Abstract
Schizophrenia patients are severely impaired in nonverbal communication, including social perception and gesture production. However, the impact of nonverbal social perception on gestural behavior remains unknown, as is the contribution of negative symptoms, working memory, and abnormal motor behavior. Thus, the study tested whether poor nonverbal social perception was related to impaired gesture performance, gestural knowledge, or motor abnormalities. Forty-six patients with schizophrenia (80%), schizophreniform (15%), or schizoaffective disorder (5%) and 44 healthy controls matched for age, gender, and education were included. Participants completed 4 tasks on nonverbal communication including nonverbal social perception, gesture performance, gesture recognition, and tool use. In addition, they underwent comprehensive clinical and motor assessments. Patients presented impaired nonverbal communication in all tasks compared with controls. Furthermore, in contrast to controls, performance in patients was highly correlated between tasks, not explained by supramodal cognitive deficits such as working memory. Schizophrenia patients with impaired gesture performance also demonstrated poor nonverbal social perception, gestural knowledge, and tool use. Importantly, motor/frontal abnormalities negatively mediated the strong association between nonverbal social perception and gesture performance. The factors negative symptoms and antipsychotic dosage were unrelated to the nonverbal tasks. The study confirmed a generalized nonverbal communication deficit in schizophrenia. Specifically, the findings suggested that nonverbal social perception in schizophrenia has a relevant impact on gestural impairment beyond the negative influence of motor/frontal abnormalities.
Key words: social cognition, negative symptoms, pantomime, imitation
Introduction
Social impairments are a central feature of schizophrenia, and social cognition has been suggested as determinant for functional outcome.1,2 Social cognition includes processes of social interaction, ie, perception, interpretation, and responding to social relevant stimuli.3 Various domains of nonverbal communication are impaired in schizophrenia, such as facial emotion recognition4 and imitation,5 recognition of emotional prosody,6 use of co-speech gestures,7,8 and imitation of hand gestures.9,10
Successful nonverbal communication relies on both correct perception and expression of information. How nonverbal perception and expression influence each other in schizophrenia is poorly understood, as are the associations with clinical phenomena. Gestures are heterogeneous, complex expressive behaviors that may accompany speech including movements of fingers, hands, and arms. They may substitute or aid language comprehension and provide clues on cognitive action representation.11–13 Here, we refer to hand and finger gestures related to transitive and intransitive symbolic information. Transitive gestures are tool related and require simulating the specific action in absence of the object (eg, signaling the use of a comb or a hammer). Intransitive gestures convey highly overlearned, emblematic information (eg, signaling stop or waving good bye). Both transitive and intransitive gestures are important components of everyday nonverbal communication. Schizophrenia patients use spontaneous hand gestures less frequently than healthy subjects.7,8 In hand imitation tasks, patients perform less accurate than controls.5,9,14 First studies report clear-cut gestural deficits in up to 67% of schizophrenia patients.14 These gestural deficits encompass spatial and temporal errors as well as body part as object errors (eg, using the index finger when asked to demonstrate how to use a tooth brush).10,14,15 Gesture deficits in schizophrenia have been linked to negative symptoms, motor abnormalities (ie, parkinsonism and catatonia), frontal lobe dysfunction, and working memory impairments.5,8–10,14
Nonverbal social perception relates to the decoding of social relevant emotional information from various nonverbal cues such as facial affect, prosody, and body gestures.16 Deficits of nonverbal social perception have been demonstrated in schizophrenia by using multimodal tasks,16 including poor recognition of hand gestures. In fact, schizophrenia patients tend to misinterpret hand gestures.17 Poor nonverbal social perception was reported to correlate with conceptual disorganization, but not with negative or positive symptoms.16
Correct gesture use is thought to rely on action representation and knowledge of the symbolic content of gestures.11 Thus, to understand gestural deficits in schizophrenia, we need to test the association between nonverbal social perception, gestural knowledge, and gesture production. As mentioned above, clinical phenomena such as working memory deficits, negative symptoms, and motor abnormalities hamper gesture production in schizophrenia. Their contribution to gestural knowledge and nonverbal social perception requires elucidation. Finally, it has to be clarified whether impairments in the performance of transitive gestures resemble deficits in symbolic representation of action rather than true impairments of action. In other words, do patients suffer from impaired simulation of tool use or from actual defective tool use? For instance, apraxic stroke patients also perform poorly when pantomiming tool use but benefit from the physical properties of the tool during demonstration and actual use.18
The present study aimed to investigate whether gesture deficits in schizophrenia were related to nonverbal social perception, gesture knowledge, or actual tool use. Furthermore, we investigated the impact of clinical symptoms such as negative symptoms, working memory impairment, and motor abnormalities (parkinsonism, neurological soft signs [NSS], dyskinesia, and catatonia) on domains of nonverbal communication. We hypothesized that schizophrenia patients were impaired in all tasks on nonverbal communication (nonverbal social perception, gesture performance, gesture knowledge, and tool use). Furthermore, we expected poor nonverbal social perception and presence of motor abnormalities to impair gesture performance in schizophrenia.
Methods
Subjects
In total, 46 patients and 44 healthy control subjects matched for age, gender, and education were included in this study. Subjects were recruited from the inpatient and outpatient departments of the University Hospital of Psychiatry, Bern, Switzerland. Healthy controls were recruited among staff and via advertisement. All subjects were right handed as determined by the Edinburgh handedness inventory.19 Exclusion criteria included substance abuse or dependence other than nicotine; past or current medical or neurological condition impairing movements, such as dystonia, idiopathic parkinsonism, or stroke; and history of head trauma with concurrent loss of consciousness. Exclusion criterion for patients was a history of electroconvulsive treatment. Exclusion criteria for controls were a history of any psychiatric disorder as well as any first-degree relatives with schizophrenia or schizoaffective disorder.
All participants were interviewed with the Mini International Neuropsychiatric Interview.20 Patients were further interviewed with the Comprehensive Assessment of Symptoms and History.21 Diagnoses were given according to Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria (n = 37 schizophrenia, n = 2 schizoaffective disorder, and n = 7 schizophreniform disorder). All but 4 patients received antipsychotic pharmacotherapy. Clinical and demographic data are given in table 1. All participants provided written informed consent. The protocol was approved by the local ethics committee.
Table 1.
Demographic and Clinical Characteristics, Mean (SD)
| Characteristic | Controls | Patients | F/X 2 | P |
|---|---|---|---|---|
| No. (%) male | 26 (59) | 28 (63) | 0.15 | .83 |
| Age (y) | 38.77 (13.58) | 37.96 (11.17) | 0.10 | .99 |
| Education (y) | 14.14 (2.66) | 13.58 (3.03) | 0.87 | .99 |
| TONI index | 110.60 (10.33) | 97.73 (11.03) | 31.09 | <.001 |
| Digit span | 5.36 (0.78) | 4.37 (1.22) | 20.47 | <.001 |
| AIMS | 0.15 (0.71) | 2.33 (3.86) | 10.19 | .02 |
| BFCRS | 0 (0) | 1.63 (3.58) | 9.12 | .03 |
| UPDRS III | 0 (0) | 7.09 (7.29) | 41.56 | <.001 |
| NES | 3.94 (4.84) | 13.59 (11.45) | 21.26 | <.001 |
| MRS | 0 (0) | 2.80 (5.21) | 12.72 | .006 |
| FAB | 17.57 (0.66) | 16.16 (2.64) | 11.90 | .009 |
| TLC | 6.20 (7.72) | |||
| SANS | 24.87 (16.93) | |||
| CAINS | 17.96 (10.67) | |||
| PANSS positive | 18.00 (6.13) | |||
| PANSS negative | 18.33 (5.13) | |||
| PANSS general | 35.43 (8.36) | |||
| CPZ (mg) | 403.22 (346.98) | |||
| Duration of illness (y) | 12.89 (12.08) |
Note: AIMS, Abnormal Involuntary Movement Scale; BFCRS, Bush Francis Catatonia Rating Scale; UPDRS III, motor part of the Unified Parkinson’s Disease Rating Scale; NES, Neurological Evaluation Scale; MRS, Modified Rogers Scale; FAB, Frontal Assessment Battery; TLC, Thought Language and Communication Scale; SANS, Scale for the Assessment of the Negative Syndrome; CAINS, Clinical Assessment Interview for Negative Symptoms; PANSS, Positive and Negative Syndrome Scale; P values adjusted for multiple comparisons.
Procedures
Nonverbal Communication Tests.
Participants underwent behavioral tests on 4 tasks on nonverbal communication related to hand gestures (for detailed description, see supplementary material). In all tasks, higher scores indicate superior performance. The test of upper limb apraxia (TULIA)22 is a comprehensive assessment of gesture production in 2 domains: following demonstration by the examiner (imitation) or on verbal command (pantomime). Performance of 48 items was videotaped. Evaluation rated content and temporal-spatial errors (for details, see supplementary material). Total scores range 0–240. All ratings were performed by a single rater blind to diagnoses and clinical presentation (S.W.), who had been trained by the test developers (T.V. and S.B.). Intraclass correlations exceeded .83.
The Tool-Use test18 additionally examines the demonstration and actual use of tools. Specifically, 3 conditions using a scoop and a hammer are evaluated: pantomime (without the tool), demonstration (with the tool), and actual use (with a recipient object). Each tool is tested in 3 trials per condition. Performance was videotaped and later evaluated considering grip formation, movement execution, movement direction, and spatial errors. Total scores range 0–72. Tool use was evaluated by 2 raters blind to diagnoses and clinical presentation, who had been extensively trained. Interrater reliability met intraclass correlations of .86.
The modified postural knowledge task (PKT)23,24 is a gesture recognition task. Participants were presented with cartoons of persons carrying out 10 intransitive and 10 transitive actions, while the distal parts of the executing limbs are not shown. Below each cartoon, 3 images of limb positions are given including the correct one. Participants have to choose the correct match. In addition, 10 images of 3 hands holding objects were presented, again with 2 versions of incorrect grip and position and 1 correct. Total scores range 0–30.
We applied the Mini Profile of Nonverbal Sensitivity (Mini-PONS)25 to test social perception. The Mini-PONS includes 64 scenes from the original PONS,26 in which short videos of 2 s each present a white woman with altering facial expression, voice intonation, and/or bodily gestures. Participants had to choose from 2 options the one that best describes the observed situation immediately after watching the video, eg, saying a prayer or talking to a lost child. The total scores range 0–64.
Clinical Assessments.
Furthermore, we investigated motor abnormalities in the participants using clinical rating scales to assess abnormal involuntary movements, parkinsonism, NSS, and catatonic behavior. We applied the Abnormal Involuntary Movement Scale (AIMS),27 the motor part of the Unified Parkinson’s Disease Rating Scale (UPDRS III),28 the Neurological Evaluation Scale (NES),29 the Bush Francis Catatonia Rating Scale (BFCRS),30 and the Modified Rogers Scale.31 In addition, we applied the Frontal Assessment Battery (FAB).32 Verbal working memory was assessed with the digit span backward from the Wechsler Memory Scale.33
In all motor rating scales, higher scores indicate the presence of motor abnormalities. In contrast, in the FAB and digit span, higher scores indicate superior performance. In all subjects, nonverbal intelligence was measured with the Test of Nonverbal Intelligence (TONI).34
In the patients, we further assessed the Positive and Negative Syndrome Scale (PANSS)35 and the Thought Language and Communication scale36 to monitor formal thought disorder and 2 scales for negative syndrome severity: the Scales for the Assessment of Negative Syndrome (SANS)37 and the Clinical Assessment Interview for Negative Symptoms (CAINS).38 The clinical assessments have been performed by a single expert psychiatrist (K.S.), who had been trained by the senior investigator to achieve interrater reliabilities of κ > .80.
The total assessment had approximately 5 hours duration for patients and approximately 4 hours for controls. In many subjects, tests were performed on 2 consecutive days.
Statistical Analyses
Demographic and clinical data were compared between groups using ANOVAs or chi-square tests where appropriate. In patients, the clinical rating scales were subject to principal component analysis (PCA) extracting components with eigenvalues > 1 and subsequent varimax rotation (for details, see supplementary material). PCA yielded 4 factors explaining a sum of 83.5% of the variance: (1) negative symptoms (30.1%, including PANSS negative, PANSS general, CAINS, and SANS), (2) motor/frontal abnormalities (25.7%, including UPDRS motor part, NES, BFCRS, FAB, and digit span backward), (3) positive symptoms/working memory (14.0%, including PANSS positive, PANSS general, and digit span backward), and (4) dyskinesia/catatonia (13.7%, including AIMS and BFCRS). Factor scores were extracted for further analyses.
First, we compared total scores of the tasks on nonverbal communication (TULIA, PKT, PONS, and Tool-Use) between groups using ANCOVAs controlling for TONI index score and digit span, as groups differed in these variables (table 1). Second, we explored whether performance in one of the nonverbal communication tasks was related to the performance of the other tasks for both groups separately. In controls, we were interested in whether associations were found in the absence of major motor or cognitive impairments. As both medication and age were shown to influence gesture performance and recognition,10,14,39 we calculated the correlations between the 4 tasks using within-group partial correlations correcting for age and chlorpromazine equivalents (CPZ) in patients and correcting for age in controls. Third, we investigated within-group associations between the 4 nonverbal communication tasks and the 4 clinical factors, age, and CPZ in stepwise regression analyses. Finally, we explored the association between nonverbal social perception and gesture performance using (1) partial correlations correcting for age, CPZ, and the factor motor/frontal abnormalities, (2) a series of regression models to test whether motor/frontal abnormalities were mediating or moderating this association, and (3) a hierarchical regression analysis, in which motor/frontal abnormalities were the first step and nonverbal social perception was the second step to determine the effect on gesture performance. All analyses were conducted in SPSS22 (IBM). Tests were corrected for multiple comparisons (P corr = P × n), with n being the number of tests.
Results
Between-Group Differences in Nonverbal Communication
Controls had superior performance in the nonverbal intelligence test and the digit span backward and less motor abnormalities and frontal lobe dysfunction compared with schizophrenia patients (table 1). Patients had inferior performance in all tasks of nonverbal communication (table 2) when controlling for nonverbal intelligence and working memory. Applying the cutoff scores,14,18 45.7% of schizophrenia patients had gesture performance deficits (47.8% pantomime and 32.6% imitation) and 37.8% were impaired in the Tool Use task (37.8% pantomime, 11.1% demonstration, and 11.1% use).
Table 2.
Group Comparisons of Task Performance, Mean (SD) With Covariates TONI and Digit Span Backward
| Controls (n = 44) | Patients (n = 46) | F (df = 3) | P | |
|---|---|---|---|---|
| TULIA | 225.67 (7.75) | 206.13 (28.12) | 13.88 | <.001 |
| PKT | 27.31 (1.44) | 24.41 (4.34) | 10.98 | <.001 |
| PONS | 46.67 (4.42) | 41.42 (6.03) | 19.88 | <.001 |
| Tool Use | 71.93 (0.46) | 69.59 (4.28) | 8.77 | <.001 |
Note: TONI, Test of Nonverbal Intelligence; TULIA, test of upper limb apraxia; PKT, postural knowledge task; PONS, Profile of Nonverbal Sensitivity; P values adjusted for multiple comparisons.
Within-Group Correlations
Controls.
In controls, none of the nonverbal communication tasks correlated significantly with any other. Partial correlations corrected for age indicated that gesture performance, gestural knowledge, and nonverbal social perception were related to higher IQ (supplementary table S2). In contrast, gestural knowledge and nonverbal social perception were correlated with longer duration of education, and gesture performance was correlated with better working memory performance. Finally, tool use performance was unrelated to any of the descriptive variables.
Patients.
In patients, the performance between each of the nonverbal communication tasks was strongly correlated (table 3), except the correlation between nonverbal social perception (PONS) and tool use at trend level. Particularly, gesture performance was closely linked to gestural knowledge, tool use, and nonverbal social perception. The correlations between communication tasks remained significant even when correcting for verbal working memory impairments, age, and CPZ (supplementary table S3). Furthermore, nonverbal communication task performances demonstrated significant partial correlations with clinical variables when correcting for age and CPZ (supplementary tables S4 and S5).
Table 3.
Partial Correlations Between Tasks in Patients (n = 46), Corrected for Age and CPZ
| PONS | PKT | Tool Use | ||||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| TULIA | .57 | <.001 | .76 | <.001 | .75 | <.001 |
| PONS | .52 | .003 | .38 | .07 | ||
| PKT | .51 | .005 | ||||
Note: Abbreviations are explained in the first footnote to table 2. P values adjusted for multiple comparisons.
Next, the contributions of age, CPZ, and the 4 clinical factors to the 4 nonverbal communication tasks were tested using stepwise linear regression models (table 4). All 4 tasks were associated with motor/frontal abnormalities. Beyond these associations, gesture performance was related to age, positive symptoms, and the dyskinesia/catatonia factor. Furthermore, nonverbal social perception was explained by positive symptoms/working memory and tool use by dyskinesia/catatonia. The negative symptom factor and CPZ were excluded in each model.
Table 4.
Factors Impacting Nonverbal Communication Tasks
| Model |
|
df | F | P | Predictor | β | T | P | |
|---|---|---|---|---|---|---|---|---|---|
| TULIA | .60 | 4, 41 | 17.80 | <.001 | Motor/frontal | −.56 | −5.39 | <.001 | |
| Age | −.27 | −2.60 | .01 | ||||||
| Positive/WM | −.22 | −2.33 | .03 | ||||||
| Dyskinesia/catatonia | −.22 | −2.28 | .03 | ||||||
| PONS | .46 | 2, 43 | 20.06 | <.001 | Motor/frontal | −.53 | −4.81 | <.001 | |
| Positive/WM | −.45 | −4.08 | <.001 | ||||||
| PKT | .23 | 1, 44 | 14.37 | <.001 | Motor/frontal | −.50 | −3.79 | <.001 | |
| Tool use | .54 | 2, 43 | 27.41 | <.001 | Motor/frontal | −.69 | −6.78 | <.001 | |
| Dyskinesia/catatonia | −.26 | −2.53 | .02 |
Note: Abbreviations are explained in the first footnote to table 2. WM, working memory. Collinearity statistics: tolerance > 0.82, variance inflation factor < 1.22.
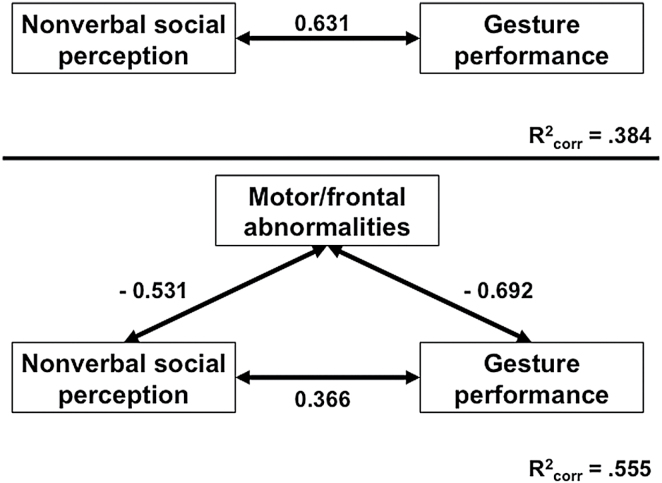
Finally, we explored whether intact nonverbal social perception was critical for correct performance of hand gestures and whether motor/frontal abnormalities may hamper this association. Partial correlations suggested that nonverbal social perception was associated with better performance of hand gestures (r = 0.34, P = .04) when correcting for age, CPZ, and the factor motor/frontal abnormalities. Figure 1 depicts the mediator analysis. In patients, superior nonverbal social perception is associated with correct performance of hand gestures (top panel). Although adding motor/frontal abnormalities to the model decreased the effect of nonverbal social perception on gesture performance, the association still remained significant, while the explained variance was increased (lower panel). In other words, motor/frontal abnormalities only partially mediated the effect of nonverbal social perception on gesture performance. In addition, there was no interaction effect of nonverbal social perception and motor/frontal abnormalities on gesture performance (data not shown). Hierarchical regression analysis indicated that nonverbal social perception had an effect on gesture performance beyond motor/frontal abnormalities (model 1: motor/frontal abnormalities R 2 = .48, F (change)[1, 44] = 40.39, P (change) < .001, motor/frontal abnormalities β = −.69, P < .001; model 2: nonverbal social perception ΔR 2 = .10, F (change)[2, 43] = 9.75, P (change) = .003, motor/frontal abnormalities β = −.50, P < .001, nonverbal social perception β = .37, P = .003).
Fig. 1.
Association of social perception, motor/frontal abnormalities, and gesture performance. Upper panel: direct association between nonverbal social perception (PONS) and gesture performance (TULIA). Lower panel: inclusion of the mediator motor/frontal abnormalities (motor/frontal factor). Numbers indicate beta-weights. Note that the association between nonverbal social perception and gesture performance is weaker in the lower panel, suggesting a partial mediator effect of motor/frontal abnormalities.
Discussion
The present study on nonverbal communication deficits in schizophrenia yielded 4 main findings. First, we confirmed impairments in nonverbal social perception and gesture production in schizophrenia.1,10,14,16,40 In addition, patients had deficits in gestural knowledge and actual tool use. Second, as hypothesized, in schizophrenia, deficits in gesture performance were associated with impairments in nonverbal social perception, gesture knowledge, and tool use. In contrast, performance in these tasks was not correlated in controls. Third, motor/frontal abnormalities were the common factors associated with poor performance in all 4 nonverbal tasks in patients. Still, beyond motor abnormalities, the tasks were associated with distinct clinical factors. Fourth, poor nonverbal social perception was associated with impaired gesture performance and this association was only partially mediated by motor/frontal abnormalities. In contrast, a smaller ecological study failed to find an association between nonverbal social perception and spontaneous gesture use in schizophrenia.8
Motor Abnormalities Impaired Nonverbal Communication
Motor abnormalities are an intrinsic dimension of schizophrenia present even before the onset of the full blown disorder and often deteriorated by antipsychotic treatment.41–44 The motor phenomena include catatonia, parkinsonism, abnormal involuntary movements, and NSS.42 We hypothesized that motor abnormalities would contribute to nonverbal social communication, particularly to expressive gestures or body posture and action imitation. Indeed, the factor motor/frontal abnormalities (including parkinsonism, NSS, catatonia, frontal dysfunction, and working memory) correlated inversely with all tasks. In addition, the dyskinesia/catatonia factor also correlated inversely with gesture production and tool use. Thus, the presence of motor/frontal abnormalities impairs perception and expression of nonverbal communication in schizophrenia. Impaired gesture performance has been linked to motor abnormalities and frontal lobe dysfunction before.10 Consequently, patients with motor abnormalities are prone to impairments in nonverbal communication. This is not only relevant for chronic schizophrenia but also for subjects at risk for psychosis and first episode patients with schizophrenia. Both groups may experience motor abnormalities45,46 and deficits in social cognition.47 Therefore, it is conceivable that some of the social cognitive impairments are related to motor abnormalities. Indeed, young patients with schizotypal disorder use gestures less frequently than controls.48 Furthermore, subjects at ultrahigh risk of psychosis demonstrate content errors of spontaneous co-speech gestures.49
Link of Social Perception and Gesture Control: Mirror Neurons and Theory of Mind
Part of the strong relationship of social perception and gesture control was independent of motor/frontal abnormalities as demonstrated by the mediation analysis. Furthermore, hierarchical regression confirmed that the impact of nonverbal social perception on gesture performance was greater than the negative impact of motor/frontal abnormalities although regression models are not suited to finally prove causal relationships. In case a patient had sufficient nonverbal social perception abilities, the presence of significant motor/frontal abnormalities would, therefore, impair but not perish gesture performance.
A possible link between social perception and gesture control may be viewed in the light of embodied cognition, in which gestures were suggested to mediate between action and its mental representation.11 Particularly, the mirror mechanism indicates that motor acts of others are understood by the same mechanism underlying the execution of these motor acts. Furthermore, the mirror mechanism of motor behavior is critical for the interpretation of goals and intentions of others.50 Another model claims that action semantics, ie, knowledge on specific actions and their meaning, is critical to understand action of others.51 Thus, the knowledge of the abstract meaning of gestures along with the action representation must, therefore, be critical for correct gesture performance.
The brain areas involved in gesture production, gesture observation, action imitation, and action observation are broadly the same: inferior frontal gyrus (IFG), insula and inferior parietal lobule (IPL).24,52–55 Imitation and observation of hand gestures activate the right IPL and the medial prefrontal cortex, thus engaging the mirror neuron system and the mentalizing system.55 In schizophrenia, aberrant neural activation was detected during the processing of metaphoric gestures in the left IFG and left superior temporal sulcus (STS).56,57 Likewise, patients had abnormal activation during imitation and observation of finger movements within the IPL and STS.58 Therefore, the cerebral motor system including cortical premotor areas may contribute to social cognitive deficits in schizophrenia during the perception, interpretation, and production of actions such as hand gestures and body postures.
Current psychological treatment programs to enhance social cognition efficiently improve emotion recognition but fail to impact more complex measures of nonverbal social perception.59 A specific add-on training of hand gestures may increase and generalize the effects of current social skills training approaches. Furthermore, our data suggest considering some aspects of motor behavior when assessing social cognition. In fact, hand gestures and head and body movements are used for nonverbal expression in social interactions.
Negative Symptoms and Working Memory in Nonverbal Communication
Negative symptoms could have impact on social interaction, thus impair social cognition.3,60 The results of our study were conflicting: the negative factor of the PCA failed to correlate with any nonverbal communication task. However, in line with previous reports, impaired gesture performance, tool use, and gestural knowledge correlated with increased ratings in CAINS, SANS, and PANSS negative.5,9,10,14 Still, nonverbal social perception (PONS) lacked correlation with negative symptoms, as in other studies.1,16 The discrepancy between tests might be due to the composition of the negative factor in the PCA. In sum, our results argue against a general impact of negative symptoms on nonverbal communication in schizophrenia. Instead, negative symptoms may impact expression but not perception of nonverbal social interaction.
Schizophrenia has been associated with a generalized supramodal impairment in working memory.61 In fact, imitation of hand gestures was linked to working memory in schizophrenia before.9 However, our results argue against a specific effect on gesture production and in favor of a generalized effect on nonverbal communication. Indeed, the test of verbal working memory correlated with each of the 4 tasks (supplementary table S4). Furthermore, working memory was part of the factor motor/frontal abnormalities that correlated with all nonverbal tasks. The correlations between the tasks remain significant in schizophrenia even when controlling for working memory (supplementary table S3).
In controls, we found no correlation between the 4 nonverbal communication tasks. This lack of association would argue for distinct processes and abilities between gesture performance, gesture knowledge, tool use, and social perception. The scores of the gesture knowledge (PKT) and the Tool Use task clearly demonstrate a ceiling effect because these tests were developed for use in elderly subjects with apraxia.18,23,39,62 However, tasks of nonverbal social perception (PONS) and gesture performance (TULIA) were designed to avoid ceiling effects and have sufficient variance in our sample. Still, nonverbal social perception and gesture performance were unrelated in healthy subjects in contrast to patients with schizophrenia.
Patients and controls were well matched for age, gender, and educational level. However, they still differed in nonverbal IQ and working memory performance. Therefore, we had to include these parameters as covariates in the group comparisons. We investigated a heterogeneous group of patients concerning age, duration of illness, and symptom severity. Therefore, gesture performance was better in this sample than in our previous study.14 In order to account for effects of treatment and age, CPZ and age were control variables in partial correlation analyses. Of course, controlling for antipsychotic medication dose will not exclude medication effects. Furthermore, although we excluded patients with current comorbid disorders based on diagnostic interviews, we cannot completely exclude comorbidity effects of subsyndromal disturbances or undeclared past disorders.
Conclusion
In conclusion, schizophrenia patients presented generalized impairments in 4 tasks of nonverbal communication. In addition, patients with motor/frontal abnormalities had more difficulties in the tasks. Finally, irrespective of the negative influence of motor/frontal impairments, there was a strong association between nonverbal social perception and gesture performance pointing to a mirror mechanism of gesture behavior. Future studies should focus on the underlying brain alterations and establish whether specific interventions on motor abnormalities may aid social cognition.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This study received funding from the Bangerter-Rhyner Foundation (to S.W.) and the Swiss National Science Foundation (SNF grant 152619/1 to S.W. and S.B.).
Supplementary Material
Acknowledgments
The authors thank Ms Nora Schaub who rated the Tool Use videos. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Green MF, Hellemann G, Horan WP, Lee J, Wynn JK. From perception to functional outcome in schizophrenia: modeling the role of ability and motivation. Arch Gen Psychiatry. 2012;69:1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schmidt SJ, Mueller DR, Roder V. Social cognition as a mediator variable between neurocognition and functional outcome in schizophrenia: empirical review and new results by structural equation modeling. Schizophr Bull. 2011;37(suppl 2):S41–S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Green MF, Penn DL, Bentall R, et al. Social cognition in schizophrenia: an NIMH workshop on definitions, assessment, and research opportunities. Schizophr Bull. 2008;34:1211–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr Bull. 2010;36:1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park S, Matthews N, Gibson C. Imitation, simulation, and schizophrenia. Schizophr Bull. 2008;34:698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bach DR, Buxtorf K, Grandjean D, Strik WK. The influence of emotion clarity on emotional prosody identification in paranoid schizophrenia. Psychol Med. 2009;39:927–938. [DOI] [PubMed] [Google Scholar]
- 7. Troisi A, Spalletta G, Pasini A. Non-verbal behaviour deficits in schizophrenia: an ethological study of drug-free patients. Acta Psychiatr Scand. 1998;97:109–115. [DOI] [PubMed] [Google Scholar]
- 8. Lavelle M, Healey PG, McCabe R. Is nonverbal communication disrupted in interactions involving patients with schizophrenia? Schizophr Bull. 2013;39:1150–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matthews N, Gold BJ, Sekuler R, Park S. Gesture imitation in schizophrenia. Schizophr Bull. 2013;39:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walther S, Vanbellingen T, Müri R, Strik W, Bohlhalter S. Impaired pantomime in schizophrenia: association with frontal lobe function. Cortex. 2013;49:520–527. [DOI] [PubMed] [Google Scholar]
- 11. Cartmill EA, Beilock S, Goldin-Meadow S. A word in the hand: action, gesture and mental representation in humans and non-human primates. Philos Trans R Soc Lond B Biol Sci. 2012;367:129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kelly SD, Ozyürek A, Maris E. Two sides of the same coin: speech and gesture mutually interact to enhance comprehension. Psychol Sci. 2010;21:260–267. [DOI] [PubMed] [Google Scholar]
- 13. Obermeier C, Dolk T, Gunter TC. The benefit of gestures during communication: evidence from hearing and hearing-impaired individuals. Cortex. 2012;48:857–870. [DOI] [PubMed] [Google Scholar]
- 14. Walther S, Vanbellingen T, Müri R, Strik W, Bohlhalter S. Impaired gesture performance in schizophrenia: particular vulnerability of meaningless pantomimes. Neuropsychologia. 2013;51:2674–2678. [DOI] [PubMed] [Google Scholar]
- 15. Martin P, Tewesmeier M, Albers M, Schmid G, Scharfetter C. Investigation of gestural and pantomime performance in chronic schizophrenic inpatients. Eur Arch Psychiatry Clin Neurosci. 1994;244:59–64. [DOI] [PubMed] [Google Scholar]
- 16. Toomey R, Schuldberg D, Corrigan P, Green MF. Nonverbal social perception and symptomatology in schizophrenia. Schizophr Res. 2002;53:83–91. [DOI] [PubMed] [Google Scholar]
- 17. Bucci S, Startup M, Wynn P, Baker A, Lewin TJ. Referential delusions of communication and interpretations of gestures. Psychiatry Res. 2008;158:27–34. [DOI] [PubMed] [Google Scholar]
- 18. Randerath J, Goldenberg G, Spijkers W, Li Y, Hermsdörfer J. From pantomime to actual use: how affordances can facilitate actual tool-use. Neuropsychologia. 2011;49:2410–2416. [DOI] [PubMed] [Google Scholar]
- 19. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- 20. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33; quiz 34–57. [PubMed] [Google Scholar]
- 21. Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49:615–623. [DOI] [PubMed] [Google Scholar]
- 22. Vanbellingen T, Kersten B, Van Hemelrijk B, et al. Comprehensive assessment of gesture production: a new test of upper limb apraxia (TULIA). Eur J Neurol. 2010;17:59–66. [DOI] [PubMed] [Google Scholar]
- 23. Mozaz M, Rothi LJ, Anderson JM, Crucian GP, Heilman KM. Postural knowledge of transitive pantomimes and intransitive gestures. J Int Neuropsychol Soc. 2002;8:958–962. [DOI] [PubMed] [Google Scholar]
- 24. Bohlhalter S, Vanbellingen T, Bertschi M, et al. Interference with gesture production by theta burst stimulation over left inferior frontal cortex. Clin Neurophysiol. 2011;122:1197–1202. [DOI] [PubMed] [Google Scholar]
- 25. Banziger T, Scherer KR, Hall JA, Rosenthal R. Introducing the MiniPONS: a short multichannel version of the Profile of Nonverbal Sensitivity (PONS). J Nonverbal Behav. 2011;35:189–204. [Google Scholar]
- 26. Rosenthal R, Hall JA, DiMatteo MR, Rogers PL, Archer D. Sensitivity to Nonverbal Communication: The PONS Test. Baltimore, MD: John Hopkins University Press; 1979. [Google Scholar]
- 27. Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education and Welfare; 1976. [Google Scholar]
- 28. Fahn S, Elton RL, Members UP. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, eds. Recent Developments in Parkinson’s Disease. Vol 2 Florham Park, NJ: Macmillan Healthcare Information; 1987. [Google Scholar]
- 29. Buchanan RW, Heinrichs DW. The Neurological Evaluation Scale (NES): a structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Res. 1989;27:335–350. [DOI] [PubMed] [Google Scholar]
- 30. Bush G, Fink M, Petrides G, Dowling F, Francis A. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93:129–136. [DOI] [PubMed] [Google Scholar]
- 31. Lund CE, Mortimer AM, Rogers D, McKenna PJ. Motor, volitional and behavioural disorders in schizophrenia. 1: assessment using the Modified Rogers Scale. Br J Psychiatry. 1991;158:323–327, 333–336. [DOI] [PubMed] [Google Scholar]
- 32. Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55:1621–1626. [DOI] [PubMed] [Google Scholar]
- 33. Wechsler D. Wechsler Memory Scale (WMS-III). San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 34. Brown L, Sherbenou RJ, Johnsen SK. Test of Nonverbal Intelligence. Austin, TX: PRO-ED; 1982. [Google Scholar]
- 35. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 36. Andreasen NC. Scale for the assessment of thought, language, and communication (TLC). Schizophr Bull. 1986;12:473–482. [DOI] [PubMed] [Google Scholar]
- 37. Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl. 1989;49–58. [PubMed] [Google Scholar]
- 38. Forbes C, Blanchard JJ, Bennett M, Horan WP, Kring A, Gur R. Initial development and preliminary validation of a new negative symptom measure: the Clinical Assessment Interview for Negative Symptoms (CAINS). Schizophr Res. 2010;124:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mozaz MJ, Crucian GP, Heilman KM. Age-related changes in arm-hand postural knowledge. Cogn Neuropsychol. 2009;26:675–684. [DOI] [PubMed] [Google Scholar]
- 40. Sergi MJ, Rassovsky Y, Nuechterlein KH, Green MF. Social perception as a mediator of the influence of early visual processing on functional status in schizophrenia. Am J Psychiatry. 2006;163:448–454. [DOI] [PubMed] [Google Scholar]
- 41. Whitty PF, Owoeye O, Waddington JL. Neurological signs and involuntary movements in schizophrenia: intrinsic to and informative on systems pathobiology. Schizophr Bull. 2009;35:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Walther S, Strik W. Motor symptoms and schizophrenia. Neuropsychobiology. 2012;66:77–92. [DOI] [PubMed] [Google Scholar]
- 43. Morrens M, Hulstijn W, Sabbe B. Psychomotor slowing in schizophrenia. Schizophr Bull. 2007;33:1038–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Walther S, Ramseyer F, Horn H, Strik W, Tschacher W. Less structured movement patterns predict severity of positive syndrome, excitement, and disorganization. Schizophr Bull. 2014;40:585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mittal VA, Neumann C, Saczawa M, Walker EF. Longitudinal progression of movement abnormalities in relation to psychotic symptoms in adolescents at high risk of schizophrenia. Arch Gen Psychiatry. 2008;65:165–171. [DOI] [PubMed] [Google Scholar]
- 46. Peralta V, Campos MS, De Jalón EG, Cuesta MJ. Motor behavior abnormalities in drug-naïve patients with schizophrenia spectrum disorders. Mov Disord. 2010;25:1068–1076. [DOI] [PubMed] [Google Scholar]
- 47. Green MF, Bearden CE, Cannon TD, et al. Social cognition in schizophrenia, Part 1: performance across phase of illness. Schizophr Bull. 2012;38:854–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mittal VA, Tessner KD, McMillan AL, Delawalla Z, Trotman HD, Walker EF. Gesture behavior in unmedicated schizotypal adolescents. J Abnorm Psychol. 2006;115:351–358. [DOI] [PubMed] [Google Scholar]
- 49. Millman ZB, Goss J, Schiffman J, Mejias J, Gupta T, Mittal VA. Mismatch and lexical retrieval gestures are associated with visual information processing, verbal production, and symptomatology in youth at high risk for psychosis. Schizophr Res. 2014;158:64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rizzolatti G, Sinigaglia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nat Rev Neurosci. 2010;11:264–274. [DOI] [PubMed] [Google Scholar]
- 51. Buxbaum LJ, Kalénine S. Action knowledge, visuomotor activation, and embodiment in the two action systems. Ann N Y Acad Sci. 2010;1191:201–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bohlhalter S, Hattori N, Wheaton L, et al. Gesture subtype-dependent left lateralization of praxis planning: an event-related fMRI study. Cereb Cortex. 2009;19:1256–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fridman EA, Immisch I, Hanakawa T, et al. The role of the dorsal stream for gesture production. Neuroimage. 2006;29:417–428. [DOI] [PubMed] [Google Scholar]
- 54. Goldenberg G. Apraxia and the parietal lobes. Neuropsychologia. 2009;47:1449–1459. [DOI] [PubMed] [Google Scholar]
- 55. Mainieri AG, Heim S, Straube B, Binkofski F, Kircher T. Differential role of the Mentalizing and the Mirror Neuron system in the imitation of communicative gestures. Neuroimage. 2013;81:294–305. [DOI] [PubMed] [Google Scholar]
- 56. Straube B, Green A, Sass K, Kirner-Veselinovic A, Kircher T. Neural integration of speech and gesture in schizophrenia: evidence for differential processing of metaphoric gestures. Hum Brain Mapp. 2013;34:1696–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Straube B, Green A, Sass K, Kircher T. Superior temporal sulcus disconnectivity during processing of metaphoric gestures in schizophrenia. Schizophr Bull. 2014;40:936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Thakkar KN, Peterman JS, Park S. Altered brain activation during action imitation and observation in schizophrenia: a translational approach to investigating social dysfunction in schizophrenia. Am J Psychiatry. 2014;171:539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Horan WP, Kern RS, Tripp C, et al. Efficacy and specificity of social cognitive skills training for outpatients with psychotic disorders. J Psychiatr Res. 2011;45:1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ventura J, Wood RC, Hellemann GS. Symptom domains and neurocognitive functioning can help differentiate social cognitive processes in schizophrenia: a meta-analysis. Schizophr Bull. 2013;39:102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol. 2005;114:599–611. [DOI] [PubMed] [Google Scholar]
- 62. Randerath J, Li Y, Goldenberg G, Hermsdörfer J. Grasping tools: effects of task and apraxia. Neuropsychologia. 2009;47:497–505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.