Abstract
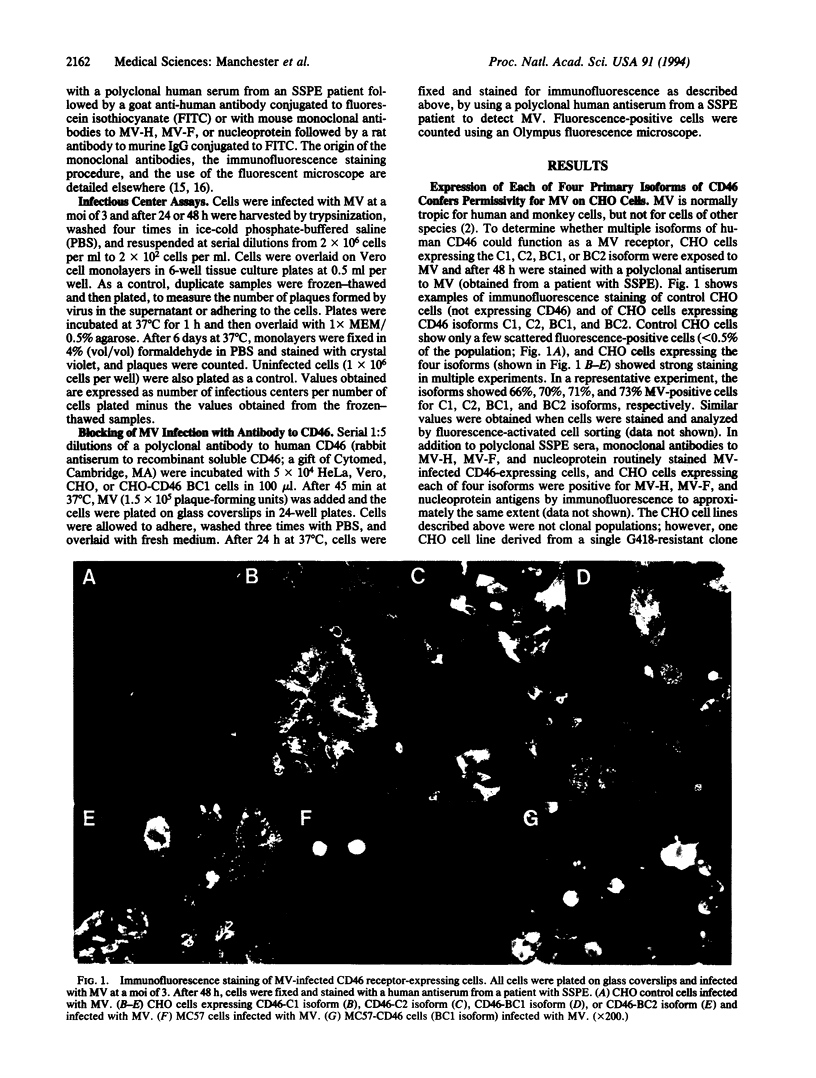
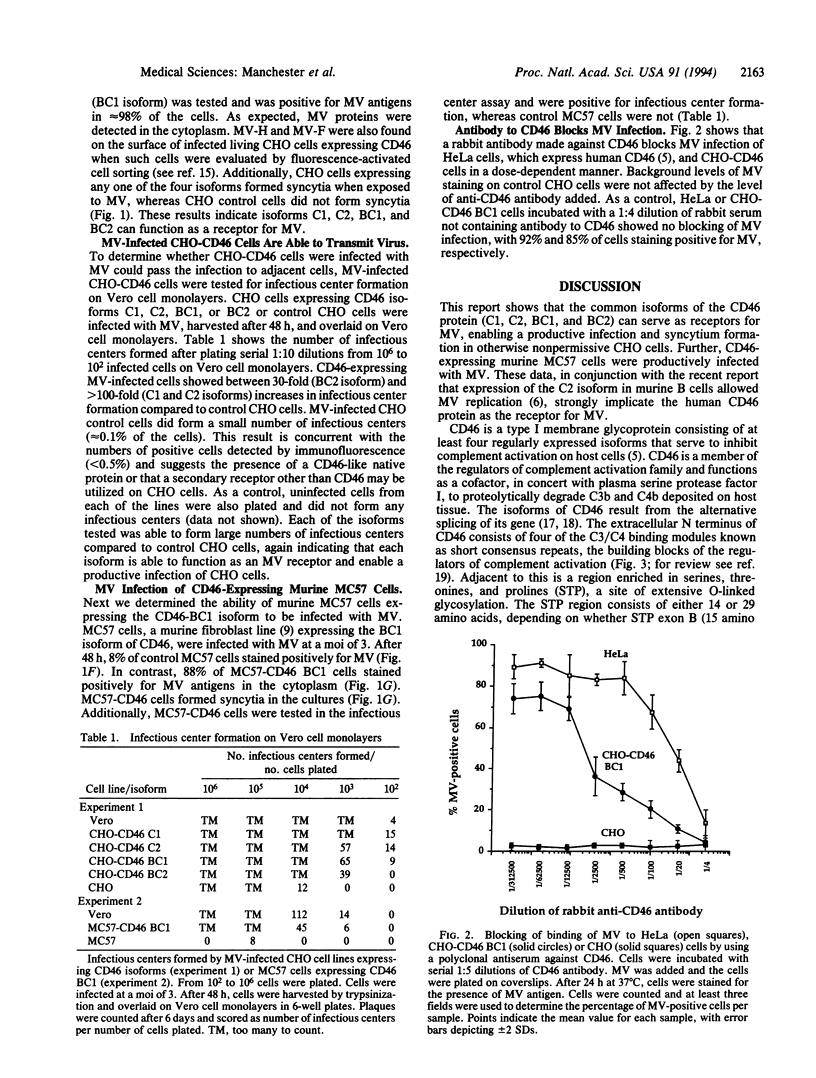
Measles virus (MV) causes a productive infection in humans and certain simian hosts. Rodent cells such as Chinese hamster ovary (CHO) and murine cell lines normally resist MV infection. Human CD46, or membrane cofactor protein, a complement regulatory protein, recently has been reported as the cellular receptor for MV. Multiple isoforms of the CD46 protein exist; four of these isoforms are commonly expressed on human cells. Expression of each of the four isoforms in CHO cells followed by exposure to MV led to the appearance of viral proteins within the cells and on the cell surface as detected by immunofluorescence. Syncytium formation also was observed in the cultures. CHO cells expressing any of the four isoforms and exposed to MV formed infectious centers when plated on Vero cell monolayers, indicating that the cells can transmit virus to uninfected cells. The murine cell line MC57 expressing the BC1 isoform of CD46 also stained positively for MV antigens and was positive in the infectious center assay after exposure to MV. Treatment of CD46-expressing cells with antibody to human CD46 inhibited MV binding in a dose-dependent manner. These observations indicate that any of the four primary isoforms of CD46 are able to serve as a receptor for MV.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom B. R. Vaccines for the Third World. Nature. 1989 Nov 9;342(6246):115–120. doi: 10.1038/342115a0. [DOI] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Dunster L. M., Schneider-Schaulies J., Löffler S., Lankes W., Schwartz-Albiez R., Lottspeich F., ter Meulen V. Moesin: a cell membrane protein linked with susceptibility to measles virus infection. Virology. 1994 Jan;198(1):265–274. doi: 10.1006/viro.1994.1029. [DOI] [PubMed] [Google Scholar]
- Dörig R. E., Marcil A., Chopra A., Richardson C. D. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell. 1993 Oct 22;75(2):295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- Fingeroth J. D., Weis J. J., Tedder T. F., Strominger J. L., Biro P. A., Fearon D. T. Epstein-Barr virus receptor of human B lymphocytes is the C3d receptor CR2. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4510–4514. doi: 10.1073/pnas.81.14.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve J. M., Davis G., Meyer A. M., Forte C. P., Yost S. C., Marlor C. W., Kamarck M. E., McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989 Mar 10;56(5):839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- Gunning P., Leavitt J., Muscat G., Ng S. Y., Kedes L. A human beta-actin expression vector system directs high-level accumulation of antisense transcripts. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4831–4835. doi: 10.1073/pnas.84.14.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsi B. L., Yeh C. J., Fénichel P., Samson M., Grivaux C. Monoclonal antibody GB24 recognizes a trophoblast-lymphocyte cross-reactive antigen. Am J Reprod Immunol Microbiol. 1988 Sep;18(1):21–27. doi: 10.1111/j.1600-0897.1988.tb00228.x. [DOI] [PubMed] [Google Scholar]
- Johnstone R. W., Russell S. M., Loveland B. E., McKenzie I. F. Polymorphic expression of CD46 protein isoforms due to tissue-specific RNA splicing. Mol Immunol. 1993 Oct;30(14):1231–1241. doi: 10.1016/0161-5890(93)90038-d. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Liszewski M. K., Post T. W., Atkinson J. P. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu Rev Immunol. 1991;9:431–455. doi: 10.1146/annurev.iy.09.040191.002243. [DOI] [PubMed] [Google Scholar]
- Lublin D. M., Liszewski M. K., Post T. W., Arce M. A., Le Beau M. M., Rebentisch M. B., Lemons L. S., Seya T., Atkinson J. P. Molecular cloning and chromosomal localization of human membrane cofactor protein (MCP). Evidence for inclusion in the multigene family of complement-regulatory proteins. J Exp Med. 1988 Jul 1;168(1):181–194. doi: 10.1084/jem.168.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNearney T., Ballard L., Seya T., Atkinson J. P. Membrane cofactor protein of complement is present on human fibroblast, epithelial, and endothelial cells. J Clin Invest. 1989 Aug;84(2):538–545. doi: 10.1172/JCI114196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naniche D., Varior-Krishnan G., Cervoni F., Wild T. F., Rossi B., Rabourdin-Combe C., Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993 Oct;67(10):6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemerow G. R., Wolfert R., McNaughton M. E., Cooper N. R. Identification and characterization of the Epstein-Barr virus receptor on human B lymphocytes and its relationship to the C3d complement receptor (CR2). J Virol. 1985 Aug;55(2):347–351. doi: 10.1128/jvi.55.2.347-351.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oglesby T. J., Allen C. J., Liszewski M. K., White D. J., Atkinson J. P. Membrane cofactor protein (CD46) protects cells from complement-mediated attack by an intrinsic mechanism. J Exp Med. 1992 Jun 1;175(6):1547–1551. doi: 10.1084/jem.175.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oglesby T. J., White D., Tedja I., Liszewski K., Wright L., Van den Bogarde J., Atkinson J. P. Protection of mammalian cells from complement-mediated lysis by transfection of human membrane cofactor protein and decay-accelerating factor. Trans Assoc Am Physicians. 1991;104:164–172. [PubMed] [Google Scholar]
- Oldstone M. B., Fujinami R. S., Tishon A., Finney D., Powell H. C., Lampert P. W. Mapping of the major histocompatibility complex and viral antigens on the plasma membrane of a measles virus-infected cell. Virology. 1983 Jun;127(2):426–437. doi: 10.1016/0042-6822(83)90155-1. [DOI] [PubMed] [Google Scholar]
- Post T. W., Liszewski M. K., Adams E. M., Tedja I., Miller E. A., Atkinson J. P. Membrane cofactor protein of the complement system: alternative splicing of serine/threonine/proline-rich exons and cytoplasmic tails produces multiple isoforms that correlate with protein phenotype. J Exp Med. 1991 Jul 1;174(1):93–102. doi: 10.1084/jem.174.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racaniello V. R. Cell receptors for picornaviruses. Curr Top Microbiol Immunol. 1990;161:1–22. doi: 10.1007/978-3-642-75602-3_1. [DOI] [PubMed] [Google Scholar]
- Reid K. B., Day A. J. Structure-function relationships of the complement components. Immunol Today. 1989 Jun;10(6):177–180. doi: 10.1016/0167-5699(89)90317-4. [DOI] [PubMed] [Google Scholar]
- Rice G. P., Casali P., Oldstone M. B. A new solid-phase enzyme-linked immunosorbent assay for specific antibodies to measles virus. J Infect Dis. 1983 Jun;147(6):1055–1059. doi: 10.1093/infdis/147.6.1055. [DOI] [PubMed] [Google Scholar]
- Seya T., Ballard L. L., Bora N. S., Kumar V., Cui W., Atkinson J. P. Distribution of membrane cofactor protein of complement on human peripheral blood cells. An altered form is found on granulocytes. Eur J Immunol. 1988 Aug;18(8):1289–1294. doi: 10.1002/eji.1830180821. [DOI] [PubMed] [Google Scholar]
- Sissons J. G., Oldstone M. B., Schreiber R. D. Antibody-independent activation of the alternative complement pathway by measles virus-infected cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):559–562. doi: 10.1073/pnas.77.1.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassini J. E., Graham D., DeWitt C. M., Lineberger D. W., Rodkey J. A., Colonno R. J. cDNA cloning reveals that the major group rhinovirus receptor on HeLa cells is intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4907–4911. doi: 10.1073/pnas.86.13.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton J. L., Oldstone M. B. Class I MHC can present an endogenous peptide to cytotoxic T lymphocytes. J Exp Med. 1989 Sep 1;170(3):1033–1038. doi: 10.1084/jem.170.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild T. F., Malvoisin E., Buckland R. Measles virus: both the haemagglutinin and fusion glycoproteins are required for fusion. J Gen Virol. 1991 Feb;72(Pt 2):439–442. doi: 10.1099/0022-1317-72-2-439. [DOI] [PubMed] [Google Scholar]