Abstract
A subset of chronic lymphocytic leukemia (CLL) B cell receptors (BCRs) interact with antigens expressed on apoptotic cells, suggesting that CLL BCRs have the potential to internalize apoptotic cell RNA or DNA-containing fragments with resultant activation of TLR7 or TLR9, respectively. By blocking cAMP degradation, type 4 cyclic nucleotide phosphodiesterase (PDE4) inhibitors activate cAMP-mediated signaling and induce apoptosis in CLL cells. Here we show that autologous irradiated leukemic cells induce proliferation in CLL cells and that such proliferation is blocked by a TLR7/8/9 inhibitor, by DNase and by the PDE4 inhibitor rolipram. Rolipram also inhibited CLL cell proliferation induced by synthetic TLR7 and TLR9 agonists, as well as TLR agonist-induced costimulatory molecule expression and TNF-α (but not IL-6 or IL-10) production. While treatment with a TLR9 agonist protected immunoglobulin heavy chain variable region (IGHV) unmutated, but not mutated, CLL cells from apoptosis, PDE4 inhibitors augmented apoptosis in both subtypes, suggesting that cAMP-mediated signaling may abrogate a TLR9-mediated survival signal in prognostically unfavorable IGHV-unmutated CLL cells. Rolipram inhibited both TLR7/8 and TLR9-induced IRF5 and NF-κB p65 nuclear translocation. PDE4 inhibitors also blocked TLR signaling in normal human immune cells. In peripheral blood whole mononuclear cells (PBMC) and CD14-positive monocytes, PDE4 inhibitors blocked IFN-α or TNF-α (but not IL-6) production, respectively, following stimulation with synthetic TLR agonists or RNA-containing immune complexes. These results suggest that PDE4 inhibitors may be of clinical utility in CLL or autoimmune diseases that are driven by TLR-mediated signaling.
Keywords: PDE4, TLR7, TLR9, CLL, cAMP
Introduction
One current hypothesis as to the origin of CLL cells is that they are derived from marginal zone B cells whose normal function includes clearance of apoptotic debris (1). Consistent with such a hypothesis, at least a subset of CLL cells have been shown to express B cell receptors (BCRs) that react with antigens expressed on apoptotic cells (2–5). Patients with CLL whose clonal “unmutated” immunoglobulin heavy chain variable region (IGHV) sequence closely resembles germline sequence (>98% homology) have a significantly poorer prognosis than those with “mutated” IGHV regions (6, 7). Amongst CLL patients whose clonal BCRs bind to apoptotic cells, there is significant enrichment for BCRs that have unmutated IGHV sequences (3).
The concept that some CLL clones may derive a positive proliferation signal from apoptotic cells in their environment focuses attention on the potential pathophysiologic importance of Toll-like receptors (TLRs) in CLL. TLRs play a key role in the response of immune cells to patterned antigens present in microorganisms, including single-stranded RNA (TLR7 and TLR8) and CpG-enriched DNA (TLR9) (8). CLL cells express TLR1, 2, 6, 7 and 9 but not TLR8 (9–13). Treatment of CLL cells with synthetic TLR ligands induces CLL proliferation (10). Although TLR7 and TLR9 agonists have been shown to up-regulate immunostimulatory molecules on CLL cells, thereby potentially rendering them more sensitive to a host immune response, trials examining TLR agonist therapy have thus far not demonstrated significant clinical responses (14, 15).
As TLR7, TLR8 and TLR9 normally respond to exogenous ligands in pathogens that have been internalized and require transfer of TLRs from the endoplasmic reticulum to an endolysosomal compartment, the relevance of TLR signaling to the pathophysiology of CLL is initially not apparent (16, 17). However, studies of autoimmunity have demonstrated that autoreactive BCRs that bind endogenous RNA or DNA or immune complexes (ICs) can internalize autoantigens derived from apoptotic cells and activate B cell TLR7 and TLR9 signaling (18–20). Similiarly, dendritic cells can internalize RNA- or DNA-containing IC via FcRs resulting in TLR7- or TLR9-dependent dendritic cell activation (21, 22). Thus, it is plausible that CLL BCRs reactive with apoptotic antigens could serve to deliver endogenous RNA or DNA to endolysosomal TLR7 and TLR9. Of note, activating mutations in the TLR adapter protein myeloid differentiation factor 88 (MyD88) have been identified in 2–10% of CLL patients and B cell activation induced by this MyD88 mutation requires TLR9 (23–26).
G protein-coupled receptors (GPCRs) are powerful modulators of signal transduction in the immune system, in part through Gs-mediated activation of adenylate cyclase and subsequent protein kinase A-mediated phosphorylation of a wide variety of critical immune cell signal transduction enzymes (27). One pharmacologic approach to mimicking the generally immunosuppressive effects of cAMP signaling in the immune system is the use of cyclic nucleotide phosphodiesterase inhibitors, drugs that block the catabolism of cAMP, thereby prolonging signaling by this second messenger. Even in the absence of specific stimulation of GPCRs, cAMP signaling through the effectors protein kinase A (PKA) and exchange protein activated by cAMP (EPAC) is strikingly activated in CLL cells by inhibitors of type 4 cAMP phosphodiesterases (PDE4) (28). In addition to activating PKA, as judged by CREB Ser 133 phosphorylation, and EPAC, as judged by Rap1 activation, the prototypic PDE4 inhibitor rolipram also induces apoptosis in CLL cells and augments glucocorticoid-mediated apoptosis (29–31).
PDE4B plays a critical role in the regulation of murine macrophage responses to lipopolysaccharide (LPS), a bacterial product that activates the plasma membrane-bound Toll-like receptor TLR4, as peripheral blood leucocytes and macrophages from mice lacking a functional PDE4B gene fail to produce TNF-α in response to LPS stimulation (32, 33). However, the precise mechanism by which PDE4B-modulated cAMP signaling regulates LPS-induced signaling remains undetermined and the generalizability of this observation to intracellular TLR receptors such as TLR7 and TLR9 is undetermined. Given the growing evidence for a potential pathophysiologic role for intracellular Toll-like receptors in CLL and in autoimmune diseases, we sought to determine whether PDE4 inhibitors could inhibit TLR7 and TLR9 mediated signaling in CLL and normal circulating hematopoietic cells.
Materials and Methods
Materials
Type A CpG ODN (2216), type B CpG ODN (2006), R848 and Pam3CSK4 were purchased from InvivoGen. Rolipram (Enzo Life Science) and roflumilast (Santa Cruz Biotechnology, Inc.) were dissolved with DMSO. The final DMSO concentration in the experiments described in this study did not exceed 0.1%. A oligonucleotide-based TLR7/8/9 antagonist was a gift from Idera Pharmaceuticals and was dissolved in sterile, endotoxin free water (34). DNase was obtained from Roche.
Cell isolation
Blood samples from flow cytometry-verified CLL patients who were untreated or at least 6 months post chemotherapy, were drawn after IRB-approved informed consent. CLL cells or peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated and cultured as previously described (35). Monocytes were isolated with human CD14 MicroBeads (Miltenyi Biotec).
Proliferation assays
One million CLL cells per well were incubated in 96-well dishes at 37°C for 3 days. Phenotypic analyses of the live cell population used for the proliferation assays typically showed greater than 95% CD5+ CD19+ lymphocytes, 3–4 % T cells and no more than 1.5% CD14+ monocytes. 18 hours before harvesting, the cells were pulsed with 1 µCi/well of 3H-thymidine. For studies involving irradiated cells, twenty-four hours after isolation, CLL cells were removed from culture dishes and irradiated using a Cesium 131 irradiator (50 Gy). After an additional 24 hours of cell culture, 0.5 to 2.0 million irradiated cells were added to one million untreated leukemic cells/well and incubated for 6 days. Proliferation was examined as described above. Apoptosis was assessed by flow cytometry (see below) in cell samples 24 hours after irradiation and, in a subset of samples, at the completion of the 6 days of culture.
Flow cytometry
After staining with fluorescent antibodies against CD19, CD40, CD54 and appropriate isotype controls (BD Pharmingen), CLL cell samples were acquired using an LSRII flow cytometer (BD Biosciences). Data were analyzed with FlowJo software (10.0.6, Tree Star).
Apoptosis assays
One million CLL cells per well were incubated in 96-well U-bottom plates at 37°C for 72 hours. Hoechst 33342 (Life Technologies) was added at 0.25 µg/mL, incubated 10 minutes at 37°C, and analyzed by flow cytometry as previously described (36).
Cytokine assays
Supernatants were collected after 24 hours of incubation with stimuli. Human TNF-α (eBioscience), IFN-α (PBL Biomedical Laboratories), IL-6 (BD Biosciences) and IL-10 (eBioscience) were quantified by ELISA following the manufacturer’s instructions. ICs were formed by combining serum from a patient with systemic lupus erythematosus (SLE) containing anti-Sm/RNP autoantibody (37) with purified Sm/RNP antigen (Arotec Diagnostics) (19).
IGHV gene sequencing
Total RNA from CLL cells was extracted (RiboPure kit, Life Technologies), used to synthesize cDNA (High Capacity RNA-to-cDNA Kit, Life Technologies) and PCR amplified using six oligonucleotides as previously described (38). The dominant PCR product was purified (QIAGEN), sequenced using both forward and reverse primers and analyzed at the IMGT/V-QUEST website (http://www.imgt.org) (39).
Immunoblotting
CLL cells were lysed with RIPA buffer and immunoblotted using antibodies specific for NF-κB p65, IRF5, phospho-IκB-α (Ser32) (Cell Signaling); IκB-α, IRAK-1, LDH (Santa Cruz Biotechnology); tubulin (Sigma) and secondary peroxidase-conjugated AffiniPure anti-rabbit or anti-mouse IgG (Jackson ImmunoResearch) as previously described (35). Membranes were developed with Lumi-Light Western Blotting Substrate (Roche).
Immunofluorescence
CLL cells were cultured in chamber slides with or without stimuli at 37°C for two hours, washed and fixed for fifteen minutes at room temperature in 4% formaldehyde, and permeabilized with staining buffer (PBS with 0.2% BSA, 0.1% Triton X-100) for 30 minutes at room temperature. Cells were incubated with mouse anti-p65 (Cell Signaling) at 4°C overnight, washed with staining buffer, incubated with Alexa Fluor 488 anti-mouse IgG (Life Technologies) at room temperature for two hours, and counterstained with TO-PRO-3 (Life Technologies). Images were acquired using a Zeiss LSM 710 confocal microscope and processed using ImageJ.
Statistical analysis
Unless otherwise indicated, statistical analysis of each set of conditions for a specific TLR agonist was performed by two-way ANOVA with multiple comparison and Tukey’s post hoc test for significance using Prism 6.0 software.
Results
PDE4 inhibitors block CLL cell proliferation induced by autologous irradiated apoptotic cells
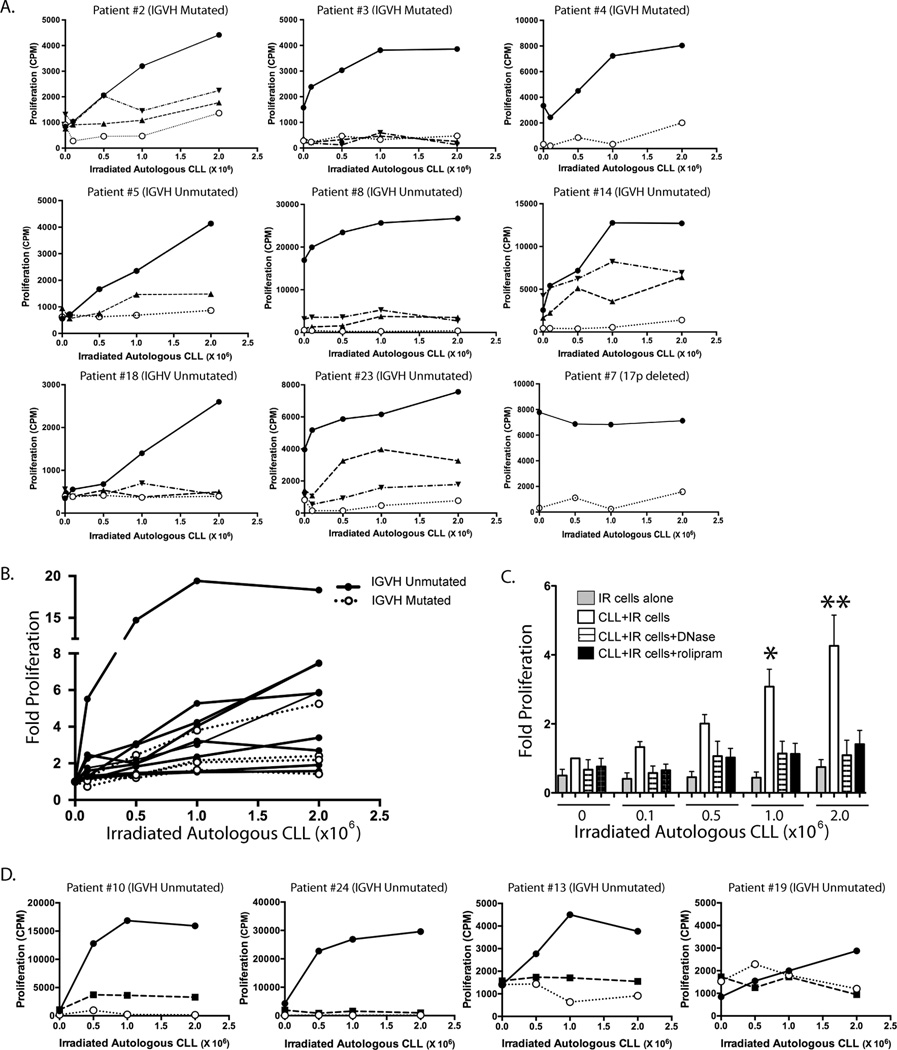
Given prior studies suggesting that CLL BCRs frequently bind to antigens expressed on apoptotic cells, we sought to determine whether apoptotic autologous leukemic cells could induce CLL cell proliferation. Freshly isolated CLL cells were cultured overnight, then transferred to new culture plates and combined with increasing numbers of autologous CLL cells that had been irradiated (50 Gy) the previous day. Proliferation was assessed six days later by tritiated thymidine assay. When measured at 24 hours, in nine CLL samples so tested, apoptosis was 11.8% +/− 11.6% (S.D.) in control cells and 49.6% +/− 21.8% in irradiated cells (p = .0004). When measured after five days of culture, 95±8% of the irradiated cells had undergone apoptosis. When cultured alone, irradiated cells demonstrated very little tritiated thymidine uptake, even at the highest cell dosage studied (Figure 1A; open circles and Figure 1C). In eight CLL patients examined, addition of irradiated cells at either a 1:1 or 2:1 irradiated/unirradiated cell ratio led to augmented levels of proliferation of the CLL cell culture (Figure 1A; closed circles and Figure 1C). When we examined leukemic cells from a CLL patient with known deletion of 17p and therefore a lack of apoptotic response to irradiation (data not shown), no increase in proliferation following the addition of irradiated autologous cells was observed, consistent with the hypothesis that apoptotic cells are required to induce proliferation of the unirradiated cells (Figure 1A, patient #7).
Figure 1.
Autologous irradiated cells induce proliferation of CLL cells and this is inhibited by treatment with either DNase or the PDE4 inhibitor rolipram. (A) One million leukemic cells/well were cultured for six days in media alone with increasing concentrations of autologous leukemic cells that had been previously irradiated (solid line, filled circles). As a control, increasing concentrations of the irradiated autologous leukemic cells were also cultured for the same time period in the absence of the one million un-irradiated cells (dotted line, open circles). One patient’s leukemic cells (lower right, Patient #7) were known to carry a 17p deletion conferring resistance to radiation-induced apoptosis. Parallel cultures were carried out in the presence of the PDE4 inhibitor rolipram at 20 µM (dashed line, upright triangles; n=7) or following overnight treatment of the irradiated cells with 20 U/mL DNase (dash/dotted line, inverted triangles; n=6). Proliferation was measured by tritiated thymidine incorporation after six days. (B) 9 IGHV unmutated and 4 IGHV mutated leukemic samples were cultured with increasing concentrations of irradiated autologous leukemic cells. Data from IGHV unmutated samples is shown with solid lines while IGHV mutated samples are shown with dotted lines. (C) Combined proliferation data from the eight CLL patients without a 17p deletion shown in panel A. Fold proliferation was calculated for each condition relative to proliferation for that individual’s CLL cells in the absence of added irradiated cells. * p< 0.05, ** p< 0.01. (D) Effect of a TLR7/8/9 antagonist on CLL proliferation induced by irradiated autologous CLL cells. Solid lines with filled circles represent proliferation of CLL cells cultured alone or with increasing numbers of autologous irradiated leukemic cells. The dotted line with open circles represents the irradiated cells cultured alone. The dashed line with filled squares represents proliferation of CLL cells treated with the TLR7/8/9 antagonist (3 µg/mL) prior to culture alone or with autologous irradiated leukemic cells.
As prior studies have suggested that amongst CLL patients whose clonal BCRs bind to apoptotic cells, there is significant enrichment for BCRs that have unmutated IGHV sequences, we sequenced the IGVH genes of the CLL patients (Table 1) (3). Among the total of thirteen CLL samples examined for proliferative responses to autologous irradiated cells (nine unmutated IGHV and four mutated IGHV), there was a trend in which the strongest proliferative response to such a stimulus occurred in IGHV-unmutated CLL cells. As shown in Figure 1B, seven out of the top eight responding CLL samples were IGHV-unmutated.
Table 1.
Clinical and molecular characteristics of CLL patients examined in this study.
| No | Age | Sex | Rai stage |
WBC | Treatment | IGHV mutation |
IGHV | IGHJ | IGHD | Molecular |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 78 | F | IV | 70000 | Chlorambucil, alemtuzumab, CHOP, FCR | Unmutated | 1-69 | 2 | 2 | Del 17p |
| 2 | 83 | F | 0 | 30,000 | CHOP | Mutated | 3-30 | 3 | 3 | Normal |
| 3 | 73 | M | 0 | 85,000 | None | Mutated | 1-3 | 4 | 3 | Del 13q |
| 4 | 62 | M | I | 59,000 | None | Mutated | 4-4 | 4 | 1 | Normal |
| 5 | 75 | M | 0 | 111,000 | FCR | Unmutated | 1-69 | 4 | 3 | Normal |
| 6 | 69 | F | III | 94,000 | None | Mutated | 1-46 | n/a | n/a | Normal |
| 7 | 64 | F | II | 76,000 | FCR, R-bendamustine, lenalidomide | Unmutated | 6-01 | n/a | n/a | Del 17p |
| 8 | 69 | F | 0 | 34,000 | None | Unmutated | 4-34 | 4 | 6 | Del 13q |
| 9 | 55 | M | I | 18,000 | None | Mutated | 1-2 | 4 | 6 | Del 13q |
| 10 | 58 | F | 0 | 19,000 | Chlorambucil, FR | Unmutated | 1-69 | n/a | n/a | Normal |
| 11 | 83 | F | 0 | 79,000 | FCR | Unmutated | 4-4 | 4 | 2 | Del 6q, Tri 12 |
| 12 | 61 | M | 0 | 16,000 | None | Mutated | 1-69 | 6 | 6 | Tri 12 |
| 13 | 53 | M | II | 32,000 | FCR | Unmutated | 1-69 | 6 | 3 | Del 11q |
| 14 | 43 | F | III | 27,000 | FR, lenalidomide | Unmutated | 1-69 | 4 | 3 | Del 13q |
| 15 | 55 | F | I | 34,000 | FCR | Unmutated | 5-51 | 2 | 1 | Normal |
| 16 | 64 | M | 0 | 27,000 | None | Unmutated | 1-69 | 6 | 3 | Tri 12 |
| 17 | 73 | M | I | 147000 | None | Unmutated | 5-a | 6 | 6 | Normal |
| 18 | 73 | M | IV | 19,000 | Chlorambucil, FC, FCR | Unmutated | 1-69 | 4 | 5 | Normal |
| 19 | 78 | F | II | 34,000 | None | Unmutated | 7-4 | 5 | 3 | Normal |
| 20 | 84 | F | 0 | 23,000 | None | Mutated | 1-69 | 6 | 2 | N.D. |
| 21 | 66 | M | 0 | 19,300 | None | Mutated | 3-23 | 5 | 4 | Normal |
| 22 | 64 | M | 0 | 19,000 | None | Mutated | 1-69 | 4 | 5 | N.D. |
| 23 | 79 | M | I | 39,600 | None | Unmutated | 1-69 | 6 | 3 | N.D. |
| 24 | 77 | F | 0 | 35,200 | None | N.D. | N.D. | N.D. | N.D. | N.D. |
CHOP: Cyclophosphamide, doxorubicin, vincristine and prednisone. FCR: Fludarabine, cyclophosphamide and rituximab. n/a: not available. N.D. Not done.
To determine whether DNA derived from apoptotic cells might play a role in the induction of proliferation by irradiated autologous leukemic cells, irradiated cells were treated with DNase overnight prior to co-culture. DNase treatment reduced the proliferative response observed following co-culture of live and irradiated cells (Figure 1A and C). An oligonucleotide-based inhibitor of TLR7, TLR8 and TLR9 signaling (3 µg/mL) also reduced CLL cell proliferation induced by autologous irradiated cells in four CLL patient samples so tested but had little or no effect on basal proliferation (Figure 1D) (34).
Prior studies have demonstrated that PDE4-regulated cAMP-mediated signaling inhibits a variety of lymphoid signal transduction pathways (40). To assess the ability of PDE4 inhibitors to alter CLL proliferation in response to autologous apoptotic cells, the prototypic PDE4 inhibitor rolipram (20 µM) was added at the initiation of the co-culture experiments. Rolipram treatment markedly reduced proliferation in the five co-culture samples examined (Figure 1A and 1C).
PDE4 inhibitors reduce TLR agonist-induced expression of CD40 and CD54 in CLL cells
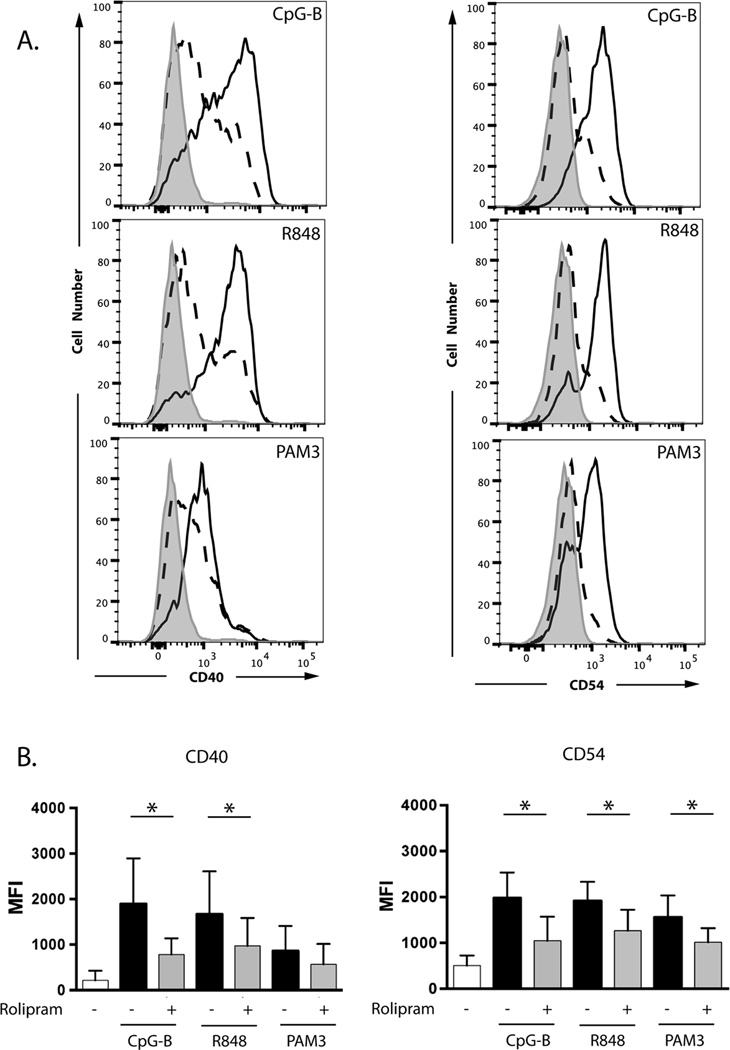
While the studies described above demonstrate that exposure to autologous apoptotic cells can drive CLL cell proliferation, at least in part through TLR-mediated pathways, and that such proliferation can be abrogated by PDE4 inhibitors, such an experimental approach does not easily address the contribution and specificity of BCR and TLR signaling in the response to this complex stimulus. We therefore next more directly examined the response of CLL cells to synthetic TLR ligands. TLR ligands augment the expression on CLL cells of costimulatory molecules including CD40 and CD54 (9–12). We assessed expression of CD40 and CD54 on CD19+ leukemic cells 72 hours after treatment with varying doses of synthetic agonists. The intracellular Toll-like receptor TLR7 was activated with the imidazoquinoline R848 (resiquimod) and the intracellular Toll-like receptor TLR9 with the oligonucleotide CpG-B (2006) (41, 42). As a control, we also treated the cells with the triacylated bacterial lipopeptide Pam3-Cys-Ser-Lys4 (Pam3CSK4), a synthetic ligand for the heterodimeric cell surface Toll-like receptor TLR1/2 (43). Each of the three TLR agonists substantially augmented expression of both CD40 and CD54 in CLL cells. Co-treatment with rolipram inhibited the induction of CD40 and CD54 expression for each of the three TLR agonists tested with the exception of Pam3CSK4-stimulated CD40 expression, where there was a non-significant trend towards inhibition (Figure 2).
Figure 2.
The PDE4 inhibitor rolipram blocks the expression of co-stimulatory molecules on CLL cells induced by TLR ligands. (A) Leukemic cells from a CLL patient were cultured for 72 hours in media with vehicle alone (DMSO; gray shaded histogram), the TLR1/2 ligand Pam3CSK4 (1 µg/mL), the TLR7/8 ligand R848 (3 µg/mL) or the TLR9 ligand CpG-B (5 µg/mL) in the absence (solid line, unshaded histogram) or presence (dashed line, unshaded histogram) of the PDE4 inhibitor rolipram (20 µM). Expression of CD40 and CD54 was analyzed by flow cytometry. Results were gated on CD19+ cells. (B) Aggregated results from five CLL patients tested using the same conditions described in panel A. * p < 0.05.
Inhibition of PDE4 reduces TLR agonist-induced proliferation in CLL cells
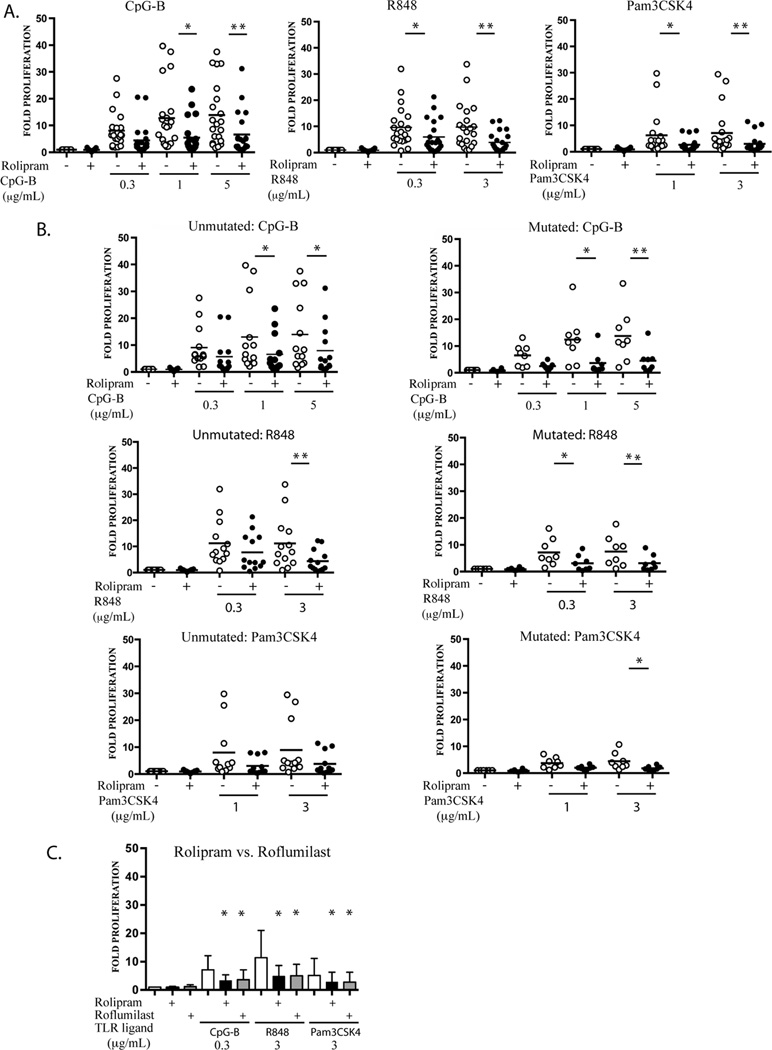
TLR ligands induce the proliferation of both normal and malignant B cells (10). To assess the effect of PDE4 inhibition on TLR-ligand-induced CLL cell growth, leukemic cells from 21 CLL patients were stimulated with graded doses of the three synthetic TLR1/2, TLR7 and TLR9 agonists in the presence or absence of rolipram (20 µM). The TLR-1/2, TLR7 and TLR9 agonists all induced CLL proliferation 72 hours after stimulation. Co-treatment with rolipram reduced such proliferation 2–3 fold for all conditions tested (Figure 3A).
Figure 3.
PDE4 inhibitors inhibit CLL cell proliferation induced by TLR1/2, TLR7 and TLR9 ligands. (A) Leukemic cells from CLL patients (n=21) were cultured for 72 hours in media with vehicle alone (DMSO), the TLR1/2 ligand Pam3CSK4 (1 or 3 µg/mL), the TLR7 ligand R848 (0.3 or 3 µg/mL) or the TLR9 ligand CpG-B (0.3, 1 or 5 µg/mL) in the absence or presence of the PDE4 inhibitor rolipram (20 µM), followed by assessment for proliferation by tritiated thymidine assay. Each patient’s level of proliferation is expressed as fold induction relative to their own basal proliferation rate (stimulus CPM/vehicle only CPM). The mean fold-induction of proliferation is shown as a horizontal bar. (B) The proliferation data presented in panel A is shown with patient samples subcategorized by their IGHV mutational status. *p < 0.05, ** p < 0.01. (C) Leukemic cells from nine CLL patients were treated with TLR1/2, TLR7 and TLR9 ligands in the presence or absence of two structurally distinct PDE4 inhibitors, rolipram (20 µM) and roflumilast (1 µM), followed by assessment for proliferation as per panel A. * p < 0.05 compared to the same stimulus without PDE4 inhibitor.
To assess the effect of IGHV mutational status on the sensitivity of CLL cells to TLR ligands and to PDE4 inhibitors, we sequenced the patients’ clonal IGHV regions (Table 1). Statistically significant reductions in proliferation following rolipram treatment were observed for 3 and 5 out of 7 TLR ligand-stimulated conditions for the unmutated and mutated IGHV samples, respectively (Figure 3B). Our results thus suggest that PDE4 inhibitors modulate TLR7 and TLR9 signaling in both IGVH mutated and unmutated CLL cells. We also sequenced leukemic cell genomic DNA for activating L265P MyD88 mutations. Such a mutation was not identified in the patients examined in this study.
To determine whether the effects observed with rolipram are specific to this compound or more generally true of PDE4 inhibitors, we performed experiments with roflumilast, a structurally distinct PDE4 inhibitor that is approved for clinical use (44). Rolipram and roflumilast inhibited TLR7 and TLR9 ligand-induced CLL cell proliferation to a similar degree (Figure 3C).
TLR9 stimulation reduces basal apoptosis in IGHV unmutated but not mutated CLL
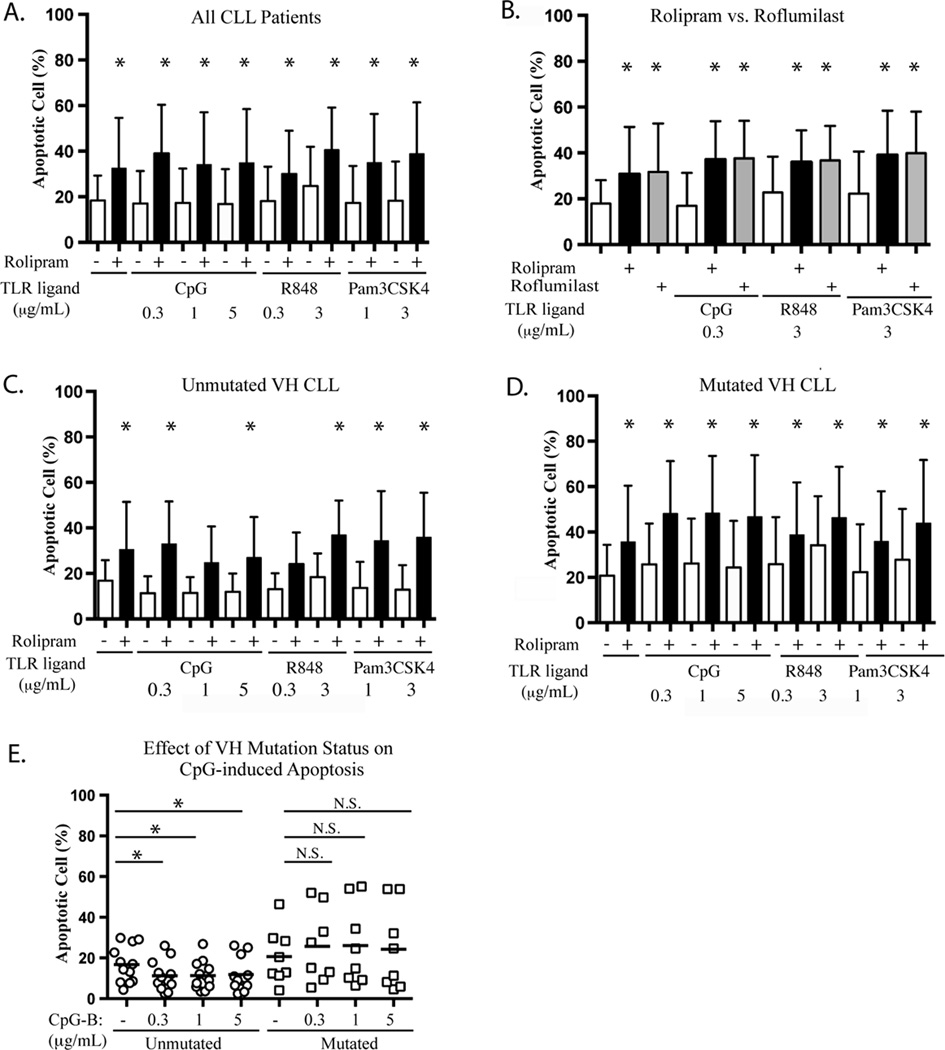
Both PDE4 inhibitors and TLR ligands have been reported to induce apoptosis in CLL cells (11, 36). To determine whether PDE4 inhibitor-induced CLL apoptosis might be altered by endogenous TLR signaling, leukemic cells from 20 CLL patients were treated with the same graded doses of TLR1/2, TLR7 and TLR9 ligands described above, with or without combined rolipram treatment, followed by assessment for apoptosis at 72 hours using a Hoechst 33342 flow cytometric assay (36). Treatment with rolipram alone or rolipram in combination with a TLR agonist modestly augmented CLL apoptosis relative to DMSO vehicle alone (Figure 4A). In contrast to some prior reports, none of the three TLR agonists tested induced apoptosis in CLL cells in the absence of rolipram and addition of the TLR ligands to rolipram had no statistically significant effect relative to rolipram alone (Figure 4A). CLL cells from nine patients were incubated with either rolipram rolipram (20 µM) or roflumilast (1 µM) in the presence or absence of TLR1/2, TLR7 or TLR9 agonists. At these doses, rolipram and roflumilast induced similar levels of apoptosis in CLL cells and again, addition of the TLR ligands had no statistically significant effect on such apoptosis (Figure 4B).
Figure 4.
Effect of IGHV mutational status on PDE4 inhibitor and TLR ligand-induced apoptosis in CLL. (A) Leukemic cells from CLL patients (n=20) were cultured with or without the PDE4 inhibitor rolipram in the presence or absence of the indicated TLR agonist for 72 hours, followed by assessment of apoptosis by Hoechst 33342 FACS analysis (36). The mean percentages of apoptotic cells from the pooled patient data were compared by ANOVA analysis. (B) Effect of two structurally distinct PDE4 inhibitors, rolipram (20 µM) and roflumilast (1 µM), on TLR ligand-induced apoptosis of CLL cells (n=9). (C, D) Basal (DMSO) and rolipram-induced apoptosis rates are compared for IGHV unmutated (C, n=12) and mutated (D, n=8) leukemic samples. (E) IGHV mutated or unmutated CLL cells were cultured with media alone or varying concentrations of CpG-B, followed by assessment for apoptosis. *p<0.05. N.S.: not statistically significant.
CLL cells with unmutated IGHV regions are more likely to have autoreactive BCRs that bind to antigens expressed on the surface of myosin heavy chain-expressing apoptotic cells, suggesting that IGHV region mutation status may influence CLL cell survival in the presence of TLR ligands (2–5). We found that the patterns of death or survival in response to treatment with TLR ligands and/or rolipram differed between these two groups of patients. Basal levels of apoptosis were not significantly affected by IGHV mutation status. Strikingly, however, IGHV region unmutated CLL cells had a statistically significant reduction in apoptosis following treatment with the TLR9 agonist CpG-B at all doses tested. No such reduction was observed in IGHV region mutated CLL samples (Figure 4E). A comparable effect was not observed following treatment with TLR7/8 or TLR1/2 agonists (Figure 4C and 4D). In some contrast to the effects of treatment with a TLR9 agonist, treatment with the PDE4 inhibitor rolipram significantly augmented apoptosis in both IGHV mutated (n = 8) and unmutated (n = 12) CLL cells (Figures 4C and D). These results suggest that IGHV unmutated CLL cells may differ from their IGHV mutated counterparts in that they derive an anti-apoptotic survival signal following TLR9 stimulation and that treatment with PDE4 inhibitors may abrogate such anti-apoptotic signaling.
PDE4 inhibitors block TLR7 and TLR9 ligand-induced NF-κB signaling
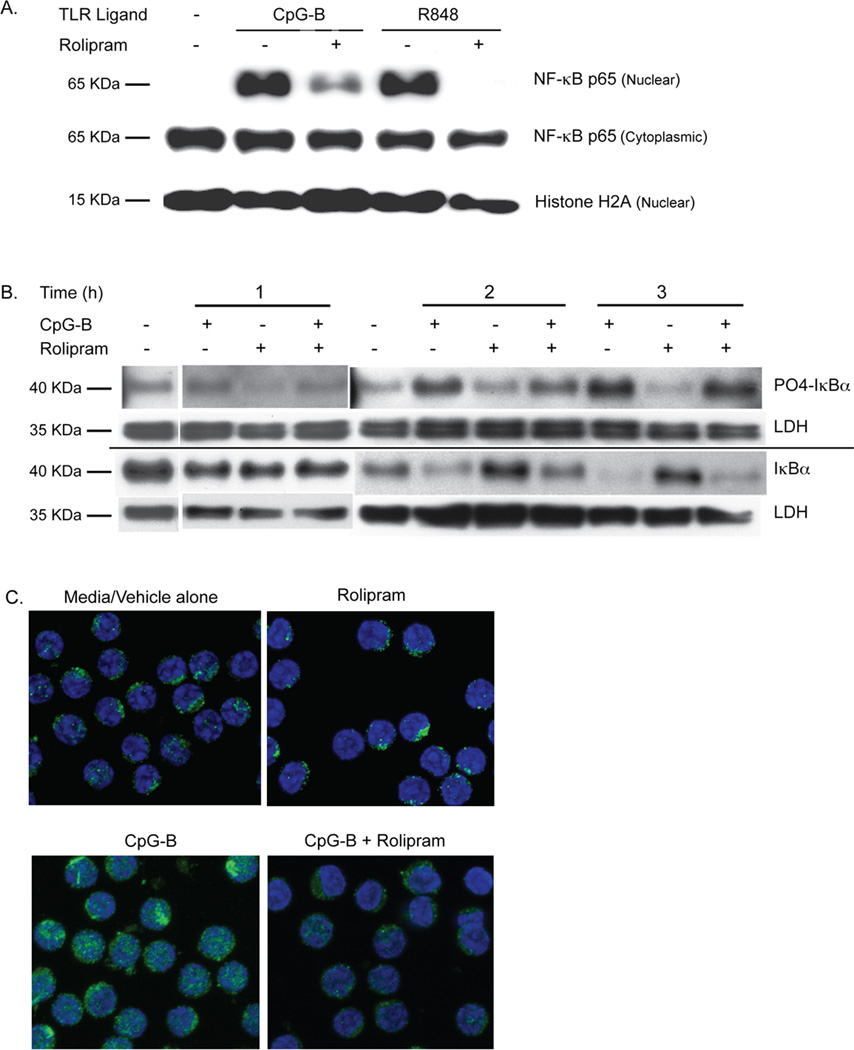
Activation of TLR7 and TLR9 induces NF-κB signaling (45). In order to determine how PDE4 inhibitors might be altering TLR ligand-mediated proliferation and survival signaling in CLL cells, we began by assessing NF-κB p65/RelA (p65) nuclear translocation. Treatment of CLL cells with either R848 or CpG-B induced p65 nuclear translocation at two hours, as judged by Western analysis of cytosolic and nuclear fractions. This translocation was markedly reduced by co-treatment with rolipram (Figure 5A).
Figure 5.
PDE4 inhibitors block TLR7 and TLR9 ligand-induced NF-κB p65 nuclear translocation in CLL. (A) Leukemic cells from a CLL patient were incubated for two hours with media alone, the TLR9 agonist CpG-B (5 µg/mL) or the TLR7 agonist R848 (3 µg/mL) either with rolipram or without rolipram (20 µM). Proteins extracted from the cytoplasmic and nuclear fractions of these cells were then Western blotted for NF- κB p65 as well as the nuclear marker histone H2A. Blots are representative of eight CLL patients tested with CpG-B and three CLL patients tested with R848. (B) Leukemic cells from a CLL patient were incubated with vehicle alone or the TLR9 agonist CpG-B (5 µg/mL), rolipram (20 µM) or a combination of these two stimuli for 1, 2 or 3 hours. Cell lysates were assessed by Western analysis for levels of total or phosphorylated IκBα. The relative loading of cell lysates for each experimental sample was established by also determining levels of LDH on the same Western blot membrane. Blots are representative of five CLL patients tested. (C) The cytoplasmic or nuclear localization of NF-κB p65 in CLL cells was determined after two hours of incubation in media with vehicle alone, rolipram (20 µM), the TLR9 agonist CpG-B (5 µg/mL) or the combination of CpG-B and rolipram. NF-κB p65 was localized by immunohistochemical analysis of permeabilized CLL cells using a confocal fluorescent microscope. In these 100× images, p65 is labeled with Alexa Fluor 488 (green) and the nucleus is counterstained with TO-PRO-3 (blue). Images are representative of three patients tested. CT = Control.
TLRs induce ‘canonical’ NF-κB activation by inducing IKKβ-induced serine phosphorylation and subsequent proteosomal degradation of IκBα, resulting in the release of p65 and other NF-κB family members for translocation to the nucleus (46). Treatment of CLL cells with CpG-B induced IκBα phosphorylation and a reduction in total IκBα levels in a time-dependent manner, with little change in either total or phosphorylated IκBα one hour after CpG-B treatment, but substantial phosphorylation and degradation apparent after two hours. When measured at two hours, rolipram modestly reduced CpG-B-induced IκBα phosphorylation in CLL cells and augmented corresponding total IκBα levels (Figure 5B). In order to verify the reduction in p65 nuclear translocation following PDE4 inhibitor treatment by another technique, we carried out confocal immunofluorescence studies. p65 was detectable in the cytosol of resting or rolipram-treated CLL cells but rarely present in the nucleus. Treatment with CpG-B induced nuclear translocation of p65 whereas rolipram co-treatment markedly reduced such translocation (Figure 5C).
PDE4 inhibitors reduce TLR7 and TLR9 ligand-induced IRF5 nuclear translocation but do not alter IRAK1 degradation
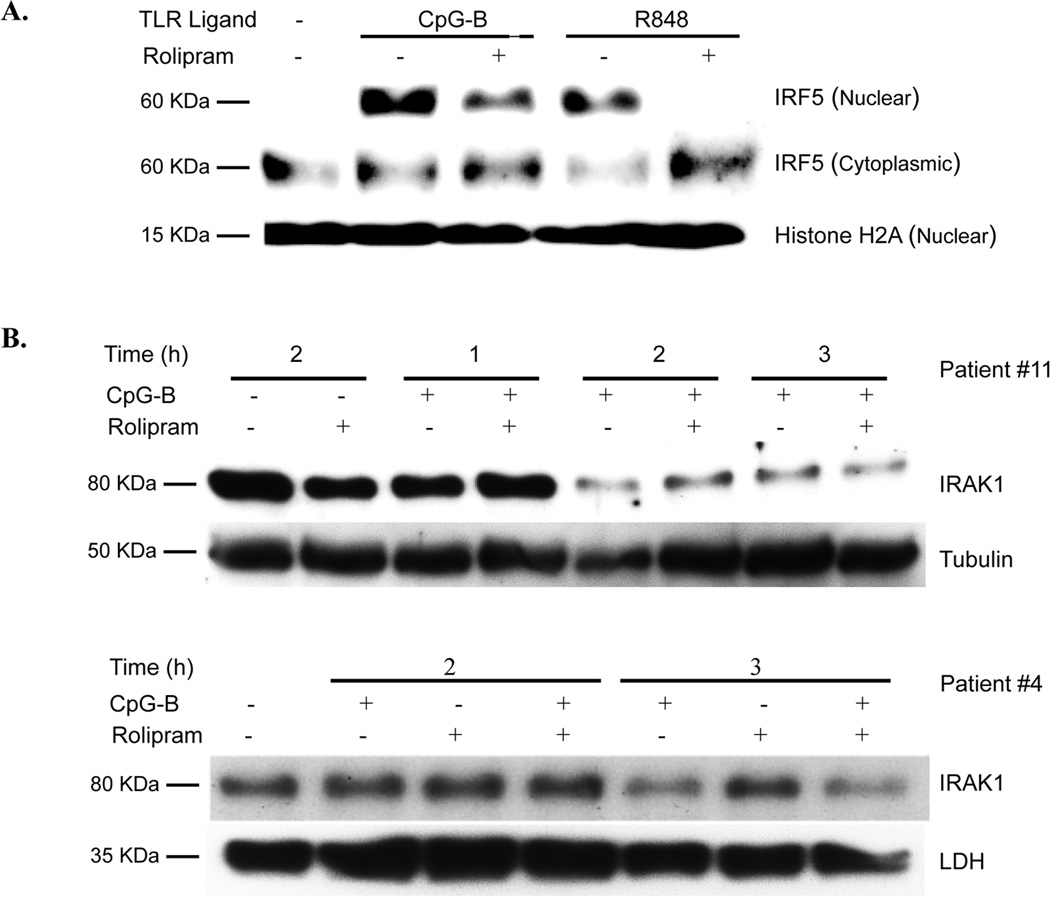
Ligands of MyD88-associated TLRs induce activation of IRAK4 and IRAK1, followed by TRAF6-associated signaling that leads to nuclear translocation of the transcription factors NF-κB and IRF5 (47). Treatment of CLL cells with R848 or CpG-B for two hours induced translocation of IRF5 to the nucleus that was markedly reduced by co-treatment with rolipram (Figure 6A). As previously reported for other TLR agonists, CpG-B treatment led to a decrease in IRAK1 levels 2–3 hours after TLR ligand stimulation (48). Rolipram had no effect on basal levels of IRAK1 nor did it alter CpG-B induced degradation of IRAK1 (Figure 6B).
Figure 6.
PDE4 inhibitors reduce TLR agonist induced IRF5 nuclear translocation but not IRAK1 degradation. (A) Leukemic cells from a CLL patient were incubated for two hours with media alone, the TLR9 agonist CpG-B (5 µg/mL) or the TLR7 agonist R848 (3 µg/mL) in the absence or presence of rolipram (20 µM). Protein extracted from the cytoplasmic and nuclear fractions of the cells were then Western blotted for IRF5. The relative amounts of nuclear lysates loaded from these samples was assessed by blotting for the nuclear protein histone H2A. Blots are representative of five CLL patients tested. (B) Leukemic cells from CLL patients were incubated with vehicle alone or the TLR9 agonist CpG-B (5 µg/mL), rolipram (20 µM) or a combination of these two stimuli for 1, 2 or 3 hours. Cell lysates were assessed by Western analysis for levels of IRAK1. The blot was then re-probed with LDH or tubulin antibodies as a control for the amount of lysate loaded. Blots are representative of three CLL patients tested.
PDE4 inhibitors block TLR7 and TLR9 ligand-induced TNF-α and IFN-α secretion by CLL cells, normal PBMC and CD14+ monocytes but do not block IL-10 or IL-6 secretion
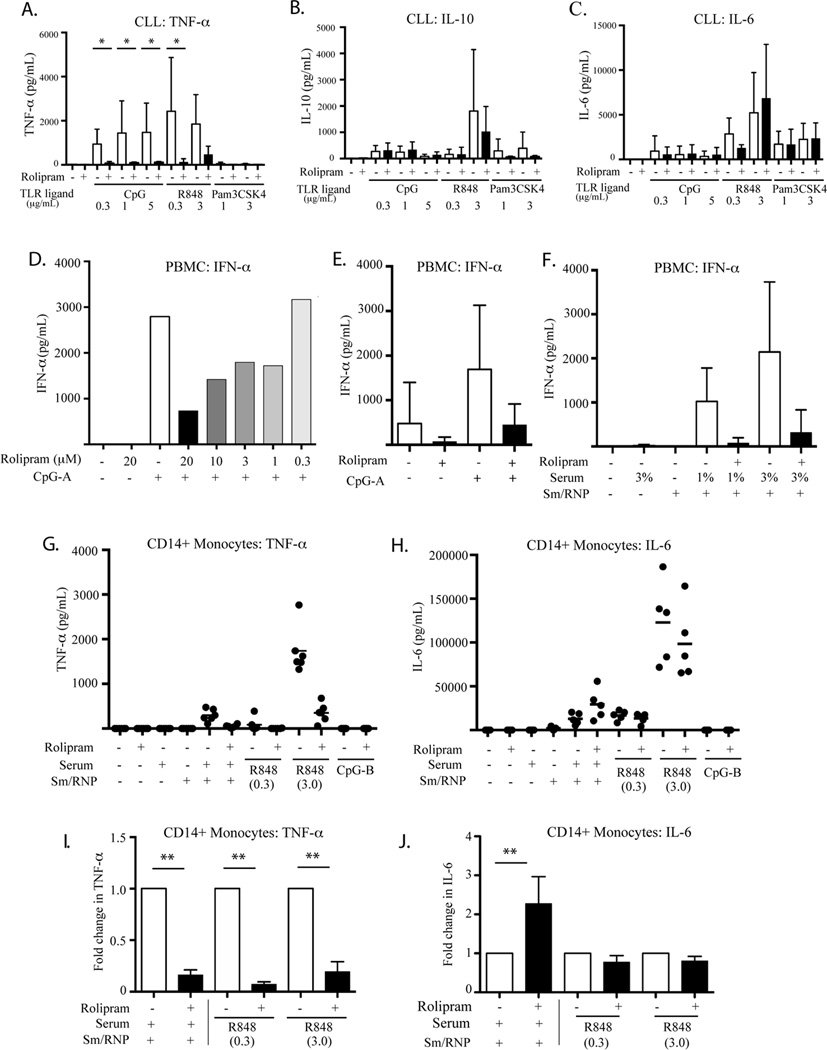
Serum levels of the pro-inflammatory cytokines TNF-α and IL-6 and the anti-inflammatory cytokine IL-10 are elevated in CLL patients and may play an important role in the biology of this disease (49, 50). R848 and CpG-B induced substantial levels of TNF-α secretion from leukemic cells while Pam3CSK4 had little or no effect. All three TLR ligands induced IL-6 and IL-10 secretion. Co-treatment with rolipram markedly reduced TLR ligand-induced TNF-α secretion but did not block IL-6 or IL-10 secretion (Figure 7A–C).
Figure 7.
TLR ligand-induced TNF-α and IFN-α secretion is inhibited by the PDE4 inhibitor rolipram in primary CLL cells and in PBMCs and CD14+ monocytes of normal donors whereas IL-6 and IL-10 secretion is not inhibited. (A–C) Secretion of TNF-α (A), IL-10 (B) and IL-6 (C) by leukemic cells from CLL patients (n = 4) was assessed by ELISA following 24 hours of culture in media/vehicle alone or varying concentrations of the TLR9 agonist CpG-B, the TLR7 agonist R848 or the TLR1/2 agonist Pam3SK4 in the absence or presence of rolipram (20 µM). * p< 0.05. (D) Secretion of IFN-α by PBMCs from a normal donor was assessed by ELISA following 24 hour of culture in media/vehicle alone or the plasmacytoid dendritic cell TLR9 agonist CpG-A (0.8 µg/mL) in the absence or presence of the indicated graded concentrations of rolipram. (E) Secretion of IFN-α by normal donors (n=4) PBMCs was analyzed by ELISA following 24 hour of culture in media/vehicle alone or the plasmacytoid dendritic cell TLR9 agonist CpG-A (0.8 µg/mL) with or without rolipram (20 µM). (F) IFN-α secretion of normal donor (n=3) PBMCs was assessed by ELISA following 24 hours of culture with media alone, 3% anti-Sm-RNP-containing SLE sera alone, Sm-RNP alone, or pre-formed anti-Sm-RNP/Sm-RNP ICs in the absence or presence of rolipram (20 µM). (G) Positively selected normal donor (n=5) peripheral blood CD14+ monocytes were assessed for TNF-α secretion by ELISA following 24 hours of culture with the TLR9 agonist CpG-B, TLR7/8 agonist R848, 3% anti-Sm-RNP-containing SLE sera alone, Sm-RNP alone, or pre-formed anti-Sm-RNP/Sm-RNP ICs, in the absence or presence of rolipram (20 µM). (H) Secretion of IL-6 by CD14+ monocytes (n=5) was assessed by ELISA following 24 hours incubation using the same conditions described in panel G. (I) Fold changes of TNF-α secretion by CD14+ monocytes caused by rolipram shown in panel G are calculated. **p<0.001. (J) Fold changes of IL-6 secretion by CD14+ monocytes caused by rolipram shown in panel H are calculated. **p<0.001.
TLR7 and TLR9 signaling is thought to play an important role in the pathogenesis of several autoimmune diseases, including systemic lupus erythematosus (SLE) (51, 52). In order to assess whether the observed PDE4 inhibitor-induced blockade of TLR7 and TLR9 signaling observed in CLL also occurred in normal hematopoietic cells and therefore might be relevant to the treatment of such autoimmune illnesses, we next examined TLR7 and TLR9 signaling in PBMC from healthy donors. When PBMC are stimulated with TLR7 ligands such as RNA-containing immune complexes (ICs) or synthetic TLR9 ligands such as CpG-A, easily detectable IFN-α production is limited to plasmacytoid dendritic cells (pDC) as this is the only circulating cell that produces this cytokine at high levels in response to these stimuli (53, 54). When stimulated with CpG-A for 24 hours, PBMC produced substantial levels of IFN-α that were reduced in a dose-dependent manner by co-treatment with the PDE4 inhibitor rolipram (Figure 7D and 7E).
Although otherwise an effective TLR7/8 agonist, R848 does not effectively induce IFN-α in PBMC (37, 55). In order to instead examine TLR7-dependent responses of PBMC to a physiologic ribonucleoprotein (RNP) IC stimulus, we incubated serum from an SLE patient containing anti-Smith (Sm)/RNP antibodies (37) with purified bovine Sm/RNP that contains endogenous mammalian RNA bound to the Sm/RNP protein in the native state (19). The resulting ICs were then added to PBMC in the presence or absence of rolipram. While the Sm/RNP alone or the serum alone had no demonstrable effect, the Sm/RNP-containing ICs induced IFN-α production that was reduced by co-treatment with rolipram (Figure 7F, N=3, p = 0.08 for 1% serum).
While circulating human monocytes do not express TLR9, they do express TLR7 and TLR8 and respond to synthetic TLR7/8 agonists. Circulating human monocytes from healthy donors were stimulated for 24 hours with CpG-B, R848, Sm/RNP, anti-Sm/RNP serum, or Sm/RNP-containing ICs. As expected, CpG-B induced neither IL-6 nor TNF-α secretion in monocytes. However, both Sm/RNP-containing IC and R848 induced monocyte TNF-α secretion and this was strongly inhibited by rolipram (Figure 7G and I). In marked contrast, rolipram treatment did not inhibit R848-induced IL-6 production and actually enhanced monocyte IL-6 production 2.27 ± 0.3 fold following stimulation with Sm/RNP-containing ICs (p = .0033) (Figure 7H and J).
Discussion
In this study, we have demonstrated that treatment with PDE4 inhibitors blocks signaling, proliferation and cytokine production induced by TLR7 or TLR9 ligands in both CLL and normal human immune cells. Work in the field of autoimmunity has established the principle that B cells and dendritic cells undergo TLR7 and TLR9-mediated activation and proliferation in response to nucleoprotein complexes derived from endogenous apoptotic cells (56). We found that autologous apoptotic CLL cells induced proliferation of live CLL cells in a manner that could be inhibited by DNase, a TLR7/8/9 antagonist or the PDE4 inhibitor rolipram. Such results are consistent with a growing body of literature suggesting that CLL cells may be derived from marginal zone B cells that normally clear apoptotic debris and bear BCRs specific for antigens expressed on apoptotic cells (1, 18, 19, 21, 22). Of interest, when CLL patient samples were assessed for IGHV mutational status and compared for their relative fold increase in proliferation in response to autologous apoptotic cells, seven out of the top eight responders were IGHV unmutated samples, consistent with studies that have documented an enrichment in IGHV unmutated sequences among CLL samples whose BCRs bind to apoptotic cells (3). Although these differences were not statistically significant in this small sample group, a larger set of mutated and unmutated CLL samples will need to be examined to determine whether the observed trend is in fact a reproducible observation.
The effect of TLR signaling on CLL cell survival is controversial, with some authors reporting that TLR agonists induce apoptosis while others report no or even anti-apoptotic effects (9, 11, 13, 57, 58). While PDE4 inhibitors themselves induced moderate apoptosis, we observed that the three TLR agonists induced little or no apoptosis in CLL samples at 72 hours. Of some note, treatment with the TLR9 agonist CpG-B significantly reduced basal apoptosis in IGHV unmutated CLL cells, while no reduction in basal apoptosis was observed in IGHV mutated cases. This result suggests that TLR9 signaling may be of particular pathophysiologic importance in IGHV unmutated CLL patients. Further, our observation that we are able to induce apoptosis in TLR9 agonist-treated IGHV unmutated cell samples by rolipram treatment is consistent with the hypothesis that PDE4 inhibitor-induced cAMP signaling results in abrogation of a TLR9 ligand-associated anti-apoptotic effect in leukemic cells from this poor prognostic subgroup of CLL patients.
Our results stand in some contrast to those of Fonte et al, who, using a different apoptosis assay and a different time point, observed no difference in the effect of a TLR9 agonist on basal apoptosis in IGHV mutated or unmutated CLL cells (59). They did however find that the TLR9 agonist preferentially protected IGHV unmutated CLL cells from the induction of apoptosis by fludarabine relative to IGHV mutated samples. While our pre-clinical data from this and prior studies suggest that PDE4 inhibitors are likely to induce only a modest degree of apoptosis in CLL cells when used alone, this class of drugs may nonetheless prove to be useful therapeutic agents for B cell malignancies when combined with other treatment regimens, particularly given their ability to augment glucocorticoid-induced apoptosis (31, 36, 60).
In order to more specifically establish whether PDE4 inhibitors might block signaling by the intracellular TLR receptors implicated in responses to apoptotic cell debris, we assessed CLL responses to synthetic TLR ligands. TLR1/2, TLR7 and TLR9 agonists induced up-regulation of CD40 and CD54 expression and proliferation of CLL cells and these responses were blocked by two structurally distinct PDE4 inhibitors, rolipram and roflumilast. PDE4 inhibitors block at least two signaling outcomes of TLR7 and TLR9 signal transduction: NF-κB p65 nuclear translocation and IRF5 nuclear translocation. Rolipram treatment reduced TLR7 or TLR9 agonist-induced IκBα phosphorylation and augmented residual total IκBα levels at two hours. When the earliest steps of TLR9 signal transduction were examined, we observed that CpG-B treatment consistently induced degradation of CLL cell IRAK1, a phenomenon previously linked to IRAK4-mediated phosphorylation of IRAK1 (48). This event was not altered by co-treatment with rolipram. These studies suggest that a PDE4 inhibitor-induced regulatory step occurs distal to IRAK1 activation and proximal to the branch point for NF-κB and IRF5 signaling. IRAK1 is known to bind to TRAF6, resulting in recruitment of TAB1/2 and TAB3 as well as TAK1. This complex regulates NF-κB activation by activating the IKK kinases. TRAF6 also binds IRF5. Thus a logical focus for future research will be to assess whether PDE4 inhibitors alter TLR ligand-induced TRAF6- mediated signaling.
Rolipram markedly reduced CpG-B and R848-induced TNF-α secretion but had no effect on TLR ligand-induced IL-10 or IL-6 secretion by CLL cells or purified monocytes. The antiinflammatory cytokine IL-10 is known to have cAMP response elements (CRE) in its promoter and PDE4 inhibitor-induced PKA activation leads to ATF1/CREB Ser 63/133 phosphorylation that would be expected to activate transcription in such CRE-containing promoters. Thus, it is plausible that persistent IL-10 secretion in rolipram-treated CLL cells may be related to the summation of inhibition of TLR ligand-induced IL-10 transcription and concomitant ATF1/CREB-mediated activation of IL-10 transcription (35, 61–63). More striking, however, is the persistence of TLR-ligand-induced IL-6 production in the face of PDE4 inhibitor treatment of CLL cells and purified monocytes. Our results are consistent with prior studies using the TLR4 ligand LPS which documented that PDE inhibitors blocked TNF-α but not IL-6 production in monocytes (64, 65). The Tec family cytoplasmic tyrosine kinase Btk has been reported as a critical mediator of LPS-induced TNF-α but not IL-6 production in macrophages, while LPS-induced IL-6 production has been reported to be regulated by another Tec family kinase, Bmx (66, 67). The discrepant inhibition of TLR agonist-induced TNF-α and IL-6 signaling by PDE4 inhibitors may reflect differential effects of cAMP-mediated signaling on TEC family kinases.
IFN-α production by PBMC following TLR7 and TLR9 stimulation with Sm/RNP-containing ICs and by CpG-A, respectively, is the result of pDC activation (53, 54). A dendritic cell-derived IFN-α "signature" has been identified as a critical pathophysiologic event in patients with active SLE and blockade of TLR7 and TLR9 function in circulating pDC from SLE patients has been shown to restore steroid sensitivity to such cells (52, 68). Abnormal synthesis of pro-inflammatory cytokines has also been observed in monocytes from SLE patients (69). NCS 613, a PDE4 inhibitor, has been reported to reduce disease activity in lupus prone-mice (70). We found that co-treatment with rolipram markedly reduced PBMC IFN-α production stimulated by TLR7 and TLR9 agonists. Rolipram also blocked both R848 and Sm/RNP-containing IC-induced synthesis of TNF-α in monocytes but actually enhanced IC-induced synthesis of IL-6. The in vitro lack of activity of PDE4 inhibition on TLR ligand-induced IL-6 synthesis documented here will need to be examined carefully in assessing the potential clinical utility of PDE4 inhibitor therapy in autoimmune disease.
Overall, our results demonstrate that in both primary leukemic cells derived from CLL patients and in normal PBMC and purified monocytes, inhibition of PDE4 enzymes potently inhibits TLR7 and TLR9 ligand-induced signal transduction (NF-κB and IRF5 activation) as well as consequent alterations in cell surface antigen expression, proliferation and inflammatory cytokine production. Although the varying stimuli utilized make it difficult to make a straight-forward comparison, TLR7-mediated signaling appears to be at least as sensitive as TLR9 to rolipram-mediated inhibition. Rolipram blocks TLR signaling in both good prognosis IGHV mutated and poor prognosis IGHV unmutated CLL patients, although the basal pathophysiologic relevance of TLR9 signaling appears to be particularly important in the IGHV unmutated subset. Finally, our results demonstrate that CLL cells can be induced to proliferate by exposure to autologous apoptotic cells and that this effect can be blocked by PDE4 inhibitors. These observations suggest that PDE4 inhibitors may be of use in the management of CLL patients and also provide further rationale for current efforts evaluating the use of PDE4 inhibitors in SLE and other TLR-driven autoimmune diseases (71, 72).
Acknowledgements
The authors wish to thank David Seldin (Hematology/Oncology Section, Boston University School of Medicine), David Sherr (Department of Environmental Health, Boston University School of Public Health), Hanni Menn-Josephy and Kei Yasuda (Section of Nephrology, Boston University School of Medicine) and Mitch White (Hematology/Oncology Section, Boston University School of Medicine) for their advice and assistance in this study.
This work was funded in part by NIH PO1 AR050256 (I.R.) and by an NHLBI T32 (HL007501) training fellowship (Y.T.).
Footnotes
The authors have nothing to disclose.
This work was presented in abstract form at the 54th annual meeting of the American Society of Hematology, Atlanta GA, December 2012.
References
- 1.Chiorazzi N, Ferrarini M. Cellular origin(s) of chronic lymphocytic leukemia: cautionary notes and additional considerations and possibilities. Blood. 2011;117:1781–1791. doi: 10.1182/blood-2010-07-155663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chu CC, Catera R, Hatzi K, Yan XJ, Zhang L, Wang XB, Fales HM, Allen SL, Kolitz JE, Rai KR, Chiorazzi N. Chronic lymphocytic leukemia antibodies with a common stereotypic rearrangement recognize nonmuscle myosin heavy chain IIA. Blood. 2008;112:5122–5129. doi: 10.1182/blood-2008-06-162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu CC, Catera R, Zhang L, Didier S, Agagnina BM, Damle RN, Kaufman MS, Kolitz JE, Allen SL, Rai KR, Chiorazzi N. Many chronic lymphocytic leukemia antibodies recognize apoptotic cells with exposed nonmuscle myosin heavy chain IIA: implications for patient outcome and cell of origin. Blood. 2010;115:3907–3915. doi: 10.1182/blood-2009-09-244251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catera R, Silverman GJ, Hatzi K, Seiler T, Didier S, Zhang L, Herve M, Meffre E, Oscier DG, Vlassara H, Scofield RH, Chen Y, Allen SL, Kolitz J, Rai KR, Chu CC, Chiorazzi N. Chronic lymphocytic leukemia cells recognize conserved epitopes associated with apoptosis and oxidation. Mol Med. 2008;14:665–674. doi: 10.2119/2008-00102.Catera. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanemo Myhrinder A, Hellqvist E, Sidorova E, Soderberg A, Baxendale H, Dahle C, Willander K, Tobin G, Backman E, Soderberg O, Rosenquist R, Horkko S, Rosen A. A new perspective: molecular motifs on oxidized LDL, apoptotic cells, and bacteria are targets for chronic lymphocytic leukemia antibodies. Blood. 2008;111:3838–3848. doi: 10.1182/blood-2007-11-125450. [DOI] [PubMed] [Google Scholar]
- 6.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–1854. [PubMed] [Google Scholar]
- 7.Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL, Buchbinder A, Budman D, Dittmar K, Kolitz J, Lichtman SM, Schulman P, Vinciguerra VP, Rai KR, Ferrarini M, Chiorazzi N. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–1847. [PubMed] [Google Scholar]
- 8.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Grandjenette C, Kennel A, Faure GC, Bene MC, Feugier P. Expression of functional toll-like receptors by B-chronic lymphocytic leukemia cells. Haematologica. 2007;92:1279–1281. doi: 10.3324/haematol.10975. [DOI] [PubMed] [Google Scholar]
- 10.Rozkova D, Novotna L, Pytlik R, Hochova I, Kozak T, Bartunkova J, Spisek R. Toll-like receptors on B-CLL cells: expression and functional consequences of their stimulation. Int J Cancer. 2009;126:1132–1143. doi: 10.1002/ijc.24832. [DOI] [PubMed] [Google Scholar]
- 11.Liang X, Moseman EA, Farrar MA, Bachanova V, Weisdorf DJ, Blazar BR, Chen W. Toll-like receptor 9 signaling by CpG-B oligodeoxynucleotides induces an apoptotic pathway in human chronic lymphocytic leukemia B cells. Blood. 2010;115:5041–5052. doi: 10.1182/blood-2009-03-213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spaner DE, Shi Y, White D, Mena J, Hammond C, Tomic J, He L, Tomai MA, Miller RL, Booth J, Radvanyi L. Immunomodulatory effects of Toll-like receptor-7 activation on chronic lymphocytic leukemia cells. Leukemia. 2006;20:286–295. doi: 10.1038/sj.leu.2404061. [DOI] [PubMed] [Google Scholar]
- 13.Ntoufa S, Vardi A, Papakonstantinou N, Anagnostopoulos A, Aleporou-Marinou V, Belessi C, Ghia P, Caligaris-Cappio F, Muzio M, Stamatopoulos K. Distinct innate immunity pathways to activation and tolerance in subgroups of chronic lymphocytic leukemia with distinct immunoglobulin receptors. Mol Med. 2012;18:1281–1291. doi: 10.2119/molmed.2011.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spaner DE, Shi Y, White D, Shaha S, He L, Masellis A, Wong K, Gorczynski R. A phase I/II trial of TLR-7 agonist immunotherapy in chronic lymphocytic leukemia. Leukemia. 2010;24:222–226. doi: 10.1038/leu.2009.195. [DOI] [PubMed] [Google Scholar]
- 15.Spaner DE, Masellis A. Toll-like receptor agonists in the treatment of chronic lymphocytic leukemia. Leukemia. 2007;21:53–60. doi: 10.1038/sj.leu.2404456. [DOI] [PubMed] [Google Scholar]
- 16.Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 17.Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452:234–238. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- 18.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 19.Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, Christensen SR, Shlomchik MJ, Viglianti GA, Rifkin IR, Marshak-Rothstein A. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viglianti GA, Lau CM, Hanley TM, Miko BA, Shlomchik MJ, Marshak-Rothstein A. Activation of autoreactive B cells by CpG dsDNA. Immunity. 2003;19:837–847. doi: 10.1016/s1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- 21.Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med. 2004;199:1631–1640. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vollmer J, Tluk S, Schmitz C, Hamm S, Jurk M, Forsbach A, Akira S, Kelly KM, Reeves WH, Bauer S, Krieg AM. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J Exp Med. 2005;202:1575–1585. doi: 10.1084/jem.20051696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puente XS, Pinyol M, Quesada V, Conde L, Ordonez GR, Villamor N, Escaramis G, Jares P, Bea S, Gonzalez-Diaz M, Bassaganyas L, Baumann T, Juan M, Lopez-Guerra M, Colomer D, Tubio JM, Lopez C, Navarro A, Tornador C, Aymerich M, Rozman M, Hernandez JM, Puente DA, Freije JM, Velasco G, Gutierrez-Fernandez A, Costa D, Carrio A, Guijarro S, Enjuanes A, Hernandez L, Yague J, Nicolas P, Romeo-Casabona CM, Himmelbauer H, Castillo E, Dohm JC, de Sanjose S, Piris MA, de Alava E, San Miguel J, Royo R, Gelpi JL, Torrents D, Orozco M, Pisano DG, Valencia A, Guigo R, Bayes M, Heath S, Gut M, Klatt P, Marshall J, Raine K, Stebbings LA, Futreal PA, Stratton MR, Campbell PJ, Gut I, Lopez-Guillermo A, Estivill X, Montserrat E, Lopez-Otin C, Campo E. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475:101–105. doi: 10.1038/nature10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Lawrence MS, Wan Y, Stojanov P, Sougnez C, Stevenson K, Werner L, Sivachenko A, DeLuca DS, Zhang L, Zhang W, Vartanov AR, Fernandes SM, Goldstein NR, Folco EG, Cibulskis K, Tesar B, Sievers QL, Shefler E, Gabriel S, Hacohen N, Reed R, Meyerson M, Golub TR, Lander ES, Neuberg D, Brown JR, Getz G, Wu CJ. SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. N Engl J Med. 2011;365:2497–2506. doi: 10.1056/NEJMoa1109016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landau DA, Carter SL, Stojanov P, McKenna A, Stevenson K, Lawrence MS, Sougnez C, Stewart C, Sivachenko A, Wang L, Wan Y, Zhang W, Shukla SA, Vartanov A, Fernandes SM, Saksena G, Cibulskis K, Tesar B, Gabriel S, Hacohen N, Meyerson M, Lander ES, Neuberg D, Brown JR, Getz G, Wu CJ. Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell. 2013;152:714–726. doi: 10.1016/j.cell.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang JQ, Jeelall YS, Beutler B, Horikawa K, Goodnow CC. Consequences of the recurrent MYD88 (L265P) somatic mutation for B cell tolerance. J Exp Med. 2014;211:413–426. doi: 10.1084/jem.20131424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lombardi MS, Kavelaars A, Heijnen CJ. Role and modulation of G protein-coupled receptor signaling in inflammatory processes. Crit Rev Immunol. 2002;22:141–163. [PubMed] [Google Scholar]
- 28.Lerner A, Epstein PM. Cyclic nucleotide phosphodiesterases as targets for treatment of haematological malignancies. Biochem J. 2006;393:21–41. doi: 10.1042/BJ20051368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moon EY, Lerner A. PDE4 inhibitors activate a mitochondrial apoptotic pathway in chronic lymphocytic leukemia cells that is regulated by protein phosphatase 2A. Blood. 2003;101:4122–4130. doi: 10.1182/blood-2002-10-3208. [DOI] [PubMed] [Google Scholar]
- 30.Tiwari S, Felekkis K, Moon EY, Flies A, Sherr DH, Lerner A. Among circulating hematopoietic cells, B-CLL uniquely expresses functional EPAC1, but EPAC1-mediated Rap1 activation does not account for PDE4 inhibitor-induced apoptosis. Blood. 2004;103:2661–2667. doi: 10.1182/blood-2003-06-2154. [DOI] [PubMed] [Google Scholar]
- 31.Meyers JA, Taverna J, Chaves J, Makkinje A, Lerner A. Phosphodiesterase 4 inhibitors augment levels of glucocorticoid receptor in B cell chronic lymphocytic leukemia but not in normal circulating hematopoietic cells. Clin Cancer Res. 2007;13:4920–4927. doi: 10.1158/1078-0432.CCR-07-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin SL, Conti M. Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-alpha responses. Proc Natl Acad Sci U S A. 2002;99:7628–7633. doi: 10.1073/pnas.122041599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin SL, Lan L, Zoudilova M, Conti M. Specific role of phosphodiesterase 4B in lipopolysaccharide-induced signaling in mouse macrophages. J Immunol. 2005;175:1523–1531. doi: 10.4049/jimmunol.175.3.1523. [DOI] [PubMed] [Google Scholar]
- 34.Henriques-Pons A, Yu Q, Rayavarapu S, Cohen TV, Ampong B, Cha HJ, Jahnke V, Van der Meulen J, Wang D, Jiang W, Kandimalla ER, Agrawal S, Spurney CF, Nagaraju K. Role of Toll-like receptors in the pathogenesis of dystrophin-deficient skeletal and heart muscle. Human molecular genetics. 2014;23:2604–2617. doi: 10.1093/hmg/ddt656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyers JA, Su DW, Lerner A. Chronic lymphocytic leukemia and B and T cells differ in their response to cyclic nucleotide phosphodiesterase inhibitors. J Immunol. 2009;182:5400–5411. doi: 10.4049/jimmunol.0804255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim DH, Lerner A. Type 4 cyclic adenosine monophosphate phosphodiesterase as a therapeutic target in chronic lymphocytic leukemia. Blood. 1998;92:2484–2494. [PubMed] [Google Scholar]
- 37.Yasuda K, Richez C, Maciaszek JW, Agrawal N, Akira S, Marshak-Rothstein A, Rifkin IR. Murine dendritic cell type I IFN production induced by human IgG-RNA immune complexes is IFN regulatory factor (IRF)5 and IRF7 dependent and is required for IL-6 production. J Immunol. 2007;178:6876–6885. doi: 10.4049/jimmunol.178.11.6876. [DOI] [PubMed] [Google Scholar]
- 38.Campbell MJ, Zelenetz AD, Levy S, Levy R. Use of family specific leader region primers for PCR amplification of the human heavy chain variable region gene repertoire. Mol Immunol. 1992;29:193–203. doi: 10.1016/0161-5890(92)90100-c. [DOI] [PubMed] [Google Scholar]
- 39.Brochet X, Lefranc MP, Giudicelli V. IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res. 2008;36:W503–W508. doi: 10.1093/nar/gkn316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bjorgo E, Tasken K. Role of cAMP phosphodiesterase 4 in regulation of T-cell function. Crit Rev Immunol. 2006;26:443–451. doi: 10.1615/critrevimmunol.v26.i5.40. [DOI] [PubMed] [Google Scholar]
- 41.Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H, Lipford G, Bauer S. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol. 2002;3:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 42.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 43.Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik SG, Lee H, Lee JO. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. 2007;130:1071–1082. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Fabbri LM, Beghe B, Yasothan U, Kirkpatrick P. Roflumilast. Nat Rev Drug Discov. 2010;9:761–762. doi: 10.1038/nrd3276. [DOI] [PubMed] [Google Scholar]
- 45.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 47.Takaoka A, Yanai H, Kondo S, Duncan G, Negishi H, Mizutani T, Kano S, Honda K, Ohba Y, Mak TW, Taniguchi T. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature. 2005;434:243–249. doi: 10.1038/nature03308. [DOI] [PubMed] [Google Scholar]
- 48.Kubo-Murai M, Hazeki K, Nigorikawa K, Omoto T, Inoue N, Hazeki O. IRAK-4-dependent degradation of IRAK-1 is a negative feedback signal for TLR-mediated NF-kappaB activation. Journal of biochemistry. 2008;143:295–302. doi: 10.1093/jb/mvm234. [DOI] [PubMed] [Google Scholar]
- 49.Ferrajoli A, Keating MJ, Manshouri T, Giles FJ, Dey A, Estrov Z, Koller CA, Kurzrock R, Thomas DA, Faderl S, Lerner S, O'Brien S, Albitar M. The clinical significance of tumor necrosis factor-alpha plasma level in patients having chronic lymphocytic leukemia. Blood. 2002;100:1215–1219. [PubMed] [Google Scholar]
- 50.Fayad L, Keating MJ, Reuben JM, O'Brien S, Lee BN, Lerner S, Kurzrock R. Interleukin-6 and interleukin-10 levels in chronic lymphocytic leukemia: correlation with phenotypic characteristics and outcome. Blood. 2001;97:256–263. doi: 10.1182/blood.v97.1.256. [DOI] [PubMed] [Google Scholar]
- 51.Celhar T, Magalhaes R, Fairhurst AM. TLR7 and TLR9 in SLE: when sensing self goes wrong. Immunol Res. 2012;53:58–77. doi: 10.1007/s12026-012-8270-1. [DOI] [PubMed] [Google Scholar]
- 52.Guiducci C, Gong M, Xu Z, Gill M, Chaussabel D, Meeker T, Chan JH, Wright T, Punaro M, Bolland S, Soumelis V, Banchereau J, Coffman RL, Pascual V, Barrat FJ. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature. 2010;465:937–941. doi: 10.1038/nature09102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krug A, Rothenfusser S, Hornung V, Jahrsdorfer B, Blackwell S, Ballas ZK, Endres S, Krieg AM, Hartmann G. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol. 2001;31:2154–2163. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 54.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 55.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 56.Liu Z, Davidson A. Taming lupus-a new understanding of pathogenesis is leading to clinical advances. Nat Med. 2012;18:871–882. doi: 10.1038/nm.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muzio M, Scielzo C, Bertilaccio MT, Frenquelli M, Ghia P, Caligaris-Cappio F. Expression and function of toll like receptors in chronic lymphocytic leukaemia cells. Br J Haematol. 2009;144:507–516. doi: 10.1111/j.1365-2141.2008.07475.x. [DOI] [PubMed] [Google Scholar]
- 58.Jahrsdorfer B, Muhlenhoff L, Blackwell SE, Wagner M, Poeck H, Hartmann E, Jox R, Giese T, Emmerich B, Endres S, Weiner GJ, Hartmann G. B-cell lymphomas differ in their responsiveness to CpG oligodeoxynucleotides. Clin Cancer Res. 2005;11:1490–1499. doi: 10.1158/1078-0432.CCR-04-1890. [DOI] [PubMed] [Google Scholar]
- 59.Fonte E, Apollonio B, Scarfo L, Ranghetti P, Fazi C, Ghia P, Caligaris-Cappio F, Muzio M. In vitro sensitivity of CLL cells to fludarabine may be modulated by the stimulation of Toll-like receptors. Clin Cancer Res. 2013;19:367–379. doi: 10.1158/1078-0432.CCR-12-1922. [DOI] [PubMed] [Google Scholar]
- 60.Tiwari S, Dong H, Kim EJ, Weintraub L, Epstein PM, Lerner A. Type 4 cAMP phosphodiesterase (PDE4) inhibitors augment glucocorticoid-mediated apoptosis in B cell chronic lymphocytic leukemia (B-CLL) in the absence of exogenous adenylyl cyclase stimulation. Biochem Pharmacol. 2005;69:473–483. doi: 10.1016/j.bcp.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 61.Platzer C, Meisel C, Vogt K, Platzer M, Volk HD. Up-regulation of monocytic IL-10 by tumor necrosis factor-alpha and cAMP elevating drugs. Int Immunol. 1995;7:517–523. doi: 10.1093/intimm/7.4.517. [DOI] [PubMed] [Google Scholar]
- 62.Eigler A, Siegmund B, Emmerich U, Baumann KL, Hartmann G, Endres S. Anti-inflammatory activities of cAMP-elevating agents: enhancement of IL-10 synthesis and concurrent suppression of TNF production. J Leuk Biol. 1998;63:101–107. doi: 10.1002/jlb.63.1.101. [DOI] [PubMed] [Google Scholar]
- 63.Brenner S, Prosch S, Schenke-Layland K, Riese U, Gausmann U, Platzer C. cAMP-induced Interleukin-10 promoter activation depends on CCAAT/enhancer-binding protein expression and monocytic differentiation. J Biol Chem. 2003;278:5597–5604. doi: 10.1074/jbc.M207448200. [DOI] [PubMed] [Google Scholar]
- 64.Bailly S, Ferrua B, Fay M, Gougerot-Pocidalo MA. Differential regulation of IL 6, IL 1 A, IL 1 beta and TNF alpha production in LPS-stimulated human monocytes: role of cyclic AMP. Cytokine. 1990;2:205–210. doi: 10.1016/1043-4666(90)90017-n. [DOI] [PubMed] [Google Scholar]
- 65.Prabhakar U, Lipshutz D, Bartus JO, Slivjak MJ, Smith EF, 3rd, Lee JC, Esser KM. Characterization of cAMP-dependent inhibition of LPS-induced TNF alpha production by rolipram, a specific phosphodiesterase IV (PDE IV) inhibitor. Int J Immunopharmacol. 1994;16:805–816. doi: 10.1016/0192-0561(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 66.Horwood NJ, Page TH, McDaid JP, Palmer CD, Campbell J, Mahon T, Brennan FM, Webster D, Foxwell BM. Bruton's tyrosine kinase is required for TLR2 and TLR4-induced TNF, but not IL-6, production. J Immunol. 2006;176:3635–3641. doi: 10.4049/jimmunol.176.6.3635. [DOI] [PubMed] [Google Scholar]
- 67.Palmer CD, Mutch BE, Workman S, McDaid JP, Horwood NJ, Foxwell BM. Bmx tyrosine kinase regulates TLR4-induced IL-6 production in human macrophages independently of p38 MAPK and NFkapp}B activity. Blood. 2008;111:1781–1788. doi: 10.1182/blood-2007-07-102343. [DOI] [PubMed] [Google Scholar]
- 68.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 69.Sule S, Rosen A, Petri M, Akhter E, Andrade F. Abnormal production of pro- and anti-inflammatory cytokines by lupus monocytes in response to apoptotic cells. PLoS One. 2011;6:e17495. doi: 10.1371/journal.pone.0017495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Keravis T, Monneaux F, Yougbare I, Gazi L, Bourguignon JJ, Muller S, Lugnier C. Disease progression in MRL/lpr lupus-prone mice is reduced by NCS 613, a specific cyclic nucleotide phosphodiesterase type 4 (PDE4) inhibitor. PLoS One. 7:e28899. doi: 10.1371/journal.pone.0028899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chugh PK. Lupus: novel therapies in clinical development. Eur J Intern Med. 2012;23:212–218. doi: 10.1016/j.ejim.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 72.Wittmann M, Helliwell PS. Phosphodiesterase 4 inhibition in the treatment of psoriasis, psoriatic arthritis and other chronic inflammatory diseases. Dermatology and therapy. 2013;3:1–15. doi: 10.1007/s13555-013-0023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]