfunctional integration of reward and effort during decision making. We asked whether apathy in particular is associated with stronger devaluation (“discounting”) of monetary rewards that require physical effort. Thirty-one patients with schizophrenia and 20 healthy control participants performed a computerized effort discounting task in which they could choose to exert physical effort on a handgrip to obtain monetary rewards. This procedure yields an individual measure for the strength of effort discounting. The degree of effort discounting was strongly correlated with apathy, but not with diminished expression. Importantly, the association between apathy and effort discounting was not driven by cognitive ability, antipsychotic medication, or other clinical and demographic variables. This study provides the first evidence for a highly specific association of apathy with effort-based decision making in patients with schizophrenia. Within a translational framework, the present effort discounting task could provide a bridge between apathy as a psychopathological phenomenon and established behavioral tasks to address similar states in animals.
Key words: negative symptoms, effort-based decision making, cost-benefit calculation
Introduction
Negative symptoms are a core feature of schizophrenia and have a strong impact on functional outcome.1–4 Although the detrimental functional consequences of negative symptoms are well recognized, causal mechanisms still remain largely unknown, hindering the development of effective treatment. Recently, a consensus has emerged that negative symptoms can be grouped into 2 factors,5–7 which we refer to as apathy and diminished expression. It has been proposed that these 2 dimensions could be caused by partly different pathophysiological mechanisms.6,8
Apathy can be defined as a reduction of motivation and/or goal-directed behavior.9 Reward is considered a driving factor for both motivation and goal-directed behavior. Accordingly, deficits in reward learning,10,11 the neural representation of reward anticipation,12 and the ability to form mental representations of prospective rewards13 have been put forward as correlates of apathy. More recently, research into negative symptoms has proposed that goal-directed behavior is not solely driven by the reward component itself, but also the effort required to obtain the reward.14–16 Consequently, an overweighing of effort costs in decision making could result in a decrease of goal-directed behavior and present clinically as apathy. Two important studies report dysfunctions of effort-based decision making in patients with schizophrenia, but the expected symptom-level link between apathy and choice behavior was not observed in patients.14,15
In this study, we used an approach informed by behavioral economics to specifically address the relationship between negative symptoms and making decisions involving widely different levels of real and pure physical effort.17,18 Specifically, we adapted a standard choice paradigm19 to provide a subjective measure of how monetary reward is devalued in proportion to a requirement for handgrip force (effort discounting).20 In other words, we measured one’s propensity to refrain from engaging in a rewarded but effortful behavior. We hypothesized that steeper effort discounting could account for the reduction of motivation and goal-directed behavior in apathetic patients relative to a healthy control (HC) group and to patients with lower apathy levels. In particular, we hypothesized that increased effort discounting would be correlated with apathy but not with diminished expression ratings.
Methods
Participants
Thirty-one individuals meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)21 criteria for schizophrenia (n = 25) or schizoaffective disorder (n = 6, no mood episode) and 20 HC participants took part in the study. The local Ethics committee approved the study, and all participants gave written informed consent. Patients were clinically and pharmacologically stable inpatients at the end of their hospitalization (n = 25) or outpatients (n = 6) treated at the Psychiatric Hospital, University of Zurich. Please note that the average inpatient stay for patients with schizophrenia in Swiss psychiatric hospitals is above 40 days,22 thus many of our inpatients would be treated as outpatients in other healthcare systems. Importantly, inpatients participated in a multimodal treatment program and were encouraged to engage in activities outside the hospital, which allowed assessment of negative symptoms. Patients were excluded if (1) daily lorazepam dosage exceeded 1mg, (2) florid positive symptoms were present (Positive and Negative Syndrome Scale; PANSS;23 any positive subscale item score >4), (3) extrapyramidal side effects were observed by the treating clinician, or (4) additional DSM-IV axis-1 or axis-2 diagnostic criteria were met (according to the treating clinician). To confirm axis-1 diagnosis in patients, exclude comorbid axis-1 disorders and ensure the absence of axis-1 disorders in the HC group, we used the Mini-International Neuropsychiatric Interview.24
Assessment of Psychopathology and Cognition
For psychopathological assessment, the following instruments were used: Brief Negative Symptom Scale (BNSS),25 Scale for the Assessment of Negative Symptoms (SANS),26 PANSS, Global Assessment of Functioning scale,27 Personal and Social Performance Scale,28 and the Calgary Depression Scale for Schizophrenia.29 The BNSS was translated into German by the senior author (see supplementary material), who trained and regularly supervised all raters. The scores for the 2 negative symptom factors in the BNSS were calculated according to the 2-factor structure proposed by the original authors (see supplementary table S1).30
A composite cognitive ability score was computed as the mean of z-transformed scores (based on HC group data) of the following cognitive tests: verbal learning (German version of the Auditory Verbal Learning Memory Test),31 verbal and visual short-term and working memory (Digit Span,32 Corsi block-tapping test)33, processing speed (Digit-Symbol Coding),34 planning (Tower of London),35 and semantic and phonemic fluency (animal naming, s-words).36
Experimental Procedure: Effort Discounting Task
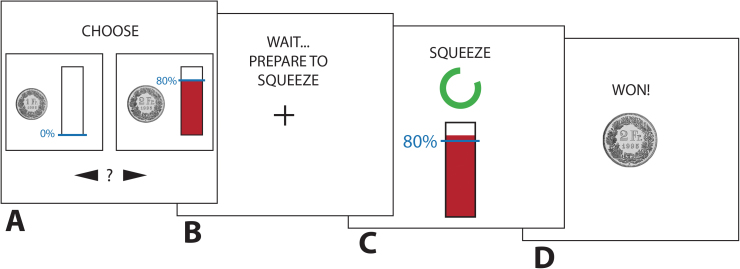
The procedure constitutes an adapted version of a recently described effort discounting task20 (figure 1). An isometric dynamometer (Sensory-Motor Systems Laboratory ETH Zurich; measuring range: 0–600 Newton) was used to assess physical effort. To determine maximum voluntary contraction (MVC), participants were asked to squeeze the handgrip with their dominant hand as hard as possible for 2 consecutive trials of 3.5 s without visual feedback of their grip strength. To approximate realistic steady-state values, MVC corresponded to the median force value of the period 1–3.5 s of these 2 maximum effort trials.
Fig. 1.
Schematic of the effort discounting task. (A) Presentation of choice options (no time limit). (B) Fixation cross (4 s). (C) Effort exertion period (3.5 s). (D) Feedback period (3 s).
During the task, participants then made a series of choices between a default small amount of money available without any effort and an alternative larger amount that was conditional on physical effort exertion. Participants indicated their preference by button-press. The effortful option was manipulated over successive trials in terms of reward (1.5, 2, 2.5, 3, 5 Swiss Francs; CHF; 1 CHF ≈ 1.09 $) and effort (40, 60, 80, 100% MVC), whereas the default effortless option always yielded 1 CHF. Each option pair was randomly presented 4 times, resulting in a total of 80 trials, which were divided in to 2 blocks. Time for choice was not restricted. For a minority of participants and effort levels, reward had to be iteratively decreased or increased in additional trials for more accurate estimation of effort discounting indices (see the “Data Processing” section).
The effort level of the chosen option had to be implemented after each choice in constant 3.5-s effort exertion periods with visual feedback (critical measurement period: 1–3.5 s). Importantly, the duration of the effort period was also implemented if the default effortless option was chosen. Thus, time costs were held constant between the effortful and effortless options. The individually adjusted effort levels assured that the participants were physically capable of performing each effort level. To exclude effects of loss aversion, participants were given the default reward of 1 CHF when failing to hold the required effort level (the number of failed trials was low and thus remained in the analyses as choice data; M = 1.2, SD = 1.68). To control for effects of fatigue, we collected an additional MVC measure (identical to the one described above) after finishing the experiment. Five of the total completed trials were randomly drawn and paid out after completion of the task. No feedback about earnings was given during the task. The task was implemented using the MATLAB toolboxes Cogent 2000 and Cogent Graphics and presented on a 19-in. computer screen.
Visual Analog Scales: Monetary Reward Pleasure and Perceived Effort
After the effort discounting task, participants provided self-report measures of anticipated monetary reward pleasure (how much pleasure they would feel when they would unexpectedly find a 50 CHF bill on the street) and effort perception (how strenuous they perceived 40, 60, 80, and 100% MVC) on visual analog scales.
Data Processing
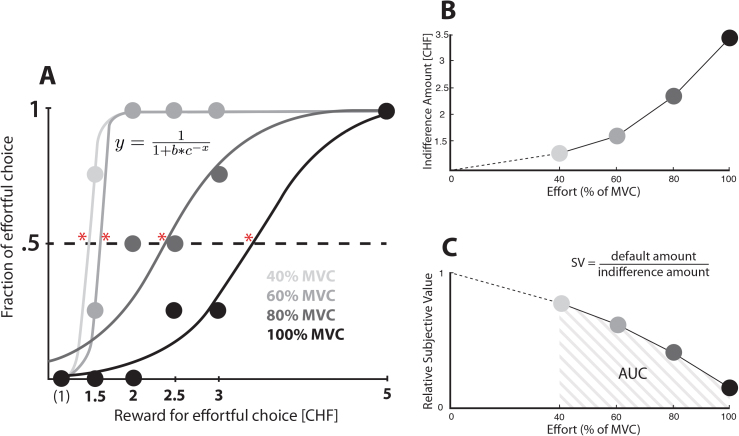
Intuitively, the effort discounting task aims to identify the minimum amount of payment each subject demands before agreeing to exert a given effort. More precisely, this is the amount of payment that makes them indifferent between the effortless and a given effortful option. To extract the indifference points, a logistic function was fitted to the fraction of effortful choices across all reward levels (figure 2A). Overall model fit (R 2) was not different between the patient and HC group (t(49) = 1.16, P = .25). These indifference values (figure 2B) then served to capture how the different effort levels (40, 60, 80, and 100% MVC) reduce (ie, “discount”) value in each participant. To do so, the default reward (1 CHF) was divided by the respective indifference amount, which yields a measure of relative subjective value (SV; figure 2C). Indifference points were estimated online during the task and if no preference reversal was observed, the reward amount for the effortful option was iteratively increased (7/10/20 CHF) or decreased (1.20/1.10/1.05 CHF) in additional 3 steps until choice behavior reversed.
Fig. 2.
Effort discounting. (A) Choice data from 1 participant and illustration of how we estimate the indifference points. In particular, we used a logistic function to interpolate the precise amount of reward that each participant required in order to be completely indifferent between the effortful and effortless options, ie, in order for them to choose each option at 50% probability (*). (B) Indifference points plotted against all effort levels in the example participant shown in (A). (C) Discount curve of the same participant. The relative subjective values are calculated by dividing the default amount (1 CHF) by the indifference amount. The area under the curve of the relative subjective values constitutes our main dependent variable of overall individual effort discounting.
In discounting paradigms, the indicator for the degree of discounting has traditionally been the fitted parameter of a model with one free parameter that modulates the steepness of the curve.37 However, debate has recently arisen about the appropriate shape of that curve in effort discounting.20 To circumvent this issue and capture individual effort discounting in an unbiased way, we computed the area under the curve (AUC) of the relative SVs over the 4 effort conditions as the measure for overall discounting (figure 2C). A smaller AUC corresponds to steeper effort discounting. This procedure is entirely driven by data but has comparable sensitivity to a one-parameter discount model.38 In sum, for each participant, we have thus a measure of overall discounting (AUC) and measures for the 4 effort levels separately (relative SVs).
Statistical Analyses
To test our main hypothesis, we computed Pearson correlations (r) between negative symptoms (apathy and diminished expression) and overall effort discounting (AUC). To further test for a significant difference between these correlations, we computed a t statistic.39 In addition, we calculated Bayes factors (BF 10) on these correlations,40 allowing us to quantify evidence in favor of the null hypotheses in the case of nonsignificant correlations. To control for confounds, partial correlations were computed.
We then pursued a categorical approach to compare effort discounting of the HC group to LOW-APATHY and HIGH-APATHY patients using the median split on the BNSS apathy score (Mdn = 16). We conducted a 4 (relative SVs for the 4 effort levels) × 3 (HC, LOW-APATHY, HIGH-APATHY) mixed design ANOVA to investigate overall group effects and additional ANOVAs to detect specific effects.
If variables were non-normally distributed according to Shapiro-Wilk tests, nonparametric statistics (Spearman correlation r s, Mann-Whitney U test) were applied.
Results
Sample Characteristics
Group characteristics and group comparisons are depicted in table 1.
Table 1.
Demographics, Clinical Variables, Composite Cognition Score, and Effort Task Performance
| Patient Group (n = 31) | HC Group (n = 20) | Test Statistic (t/χ2 /U) | P | LOW-APATHY (n = 15) | HIGH-APATHY (n = 16) | Test Statistic (t/χ2 /U) | P | |
|---|---|---|---|---|---|---|---|---|
| Age in years | 30.42 (8.69) | 32.10 (6.79) | U = 362.00 | .32 | 30.33 (6.32) | 30.50 (10.67) | t = 0.05 | .96 |
| Gender (male/female) | 25/6 | 15/5 | χ2 = 0.23 | .63 | 11/4 | 14/2 | χ2 = 0.99 | .32 |
| Handedness (r/l) | 29/2 | 16/4 | χ2 = 4.24 | .14 | 14/1 | 15/1 | χ2 = 0.002 | .96 |
| Formal education in yearsa | 9.79 (1.66) | 12.27 (3.88) | U = 428.00 | <.01 | 9.93 (1.67) | 9.66 (1.70) | U = 108.50 | .65 |
| Number of hospitalizations | 4.10 (2.74) | — | — | — | 4.33 (3.13) | 3.88 (2.39) | t = 0.45 | .64 |
| Chlorpromazine equivalents (mg/day) | 568.23 (409.97) | — | — | — | 544.40 (352.60) | 590.56 (487.98) | t = 0.31 | .76 |
| Psychopathology | ||||||||
| BNSS apathy | 16.55 (7.50) | — | — | — | 10.73 (3.58) | 22.00 (5.91) | U = 240.00 | <.001 |
| BNSS diminished expression | 10.42 (6.97) | — | — | — | 7.93 (5.23) | 12.75 (7.72) | t = 2.02 | .05 |
| SANS apathy b | 12.68 (5.87) | — | — | — | 8.73 (3.62) | 16.38 (5.15) | t = 4.75 | <.001 |
| SANS diminished expression b | 13.32 (9.46) | — | — | — | 10 (6.96) | 16.44 (10.59) | U = 162.00 | .10 |
| PANSS positive factor c | 11.29 (2.81) | — | — | — | 6.53 (2.70) | 7.88 (2.75) | U = 154.50 | .18 |
| PANSS negative factor c | 16.06 (6.01) | — | — | — | 11.60 (3.48) | 16.75 (5.79) | U = 189.00 | <.01 |
| GAF | 50.65 (9.71) | — | — | — | 56.33 (6.11) | 45.31 (9.55) | t = 3.80 | .001 |
| PSP (total) | 53.51 (10.61) | — | — | — | 60.60 (5.60) | 46.88 (9.93) | t = 4.69 | <.001 |
| CDSS (total) | 2.42 (2.41) | — | — | — | 2.20 (2.16) | 2.63 (2.15) | U = 139.50 | .45 |
| Cognitiond | ||||||||
| Composite cognitive ability | −0.87 (0.67) | 0 (0.60) | t = 4.72 | <.001 | −0.73 (0.62) | −1.00 (0.70) | t = 1.16 | .26 |
| Effort task performance | ||||||||
| MVC (N) | 184.96 (58.88) | 202.91 (65.26) | t = 1.02 | .31 | 177.55 (54.75) | 191.91 (63.47) | t = 0.67 | .51 |
| Time to reach MVC (s) | 0.81 (0.17) | 0.78 (0.16) | t = 0.75 | .46 | 0.79 (0.18) | 0.83 (0.15) | t = 0.66 | .51 |
| Fatigue (MVC1–MVC2) | 8.95 (40.34) | 10.22 (50.25) | t = 0.10 | .92 | 21.08 (38.52) | −2.42 (39.99) | t = 1.66 | .11 |
| Final payout (in CHF) | 10.84 (3.70) | 12.15 (3.05) | t = 1.32 | .19 | 11.70 (3.80) | 10.03 (3.53) | t = 1.27 | .22 |
| Total trial number | 83.90 (3.52) | 82.13 (3.02) | U = 404.00 | .05 | 82.40 (3.44) | 81.88 (2.66) | U = 113.00 | .80 |
Note: Data are presented as means and standard deviations. Potential group differences were investigated using 2-sample t tests and chi-square for continuous and categorical data, respectively. For non-normally distributed data, Mann-Whitney U tests were applied. All patients were receiving atypical antipsychotics at the time of testing. Three individuals were additionally medicated with low doses of typical antipsychotics. Seven patients were receiving an SSRI, 3 were receiving low doses of benzodiazepine, 1 was receiving a mood stabiliser, and 2 were receiving zolpidem against insomnia. BNSS, Brief Negative Symptom Scale; CDSS, Calgary Depression Scale for Schizophrenia; CHF, Swiss Francs; GAF, Global Assessment of Functioning; HC, healthy control; MVC, Maximum Voluntary Contraction; N, Newton; PANSS, Positive and Negative Syndrome Scale; PSP, Personal and Social Performance Scale; s, seconds; SANS, Scale for the Assessment of Negative Symptoms.
P values lower than .05 are in bold.
aCompulsory education in Switzerland is 9 years.
b Apathy = Avolition/Apathy, Anhedonia/Asociality; diminished expression = Affective Flattening or Blunting, Alogia.
c Positive factor = P1, P3, P5, G9; negative factor = N1, N2, N3, N4, N6, G7.
dCognition data have been z-transformed based on the data of the HC group for each test separately. The composite cognitive ability score was computed as the mean of the z-transformed test scores on subject level.
Effort Task Performance
All participants demonstrated preference reversal for all effort levels and showed overall effort discounting (decreasing relative SVs with increasing effort), which indicates that they processed both effort and reward information. For raw choice data, see supplementary figure S1. The HC and the patient group did not differ significantly with regard to MVC before the experiment, time to reach MVC, fatigue, and final payout (table 1). None of the groups showed significant fatigue (decline in MVC before vs after the experiment; P > .23). There was no significant correlation of apathy with MVC before the experiment (r(29) = .12, P = .51), fatigue (r(29) = −.18, P = .34), final payout (r(29) = −.30, P = .10), and total number of trials completed (r s(29) = −.17, P = .36).
Association of Negative Symptoms With Effort Discounting
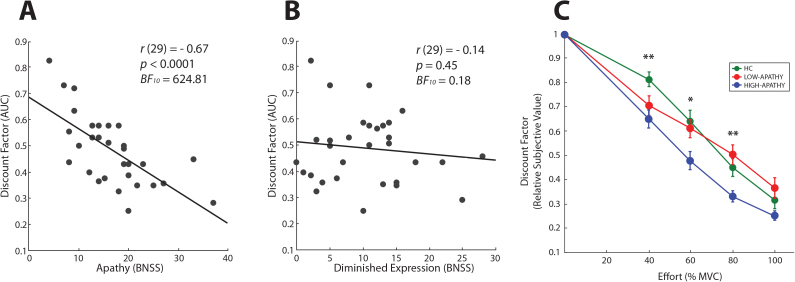
We used AUC of the relative SVs to determine overall effort discounting. Regarding our main hypothesis, we found a highly significant correlation between apathy and effort discounting (r(29) = −.67, P < .0001; figure 3A). In contrast, the correlation between diminished expression and effort discounting was not significant (r(29) = −.14, P = .45; figure 3B). Importantly, these 2 correlations between symptomatology (apathy vs diminished expression) and effort discounting were significantly different (t(28) = 4.57, P < .0001). Strikingly, the differential correlations arose even though, in line with prior studies on the structure of negative symptoms.5,7,41,42, apathy and diminished expression were significantly correlated with each other (r(29) = .58, P < .01). Thus, our results indicate that effort discounting is more strongly associated with apathy than diminished expression.
Fig. 3.
Bivariate Pearson correlation (including significance test and Bayes factor) of apathy (A) and diminished expression (B) with the effort discount factor, measured as the area under the curve of the relative subjective values plotted against the 4 effort levels. (C) Group level effort discounting plotted against all effort levels (*P < .05, **P < .01).
To quantify the relative evidence for the null (H 0) or the alternative hypothesis (H 1) in these correlations, we performed “Bayesian hypothesis tests”.40 These analyses revealed a BF 10 of 624.81 in the correlation between apathy and effort discounting and a BF 10 of 0.18 in the correlation between diminished expression and effort discounting. By accepted convention,43 the first implies “decisive evidence” for the H 1 (BF 10 > 100), whereas the latter implies “substantial evidence” for the H 0 (BF 10: 0.1–0.33). In other words, there is decisive evidence for the association between apathy and effort discounting and substantial evidence for the lack of an association between diminished expression and effort discounting.
We next computed a nonparametric partial correlation between apathy and effort discounting, controlling for depressive symptoms, MVC, fatigue, chlorpromazine equivalents, cognitive ability, age, education, and income. Importantly, the association between clinically assessed apathy and our measure of effort discounting remains highly significant even when we control for variance in all the considered factors (r s(21) = −.59, P < .01) indicating that these factors cannot account for the observed association.
Association of Covariates With Effort Discounting
We also conducted further correlational analyses between the covariates in our study and effort discounting (table 2; see supplementary table S2 for correlations with SVs at each effort level). Three main results from these analyses have to be highlighted: first, the main finding of the study—apathy but not diminished expression is associated with effort discounting—also holds when SANS is used to quantify symptoms. Second, no significant correlations with positive symptoms, depression, and chlorpromazine equivalents44 were obtained. Finally, in both, patient and HC groups, cognition, education, income, and MVC were not significantly associated with effort discounting.
Table 2.
Bivariate Correlations
| Effort Discounting (AUC) | ||
|---|---|---|
| Psychopathology | ||
| BNSS apathy | −0.67*** | |
| BNSS diminished expression | −0.14 | |
| SANS apathya | −0.56** | |
| SANS diminished expressiona | −0.17 | |
| PANSS positive factorb | −0.26 | |
| PANSS negative factorb | −0.25 | |
| GAF | 0.51** | |
| PSP (total) | 0.58** | |
| CDSS (total) | −0.11d | |
| Number of hospitalizations | 0.01d | |
| Chlorpromazine equivalents (mg/day) | −0.12 | |
| Cognitionc | ||
| Composite cognitive ability | SZ | 0.03 |
| HC | −0.04 | |
| Income | SZ | 0.10d |
| HC | −0.09 | |
| Maximum voluntary contraction (MVC) | SZ | −0.29 |
| HC | −0.35 | |
| Anticipatory reward pleasure (VAS) | SZ | 0.41* |
| HC | −0.30 | |
| Perceived effort (overall, VAS) | SZ | −0.26 |
| HC | 0.04 | |
Note: Abbreviations are explained in the first footnote to tables 1. AUC, area under the curve; SZ, schizophrenia patient group; VAS, Visual Analogue Scale.
a Apathy = Avolition/Apathy, Anhedonia/Asociality; diminished expression = Affective Flattening or Blunting, Alogia.
b Positive factor = P1, P3, P5, G9; negative factor = N1, N2, N3, N4, N6, G7.
cCognition data have been z-transformed based on the data of the HC group for each test separately. The composite cognitive ability score was computed as the mean of the z-transformed test scores on subject level.
dSpearman correlations (r s).
*P < .05, **P < .01, ***P < .001.
Self-report Measures of Monetary Reward Valuation and Perceived Effort
There were no significant effects of group (HC, LOW-APATHY, HIGH-APATHY) on self-report measures of either monetary reward valuation or perceived effort (see supplementary figures S2A and S2B). To investigate possible antecedents of increased effort discounting in apathetic patients, we correlated effort discounting and apathy with the perceived effort in the 4 effort levels (AUC) and the self-report measure of anticipated monetary reward pleasure. First, none of the measures for perceived effort were associated with apathy in the patient group (all P > .46, all BF 10 < 0.18). This indicates that altered effort-based decision making in apathetic states does not seem to be primarily driven by changes in the pure psychophysical translation of physical force to sensation. Second, anticipated pleasure derived from monetary reward was negatively correlated with apathy (r(29) = −.43, P = .02). Furthermore, effort discounting was associated with less anticipated pleasure, but not with perceived effort (see table 2). These data suggest that the relationship between apathy and effort discounting in patients might be partly driven by a reduction in anticipated pleasure. However, when controlling for reward pleasure in a partial correlation between apathy and effort discounting, the resulting coefficient remains highly significant (r(28) = −.59, P = .001), suggesting that reward pleasure fails to completely account for the relation between apathy and effort discounting.
Group Differences in Effort Discounting
To assess how effort discounting in high and low apathy patients compares to, and differs from, effort discounting in HC, we median-split the patient group. Group level results are depicted in figure 3C. In the 4 (relative SVs for the 4 effort levels) × 3 (HC, LOW-APATHY, HIGH-APATHY) mixed design ANOVA, we observed a significant main effect of group (F(2,48) = 5.81, P < .01) and effort (F(3,48) = 119.33, P < .0001), and a nearly significant interaction term (F(6,48) = 2.12, P = .054). Follow-up 4×2 mixed design ANOVAs showed that the HC and the LOW-APATHY group did not significantly differ (F(1,33) = 0.04, P = .84), whereas the HIGH-APATHY group was significantly different from both the LOW-APATHY and HC group (F(1,29) = 8.40, P < .01; F(1,34) = 10.90, P < .01).
We further computed 1-way ANOVAs for all the effort levels (factor group: HC, LOW-APATHY, HIGH-APATHY) and found significant group effects in the 40, 60, and 80% effort levels (F(1,33) = 5.22, P < .01; F(1,33) = 4.15, P < .05; F(1,33) = 5.45, P < .01). Post hoc comparisons using the Fisher LSD test further revealed significant differences (P < .05) indicative of stronger effort discounting with more apathy in the 60% and 80% condition (HIGH-APATHY group vs. both the HC and LOW-APATHY group). Moreover, in the 40% condition, both patient groups discounted significantly more than the HC group. In sum, the HIGH-APATHY group shows stronger effort discounting than the LOW-APATHY and HC group across a broad effort range, whereas the only effort level where both patient groups can be statistically distinguished from the HC group is the low 40% effort level.
To investigate whether effort discounting was stable over the course of the experiment, we split choice data into 4 blocks and computed a mixed-design ANOVA on fraction of effortful choice. This analysis revealed no significant main effect of group (F(2,48) = 2.08, P = .14), a trend-level main effect of block (F(3,48) = 2.55, P = .07), and a trend-level block × group interaction (F(6,48) = 2.02, P = .07).
Discussion
In the current study, we adapted a paradigm from behavioral economics to investigate how the discounting of monetary rewards by physical effort requirements is associated with the 2 factors of negative symptoms in schizophrenia—apathy and diminished expression. We have several key findings to report. First and most importantly, increased effort discounting was strongly correlated with apathy but not with diminished expression. This effect was not due to depressive symptoms, grip strength, fatigue, antipsychotic medication dosage, cognitive impairment, age, education, and income. Second, our data suggest that increased effort discounting in apathy is due to deficits in both weighing of effort cost in decision making and the anticipated value of reward. Third, group comparisons revealed that only HIGH-APATHY patients showed overall differences in effort discounting compared with the HC participants.
To our knowledge, this is the first study to show that a decreased willingness to exert physical effort for a secondary reward is negatively correlated with apathy but not with diminished expression within a schizophrenia sample. Two important recent studies also reported a decreased willingness to exert effort for monetary rewards in schizophrenia.14,15 Our results are generally in line with those 2 studies, but some differences have to be pointed out. In both studies, the effect mainly surfaced in group comparisons, either between a patient and a HC group,15 or 2 patient groups (high and low negative symptoms) and a HC group.14 It is of note that Fervaha and colleagues15 computed across-group correlations (pooling HC and patient groups) and found significant results in the association between apathy and effort-based decision making using this approach. However, they reported no significant correlations within the patient group. Interestingly, Gold and colleagues14 also applied the BNSS, but they found a significant effect only when the group median split was performed with the total negative symptom score. No group differences were apparent when the split was based on the apathy factor. The authors considered this as surprising, because their theoretical framework predicted that apathy in particular would be associated with effort-based decision making. There are several differences in the experimental task between these studies, and our current study that might explain the partial discrepancies in the results. First, instead of operationalizing effort as number of button presses on a computer device, we used different levels of physical force exerted on a handgrip that was calibrated according to the participant’s maximum grip strength. This procedure has the advantage that we keep time costs constant and are thus able to interpret our results as pure effort discounting independent of delay discounting. Moreover, handgrip effort exertion is less likely to be susceptible to an influence of motor symptoms, because to our knowledge, deficits in pure force application have not been observed in patients with schizophrenia.45 In line with this notion, we found no difference in MVC and time to reach MVC between patients and HCs. Second, we aimed for a task structure with easily understandable choice options and consequently restricted our cost manipulation to physical effort. In the previous studies, both effort and probability costs were manipulated, which might lead to a different pattern of associations with psychopathology. This difference between studies could also account for the lack of association between effort discounting and cognitive ability in our study. Third, our present task incorporates a wide range of effort levels from small to maximum, which is likely to increase overall sensitivity.
In our group analyses, the combined patient group differed significantly from HCs only in the 40% effort condition (figure 3C). HCs discount less in lower effort levels, which is consistent with data from a previous study of our group.20 Because this pattern is absent in the patient group, it can be hypothesized that groups not only show differences in overall discounting, but also in the distinct form of discounting. It is also noteworthy that group differences at the highest effort level are not significant. In other words, choice variance and intergroup differences seem to decrease with increasing effort.
A decision whether to pursue a potentially rewarding behavior when effort is involved is mainly determined by subjectively weighing reward against effort costs. In this study, we show that, based on choice data, apathy is associated with stronger effort discounting. Post-test self-report assessments of monetary reward and the performed effort levels provide us with additional information about how these 2 decision components are perceived. These measures do not reflect in-the-moment experience of effort and reward. The perceived effort for the 4 subjectively calibrated levels seems to be comparable across groups and not associated with symptoms or effort discounting. Self-reported anticipated reward pleasure as a measure of reward representation was associated with both apathy and effort discounting. This is in line with the notion that negative symptoms are linked to aberrant mental representation of anticipated reward,13 but stands in contrast to results reported in the discussed study by Fervaha and colleagues,15 who used a similar measure but did not report any associations with symptoms. The significant partial correlation between apathy and effort discounting, controlling for perceived reward, indicates that the strong relationship between apathy and effort discounting can only partially be accounted for by degraded reward representations.
Some limitations should be noted in relation to the current study. Most of our patients were inpatients with moderate levels of negative symptoms. Although all inpatients were well stabilized and had the opportunity to engage in a variety of activities, it would be important to assess generalizability in an outpatient sample. Moreover, sample size was modest (n = 31). Although our main effects are strong, one has to consider this in particular regarding the missing association between perceived effort and effort discounting or apathy. It has to be further mentioned that our effort perception measure was assessed post-test, which constitutes a retrospective estimation of in-the-moment experience that might be influenced by effort expenditure during the task. Future studies should assess cigarette smoking characteristics of participants because this might affect effort discounting.46 Finally, our study design only included money, which is a secondary reward. Thus, we are not able to generalize our results to the discounting of primary rewards (eg, food, sex) by effort, which have been shown to be partially processed by different brain regions.47 Future studies should also investigate how cognitive effort costs are processed in relation to apathetic states. It has been suggested that cognitive and physical effort might be driven by common neural systems.48 We would thus hypothesize that apathy is also associated with stronger cognitive effort discounting.
The strong link between effort discounting and the negative symptom dimension apathy contributes to a translational approach to the symptoms of schizophrenia.49,50 Within this framework, a human behavioral task as used in the current study provides an essential bridge between human psychopathology and behavioral tasks to assess related phenomena in animals. For this bridging role, our task seems to be well suited for 2 main reasons: First, effort discounting in our binary choice task shows a strong and specific relationship with the apathy dimension, which is not affected by the major possible confounds. Second, although our task is not equivalent to rodent tasks, it provides a close approximation. Importantly, similar to T-maze tasks in rodents,51 we use a simple binary choice independent of probability costs. A translational framework including human and animal tasks for a specific psychopathological dimension can be used to investigate pathophysiological mechanisms and pharmacologic compounds for specific symptoms. There are already promising causal models for negative symptoms—for example, D2 receptor overexpression52—that could be investigated with available animal analogs of our effort-based choice task. Importantly, human and animal effort-based decision-making tasks could contribute to a model for preclinical testing of drugs aiming to reduce negative symptoms. Currently, most preclinical tests used in drug development for schizophrenia are unrelated to negative symptoms, such as prepulse inhibition53 or amphetamine-induced hyperlocomotion54. In line with other authors, we believe that new compounds51,55 should be developed in preclinical and clinical studies with tasks that have shown a strong relationship with the target negative symptom50—as exemplified by the relationship between effort-based decision making and apathy.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Volkswagen foundation (Hannover, Germany) through the European Platform for Life Sciences, Mind Sciences and the Humanities (85061); Swiss National Science Foundation (PP00P1_128574, CRSII3_141965); Swiss National Centre of Competence in Research in Affective Sciences (51NF40-104897).
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Milev P, Ho BC, Arndt S, Andreasen NC. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162:495–506. [DOI] [PubMed] [Google Scholar]
- 2. Rabinowitz J, Levine SZ, Garibaldi G, Bugarski-Kirola D, Berardo CG, Kapur S. Negative symptoms have greater impact on functioning than positive symptoms in schizophrenia: analysis of CATIE data. Schizophr Res. 2012;137:147–150. [DOI] [PubMed] [Google Scholar]
- 3. Fervaha G, Foussias G, Agid O, Remington G. Amotivation and functional outcomes in early schizophrenia. Psychiatry Res. 2013;210:665–668. [DOI] [PubMed] [Google Scholar]
- 4. Faerden A, Finset A, Friis S, et al. Apathy in first episode psychosis patients: one year follow up. Schizophr Res. 2010;116:20–26. [DOI] [PubMed] [Google Scholar]
- 5. Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr Bull. 2006;32:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Messinger JW, Trémeau F, Antonius D, et al. Avolition and expressive deficits capture negative symptom phenomenology: implications for DSM-5 and schizophrenia research. Clin Psychol Rev. 2011;31:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Foussias G, Remington G. Negative symptoms in schizophrenia: avolition and Occam’s razor. Schizophr Bull. 2010;36:359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liemburg E, Castelein S, Stewart R, et al. Two subdomains of negative symptoms in psychotic disorders: established and confirmed in two large cohorts. J Psychiatr Res. 2013;47:718–725. [DOI] [PubMed] [Google Scholar]
- 9. Brown RG, Pluck G. Negative symptoms: the ‘pathology’ of motivation and goal-directed behaviour. Trends Neurosci. 2000;23:412–417. [DOI] [PubMed] [Google Scholar]
- 10. Waltz JA, Frank MJ, Robinson BM, Gold JM. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol Psychiatry. 2007;62:756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koch K, Schachtzabel C, Wagner G, et al. Altered activation in association with reward-related trial-and-error learning in patients with schizophrenia. Neuroimage. 2010;50:223–232. [DOI] [PubMed] [Google Scholar]
- 12. Simon JJ, Biller A, Walther S, et al. Neural correlates of reward processing in schizophrenia—relationship to apathy and depression. Schizophr Res. 2010;118:154–161. [DOI] [PubMed] [Google Scholar]
- 13. Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 2008;34:835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ. Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol Psychiatry. 2013;74:130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fervaha G, Graff-Guerrero A, Zakzanis KK, Foussias G, Agid O, Remington G. Incentive motivation deficits in schizophrenia reflect effort computation impairments during cost-benefit decision-making. J Psychiatr Res. 2013;47:1590–1596. [DOI] [PubMed] [Google Scholar]
- 16. Fervaha G, Foussias G, Agid O, Remington G. Neural substrates underlying effort computation in schizophrenia. Neurosci Biobehav Rev. 2013;37:2649–2665. [DOI] [PubMed] [Google Scholar]
- 17. Burke CJ, Brünger C, Kahnt T, Park SQ, Tobler PN. Neural integration of risk and effort costs by the frontal pole: only upon request. J Neurosci. 2013;33:1706–1713a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hasler G. Can the neuroeconomics revolution revolutionize psychiatry? Neurosci Biobehav Rev. 2012;36:64–78. [DOI] [PubMed] [Google Scholar]
- 19. Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hartmann MN, Hager OM, Tobler PN, Kaiser S. Parabolic discounting of monetary rewards by physical effort. Behav Processes. 2013;100:192–196. [DOI] [PubMed] [Google Scholar]
- 21. APA. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 22. BFS. Medizinische Statistik der Krankenhäuser: Anzahl Fälle und durchschnittliche Aufenthaltsdauer (DAD) nach Altersklasse und Diagnosekode. Neuchatel, Switzerland: BFS; 2012. [Google Scholar]
- 23. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 24. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20:22–33; quiz 34–57. [PubMed] [Google Scholar]
- 25. Kirkpatrick B, Strauss GP, Nguyen L, et al. The brief negative symptom scale: psychometric properties. Schizophr Bull. 2011;37:300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Iowa City, IA: University of Iowa; 1983. [Google Scholar]
- 27. Frances A, Pincus HA, First MB. The Global Assessment of Functioning Scale (GAF). Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 28. Schaub D, Juckel G. [PSP Scale: German version of the Personal and Social Performance Scale: valid instrument for the assessment of psychosocial functioning in the treatment of schizophrenia]. Nervenarzt. 2011;82:1178–1184. [DOI] [PubMed] [Google Scholar]
- 29. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3:247–251. [DOI] [PubMed] [Google Scholar]
- 30. Strauss GP, Hong LE, Gold JM, et al. Factor structure of the Brief Negative Symptom Scale. Schizophr Res. 2012;142:96–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Helmstaedter C, Lendt M, Lux S. VLMT. Verbaler Lern- und Merkfähigkeitstest. Göttingen, Germany: Beltz Test GmbH; 2001. [Google Scholar]
- 32. Härting C, Markowitsch HJ, Neufeld H, Calabrese P, Deisinger K, Kessler J. Wechsler Memory Scale - Revised Edition, German Edition. Manual. Bern, Switzerland.: Huber; 2000. [Google Scholar]
- 33. Kessels RP, van Zandvoort MJ, Postma A, Kappelle LJ, de Haan EH. The Corsi Block-Tapping Task: standardization and normative data. Appl Neuropsychol. 2000;7:252–258. [DOI] [PubMed] [Google Scholar]
- 34. Von Aster M, Neubauer A, Horn R. Wechsler Intelligenztest für Erwachsene WIE. Deutschsprachige Bearbeitung und Adaption des WAIS-III von David Wechsler. Frankfurt, Germany: Pearson Assessment; 2006. [Google Scholar]
- 35. Shallice T. Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci. 1982;298:199–209. [DOI] [PubMed] [Google Scholar]
- 36. Delis DC, Kaplan E, Kramer J. Delis Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 37. Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96:73–86. [DOI] [PubMed] [Google Scholar]
- 38. Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. J Exp Anal Behav. 2001;76:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen PY, Popovich PM. Correlation: Parametric and Nonparametric Measures. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- 40. Wetzels R, Wagenmakers EJ. A default Bayesian hypothesis test for correlations and partial correlations. Psychon Bull Rev. 2012;19:1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mueser KT, Sayers SL, Schooler NR, Mance RM, Haas GL. A multisite investigation of the reliability of the scale for the assessment of negative symptoms. Am J Psychiatr. 1994;151:1453–1462. [DOI] [PubMed] [Google Scholar]
- 42. Sayers SL, Curran PJ, Mueser KT. Factor structure and construct validity of the scale for the assessment of negative symptoms. Psychological Assessment. 1996;8:269–280. [Google Scholar]
- 43. Jeffreys H. Theory of Probability. Oxford, England: Oxford University Press; 1961. [Google Scholar]
- 44. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. [DOI] [PubMed] [Google Scholar]
- 45. Walther S, Strik W. Motor symptoms and schizophrenia. Neuropsychobiology. 2012;66:77–92. [DOI] [PubMed] [Google Scholar]
- 46. Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl). 1999;146:455–464. [DOI] [PubMed] [Google Scholar]
- 47. Sescousse G, Redouté J, Dreher JC. The architecture of reward value coding in the human orbitofrontal cortex. J Neurosci. 2010;30:13095–13104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schmidt L, Lebreton M, Clery-Melin ML, Daunizeau J, Pessiglione M. Neural mechanisms underlying motivation of mental versus physical effort. Plos Biology. 2012;10:e1001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Barnes SA, Der-Avakian A, Markou A. Anhedonia, avolition, and anticipatory deficits: assessments in animals with relevance to the negative symptoms of schizophrenia. Eur Neuropsychopharmacol. 2014;24:744–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Markou A, Salamone JD, Bussey TJ, et al. Measuring reinforcement learning and motivation constructs in experimental animals: relevance to the negative symptoms of schizophrenia. Neurosci Biobehav Rev. 2013;37:2149–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Salamone JD, Correa M, Nunes EJ, Randall PA, Pardo M. The behavioral pharmacology of effort-related choice behavior: dopamine, adenosine and beyond. J Exp Anal Behav. 2012;97:125–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Simpson EH, Waltz JA, Kellendonk C, Balsam PD. Schizophrenia in translation: dissecting motivation in schizophrenia and rodents. Schizophr Bull. 2012;38:1111–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl). 2008;199:331–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Alberati D, Moreau JL, Mory R, Pinard E, Wettstein JG. Pharmacological evaluation of a novel assay for detecting glycine transporter 1 inhibitors and their antipsychotic potential. Pharmacol Biochem Behav. 2010;97:185–191. [DOI] [PubMed] [Google Scholar]
- 55. Simpson EH, Kellendonk C, Ward RD, et al. Pharmacologic rescue of motivational deficit in an animal model of the negative symptoms of schizophrenia. Biol Psychiatry. 2011;69:928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.