Abstract
Little is known about the neurobiological factors that determine functional outcome in people at high risk for psychosis. We use multimodal neuroimaging to investigate whether cortical responses during a cognitive task and thalamic glutamate levels were associated with subsequent functional outcome. Sixty subjects participated: 27 healthy controls (CTRL) and 33 at ultrahigh risk (UHR) for psychosis. At baseline, cortical responses during a verbal fluency task were measured using functional Magnetic Resonance Imaging (fMRI) and proton Magnetic Resonance Spectroscopy (1H-MRS) was used to measure thalamic glutamate levels. The UHR subjects were then followed clinically for a mean duration of 18 months, and subdivided into “good” and “poor” functional outcome subgroups according to their Global Assessment of Function score at follow-up. UHR subjects with a poor functional outcome showed greater cortical and subcortical activation than UHR subjects with a good functional outcome. They also had lower levels of thalamic glutamate and showed a negative relationship between thalamic glutamate levels and prefrontal-striatal activation that was not present in the good functional outcome or control groups. In people at high risk for psychosis, their subsequent level of functioning may depend on the extent to which neurophysiological and neurochemical function is perturbed when they first present to clinical services.
Key words: psychosis, ultra-high risk, functional outcomes, functional MRI, spectroscopy, glutamate
Introduction
Psychotic disorders are usually preceded by a clinical syndrome that includes attenuated psychotic symptoms, and a marked decline in social and occupational function.1 These features comprise an “ultrahigh risk” (UHR) state that is associated with an approximately 30% risk of developing psychosis in the following 2 years.2 To date, the primary outcome of interest in almost all UHR research has been the onset of psychosis, which has been associated with alterations in grey matter volume,3 prefrontal activation4, and dopamine function5 at presentation. However, the threshold at which psychotic symptoms are regarded as diagnostic of a psychotic disorder, rather than a high-risk state, does not always reflect the level of functioning in UHR cases.6 Furthermore, around 70% of UHR individuals will not develop psychosis,7 despite many of this subgroup having poor functional outcomes and requiring long-term clinical care.8
Poor functional outcomes in UHR cohorts have recently been associated with a long duration of high-risk symptoms before presentation9 and poor neurocognitive performance,10 especially on tasks of verbal memory and fluency.8 Furthermore, longitudinal studies have shown that initial UHR classification is associated with persistent and long-standing functional difficulties in a subset of cases.8,11 However, poor functional outcomes do not seem to be entirely dependent on the level of positive symptoms and the development of psychosis in UHR cohorts.8,10
One of most robust functional neuroimaging findings in UHR subjects is altered regional brain activation during tasks of executive functions, such as verbal fluency (VF).4,12,13 Moreover, in UHR subjects the prefrontal cortex (PFC) and midbrain response during VF is particularly increased in the subgroup of subjects who subsequently develop psychosis.4 This alteration in prefrontal responses during VF in UHR subjects has been associated with a reduction in glutamate levels in the thalamus,14 raising the possibility that the abnormal activation may be driven by changes in subcortical glutamate function. This is in line with experimental models in which administration of ketamine, an N-methyl-d-aspartate (NMDA) receptor antagonist, in healthy volunteers can produce impairments on tasks of executive function15,16 and alterations in prefrontal cortical activation comparable to those seen in schizophrenia.17 Low thalamic glutamate levels in UHR subjects have also recently been linked to a decline in global functioning subsequent to clinical presentation.18
The aim of this study was to assess whether neuroimaging measures in high-risk subjects could be used to predict their functional outcome after clinical presentation. We tested the hypothesis that functional outcome would be related to increased prefrontal activation during a VF task, and to low thalamic glutamate levels at baseline. A further prediction was that the relationship between these two measures would be perturbed in subjects with a poor functional outcome.
Methods
Participants
Sixty subjects (27 healthy controls [CTRL] and 33 at UHR of psychosis) participated in the study. None of the functional Magnetic Resonance Imaging (MRI) data from these 60 participants have been reported previously. Specifically, there is no overlap between these data and the data reported in our earlier studies using VF tasks in UHR subjects (Broome et al13; Allen et al4; Fusar-Poli et al.14,19). The subjects in this study from whom Magnetic Resonance Spectroscopy (MRS) glutamate data were also acquired represent a subset of a larger sample described in a separate MRS study by Egerton et al.18
Subjects were fluent in the English language and had no history of neurological illness, drug, or alcohol dependence. The study had National Research Ethics Service (NRES) approval and all participants gave their written, informed consent to participate. All participants had an estimated pre-morbid IQ in the normal range (table 1) as assessed using the National Adult Reading Scale (NART).20 Handedness was assessed using the Annet Handedness Scale.21 UHR subjects were recruited via OASIS (Outreach and Support in South London).22 An UHR state was established using the Comprehensive Assessment of At Risk Mental States (CAARMS).23 Subjects met one or more of the following criteria: (a) attenuated psychotic symptoms (b) brief limited intermittent psychotic symptoms (a history of one or more episodes of frank psychotic symptoms that resolved spontaneously within 1 week in the past year), or (c) a recent decline in function, together with either the presence of schizotypal personality disorder or a family history of psychosis in a first degree relative.
Table 1.
Demographic and Clinical Characteristics in CTRL and UHR Subjects at Follow-up and Baseline
| CTRL (n = 27) | UHR-GFO (n = 18) | UHR-PFO (n = 15) | Analysis | |
|---|---|---|---|---|
| Follow-up | ||||
| Age (years) | — | 22.83 (3.31) | 22.75 (3.62) | t = 0.06 P = .94 |
| Length of follow-up (months) | — | 19 (10) | 16 (3) | t = 1.28 P = .21 |
| GAF | — | 76.16 (6.28) | 48.93 (11.09) | t = 8.42 P < .001 |
| CAARMS totala | — | 9 (12.08) | 47.07 (22.62) | t = −5.85 P < .001 |
| CAARMS positivea | — | 1.93 (2.5) | 8.71 (4.96) | t = −6.7 P < .001 |
| CAARMS negativea | 0.70 (1.77) | 6.70 (4.11) | t = −5.2 P < .001 | |
| Digit spanb | — | 18.13 (5.20) | 15.34 (4.89) | t = 1.43 P = .16 |
| Verbal fluency (FAS)b | — | 42.26 (11.06) | 40.16 (9.96) | t = 0.51 P = .61 |
| Baseline | ||||
| Age (years) | 24.41 (4.64) | 21.11 (5.07) | 21.87 (3.70) | F = 2.16 P = .13 |
| Gender | 18M: 9F | 11M: 7F | 8M: 7F | X 2 = 1.59 P = .45 |
| Handedness | 23R: 4L | 17R: 1L | 12R: 3L | X 2 = 0.43 P = .80 |
| GAF | 82.69 (7.67) | 59.23 (10.40) | 55.26 (6.2) |
F = 65.41 P < .01 CTRL > GFO + PFO |
| CAARMS totalc | — | 39.24 (13.20) | 45.23 (17.22) | t = −1.08 P = .289 |
| CAARMS positivec | — | 6.29 (3.91) | 8.93 (3.33) | t = −1.98 P = .06 |
| CAARMS negativec | 7.88 (3.44) | 9.31 (4.57) | t = −0.97 P = .33 | |
| CAARMS criteria | 14 AP | 11 AP | X 2 = 0.50 P = .82 | |
| 2 AP + B | 1 AP + B | |||
| 1 AP + V | 2 AP + V | |||
| 1 AP + B + V | ||||
| NART FSIQd | 110 (10) | 107 (8) | 103 (12) | F =2.73 P = .07 |
| Digit span | 16.63 | 17.28 (4.06) | 15.33 (4.46) | F =1.05 P = .10 |
| Verbal fluency (FAS) | 40.63 (9.77) | 39.56 (14.34) | 34.60 (11.53) | F =1.32 P = .27 |
| Medication at baseline (no. of cases) | 5 | 4 | Z = 0.77 P = .85 | |
| Chlorpromazine equivalents | 22.75 | 21.37 | t = 0.07 P = .93 | |
| Cannabis use at baseline (median [range]) | 1 (0–4) | 2 (0–4) | 1 (0–4) | Z = −1.2 P = .24 |
| CBT sessions at follow-up (mean no. of hours) | — | 14 | 26 | t = −2.3 P = .02 |
| Remission at follow-up (no longer meets CAARMS criteria) | — | 12 | 3 | Z = −2.85 P = .01 |
| In employment or education at follow-up (no. of cases) | — | 12 | 5 | Z = −2.20 P = .02 |
Note: Medication at baseline (UHR-GFO: 3 = Citalopram, 2 = Olanzapine, UHR-PFO: 2 = Citalopram, 1 = Quetiapine and Citalopram, 1 = Risperidone). Cannabis (0 = no use, 1 = experimental use, 2 = occasional use, 3 = moderate use, 4 = severe use). AP, Attenuated Psychotic Symptoms; B, BLIP; CAARMS, Comprehensive Assessment of At Risk Mental States; CBT, cognitive behavioral therapy; CTRL, healthy controls; GAF, Global Assessment of Function; GFO, good functional outcome; NART, National Adult Reading Scale; PFC, prefrontal cortex; PFO, poor functional outcome; UHF, ultrahigh risk; V, Vulnerability; VF, verbal fluency.
aInformation in two of the UHR-GFO and in one of the UHR-PFO group was missing.
bInformation in three of the UHR-GFO and in two of the UHR-PFO group was missing.
cInformation in one of the UHR-GFO and in two of the UHR-PFO group was missing.
dInformation in one of the UHR-PFO group was missing.
eInformation in two of the UHR-GFO and one of the UHR-PFO group was missing.
Nine UHR subjects were being treated with low doses of antipsychotics at baseline (see table 1 legend). Antipsychotic treatment and cognitive behavioral therapy received at follow-up is also reported in table 1. The healthy controls (CTRL n = 27) were recruited from the local community. Participants with a history of medical or psychiatric disorders or who were receiving prescription medications were excluded from the study. Any participants reporting recent recreational drug use (cannabis, stimulants, hallucinogens, or opiates in the 2 weeks before the MRI scan) were excluded. Previous cannabis use (prior to the 2-week period before scanning) by UHR and CTRL subjects is reported in table 1. Other recreational drugs are reported in supplementary table s1.
Clinical Assessment at Follow-up
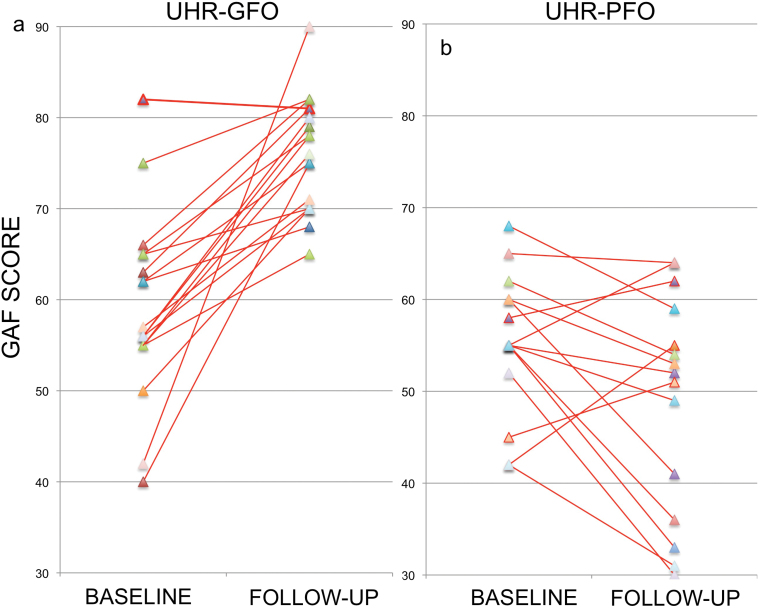
In UHR subjects, clinical follow-up occurred on average 18.1±8.6 months (range = 11.8–58.7 months) after their baseline MRI scans. During the follow-up period, three UHR subjects (9%) made a transition to a first episode psychosis, according to CAARMS criteria.23 UHR subjects were divided into two functional outcome groups on the basis of their Global Assessment of Function (GAF)24 scores at follow-up: (a) a good functional outcome group (UHR-GFO n = 18; GAF score ≥ 65) and (b) a poor functional outcome group (UHR-PFO n = 15; GAF score ≤ 64) (see figure 1). The group split at a GAF score of 65 was chosen because the 60–70 range corresponds to the presence of “some difficulty in social or occupational functioning but [the subject] generally functions pretty well.”24 GAF scores below 60 indicate “moderate to severe impairment,” whilst scores above 70 correspond to “slight impairment to good function.” Two UHR subjects that developed psychosis both had GAF scores below 65 and were included in the UHR-PFO group, the third transition had a follow-up GAF score of 78 and was included in the UHR-GFO group.
Fig. 1.
GAF scores at baseline and follow-up for each UHR subject in the good (a) and poor (b) outcome subgroups.
Verbal Fluency Task and Image Acquisition
All image acquisition parameters are described in supplementary material. Functional MRI data were acquired while subjects performed a VF task as used previously.4,13,19,25 In the experimental condition, subjects were instructed to overtly generate a single word in response to a visually presented letter in either an easy VF condition (e.g. C, S, L) or a hard VF condition (e.g. F, I, O). Experimental conditions were presented in blocks lasting 32 s, with eight presentations of a given letter per block and four blocks in each condition. The experimental condition alternated with a control condition, in which the word “REST” was presented at the same rate and participants were asked to repeat this word overtly (Word Repetition). Eight blocks of REST were interspersed with the experimental condition. Incorrect responses were defined as pass responses, as were words that were proper nouns, repetitions or grammatical variations of previous words. To ensure that subjects heard their responses clearly, their speech was transmitted by an MRI-compatible microphone, amplified by a computer sound card, and relayed back through an acoustic MRI sound system and noise-insulated, stereo headphones. No subjects reported any difficulty performing the task.
Proton Magnetic Resonance Spectroscopy
Proton Magnetic Resonance Spectroscopy (1H-MRS) data were acquired as detailed previously18,26 during the same scanning session as the fMRI VF task. 1H-MRS data in this cohort are also reported as part of a larger study.18 The 1H-MRS procedure and spectral quality is described fully in supplementary material.
fMRI Analysis
Pre-processing of functional data was performed using SPM8 software (http//www.fil.ion.ucl.ac.uk/spm), running in Matlab (Mathworks Inc, Sherbon, MA, USA). The full preprocessing and statistical procedures for fMRI analysis are described in supplementary material.
A random effects 2 × 3 factorial ANOVA was used to examine the main effects of task (Easy vs Hard VF) and group (CTRL, UHR-GFO, and UHR-PFO), and the interaction effects. Baseline GAF scores were used as a regressor in an analysis of covariance (ANCOVA) model to identify areas in which there was a relationship between task activation and global functioning levels at a voxel-wise level. Mean parameter estimates (across all significant voxels identified by ANCOVA as significantly correlated with GAF scores) were calculated and used in a correlation analysis. The relationship between BOLD response and glutamate levels was investigated by entering individual glutamate levels as subject-specific covariates in a one-way ANCOVA, allowing the covariate to interact with the group factor (CTRL, UHR-GFO, UHR-PFO). All statistical inferences from random effects models were made at a cluster corrected family-wise error (FWE) corrected level (P < .05, with a standard voxel-level threshold of P < .001). Uncorrected results (P < .001 k(cluster extent) > 100) are also reported in table 2 but are not discussed.
Table 2.
Task, Group and Interaction Effects During Verbal Fluency fMRI Task. The x, y, z Coordinates of Local Maxima Are Listed According to the MNI Coordinate System
| Side | Z | X | y | z | Region | |
|---|---|---|---|---|---|---|
| Task effects | ||||||
| VF > WR | L | 7.46 | −50 | 8 | 28 | Middle frontal gyrus/frontal operculum* |
| L | 7.46 | −34 | 21 | −4 | Insula* | |
| L | 7.51 | −4 | 20 | 40 | Superior frontal gyrus* | |
| L | 6.29 | −42 | −60 | −28 | Cerebellum | |
| L | 6.27 | −16 | −4 | 16 | Caudate head/thalamus* | |
| L | 5.79 | −46 | −54 | −16 | Fusiform gyrus* | |
| L | 5.54 | −2 | −34 | −4 | Brainstem/thalamus* | |
| L | 5.36 | −42 | −44 | 42 | Inferior parietal gyrus* | |
| R | 5.32 | 40 | 18 | −4 | Insula* | |
| R | 5.12 | 16 | 0 | 16 | Caudate body/thalamus* | |
| Easy VF vs hard VF | No supra threshold effect | |||||
| GAF correlation (positive) | No supra threshold effect | |||||
| GAF correlation (negative) | L | 4.63 | −18 | 6 | 14 | Caudate nucleus/thalamus* |
| R | 4.42 | 24 | 0 | −8 | Putamen/Insula* | |
| L | −8 | 14 | −17 | Brainstem | ||
| R | 4.42 | 10 | −14 | −16 | Brainstem/hippocampus* | |
| L | 4.42 | −32 | 5 | −7 | Insula/inferior frontal gyrus* | |
| Group effects | ||||||
| CTRL > UHR | No supra threshold effect | |||||
| CTRL < UHR | R | 3.75 | 26 | 4 | −24 | Parahippocampal gyrus |
| L | 3.42 | −34 | 4 | −26 | Superior temporal gyrus | |
| L | 3.31 | −48 | 12 | 6 | Inferior frontal gyrus (pars opercularis) | |
| L | 3.05 | −42 | 6 | −2 | Insula | |
| R | 3.05 | 44 | 2 | 0 | Insula | |
| UHR-GFO > UHR-PFO | No supra threshold effect | |||||
| UHR-GFO < UHR-PFO | L | 3.93 | −36 | 13 | 4 | Insula/inferior frontal gyrus* |
| L | 3.92 | −30 | −8 | 14 | Insula/putamen* | |
| L | 3.83 | −20 | −10 | 8 | Thalamus* | |
| R | 3.48 | 36 | −4 | −22 | Hippocampus | |
| R | 3.39 | 40 | −12 | 16 | Insula | |
| CTRL + UHR-GFO > UHR-PFO | No supra threshold effect | |||||
| CTRL+UHR-GFO < UHR-PFO | L | 3.95 | −46 | 10 | 6 | Inferior frontal gyrus (pars opercularis)* |
| L | 3.33 | −36 | 4 | −24 | Superior temporal gyrus | |
| L | 3.95 | −32 | 14 | 20 | Insula/frontal operculum* | |
| R | 3.34 | 44 | 2 | 0 | Insula | |
| R | 3.34 | 30 | 10 | 6 | Putamen | |
| R | 3.33 | 26 | 4 | −24 | Parahippocampal gyrus | |
| R | 3.33 | 26 | −8 | −16 | Hippocampus | |
| Thalamic glutamate effects | ||||||
| Correlation in CTRL subjects | ||||||
| Positive | R | 3.84 | 16 | 32 | 32 | Superior frontal gyrus/sulcus* |
| R | 3.76 | 8 | 40 | 32 | Cingulate sulcus* | |
| Negative | No supra threshold effect | |||||
| Correlation in UHR subjects | ||||||
| Positive | No supra threshold effect | |||||
| Negative | No supra threshold effect | |||||
| Group × Thal Glu interaction | R | 4.10 | 8 | 64 | 0 | Frontopolar gyrus* |
| L | 3.99 | −10 | 62 | 8 | Frontopolar gyrus* | |
| R | 3.99 | 20 | 66 | 0 | Superior frontal gyrus* | |
Note: All results reported at p < .001 uncorrected k > 100. VF, verbal fluency; GAF, Global Assessment of Function; CTRL, healthy controls; UHF, ultrahigh risk; GFO, good functional outcome; PFO, poor functional outcome.
* P < .05 family-wise error cluster corrected.
Statistical Analysis of Behavioral, Demographic, Clinical, and MRS Data
Statistical analysis was performed in SPSS version 18.0 (Chicago, IL). Group differences in demographic and clinical variables, spectral quality, and glutamate levels were explored using independent samples or paired t-tests as appropriate or non-parametric tests for nominal data. Levene’s test was used to check for equality of variance across groups. Logistic regression was used to determine the value of baseline activation and GAF scores in predicting functional outcome. Linear regression tests were used to determine the value of baseline variable on outcome variable. Statistical significance was defined as P < .05.
Results
Functional Outcome in the UHR Group
At baseline, the mean GAF score of the UHR group as a whole was 57.37 (S D = 9.30) and their mean CAARMS total symptom score was 40.49 (S D = 15.47). At the follow-up assessment the groups’ mean GAF score had increased significantly to 63.78 (t(31) = −0.208 P =.04), while the mean CAARMS total score had decreased significantly to 24.44 (22.59) (t(31)= −3.98 P <.001). At follow-up, GAF scores and CAARMS total scores were negatively correlated (r = −0.80 P < .01)
The UHR-GFO and UHR-PFO groups did not differ significantly in terms of baseline symptom scores or level of cannabis use although there were trends for higher baseline positive symptoms, lower premorbid IQ and Wechsler Adult Intelligence Scale (WAIS) digit span performance in the UHR-PGO group (see table 1). Only positive symptoms at baseline predicted GAF scores at the follow-up time point (B = −1.98 P = .02). At follow-up, the UHR-PFO group had significantly lower GAF scores, higher CAARMS symptoms scores and had received significantly more CBT treatment than the UHR-GFO group (table 1). GAF scores over the follow-up period showed a significant group (UHR-GFO vs UHR-PFO) by time (baseline vs follow-up) interaction (F(1,31) P < .001) which reflected a significant difference in the GAF scores between the groups at follow-up that was not significant at baseline (t(31) = 1.24 P = .23). The interaction remained significant after covarying for change in CAARMS total scores over the same period.
fMRI Results
Effect of Task
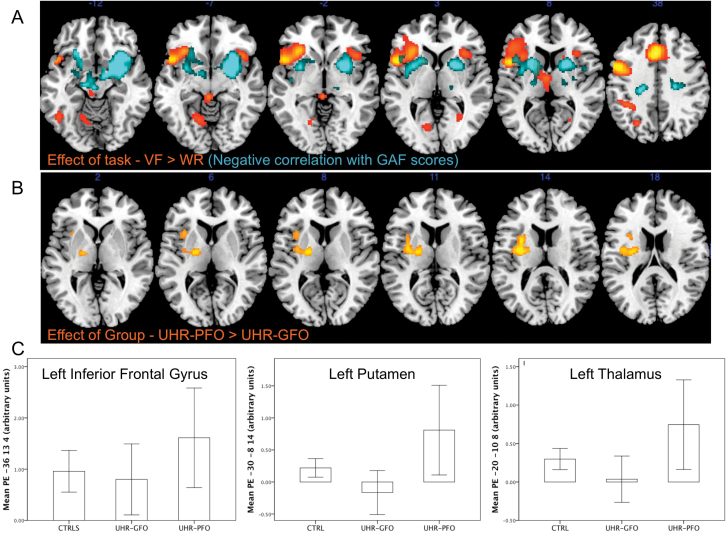
Across all subjects, VF trials relative to word repetition trials were associated with activation in the left middle frontal gyrus extending ventrally to the frontal operculum and insula, the left medial superior frontal gyrus, the left caudate body extending to the ventral thalamic nucleus, the left fusiform gyrus, the brain stem extending to the thalamus, the right insula, and the right caudate body/thalamus (P < .05 FWE; table 2 and figure 2A). The within-task contrast of easy vs hard VF was non-significant. Across all subjects there was a negative correlation between baseline GAF scores and activation in the midbrain, extending to the right hippocampus and parahippocampal gyrus, the right putamen and insula, the left insula, and inferior frontal gyrus, with a further cluster encompassing the left caudate nucleus and thalamus (P < .05 FWE; table 2; figure 2A).
Fig. 2.
(A) Statistical parametric maps showing activation during verbal fluency task in all subjects. Areas in yellow/red and blue represent VF task related activation and activation negatively correlated with GAF scores, respectively. The left side of the brain is on the left side of the image. (B) Brain areas where UHR subjects with a poor functional outcome showed greater activation than those with a good outcome and controls. (C) Plots of mean parameter estimates (PE) in each group from foci in the left insula/left inferior frontal gyrus (−36, 13, 4), left insula/putamen (−30, −8, 14), and left thalamus (−20, −10, 8).
Effect of Group
CTRL vs UHR
Relative to the CTRL group, the total UHR group showed greater activation in the left inferior frontal and superior temporal gyri, the insula bilaterally, and the right parahippocampal gyrus (P < .001 uncorrected k > 100).
CTRL vs UHR-GFO vs UHR-PFO
Relative to both the CTRL and the UHR-GFO groups, the UHR-PFO group showed greater activation in the left inferior frontal and insula (P < .05 FWE; table 2; figure 2B and 2C), and in the left superior temporal gyrus and the right putamen, parahippocampal gyrus and hippocampus at an uncorrected threshold (P < .001). There were no areas where the CTRL or UHR-GFO groups showed greater activation than the UHR-PFO group. When the analysis was restricted to the UHR-GFO and UHR-PFO groups (without the CTRL group), a similar result emerged with the UHR-PFO group showing greater activation in the left inferior frontal gyrus, putamen, thalamus, and insula (P < .05 FWE table 2). There were no areas where the UHR-GFO group showed more activation than the UHR-PFO group.
MRS—Thalamic Glutamate Levels
Data relating to spectral quality are presented in the supplementary material and did not differ across groups. There was no significant difference in thalamic glutamate levels between the total UHR group and CTRL subjects (t(57) = .87 P = .38; effect size (d) = 0.19).
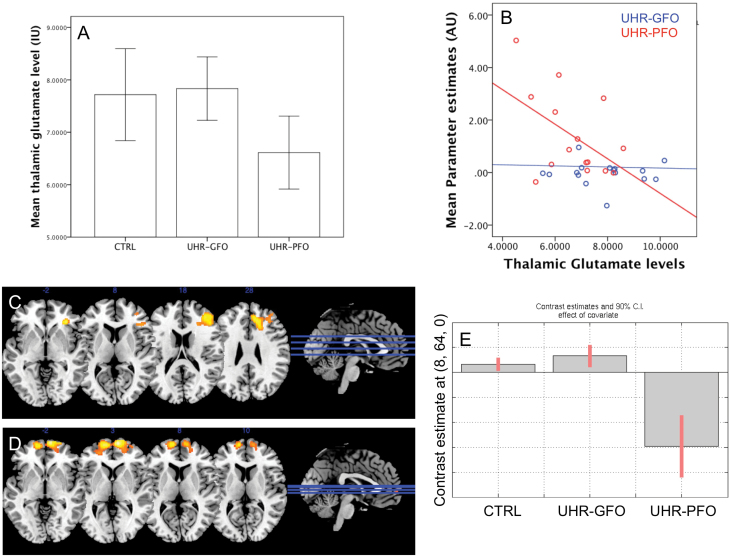
However, within the UHR sample, glutamate levels were lower in the UHR-PFO than in the UHR-GFO subgroup (t(31) = 2.57 P =.01; figure 3A). In UHR subjects, thalamic glutamate levels were negatively correlated with activation in the midbrain-striatal areas negatively associated with GAF scores identified above (figure 2A) (r = −0.37 P = .04; see figure 3B).
Fig. 3.
(A) Bar chart showing reduced thalamic glutamate levels in the UHR subgroup with a poor outcome. (B) Scatter plot showing association between thalamic glutamate levels and activation in cortico-striatal-midbrain circuit. (C) Statistical parametric maps showing a positive correlation between right PFC activation and thalamic glutamate levels in CTRL group (D) interaction between group activation and thalamic glutamate levels in frontal polar cortex bilaterally. (E) Plot showing interaction between frontopolar activation (8, 64, 0) and thalamic glutamate (driven by negative interaction effect in UHR-PFO group).
fMRI Group × Glutamate Interactions
In CTRL subjects, there was a significant positive association between thalamic glutamate levels and the BOLD signal in a region that spanned the adjacent parts of the right superior frontal, anterior cingulate, and middle frontal gyri (P < .05 FWE; table 2; and figure 3C). There was a significant interaction between thalamic glutamate levels, activation and group in the frontopolar gyrus bilaterally, the right superior frontal gyrus (P < .05 FWE; table 2; figure 3D). The interaction was driven by a negative association between activation and thalamic glutamate levels in theses regions in the UHR-PFO group. This association was not seen in the UHR-GFO or CTRL groups (figure 3E).
Discussion
Our main findings were that in subjects at high risk of psychosis, functional outcome was related to both the magnitude of activation in prefrontal and subcortical areas during a VF task, and to glutamate levels in the thalamus. Overall, the UHR cohort showed functional and symptomatic improvement at follow-up. Despite this, approximately 45% of the UHR group had poor functional outcomes according to their GAF score at follow-up, with the majority of this subgroup (10 out of 15) not in employment or education. Broadly consistent with a previous study in a larger UHR cohort,8 only baseline symptoms scores predicted GAF scores at follow-up, while baseline GAF scores, premorbid IQ and WAIS digit span performance did not.
Consistent with previous functional imaging studies using VF tasks, across all subjects there was activation in a network including the left middle and medial superior frontal gyri, the frontal operculum, and insula27–29 the left inferior parietal and fusiform gyri, the caudate nuclei, thalamus and brain stem.4 Across all subjects, there was a negative association between baseline GAF scores and activation in the inferior frontal gyrus and insula, hippocampus, thalamus, striatum, and midbrain. This suggests that the lower the level of global functioning, the more these regions are activated, perhaps in order to maintain task performance.
Previous studies in UHR samples have reported altered PFC activation during VF,4,13 language,30 working memory31–33 and executive tasks.12 In this study, PFC activation was greater in the total UHR group compared to control group, although this did not survive correction for multiple statistical comparisons. However, when the UHR subjects were subdivided according to functional outcome, a robust group effect emerged, with increased activation seen in those with a poor outcome in the left inferior frontal gyrus, insula, putamen, and thalamus. These differences in activation are unlikely to reflect differences in task performance as error rates were not significantly different between the UHR functional outcome groups and error trials were included as a nuisance covariate in all first level statistical models. Greater activation in this context may reflect inefficient cortical function,34 or a compensatory response to impaired executive function.35 These findings are consistent with our previous finding in a completely independent UHR sample in which poor clinical outcome (as defined by transition to psychosis) was associated with increased PFC and subcortical activation.4
Although there was no significant difference in thalamic glutamate levels between control and UHR groups, poor functional outcome was related to reduced thalamic glutamate levels in UHR subjects. Significantly lower thalamic glutamate levels have previously been reported in UHR subjects at presentation regardless of outcomes,26 and although we did not replicate this finding in the present cohort, the UHR group did exhibit numerically lower thalamic glutamate than healthy controls. Furthermore, a recent follow-up study in an extended sample of the UHR cohort reported here suggests that low thalamic glutamate levels at baseline were associated with a longitudinal decline in global functioning.18 Elevated thalamic glutamate/glutamine levels have been reported in patients with schizophrenia36–38 so it is unclear why decreased thalamic glutamate levels are seem in people at UHR for psychosis. It is possible that glutamate may change with stage of the disorder, and there is some evidence for a recent meta-analysis39 which showed age-dependent effects (which could be a proxy for length or stage of illness and/or length of medication).
A further finding was that the relationship between thalamic glutamate levels and activation in the cortico-striatal-midbrain regions (associated with low GAF scores) that was largely driven by a negative association in subjects with poor outcomes. The relationship between decreased thalamic glutamate levels and increased activation in the caudate nucleus/midbrain is interesting given that these are region where elevated dopamine synthesis capacity is most marked in UHR subjects,40 and linked to transition to psychosis.5 We also observed a significant group by thalamic glutamate interaction in the bilateral frontopolar gyri and the right superior frontal gyrus. In these regions, there was again a negative association between task-related activation and thalamic glutamate levels in UHR with poor functional outcomes that were not seen in either the good functional outcome or controls groups. We have previously shown in an independent UHR cohort, the alteration in PFC activation is related to the reduction in thalamic glutamate levels, and that this relationship is significantly different from that in healthy controls.14 This study extends these findings by showing that this altered relationship may be specific to UHR subjects with poor functional outcomes.
Cognitive dysfunction in schizophrenia is thought to reflect impairments in PFC and in its interactions with other brain regions, such as the thalamus. Because the pyramidal neurons that project from the thalamus to the PFC are glutamatergic, alterations in glutamate and NMDA receptor function in schizophrenia may be particularly relevant to this pathway.41 However, the data from this study cannot determine whether there is a causal relationship between alterations in thalamic glutamate levels and prefrontal activation. Nevertheless, PFC-thalamic interactions mediate attentional and executive functions41,42 via direct connections from the thalamus to the PFC that loop back to the thalamus via the striatum and globus palidus.43 Moreover, a recent study describes reduced PFC-thalamic connectivity in schizophrenia.44 Our findings, which suggest that dysfunctional PFC-thalamic interactions also occur in the UHR state, are thus in line with these findings. Longitudinal studies in UHR subjects are needed to establish the chronology of these changes in relation to the risk of psychosis.
Our study has some other limitations. First, the UHR cohort was divided according to whether follow up GAF scores were either above or below 65. Although arbitrary, this cut-off point reflects a clinically relevant divide, as the 60–70 range corresponds to the transition between poor and good levels of functioning.
Second, the GAF instrument is a measure of both functional (social, occupational etc.) and symptom levels, thus potentially conflating these two outcome variables. Indeed functional and symptoms levels may be largely collinear phenomena meaning that any putative imaging surrogates of poor functional outcome may also represent a marker for clinical outcome along the psychosis trajectory. However, the interaction between change in GAF scores over the follow-up period and outcome group remained significant after covarying for the change in total symptom score, suggesting that changes in global functioning are not entirely explained by changes in the severity of symptoms.
Third, the psychosis transition rate in our sample was lower that those reported elsewhere. However, the 18-month follow-up duration in this study may not have been sufficiently long to identify all UHR cases that made a transition to first episode psychosis.22 The treatment interventions in our UHR subjects also means that their transition rates may not reflect the natural course of the UHR state. Indeed, although well matched at baseline, the poor outcome group as a whole received more CBT treatment during the follow-up period than the good outcome group, perhaps reflecting a greater need for treatment. There is evidence from a recent and independent meta-analyses suggesting that CBT may be effective in reducing psychosis risk45; this may partly explain the low psychosis transition rate in our cohort. However, all CBT sessions occurred subsequent to the collection of functional and MRS imaging scans so could not have a directly affected these data.
Our findings suggest that alterations in prefrontal and striatal neurophysiology and glutamate function in the UHR state may be specific to individuals with poor subsequent outcomes. This is in line with other neurobiological findings3,5,46,47 which suggest that alterations in UHR subjects are particularly marked in the subgroup that later develop psychosis. Taken together, these data suggest that neuroimaging measures could provide a means of predicting clinically meaningful outcomes in high-risk subjects. The involvement of the glutamate system in this study also raises the possibility that treatments that act on glutamate function may be useful in the management of the early phase of psychosis.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Medical Research Council UK Project (G0700995).
Supplementary Material
Acknowledgments
We thank members of the Outreach and Support in South London (OASIS) team who were involved in the recruitment, management, and clinical follow-up of the subjects reported in this manuscript. P.A., C.A.C., A.E., O.D.H., I.B., P.F.P. reports no competing interests. P.M. has provided consultation for Janssen, GSK, Astra-Zeneca, GW, Roche, Alkermes and Sunovion, and research grant support from GW, Roche, GSK, and Astra-Zeneca. G.J.B. receives honoraria for teaching from General Electric, and acts as a consultant for IXICO. R.M. has received honoraria for Janssen, Lilly, BMS, Otsuka, Roche. A.E. receives funding support from the National Institute for Health Research (NIHR) and this study presents independent research supported by NIHR Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
References
- 1. Hafner H. Onset and early course as determinants of the further course of schizophrenia. Acta Psychiatr Scand Suppl. 2000;407:44–48. [PubMed] [Google Scholar]
- 2. Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69:220–229. [DOI] [PubMed] [Google Scholar]
- 3. Mechelli A, Riecher-Rössler A, Meisenzahl EM, et al. Neuroanatomical abnormalities that predate the onset of psychosis: a multicenter study. Arch Gen Psychiatry. 2011;68:489–495. [DOI] [PubMed] [Google Scholar]
- 4. Allen P, Luigjes J, Howes OD, et al. Transition to psychosis associated with prefrontal and subcortical dysfunction in ultra high-risk individuals. Schizophr Bull. 2012;38:1268–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Howes OD, Bose SK, Turkheimer F, et al. Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. Am J Psychiatry. 2011;168:1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin A, Nelson B, Yung AR. ‘At-risk’ for psychosis research: where are we heading? Epidemiol Psychiatr Sci. 2012;30:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yung AR, Yuen HP, Berger G, Francey S, Hung TC, Nelson B, Phillips L, McGorry P. Declining transition rate in ultra high risk (prodromal) services: dilution or reduction of risk? Schizophr Bull. 2007;33:673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin A, Wood SJ, Nelson B, et al. Neurocognitive predictors of functional outcome two to 13 years after identification as ultra-high risk for psychosis. Schizophr Res. 2012;132:1–7. [DOI] [PubMed] [Google Scholar]
- 9. Fusar-Poli P, Meneghelli A, Valmaggia L, et al. Duration of untreated prodromal symptoms and 12-month functional outcome of individuals at risk of psychosis. Br J Psychiatry. 2009;194:181–182. [DOI] [PubMed] [Google Scholar]
- 10. Carrión RE, McLaughlin D, Goldberg TE, et al. Prediction of functional outcome in individuals at clinical high risk for psychosis. JAMA Psychiatry. 2013;70:1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carrión RE, Goldberg TE, McLaughlin D, Auther AM, Correll CU, Cornblatt BA. Impact of neurocognition on social and role functioning in individuals at clinical high risk for psychosis. Am J Psychiatry. 2011;168:806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morey RA, Inan S, Mitchell TV, Perkins DO, Lieberman JA, Belger A. Imaging frontostriatal function in ultra-high-risk, early, and chronic schizophrenia during executive processing. Arch Gen Psychiatry. 2005;62:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Broome MR, Matthiasson P, Fusar-Poli P, et al. Neural correlates of executive function and working memory in the ‘at-risk mental state’. Br J Psychiatry. 2009;194:25–33. [DOI] [PubMed] [Google Scholar]
- 14. Fusar-Poli P, Stone JM, Broome MR, et al. Thalamic glutamate levels as a predictor of cortical response during executive functioning in subjects at high risk for psychosis. Arch Gen Psychiatry. 2011;68:881–890. [DOI] [PubMed] [Google Scholar]
- 15. Krystal JH, Karper LP, Seibyl JP, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. [DOI] [PubMed] [Google Scholar]
- 16. Morgan CJ, Curran HV. Acute and chronic effects of ketamine upon human memory: a review. Psychopharmacology (Berl). 2006;188:408–424. [DOI] [PubMed] [Google Scholar]
- 17. Fu CH, Abel KM, Allin MP, et al. Effects of ketamine on prefrontal and striatal regions in an overt verbal fluency task: a functional magnetic resonance imaging study. Psychopharmacology (Berl). 2005;183:92–102. [DOI] [PubMed] [Google Scholar]
- 18. Egerton AS JM., Chaddock CA., Barker G., Bonoldi I., Allen P., Howes H., McGuire P. Relationship between brain glutamate levels and clinical outcome in individuals at ultra high risk of psychosis [published online ahead of print June 11, 2014]. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fusar-Poli P, Howes OD, Allen P, et al. Abnormal prefrontal activation directly related to pre-synaptic striatal dopamine dysfunction in people at clinical high risk for psychosis. Mol Psychiatry. 2011;16:67–75. [DOI] [PubMed] [Google Scholar]
- 20. Nelson HE, O’Connell A. Dementia: the estimation of premorbid intelligence levels using the New Adult Reading Test. Cortex. 1978;14:234–244. [DOI] [PubMed] [Google Scholar]
- 21. Coren S. Measurement of handedness via self-report: the relationship between brief and extended inventories. Percept Mot Skills. 1993;76:1035–1042. [DOI] [PubMed] [Google Scholar]
- 22. Fusar-Poli P, Byrne M, Badger S, Valmaggia LR, McGuire PK. Outreach and support in south London (OASIS), 2001–2011: ten years of early diagnosis and treatment for young individuals at high clinical risk for psychosis. Eur Psychiatry. 2013;28:315–326. [DOI] [PubMed] [Google Scholar]
- 23. Yung AR, Phillips LJ, McGorry PD, et al. Prediction of psychosis. A step towards indicated prevention of schizophrenia. Br J Psychiatry Suppl. 1998;172:14–20. [PubMed] [Google Scholar]
- 24. Hall RC. Global assessment of functioning. A modified scale. Psychosomatics. 1995;36:267–275. [DOI] [PubMed] [Google Scholar]
- 25. Fu CH, Morgan K, Suckling J, et al. A functional magnetic resonance imaging study of overt letter verbal fluency using a clustered acquisition sequence: greater anterior cingulate activation with increased task demand. Neuroimage. 2002;17:871–879. [PubMed] [Google Scholar]
- 26. Stone JM, Day F, Tsagaraki H, et al. OASIS. Glutamate dysfunction in people with prodromal symptoms of psychosis: relationship to gray matter volume. Biol Psychiatry. 2009;66:533–539. [DOI] [PubMed] [Google Scholar]
- 27. Takizawa R, Kasai K, Kawakubo Y, et al. Reduced frontopolar activation during verbal fluency task in schizophrenia: a multi-channel near-infrared spectroscopy study. Schizophr Res. 2008;99:250–262. [DOI] [PubMed] [Google Scholar]
- 28. Moss HE, Abdallah S, Fletcher P, et al. Selecting among competing alternatives: selection and retrieval in the left inferior frontal gyrus. Cereb Cortex. 2005;15:1723–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smith EE, Jonides J. Neuroimaging analyses of human working memory. Proc Natl Acad Sci U S A. 1998;95:12061–12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sabb FW, van Erp TG, Hardt ME, et al. Language network dysfunction as a predictor of outcome in youth at clinical high risk for psychosis. Schizophr Res. 2010;116:173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Allen P, Seal ML, Valli I, et al. Altered prefrontal and hippocampal function during verbal encoding and recognition in people with prodromal symptoms of psychosis. Schizophr Bull. 2011;37:746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schmidt A, Smieskova R, Aston J, et al. Brain connectivity abnormalities predating the onset of psychosis: correlation with the effect of medication. JAMA Psychiatry. 2013;70:903–912. [DOI] [PubMed] [Google Scholar]
- 33. Yaakub SN, Dorairaj K, Poh JS, et al. Preserved working memory and altered brain activation in persons at risk for psychosis. Am J Psychiatry. 2013;170:1297–1307. [DOI] [PubMed] [Google Scholar]
- 34. Schlösser RG, Koch K, Wagner G, et al. Inefficient executive cognitive control in schizophrenia is preceded by altered functional activation during information encoding: an fMRI study. Neuropsychologia. 2008;46:336–347. [DOI] [PubMed] [Google Scholar]
- 35. Tan HY, Sust S, Buckholtz JW, et al. Dysfunctional prefrontal regional specialization and compensation in schizophrenia. Am J Psychiatry. 2006;163:1969–1977. [DOI] [PubMed] [Google Scholar]
- 36. Théberge J, Bartha R, Drost DJ, et al. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry. 2002;159:1944–1946. [DOI] [PubMed] [Google Scholar]
- 37. Théberge J, Williamson KE, Aoyama N, et al. Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. Br J Psychiatry. 2007;191:325–334. [DOI] [PubMed] [Google Scholar]
- 38. Aoyama N, Théberge J, Drost DJ, et al. Grey matter and social functioning correlates of glutamatergic metabolite loss in schizophrenia. Br J Psychiatry. 2011;198:448–456. [DOI] [PubMed] [Google Scholar]
- 39. Marsman A, van den Heuvel MP, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE. Glutamate in schizophrenia: a focused review and meta-analysis of ¹H-MRS studies. Schizophr Bull. 2013;39:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Howes OD, Montgomery AJ, Asselin MC, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. [DOI] [PubMed] [Google Scholar]
- 41. Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci. 2012;16:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zikopoulos B, Barbas H. Parallel driving and modulatory pathways link the prefrontal cortex and thalamus. PLoS One. 2007;2:e848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. [DOI] [PubMed] [Google Scholar]
- 44. Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169:1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stafford MR, Jackson H, Mayo-Wilson E, Morrison AP, Kendall T. Early interventions to prevent psychosis: systematic review and meta-analysis. BMJ. 2013;346:f185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Borgwardt SJ, Riecher-Rössler A, Dazzan P, et al. Regional gray matter volume abnormalities in the at risk mental state. Biol Psychiatry. 2007;61:1148–1156. [DOI] [PubMed] [Google Scholar]
- 47. Carletti F, Woolley JB, Bhattacharyya S, et al. Alterations in white matter evident before the onset of psychosis. Schizophr Bull. 2012;38:1170–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.