Abstract
Background
Retrospective series report varied rates of bleeding and infection with external ventricular drainage (EVD). There have been no prospective studies of these risks with systematic surveillance, threshold definitions, or independent adjudication.
Objective
We analyzed the rate of complications in the ongoing CLEAR III trial, providing a comparison with a systematic review of complications of EVD in the literature.
Methods
Cases were prospectively enrolled in the CLEAR III trial after placement of EVD for obstructive intraventricular hemorrhage (IVH) and randomized to receive recombinant tissue plasminogen activator (rt-PA) or placebo. We counted any detected new hemorrhage (catheter tract hemorrhage or any other distant hemorrhage) on CT scan within 30 days from the randomization. Meta-analysis of published series of EVD placement was compiled using STATA software.
Results
Growing or unstable hemorrhage was reported as a cause of exclusion from the trial in 74 of 5707 cases (1.3%) screened for CLEAR III. The first 250 cases enrolled have completed adjudication of adverse events. Forty-two subjects (16.8%) experienced one or more new bleeds or expansions, and 6 of 250 subjects (2.4%) suffered symptomatic hemorrhages. Eleven cases (4.4%) had culture-proven bacterial meningitis or ventriculitis.
Conclusion
Risks of bleeding and infection in the ongoing CLEAR III trial are comparable to those previously reported in EVD case series. In the current study, rates of new bleeds and bacterial meningitis/ventriculitis are very low, despite multiple daily injections, blood in the ventricles, the use of thrombolysis in half the cases, and generalization to > 60 trial sites.
Keywords: External ventricular drain, EVD, EVD complications, intraventricular hemorrhage, hemorrhage, infection
Intraventricular hemorrhage (IVH) is a debilitating form of hemorrhagic stroke affecting nearly 1 million people worldwide each year1. IVH has a varied etiology including head trauma, vascular malformation, aneurysm, tumor, hypertension, and clotting disorders, and affects individuals of all ages, with morbidity and mortality rates up to 90% and 78%, respectively2, 3. The pathologic consequences of IVH, which lead to significant morbidity and mortality, include obstructive hydrocephalus and delayed communicating hydrocephalus. Obstructive hydrocephalus occurs when blood clots block cerebrospinal fluid (CSF) conduits, leading to an increase in intracranial pressure (ICP). This is a life-threatening condition, which requires immediate management. The current treatment for obstructive hydrocephalus is insertion of an intraventricular catheter for external ventricular drainage (EVD). This procedure allows drainage of CSF resulting in a decrease in ICP, and can allow simultaneous measurement of ICP. Other major consequences of IVH are the thrombotoxicity and inflammation in reaction to ventricular blood, negatively impacting outcome. Despite EVD, reported rates of morbidity and mortality in IVH are still up to 89% and 58%, respectively3–5 These findings indicate that EVD has a modest effect on mortality but nearly no effect on the rate of poor outcomes (death or dependence) associated with IVH, leaving us with the need for a more effective treatment3
Intraventricular thrombolysis such as the use of recombinant tissue plasminogen activator (rt-PA) in conjunction with EVD has been optimized in the CLEAR II trial, and has shown promising prospect of accelerated clearance of blood from the ventricles and a positive impact on morbidity and mortality4, 6. The Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage Phase III (CLEAR III) trial is an ongoing double blind, international, multicenter, randomized clinical trial assessing the efficacy of EVD + rt-PA for clearance of intraventricular hemorrhage7
Common complications of EVD treatment include infection and catheter-related hemorrhage. Retrospective series report varied rates of bleeding and infection with external ventricular drainage (EVD). There have been no prospective studies of these risks with systematic surveillance, threshold definitions, or independent adjudication. And it is not known if these are increased in the setting of IVH or with administration of intraventricular thrombolysis. We analyze the rate of complications in the ongoing CLEAR III trial, providing a comparison with a systematic review of complications of EVD in the literature.
METHODS
CLEAR III Patient Population, Clinical protocol and Study Characteristics
CLEAR III is enrolling patients age 18–80 with spontaneous ICH ≤ 30 ml and IVH obstructing the 3rd and/or 4th ventricles, verified by CT scan performed within 24 hours of symptom onset, requiring EVD placement, in the study. We analyzed data from the first 250 patients enrolled in the CLEAR III trial. Exclusion criteria for the trial include patients with ruptured aneurysm, tumors, and other brain lesions, which may contribute to ICH, and patients with clotting disorders. Eligible patients undergo a stability scan performed at least 6 hours after EVD placement. If there is no new or expanded (difference ≤ 5 ml) hemorrhage on the stability scan, patients are enrolled in the study; otherwise another CT scan is acquired at subsequent 6 hour intervals until the bleed stabilizes (ICH ≤ 35 ml and difference between two scans ≤ 5 ml) or 72 hours passed since the diagnostic CT scan, closing the window for enrollment. Once enrolled, patients are randomized to either rt-PA or placebo group, and are administered the test article once every 8 hours for up to 12 doses, until the obstructive ventricular blood has cleared7. CT scans are acquired daily or no less than once every 3rd administration of the test article. A CT scan is also acquired 24 and 72 hours post last dose and at 30 and 365 days post enrollment for follow-up. Daily white blood cell (WBC) count, hematocrit, platelet count, activated partial thromboplastin time (PTT), International Normalized Ratio (INR), CSF cultures, cell counts, protein, and glucose are documented.
The ICH hemorrhage volume was calculated by well established formula ABC/28. For the CLEAR III study, hemorrhage was prospectively defined as a new or expansion of prior ICH or catheter track hemorrhage on CT scan by >5 ml in volume or > 5 mm in any diameter, after placement of the EVD until 30 days after its removal. Expansion of IVH is defined as any measurable enlargement in diameter of IVH on axial CT scan slices by > 2mm, or actual volumetric enlargement of IVH by > 5 ml as assessed by the trial’s Reading Center. Hemorrhage as defined by these criteria was required to be reported as an adverse event by the study site, and as verified by independent reviews and volumetric assessment of each CT scan and adjudication by the trial’s central Reading Center. A symptomatic bleed was defined as a hemorrhage associated with a drop by two or more points on concurrently documented Glasgow Coma Scale. We included new or expanded hemorrhage seen on head CT following EVD placement, occurring within 30 days of randomization, in line with the time window in most surgical series. Based on these articulated criteria, the trial’s Reading Center adjudicated every reported bleed, and an Adverse Events Committee independently adjudicated all symptomatic bleeds.
Bacterial ventriculitis, meningitis and non-bacterial ventriculitis were prospectively surveyed and adjudicated, and we included in this study all cases with positive bacterial culture of CSF obtained through the EVD, or of EVD catheter tip. Since there is wide variability in the literature regarding definition of infection related to EVD9, this threshold criterion facilitated comparison with published literature and minimized the risk of bias. Complication rates were calculated per subject, even though 83 of the 250 subjects (33.2 %) harbored more than one EVD catheter.
Literature Review
We performed an OVID Medline search most recently on June 1, 2013 to identify all published articles in English between the years of 1970 to June 2013 reporting infections and hemorrhages associated with EVD using the following search terms: “external ventricular drain,” “ventricular drain,” “ventriculostomy,” and “external ventricular catheter.” Our initial search yielded 2,097 articles for the external ventricular drain or ventricular drain or ventriculostomy or external ventricular catheter and infection search criteria, and 2955 articles for the external ventricular drain or ventricular drain or ventriculostomy or external ventricular catheter and hemorrhage search criteria. We reviewed the abstracts of each of these articles for relevance. Studies of interest for inclusion in our analysis were those reporting or permitting calculation of rates of infection or hemorrhagic complications. Only studies with ≥ 30 subjects that included one or more post procedural CT scan for reporting of hemorrhagic complications and those defining infection as a positive CSF culture acquired through the catheter or by culture of the catheter tip were included in the analysis. For the studies included in the systematic review, we defined hemorrhage as any evidence of new or expanded bleeding on a CT scan, and symptomatic hemorrhage as hemorrhage associated with new neurological symptoms or other clinical sequelae. Criteria for exclusion and inclusion of studies from our systematic review are summarized in Table 1. For the meta-analysis we strictly followed AMSTAR and PRISMA criteria10.
TABLE 1.
Exclusion/Inclusion Criteria of Published Studies on EVD Hemorrhage and Infection
| Exclusion Criteria |
| Procedure |
| -Cerebrospinal Fluid Shunting (Permanent Shunt) |
| -Endoscopic Third Ventriculostomy |
| -Lumbar Drainage |
| -EVD that were later converted to long tunneled catheter |
| Device |
| -Intracranial Pressure Monitors (Parenchymal) |
| -Rickham or Ommaya Reservoirs |
| Study Size |
| - < 30 subjects |
| Study Type |
| -Editorials |
| -Surveys |
| -In Vitro Studies |
| -Murine Studies |
| Study Population |
| -Pediatric (limited to < 18 years old/neonatal cases) |
| Other Exclusions |
| -Studies not in English |
| -Studies where incidence of complications were not reported |
| -Catheter revisions due to dislocation, obstruction, or otherwise not related to ventriculostomy procedure, choice of EVD catheter vs. other devices not clearly defined. |
| -Other: did not meet inclusion criteria |
| Inclusion Criteria |
| Procedure |
| -Primary EVD placed due to ICH |
| Device |
| -EVD |
| Study Size |
| - > 30 subjects |
| Study Type |
| -Retrospective analysis |
| -Prospective analysis |
| -Randomized controlled |
| Study Population |
| -Adult (> 18 years old) |
Statistical Analysis
Meta-analyses of published rate of infection, hemorrhage and symptomatic hemorrhage after ventriculostomy were carried out using STATA12 (StataCorp LP, College Station, Texas). When the count of symptomatic hemorrhage was zero, a correction of 0.5 was added to the number of symptomatic hemorrhage and total cases, prior to calculation11. Before pooling the results, heterogeneity between studies was assessed using χ2 and I2 test. Due to differences in the design of the trials evaluated, an inverse-variance weighted random-effects model, described by Dersimonian and Laird, was used to calculate pooled estimate of complication rates as well as 95% confidence intervals12. Publication bias was assessed graphically using funnel plot (Figure 1A, 1B, 1C) and statistically using both Begg rank correlation test and Egger linear regression test. Specific calculations in STATA were performed using the analysis package sbe24_3 (http://www.stata-journal.com/software/sj9-2). Inputs of complications rates and study weights were calculated as and respectively for each study.
Figure 1.


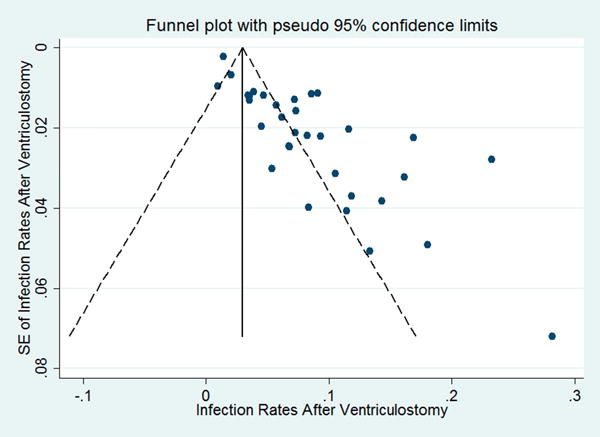
Funnel plots of A) hemorrhage rate, B) symptomatic hemorrhage rate and C) infection rate against their respective standard errors.
RESULTS
Adverse Events in CLEAR III
Data from the first 250 patients enrolled in the CLEAR III trial were analyzed. The demographics of the enrolled patients are described in Table 2. All had an external ventricular drain placed before randomization and received regular CT scans and CSF cultures. Of the 250 cases, forty-two subjects (16.8%) experienced one or more new bleeds or expansions and 6 of them suffered symptomatic hemorrhages (2.4%) within 30 days after randomization in the study. Three of the 6 symptomatic bleeds occurred during the dosing phase, and the other 3 post-dosing (Table 3). Three of 6 symptomatic bleeds (50%) were catheter tract hemorrhage and the other 3 were new distant hemorrhage. An additional 3 cases (1.2%) had suffered a new bleed or expansion after EVD insertion and prior to enrollment in the trial none of these were symptomatic.
TABLE 2.
Subject Demographic and Clinical Characteristics of 250 Subjects Enrolled in CLEAR III
| Number of Patients | |||
|---|---|---|---|
| N | (%) | ||
| Gender | Male | 134 | 53.6 |
| Female | 116 | 46.4 | |
|
| |||
| Race | White/Caucasian | 149 | 59.6 |
| Black/African American | 86 | 34.4 | |
| American Indian/Alaskan Native | 1 | 0.4 | |
| Asian | 5 | 2.0 | |
| Native Hawaiian/Pacific Islander | 3 | 1.2 | |
| Not Specified | 5 | 2.0 | |
| Multi-Racial | 1 | 0.4 | |
|
| |||
| Ethnicity | Not Hispanic/Latino | 209 | 83.6 |
| Hispanic/Latino | 41 | 16.4 | |
|
| |||
| Primary Diagnosis | Primary IVH | 42 | 16.8 |
| ICH with IVH | 208 | 83.2 | |
|
| |||
| Tobacco Use | No | 180 | 72.0 |
| Yes | 70 | 28.0 | |
|
| |||
| Cocaine Use | No | 237 | 94.8 |
| Yes | 13 | 5.2 | |
|
| |||
| On Anti-coagulation | No | 224 | 89.6 |
| Yes | 26 | 10.4 | |
|
| |||
| On HRT | No | 110 | 44.0 |
| Yes | 6 | 2.4 | |
| NA | 134 | 53.6 | |
|
| |||
| Antihypertensive Med Compliant | No | 65 | 26.0 |
| Yes | 185 | 74.0 | |
|
| |||
| Hyperlipidemia Med Compliant | No | 9 | 3.6 |
| Yes | 241 | 96.4 | |
|
| |||
| On Antiplatelet | No | 193 | 77.2 |
| Yes | 57 | 22.8 | |
Table 3.
Symptomatic Hemorrhages Within 30 Days of EVD Insertion Among 250 Cases Enrolled in CLEAR III
| Patient # | Bleed | Timing | Anticoagulation |
|---|---|---|---|
| 1 | Catheter track (during dosing) | 5hrs. (after 3 doses) | No prophylactic anticoagulation |
| 2 | Catheter track (during dosing) | 31hrs. (after 2 doses) | No prophylactic anticoagulation |
| 3 | Catheter track (during dosing) | 48hrs | DVT prophylaxis (SQ Heparin) |
| 4 | New catheter placed for hydrocephalus after protocol; New ICH (new pontine hemorrhage) | Day 16 | Full anti-coagulation (site entered reason as ‘prevention of thrombosis’ |
| 5 | New catheter placed for hydrocephalus after protocol; (new massive IVH in left lateral ventricle) | Day 22 | On Enoxaparin (DVT prophylaxis) |
| 6 | Spontaneous new bleed; New ICH (different location than original stroke) | Day 16 | On Enoxaparin (DVT prophylaxis) |
There were eleven cases (4.4%) of culture-proven bacterial meningitis or ventriculitis, although many manifested varying sterile CSF pleocytosis in reaction to IVH. Of the eleven cases of infection, ten were meningitis and one case was due to brain abscess. The pathogens consisted of gram positive cocci in 6 cases, gram negative cocci in 2 cases, and gram negative bacilli in 3 cases. There was also one case of gram stain positive CSF; however, it remained culture negative and thus was not included in the infection calculation. All patients in the trial were receiving prophylactic intravenous antibiotics per trial protocol. The infections occurred despite being on intravenous prophylaxis against gram positive organisms, during the entire duration of the EVD.
Literature Review
Hemorrhage
Per our literature inclusion/exclusion criteria, we identified 18 studies with a cumulative total of 2829 cases (Table 4). Of the total 2829 identified cases of EVD placement, there were 270 cases of CT-verified reported hemorrhage. The pooled hemorrhage incidence rate from these studies was 8.4% [5.7%, 11.1%] compared to 16.8% [12.5%, 21.9%] in CLEAR III (Test for heterogeneity: χ2=260.16, df=17, p<0.001; I2=93.5% and test for publication bias: Egger’ test: p<0.001; Begg’s test: p=0.001) (Figure 2). Of the 18 studies, 10 studies reported clinically significant symptomatic hemorrhages with a tally of 25 cases. The pooled symptomatic hemorrhage incidence rate was 0.7% [0.4%, 1.1%] compared to 2.4% [0.5%, 4.3%] in CLEAR III (Test for heterogeneity: χ2=12, df=12, p=0.45; I2=0% and publication bias: Egger’ test: p=0.001; Begg’s test: p=0.012) (Figure 3).
Table 4.
Rates of New or Expanded Hemorrhage after Ventriculostomy
| Study | Type | Cases | Hemorrhages | Hemorrhage Rate | Symptomatic Hemorrhages | Symptomatic Hemorrhage Rate | |
|---|---|---|---|---|---|---|---|
| 1 | Gardner et al., 200913 | R | 188 | 77 | 41.0% | 1 | 0.5% |
| 2 | Guyot et al., 199822 | R | 274 | 9 | 3.28% | 2 | 0.72% |
| 3 | Hoh et al., 200517 | R | 370 | 37 | 10.0% | 4 | 1.1% |
| 4 | Huyette et al., 200818 | R | 98 | 18 | 18.4% | — | — |
| 5 | Kakarla et al., 200823 | R | 346 | 17 | 4.9% | 4 | 1.2% |
| 6 | Maniker et al., 200614 | R | 160 | 52 | 32.5% | 4 | 2.5% |
| 7 | McIver et al., 200224 | R | 45 | 2 | 4.4% | — | — |
| 8 | Naff et al., 201115 | RCT | 48 | 13 | 27.1% | 7 | 14.6% |
| 9 | Paramore et al., 199425 | R | 161 | 4 | 2.5% | 2 | 1.2% |
| 10 | Wiesmann et al., 200126 | R | 92 | 6 | 6.5% | 0 | — |
| 11 | Woernle et al., 201127 | R | 137 | 5 | 3.6% | — | — |
| 12 | Zeng et al., 201028 | P | 136 | 1 | 0.7% | 0 | 0.0% |
| 13 | Patil et al., 201329 | R | 111 | 2 | 1.8% | 0 | 0.0% |
| 14 | Wang et al., 201330 | P | 45 | 3 | 6.6% | – | – |
| 15 | Park et al., 201131 | R | 250 | 16 | 6.4% | – | – |
| 16 | Abdoh et al., 201232 | R | 66 | 5 | 7.6% | 0 | 0.0% |
| 17 | North et al. 198633 | R | 199 | 2 | 1% | 1 | 0.5% |
| 18 | Roitberg et al. 200120 | R | 103 | 1 | 0.97% | 0 | — |
| 19 | CLEAR III (ongoing) | P | 250 | 43 | 16.8% | 6 | 2.4% |
P, prospective; R, retrospective; RCT, randomized clinical trial
Figure 2.

Forest plot of random-effects meta-analysis of the incidence rate of hemorrhages. ES, hemorrhage rate; CI, confidence interval.
Figure 3.

Forest plot of random-effects meta-analysis of the incidence rate of symptomatic hemorrhages. ES, symptomatic hemorrhage rate; CI, confidence interval.
Infection
Thirty-three studies reported infection rates (Table 5). Of the 9667 cases identified from these 33 studies, there were 568 cases of reported infection. The pooled infection incidence rate was 7.9% [6.3%, 9.4%] compared to 4.4% [1.9%, 6.9%] in CLEAR III trial (Test of heterogeneity: χ2=342.01, df=32, p<0.001; I2=90.6% and publication bias: Egger’ test: p<0.001; Begg’s test: p=0.016).
Table 5.
Infection Rates After Ventriculostomy
| Study | Type | Cases | Infections | Infection Rate | Procedure Location | Culture Frequency | |
|---|---|---|---|---|---|---|---|
| 1 | Alleyne et al., 200034 | R | 308 | 12 | 3.9% | Not specified | Twice weekly |
| 2 | Arabi et al., 200535 | R | 84 | 12 | 14.3% | Multiple locations | Daily |
| 3 | Bota et al., 200536 | P | 638 | 58 | 9.1% | Operating Room | Daily |
| 4 | Hoefnagel et al., 200837 | R | 228 | 53 | 23.2% | Operating room | 3 times/week |
| 5 | Kaufmann et al., 200438 | P | 158 | 13 | 8.2% | Operating room | Daily |
| 6 | Khan et al., 199839 | R | 104 | 7 | 6.7% | Not specified | Not Specified |
| 7 | Kim et al., 199540 | R | 61 | 7 | 11.5% | Operating room | Daily |
| 8 | Lackner et al., 200819 | P/R | 39 | 11 | 28.2% | Operating room | Daily in treatment group; 3 times/week in control group |
| 9 | Leung et al., 200741 | R | 103 | 7 | 6.8% | Operating room | Not routine |
| 10 | Lyke et al., 2001l42 | P | 151 | 11 | 7.3% | Multiple locations | At & 2 days after EVD placement |
| 11 | Mayhall et al., 198443 | P | 172 | 16 | 9.3% | Multiple locations | Not Specified |
| 12 | Naff et al., 201115 | RCT | 48 | 4 | 8.3% | Intensive care unit | Daily |
| 13 | Park et al., 200431 | R | 595 | 51 | 8.6% | Multiple locations | Not routine |
| 14 | Pfisterer et al., 200344 | P | 130 | 21 | 16.2% | Intensive care unit | 3 times/week |
| 15 | Roitberg et al., 200120 | R | 103 | 1 | 1.0% | Intensive care unit | Daily |
| 16 | Smith et al., 197621 | R | 56 | 3 | 5.4% | Multiple locations | Not routine |
| 17 | Tse et al., 201045 | R | 319 | 15 | 4.7% | Operating room | Not specified |
| 18 | Zentner et al., 199546 | P | 226 | 8 | 3.5% | Not specified | Not specified |
| 19 | Patil et al., 201329 | R | 111 | 5 | 4.5% | Not specified | Not specified |
| 20 | Wang et al., 201330 | P | 45 | 6 | 13.3% | Not specified | Not Specified |
| 21 | Flint et al., 201347 | R | 262 | 15 | 5.7% | Intensive care unit | Only if concern for infection |
| 22 | Lajcak et al., 201348 | R | 403 | 29 | 7.1% | Operating room | Routine monitoring |
| 23 | Winkler et al., 201349 | RTC | 61 | 11 | 18% | Not Specified | Every third day |
| 24 | Camacho et al., 201350 | P | 194 | 12 | 6.2% | Operating room | Only if concern for infection |
| 25 | Kubilay et al., 201351 | P | 2928 | 41 | 1.4% | Not specified | Only if concern for infection |
| 26 | Keong et al., 201252 | RTC | 278 | 47 | 16.9% | Not specified | Routine daily monitoring |
| 27 | Lwin et al., 201253 | R | 234 | 8 | 3.4% | Not Specified | Only if concern for infection |
| 28 | Lemcke et al., 201254 | R | 95 | 10 | 11% | Operating room | Only if concern for infection |
| 29 | Pople et al., 201255 | RTC | 434 | 9 | 2.5% | Not specified | Day of EVD placement, 3 days post EVD placement, upon EVD removal and in between if CSF infection suspected |
| 30 | Park et al., 201156 | R | 250 | 29 | 11.6% | Not specified | Not specified |
| 31 | Rafiq et al., 201157 | P | 76 | 9 | 11.8% | Operating room | Only if concern for infection |
| 32 | Guyot et al., 199822 | R | 274 | 20 | 7.29 | Not specified | Not Specified |
| 33 | North et al., 198633 | R | 199 | 7 | 3.5% | Not specified | Only if concern for infection |
| 32 | CLEAR III (ongoing) | RTC | 250 | 11 | 3.6% | Not specified | Daily |
P, prospective; R, retrospective; RCT, randomized clinical trial
DISCUSSION
Analysis of first 250 patients enrolled in CLEAR III trial showed 16.8% hemorrhage rate, 2.4% symptomatic hemorrhages rate and 4.4% infection rate compared to the 8.4% hemorrhage rate, 0.7% symptomatic hemorrhages rate and 7.9% infection rate calculated from the literature meta-analysis (Figures 2–4).
Figure 4.
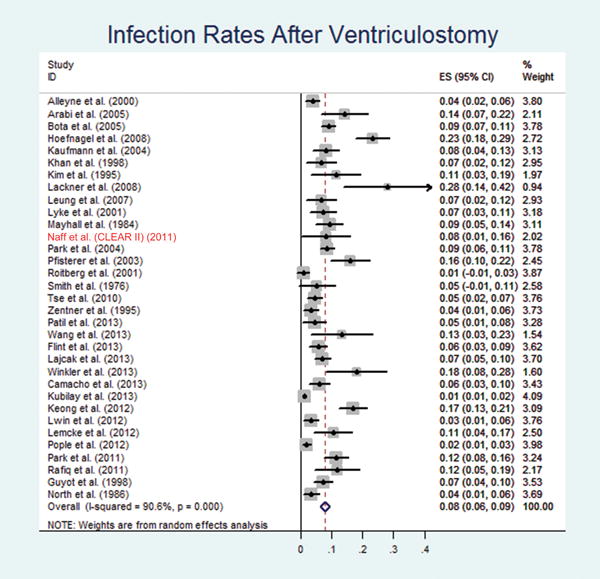
Forest plot of random-effects meta-analysis of the infection rates. ES, infection rate; CI, confidence interval.
Hemorrhage
The studies analyzed in our literature review included post-procedure head CT scans, but none with as frequent or systematic imaging surveillance as CLEAR, and most studies did not report bleeds as late as 30 days post EVD placement. In the literature there was wide variation in the stringency and frequency of CT scans after EVD placement. With the exception of the paper by Naff et al.15, the definitions of symptomatic bleed also differed among published studies, with retrospective reports lacking GCS data or other criteria defining clinical deterioration. The various studies also do not consider potential interventions, such as correction of coagulopathy, that may or may not have been deployed to prevent the conversion of asymptomatic bleeds to symptomatic ones.
Two of the studies that reported higher rate of hemorrhage after EVD placement than the other 16 studies, Gardner et al.13 (41%) and Manikar et al.14 (32.5%), also had very stringent post-procedural CT scan criteria comparable to the criteria used in CLEAR III trial. The study by Gardner et al.13 used both CT or MRI, which is known to be more sensitive in detecting blood, to detect hemorrhage. The study by Naff et al.15 (27.1%), which also reported a higher rate of hemorrhage, was a thrombolytic dose escalation study. Thus, comparing similar studies, the hemorrhage rate of the CLEAR III trial is lower.
It is likely that most published rates are an underestimate, due to variability in definition of catheter-related hemorrhage and limited information on, or lack of adjudicated assessment of, new bleeds. The study reported by Naff et al. (CLEAR Phase II trials) had one of the three highest published rates of hemorrhage. Not surprisingly, this included patients receiving thrombolysis, with prospectively articulated definitions and systematic imaging with third party adjudication of new bleeding and symptomatic bleeding (tracked as adverse events). The Naff et al. study included dose escalation, with some subjects receiving a higher dose (3 mg) of rt-PA. The protocol has been modified to 1mg rt-PA thrice daily for the CLEAR-III trial. The lower rates of hemorrhage in CLEAR III compared to those reported by Naff et al. likely reflect optimized dosing based on the Phase II trial and other lessons learned requiring demonstrated stability of hemorrhage before administration of rt-PA, and avoiding any EVD replacement or manipulation within a day of administering thrombolytic agent16. These practices have been incorporated in CLEAR III.
Beside the report by Naff, et al., four other studies reported asymptomatic hemorrhage rates higher than 10%13, 14, 17, 18. The higher than average hemorrhage rate in these studies can be attributed to certain inherent characteristics. The study by Gardner et al. defined hemorrhage as any evidence of blood on either CT or gradient echo MRI13. Higher sensitivity of gradient echo MRI to detect blood most likely attributed to very high reported asymptomatic bleed in this study. Maniker et al. also defined hemorrhage as any evidence of blood on CT scan including punctate hemorrhages14. In the study by Hoh et al. the authors were evaluating the safety of heparinization for aneurysm coiling after EVD placement17. The majority of the patients included in the retrospective study by Huyette et al. were head trauma patients, and this series also reported several multiple passes for EVD placement18.
Three of the 6 symptomatic bleeds among the first 250 subjects in CLEAR III occurred after completion of dosing of study agent, all in patients receiving thromboprophylactic or therapeutic doses of anticoagulants. With so few symptomatic hemorrhages, and many other patients without symptomatic hemorrhage receiving similar therapies, we cannot comment upon any significant contribution of these factors. Three patients suffered symptomatic bleeding during dosing, and we cannot comment at this time whether they were receiving placebo or thrombolytic agent.
Infection
The overall mean rate of EVD-related infection reported in the literature is 7.9%, which is higher than the documented infection rate of 4.4% in CLEAR III trial. The majority of the studies included in the analysis are retrospective studies. There is a large amount of variability in the reported infection rate in the literature, ranging anywhere from 1% to 28.2%, as well as variation in the definition used for defining infection. There is no CDC-provided catheter induced ventriculitis definition to provide guidance in this matter. Even though in all the studies infection is defined as positive CSF culture, the frequency of culture is highly variable. Most studies performed daily CSF sampling; however, in some studies CSF sampling was only done if there was concern for infection. There were some studies that did not specify the culture frequency. There was no obvious difference in the infection rate between retrospective vs. prospective, both the highest19 and the lowest20 reporting infection rate studies were retrospective analyses. The location of EVD placement varied from intensive care unit to operating room in CLEAR III and in the various reported studies, and this did not appear to influence infection rates. Most studies in the literature did not report pleocytosis, protein or glucose concentrations in the CSF in association with infection, and many do not mention the cultured pathogens, nor associated fever or specific treatments. And the various studies do not reveal information about these CSF parameters in cases without culture proven infection, so the rates of aseptic or “partially treated” meningitis remain unknown.
Patients in CLEAR III underwent daily CSF sampling and an independent committee adjudicated the presence of infection. It has been suggested that the presence of blood may act as a culture medium, allowing bacterial growth, and thus increase the risk of infection9, or that frequent instrumentation of the closed CSF drainage system may increase infection rates21. These are not substantiated, with fewer infections in the setting of CLEAR III. It is unclear if specific protocols in CLEAR III contributed to the lower than expected infection rates, including the mandated sterile tunneling and prophylactic intravenous antibiotics from the insertion until removal of the EVD. The administration of thrombolytic or placebo required thrice daily invasion of the closed catheter drainage system for up to 72 hours, and the protocol required strict sterile conditions for each such dosing, including full sterile draping, antiseptic preparation of the tubing, closed double three-way stopcock syringe technique (http://braininjuryoutcomes.com/component/jdownloads/viewdownload/14/92?Itemid=0), mask, hat and gown by a trained operator. The generalization of these practices would seem warranted in clinical practice. The EVDs were placed under full sterile technique in the operating room, emergency room or intensive care settings, and antibiotic impregnated catheters were used in some instances at the operator’s discretion. The low rates of overall infections will not likely inform further refinement of these practices.
This study did not address the CSF profile in cases with or without proven infection, and cases of potential aseptic or partially treated meningitis. A detailed analysis of CSF inflammatory response in CLEAR III is currently underway, defining the range of inflammatory response with various volumes of IVH, and this will be published separately.
Limitations
Our study has limitations. The most important is that the results of the meta-analysis were based on mostly retrospective studies. Variability in defining infection and assessing hemorrhage added to the heterogeneity of the studies included from the literature review, and the studies did not typically include demographic or other patient and disease parameters that may have influenced complication rates. As with all systematic reviews, there was likely an important publication bias, and this was statistically suggested by the results in the funnel plots (Figure 1A, B, C). The impact of publication bias cannot be underestimated, hence the importance of standardizing reporting standards going forward, as we attempted to do in CLEAR III. And there were heterogeneities related to sample size of the various studies, possibly affecting the reported results.
Our reported interim rates in CLEAR III included cases receiving IVH thrombolysis or placebo, so the actual hemorrhage rates in cases receiving thrombolysis may be higher. Hemorrhage rates in the literature and in CLEAR III that were considered “asymptomatic” are not necessarily “insignificant”, as the studies do not specifically assess the potential clinical impact of such bleeds. And the CLEAR III criterion for symptomatic bleed as associated with a GCS drop by 2 or more points is arbitrary, and may be insensitive to relevant clinical sequelae, particularly among patients in poor neurologic condition.
Conclusion
Our study compared the two major complication rates, hemorrhage and infection, following EVD placement from the well-designed and monitored CLEAR III trial with the meta-analysis of the rates published in the literature. Despite thrombolytic administration in half the cases, the presence on IVH, and frequent instrumentation of the CSF drainage system for injection of drug or placebo, the EVD-related complications appear low, and in the same range as the rates reported in the literature. Protocolized practices contributing to these outcomes, and the new standards for monitoring and reporting EVD complications, may be useful in clinical practice.
Acknowledgments
Disclosure: This work is supported by NIH/NINDS Grant U01-NS062851. rt-PA in CLEAR III is generously donated by Genentech.
Footnotes
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
References
- 1.Staykov D, Bardutzky J, Huttner HB, Schwab S. Intraventricular fibrinolysis for intracerebral hemorrhage with severe ventricular involvement. Neurocrit Care. 2011 Aug;15(1):194–209. doi: 10.1007/s12028-010-9390-x. [DOI] [PubMed] [Google Scholar]
- 2.Engelhard HH, Andrews CO, Slavin KV, Charbel FT. Current management of intraventricular hemorrhage. Surg Neurol. 2003 Jul;60(1):15–21. doi: 10.1016/s0090-3019(03)00144-7. discussion 21–12. [DOI] [PubMed] [Google Scholar]
- 3.Nieuwkamp DJ, de Gans K, Rinkel GJ, Algra A. Treatment and outcome of severe intraventricular extension in patients with subarachnoid or intracerebral hemorrhage: a systematic review of the literature. J Neurol. 2000 Feb;247(2):117–121. doi: 10.1007/pl00007792. [DOI] [PubMed] [Google Scholar]
- 4.Nyquist P, LeDroux S, Geocadin R. Thrombolytics in intraventricular hemorrhage. Curr Neurol Neurosci Rep. 2007 Nov;7(6):522–528. doi: 10.1007/s11910-007-0080-9. [DOI] [PubMed] [Google Scholar]
- 5.Naff NJ. Intraventricular Hemorrhage in Adults. Curr Treat Options Neurol. 1999 Jul;1(3):173–178. doi: 10.1007/s11940-999-0001-0. [DOI] [PubMed] [Google Scholar]
- 6.Morgan T, Awad I, Keyl P, Lane K, Hanley D. Preliminary report of the clot lysis evaluating accelerated resolution of intraventricular hemorrhage (CLEAR-IVH) clinical trial. Acta Neurochir Suppl. 2008;105:217–220. doi: 10.1007/978-3-211-09469-3_41. [DOI] [PubMed] [Google Scholar]
- 7.Ziai WC, Tuhrim S, Lane K, et al. A multicenter, randomized, double-blinded, placebo-controlled phase III study of Clot Lysis Evaluation of Accelerated Resolution of Intraventricular Hemorrhage (CLEAR III) International journal of stroke : official journal of the International Stroke Society. 2013 Aug 28; doi: 10.1111/ijs.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke; a journal of cerebral circulation. 1996 Aug;27(8):1304–1305. doi: 10.1161/01.str.27.8.1304. [DOI] [PubMed] [Google Scholar]
- 9.Lozier AP, Sciacca RR, Romagnoli MF, Connolly ES., Jr Ventriculostomy-related infections: a critical review of the literature. Neurosurgery. 2008 Feb;62(Suppl 2):688–700. doi: 10.1227/01.neu.0000316273.35833.7c. [DOI] [PubMed] [Google Scholar]
- 10.Klimo P, Jr, Thompson CJ, Ragel BT, Boop FA. Methodology and reporting of meta-analyses in the neurosurgical literature. Journal of neurosurgery. 2014 Apr;120(4):796–810. doi: 10.3171/2013.11.JNS13195. [DOI] [PubMed] [Google Scholar]
- 11.Schwarzer G. Methods for Meta-analysis in Medical Research. Alex J. Sutton, Keith R. Abrams, David R. Jones, Trevor A. Sheldon and Fujian Song, Wiley, Chichester, U.K., 2000. No. of pages: xvii+317. ISBN 0-471-49066-0. Statistics in Medicine. 2003;22(19):3112–3114. [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986 Sep;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 13.Gardner PA, Engh J, Atteberry D, Moossy JJ. Hemorrhage rates after external ventricular drain placement. J Neurosurg. 2009 May;110(5):1021–1025. doi: 10.3171/2008.9.JNS17661. [DOI] [PubMed] [Google Scholar]
- 14.Maniker AH, Vaynman AY, Karimi RJ, Sabit AO, Holland B. Hemorrhagic complications of external ventricular drainage. Neurosurgery. 2006 Oct;59(4 Suppl 2):ONS419–424. doi: 10.1227/01.NEU.0000222817.99752.E6. discussion ONS424–415. [DOI] [PubMed] [Google Scholar]
- 15.Naff N, Williams MA, Keyl PM, et al. Low-dose recombinant tissue-type plasminogen activator enhances clot resolution in brain hemorrhage: the intraventricular hemorrhage thrombolysis trial. Stroke. 2011 Nov;42(11):3009–3016. doi: 10.1161/STROKEAHA.110.610949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dey M, Stadnik A, Awad IA. Spontaneous intracerebral and intraventricular hemorrhage: advances in minimally invasive surgery and thrombolytic evacuation, and lessons learned in recent trials. Neurosurgery. 2014 Feb;74(Suppl 1):S142–150. doi: 10.1227/NEU.0000000000000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoh BL, Nogueira RG, Ledezma CJ, Pryor JC, Ogilvy CS. Safety of heparinization for cerebral aneurysm coiling soon after external ventriculostomy drain placement. Neurosurgery. 2005 Nov;57(5):845–849. doi: 10.1227/01.neu.0000180814.95032.07. discussion 845–849. [DOI] [PubMed] [Google Scholar]
- 18.Huyette DR, Turnbow BJ, Kaufman C, Vaslow DF, Whiting BB, Oh MY. Accuracy of the freehand pass technique for ventriculostomy catheter placement: retrospective assessment using computed tomography scans. J Neurosurg. 2008 Jan;108(1):88–91. doi: 10.3171/JNS/2008/108/01/0088. [DOI] [PubMed] [Google Scholar]
- 19.Lackner P, Beer R, Broessner G, et al. Efficacy of silver nanoparticles-impregnated external ventricular drain catheters in patients with acute occlusive hydrocephalus. Neurocrit Care. 2008;8(3):360–365. doi: 10.1007/s12028-008-9071-1. [DOI] [PubMed] [Google Scholar]
- 20.Roitberg BZ, Khan N, Alp MS, Hersonskey T, Charbel FT, Ausman JI. Bedside external ventricular drain placement for the treatment of acute hydrocephalus. Br J Neurosurg. 2001 Aug;15(4):324–327. doi: 10.1080/02688690120072478. [DOI] [PubMed] [Google Scholar]
- 21.Smith RW, Alksne JF. Infections complicating the use of external ventriculostomy. J Neurosurg. 1976 May;44(5):567–570. doi: 10.3171/jns.1976.44.5.0567. [DOI] [PubMed] [Google Scholar]
- 22.Guyot LL, Dowling C, Diaz FG, Michael DB. Cerebral monitoring devices: analysis of complications. Acta Neurochir Suppl. 1998;71:47–49. doi: 10.1007/978-3-7091-6475-4_15. [DOI] [PubMed] [Google Scholar]
- 23.Kakarla UK, Kim LJ, Chang SW, Theodore N, Spetzler RF. Safety and accuracy of bedside external ventricular drain placement. Neurosurgery. 2008 Jul;63(1 Suppl 1):ONS162–166. doi: 10.1227/01.neu.0000335031.23521.d0. discussion ONS166–167. [DOI] [PubMed] [Google Scholar]
- 24.McIver JI, Friedman JA, Wijdicks EF, et al. Preoperative ventriculostomy and rebleeding after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2002 Nov;97(5):1042–1044. doi: 10.3171/jns.2002.97.5.1042. [DOI] [PubMed] [Google Scholar]
- 25.Paramore CG, Turner DA. Relative risks of ventriculostomy infection and morbidity. Acta Neurochir (Wien) 1994;127(1–2):79–84. doi: 10.1007/BF01808552. [DOI] [PubMed] [Google Scholar]
- 26.Wiesmann M, Mayer TE. Intracranial bleeding rates associated with two methods of external ventricular drainage. J Clin Neurosci. 2001 Mar;8(2):126–128. doi: 10.1054/jocn.2000.0749. [DOI] [PubMed] [Google Scholar]
- 27.Woernle CM, Burkhardt JK, Bellut D, Krayenbuehl N, Bertalanffy H. Do iatrogenic factors bias the placement of external ventricular catheters?–a single institute experience and review of the literature. Neurol Med Chir (Tokyo) 2011;51(3):180–186. doi: 10.2176/nmc.51.180. [DOI] [PubMed] [Google Scholar]
- 28.Zeng T, Gao L. Management of patients with severe traumatic brain injury guided by intraventricular intracranial pressure monitoring: a report of 136 cases. Chin J Traumatol. 2010 Jun 1;13(3):146–151. [PubMed] [Google Scholar]
- 29.Patil V, Lacson R, Vosburgh KG, et al. Factors associated with external ventricular drain placement accuracy: data from an electronic health record repository. Acta Neurochir (Wien) 2013 Sep;155(9):1773–1779. doi: 10.1007/s00701-013-1769-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang K, Du HG, Yin LC, He M, Hao BL, Chen L. Which side of lateral ventricles to choose during external ventricular drainage in patients with intraventricular hemorrhage: ipsilateral or contralateral? The Journal of surgical research. 2013 Aug;183(2):720–725. doi: 10.1016/j.jss.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Park P, Garton HJ, Kocan MJ, Thompson BG. Risk of infection with prolonged ventricular catheterization. Neurosurgery. 2004 Sep;55(3):594–599. doi: 10.1227/01.neu.0000134289.04500.ee. discussion 599–601. [DOI] [PubMed] [Google Scholar]
- 32.Abdoh MG, Bekaert O, Hodel J, et al. Accuracy of external ventricular drainage catheter placement. Acta Neurochir (Wien) 2012 Jan;154(1):153–159. doi: 10.1007/s00701-011-1136-9. [DOI] [PubMed] [Google Scholar]
- 33.North B, Reilly P. Comparison among three methods of intracranial pressure recording. Neurosurgery. 1986 Jun;18(6):730–732. doi: 10.1227/00006123-198606000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Alleyne CH, Jr, Hassan M, Zabramski JM. The efficacy and cost of prophylactic and perioprocedural antibiotics in patients with external ventricular drains. Neurosurgery. 2000 Nov;47(5):1124–1127. doi: 10.1097/00006123-200011000-00020. discussion 1127–1129. [DOI] [PubMed] [Google Scholar]
- 35.Arabi Y, Memish ZA, Balkhy HH, et al. Ventriculostomy-associated infections: incidence and risk factors. Am J Infect Control. 2005 Apr;33(3):137–143. doi: 10.1016/j.ajic.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Bota DP, Lefranc F, Vilallobos HR, Brimioulle S, Vincent JL. Ventriculostomy-related infections in critically ill patients: a 6-year experience. J Neurosurg. 2005 Sep;103(3):468–472. doi: 10.3171/jns.2005.103.3.0468. [DOI] [PubMed] [Google Scholar]
- 37.Hoefnagel D, Dammers R, Ter Laak-Poort MP, Avezaat CJ. Risk factors for infections related to external ventricular drainage. Acta Neurochir (Wien) 2008 Mar;150(3):209–214. doi: 10.1007/s00701-007-1458-9. discussion 214. [DOI] [PubMed] [Google Scholar]
- 38.Kaufmann AM, Lye T, Redekop G, et al. Infection rates in standard vs. hydrogel coated ventricular catheters. Can J Neurol Sci. 2004 Nov;31(4):506–510. doi: 10.1017/s0317167100003723. [DOI] [PubMed] [Google Scholar]
- 39.Khan SH, Kureshi IU, Mulgrew T, Ho SY, Onyiuke HC. Comparison of percutaneous ventriculostomies and intraparenchymal monitor: a retrospective evaluation of 156 patients. Acta Neurochir Suppl. 1998;71:50–52. doi: 10.1007/978-3-7091-6475-4_16. [DOI] [PubMed] [Google Scholar]
- 40.Kim DK, Uttley D, Bell BA, Marsh HT, Moore AJ. Comparison of rates of infection of two methods of emergency ventricular drainage. J Neurol Neurosurg Psychiatry. 1995 Apr;58(4):444–446. doi: 10.1136/jnnp.58.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leung GK, Ng KB, Taw BB, Fan YW. Extended subcutaneous tunnelling technique for external ventricular drainage. Br J Neurosurg. 2007 Aug;21(4):359–364. doi: 10.1080/02688690701392881. [DOI] [PubMed] [Google Scholar]
- 42.Lyke KE, Obasanjo OO, Williams MA, O’Brien M, Chotani R, Perl TM. Ventriculitis complicating use of intraventricular catheters in adult neurosurgical patients. Clin Infect Dis. 2001 Dec 15;33(12):2028–2033. doi: 10.1086/324492. [DOI] [PubMed] [Google Scholar]
- 43.Mayhall CG, Archer NH, Lamb VA, et al. Ventriculostomy-related infections. A prospective epidemiologic study. N Engl J Med. 1984 Mar 1;310(9):553–559. doi: 10.1056/NEJM198403013100903. [DOI] [PubMed] [Google Scholar]
- 44.Pfisterer W, Muhlbauer M, Czech T, Reinprecht A. Early diagnosis of external ventricular drainage infection: results of a prospective study. J Neurol Neurosurg Psychiatry. 2003 Jul;74(7):929–932. doi: 10.1136/jnnp.74.7.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tse T, Cheng K, Wong K, Pang K, Wong C. Ventriculostomy and Infection: A 4-year-review in a local hospital. Surg Neurol Int. 2010;1:47. doi: 10.4103/2152-7806.69033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zentner J, Duffner F, Behrens E. Percutaneous needle trephination for external CSF drainage: experience with 226 punctures. Neurosurg Rev. 1995;18(1):31–34. doi: 10.1007/BF00416475. [DOI] [PubMed] [Google Scholar]
- 47.Flint AC, Rao VA, Renda NC, Faigeles BS, Lasman TE, Sheridan W. A simple protocol to prevent external ventricular drain infections. Neurosurgery. 2013 Jun;72(6):993–999. doi: 10.1227/NEU.0b013e31828e8dfd. ; discussion 999. [DOI] [PubMed] [Google Scholar]
- 48.Lajcak M, Heidecke V, Haude KH, Rainov NG. Infection rates of external ventricular drains are reduced by the use of silver-impregnated catheters. Acta Neurochir (Wien) 2013 May;155(5):875–881. doi: 10.1007/s00701-013-1637-9. [DOI] [PubMed] [Google Scholar]
- 49.Winkler KM, Woernle CM, Seule M, Held U, Bernays RL, Keller E. Antibiotic-impregnated versus silver-bearing external ventricular drainage catheters: preliminary results in a randomized controlled trial. Neurocrit Care. 2013 Apr;18(2):161–165. doi: 10.1007/s12028-013-9816-3. [DOI] [PubMed] [Google Scholar]
- 50.Camacho EF, Boszczowski I, Freire MP, et al. Impact of an educational intervention implanted in a neurological intensive care unit on rates of infection related to external ventricular drains. PloS one. 2013;8(2):e50708. doi: 10.1371/journal.pone.0050708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kubilay Z, Amini S, Fauerbach LL, Archibald L, Friedman WA, Layon AJ. Decreasing ventricular infections through the use of a ventriculostomy placement bundle: experience at a single institution. J Neurosurg. 2013 Mar;118(3):514–520. doi: 10.3171/2012.11.JNS121336. [DOI] [PubMed] [Google Scholar]
- 52.Keong NC, Bulters DO, Richards HK, et al. The SILVER (Silver Impregnated Line Versus EVD Randomized trial): a double-blind, prospective, randomized, controlled trial of an intervention to reduce the rate of external ventricular drain infection. Neurosurgery. 2012 Aug;71(2):394–403. doi: 10.1227/NEU.0b013e318257bebb. discussion 403–394. [DOI] [PubMed] [Google Scholar]
- 53.Lwin S, Low SW, Choy DK, Yeo TT, Chou N. External ventricular drain infections: successful implementation of strategies to reduce infection rate. Singapore medical journal. 2012 Apr;53(4):255–259. [PubMed] [Google Scholar]
- 54.Lemcke J, Depner F, Meier U. The impact of silver nanoparticle-coated and antibiotic-impregnated external ventricular drainage catheters on the risk of infections: a clinical comparison of 95 patients. Acta Neurochir Suppl. 2012;114:347–350. doi: 10.1007/978-3-7091-0956-4_67. [DOI] [PubMed] [Google Scholar]
- 55.Pople I, Poon W, Assaker R, et al. Comparison of infection rate with the use of antibiotic-impregnated vs standard extraventricular drainage devices: a prospective, randomized controlled trial. Neurosurgery. 2012 Jul;71(1):6–13. doi: 10.1227/NEU.0b013e3182544e31. [DOI] [PubMed] [Google Scholar]
- 56.Park YG, Woo HJ, Kim E, Park J. Accuracy and Safety of Bedside External Ventricular Drain Placement at Two Different Cranial Sites : Kocher’s Point versus Forehead. Journal of Korean Neurosurgical Society. 2011 Oct;50(4):317–321. doi: 10.3340/jkns.2011.50.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rafiq MF, Ahmed N, Ali S. Effect of tunnel length on infection rate in patients with external ventricular drain. Journal of Ayub Medical College, Abbottabad : JAMC. 2011 Oct-Dec;23(4):106–107. [PubMed] [Google Scholar]


