Abstract
We previously reported that upregulation of mortalin, an Hsp70 family chaperone, is important for B-RafV600E tumor cells to bypass p21CIP1 expression, which is activated as a tumor suppressive mechanism in response to aberrant MEK/ERK activation (Wu et al., 2013). Interestingly, mortalin depletion induced p21CIP1 transcription not only in TP53-wild type but also TP53-mutated B-RafV600E cancer cells, suggesting the presence of an additional mechanism for p21CIP1 regulation. In the present study, using luciferase reporter truncation analysis in a TP53-mutated B-RafV600E cancer cell line, SK-MEL28, we identified a proximal p21CIP1 promoter region responsive to mortalin depletion. Interestingly, when Sp1-like cis-elements in this promoter region were mutagenized, the p21CIP1 promoter luciferase reporter was no longer responsive to mortalin depletion. Consistent with this, our ChIP analysis revealed that mortalin knockdown could induce Sp1 binding to p21CIP1 promoter in a MEK/ERK-dependent manner. Moreover, RNA interference of Sp1 substantially attenuated p21CIP1 expression induced by mortalin depletion in SK-MEL28 cells. Consistent with this observation in SK-MEL28 cells, Sp1 was necessary for the tamoxifen-regulated ΔRaf-1:ER to induce p21CIP1 transcription in U251 cells, in which TP53 is mutated. However, in contrast, Sp1 was not necessary for ΔRaf-1:ER to induce p21CIP1 transcription in LNCaP cells, in which TP53 is wild type. These data suggest that Sp1 may address TP53-independent p21CIP1 transcription in Raf/MEK/ERK-activated cancer cells and that its requirement in Raf/MEK/ERK-induced p21CIP1 transcription is subject to TP53 status.
Keywords: Mortalin, p21CIP1, Sp1, TP53, Raf/MEK/ERK
1. Introduction
p21CIP1/WAF (encoded by CDKN1A) is a cyclin-dependent kinase inhibitor that negatively regulates the Rb/E2F1 cell cycle regulatory machinery mainly through cyclin-dependent kinases 1 and 2 [1]. Therefore, p21CIP1 upregulation is usually associated with cell cycle arrest in the G0/G1 phase although it is also associated with G2/M phase arrest under certain conditions such as DNA damage responses [2]. Initially identified as a mediator of TP53 tumor suppression [3, 4], p21CIP1 is now appreciated as an important tumor suppressor along with other cyclin-dependent kinase inhibitors such as p16INK4A (encoded by CDKN2A) and p27KIP (encoded by CDKN1B) in numerous tumor biological contexts [5]. Particularly, p21CIP1 has been identified as a key mediator of cellular senescence induced upon sustained activation of the Raf/MEK/ERK pathway in different cell types [6-10]. Of note, this senescence phenomenon is understood as a potential tumor suppressive mechanism, which is triggered in response to aberrant activation of cell proliferative signaling, e.g., oncogenic alterations of Raf or its upstream activator Ras [11]. Deregulated Ras/Raf signaling is detected in a wide variety of cancers. Therefore, it is important to elucidate the mechanisms by which p21CIP1 expression is regulated in the context of Raf/MEK/ERK signaling.
Recently, we have reported that mortalin (HSPA9/GRP75/PBP74), a member of the heat shock protein 70 family [12], is increasingly expressed in the tumor tissues of melanoma patients and that mortalin upregulation down-modulates MEK/ERK activity in B-RafV600E melanoma cells so that the tumor cells can avoid senescence-like growth arrest induced by high MEK/ERK activity [13]. Importantly, induction of p21CIP1 transcription was a common response of these tumor cells to mortalin depletion, which was sufficient to mediate the senescence-like growth arrest [13]. This observation suggests that mortalin upregulation may be a mechanism that enables B-RafV600E tumor cells to bypass the tumor suppressive response triggered upon aberrant Raf/MEK/ERK activation. Very intriguingly, mortalin depletion induced p21CIP1 transcription not only in TP53-wild type but also TP53-mutated B-RafV600E melanoma cells [13], leading us to suspect the presence of an additional mechanism other than TP53 underlying the p21CIP1 regulation.
In this study, we sought to identify the TP53-independent mechanism that regulates p21CIP1 transcription induced by mortalin depletion in the human B-RafV600E melanoma cell line SK-MEL28. Using promoter truncation analysis and site-directed mutagenesis, we demonstrate that the consensus sequences for DNA binding of specificity protein 1 (Sp1), a zinc finger transcription factor that binds to GC-rich motifs of gene promoters [14], in the proximal p21CIP1 promoter are critical for the TP53-independent p21CIP1 transcription. Subsequently, we identify Sp1 as a transcription factor required for the p21CIP1 transcription using RNA interference and chromatin immunoprecipitation (ChIP) assays. Moreover, we evaluate the significance of the Sp1 consensus sequences and Sp1 in the context of Raf/MEK/ERK-mediated p21CIP1 transcription using different cell lines harboring wild type or mutated TP53. Our study demonstrates a novel mechanism that may address TP53-independent p21CIP1 transcription in Raf/MEK/ERK-activated cancer cells.
2. Materials and Methods
2.1. Cell culture and reagents
The human melanoma line SK-MEL28 (ATCC) was cultured in minimum essential medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum, 100 U penicillin and 100 μg streptomycin per ml. The human prostate cancer line LNCaP (ATCC) was cultured in phenol red-deficient RPMI1640 (Invitrogen) with 10% fetal bovine serum. The human glioma line U251 was maintained in minimum Eagle’s medium (Invitrogen) supplemented with 10% fetal bovine serum. The LNCaP and U251 cells stably expressing ΔRaf-1:ER (LNCaPRaf and U251Raf respectively) were previously described [15]. ΔRaf-1:ER was activated with 1 μM 4-hydroxytamoxifen (Sigma-Aldrich, St. Louis, MO).
2.2. RNA interference
Construction of pLL3.7 (ATCC) lentiviral small hairpin RNA (shRNA) expression systems that target mortalin was previously described [13]. Briefly, pLL3.7-shMort #1 and #2 target two different regions of human mortalin mRNA, GGCGAUAUGAUGAUCCUGAA and GCACAUUGUGAAGGAGUUCAA, respectively. Sp1 was depleted using two independent pLKO.1 lentiviral shRNA systems (Thermo Fisher Scientific, Waltham, MA, TRCN0000020444 and TRCN0000020447). TP53 was depleted using two independent pLKO.1 lentiviral shRNA systems (Sigma-Aldrich, TRCN0000003754 and TRCN0000003755). Specific knockdown of target proteins was confirmed by Western blot analysis.
2.3. Viral infection
For lentivirus production, 293T cells were co-transfected with pLKO.1 or pLL3.7, and packaging vectors, as previously described [15]. Viral supernatants were collected after 72 h and mixed with polybrene (Sigma-Aldrich) at 4-8 μg/ml before use. Viral titer was determined by scoring cells expressing GFP as previously described.
2.4. Luciferase reporters of p21CIP1 promoter
The p21CIP1 promoter luciferase reporters H2320 and S2260 contain p21CIP1 promoter DNA fragments spanning −2320 to +16 base pairs and −2260 to +16 base pairs, respectively [16]. The p21CIP1 promoter in S2260 was subject to SmaI partial digestion to generate the truncated reporters S1 (containing −1,008 to +16 base pairs), S2 (containing −2260 to −1008 and −127 to +16 base pairs), S3 (containing −127 to +16 base pairs), S4 (containing −112 to +16 base pairs), and S5 (containing −63 to +16 base pairs). Expected truncation was confirmed by sequencing. To measure reporter activity, cells transfected in 24-well plates using Lipofectamine LTX (Invitrogen) were analyzed using the Luciferase® Assay System (Promega, Madison, WI) according to the manufacturer’s instructions.
2.5. Site-directed mutagenesis of p21CIP1 promoter
Sp1, AP2 and E2F1 responsive elements in p21CIP1 promoter (−112 to −63 base pairs) were mutated using QuikChange Lightning site-directed mutagenesis kit (Stratagene, La Jolla, CA) and the following primers: GAGCTAACATAACCCGAATGGCGCGGTGGGCCGAGC and GCTCGGCCCACCGCGCCATTCGGGTTATGTTAGCTC for Sp1 responsive element #2 (Sp1-2); GTGGGCCGAGCGCGGGTCTTACCTCCTTGAGGCGGGCC and GGCCCGCCTCAAGGAGGTAAGACCCGCGCTCGGCCCAC for Sp1 responsive element #3 (Sp1-3); CCCGCCTCCTTGAGGTAAGCCCGGGCGGGGCGGTTG and CAACCGCCCCGCCCGGGCTTACCTCAAGGAGGCGGG for Sp1 responsive element #4 (Sp1-4); GTGGGCCGAGCGCGGGTCTTACCTCCTTGAGGTAAGCC and GGCTTACCTCAAGGAGGTAAGACCCGCGCTCGGCCCAC for Sp1-3 and Sp1-4; CCCGGGCGGCGCGGTGTTCCGAGCGCGGGTCCCG and CGGGACCCGCGCTCGGAACACCGCGCCGCCCGGG for AP2 responsive element; CTAACATAACCCGGGCGGCTAGGTGGGCCGAGCGCGGG and CCCGCGCTCGGCCCACCTAGCCGCCCGGGTTATGTTAG for E2F1 responsive element.
2.6. RT-PCR
Total RNA was isolated from cells using TRIzol® reagent (Invitrogen) and was reverse-transcribed using Superscript III (Invitrogen) and oligo-dT according to the manufacturer’s protocols. Resulting cDNA was amplified by PCR using the following primers: CTGGAGACTCTCAGGGTCGAA and CCAGCACTCTTAGGAACCTCTCA for p21CIP1; ACAGGTGAGCTTGACCTCAC and GTTGGTTTGCACCTGGTATG for Sp1; GTCCTCTCCCAAGTCCACAC and GGGAGACCAAAAGCCTTCAT for β-actin.
2.7. Immunoblot analysis
Cells harvested at various times were lysed and analyzed using the BCA reagent to determine the protein concentration (Pierce,Rockford, IL). Immunoblotting was performed as previously described [15]. Briefly, 50-100 ug protein was resolved by SDS-PAGE, transferred to a polyvinylidene difluoride membrane filter (Bio-Rad, Hercules, CA), and stained with Fast Green reagent (Thermo Fisher Scientific). Membrane filters were then blocked in 0.1 M Tris (pH 7.5)-0.9% NaCl-0.05% Tween 20 with 5% nonfat dry milk, and incubated with appropriate antibodies. Antibodies were diluted as follows: p21CIP1, 1:2,500; Sp1, 1:500; ERK1/2, 1:2,500; TP53, 1:2,000 (Santa Cruz Biotech, Santa Cruz, CA); β-tubulin, 1:5,000; phospho-ERK1/2 (T202/Y204 for ERK1 and T183/Y185 for ERK2), 1:2,500 (Cell Signaling Technology, Danvers, MA); β-actin, 1:20,000 (Sigma).
2.8. Chromatin Immunoprecipitation (ChIP)
ChIP was performed according to Weinmann and Farnham [17] and Metivier et al [18], with minor modification. Briefly, SK-MEL28 cells seeded on 100-cm dishes for 12-18 h were infected with pLL3.7 lentivirus. Cells were cross-linked with 1% formaldehyde for 10 min and then the reactions were quenched by adding 1 M glycine to the final concentration, 0.125 M. Cells were then scraped, washed in 1X PBS, and lysed in the lysis buffer containing 5 mM PIPES [pH 8], 85 mM KCl, 0.5% NP40, and 15 mM sodium butyrate. Nuclei were then collected and lysed in the lysis buffer containing 50 mM Tris-HCl [pH 8], 10 mM EDTA, 1% SDS, and 15 mM sodium butyrate. Chromatin was sonicated to an average size of approximately 500 bp, diluted in immunoprecipitation (IP) dilution buffer (0.92% TritonX-100, 0.008% SDS, 1 mM EDTA, 13.9 mM Tris-HCl [pH 8], 13.9 mM NaCl, 12.5 mM sodium butyrate), and precleared with protein A/G-agarose beads (Santa Cruz Biotechnology). Protein was quantified using Bradford assay (Biorad, Hercules, CA). 1% of starting chromatin was used as input. Overnight IPs were performed using Sp1-specific antibody (Santa Cruz Biotechnology, sc-59X) and IgG control (Santa Cruz Biotechnology, sc-2027). All beads were preblocked with 1 mg/ml bovine serum albumin (Fisher scientific, Waltham, MA) and 1 mg/ml salmon sperm DNA (Invitrogen) prior to IP. Immunoprecipitates were incubated at 67°C for approximately 4 h to reverse the formaldehyde crosslinking. DNA was then purified using the DNA purification kit (Fermentas, Waltham, MA) and subjected to qPCR using AGCCAGAAAGGGGGCTCA and GAACCTCGCGTGCTGCA, which amplify −374 to −199 base pairs of p21CIP1 promoter. Standard and melt curves were plotted to validate primer pairing and PCR reaction. Percent input method [19] was used to normalize ChIP-qPCR data.
2.9. Statistical analysis
Unless otherwise indicated, two-tailed unpaired student’s t-test was used to assess the statistical significance of two data sets. Multiple data sets obtained from the ChIP assay were analyzed by two-way ANOVA (analysis of variance) with Bonferroni post-tests using GraphPad Prism software. p values of < 0.05 were considered statistically significant.
3. Results
3.1. Mortalin depletion activates the −112 to −63 base pair region of p21CIP1 promoter in SK-MEL28 cells
To study the possibility that a transcription factor other than TP53 can mediate p21CIP1 transcription in response to mortalin depletion in B-RafV600E cancer cells, we used the human melanoma line SK-MEL28. SK-MEL28 cells harbor B-RafV600E and a homozygous L145R mutation in the DNA binding domain of TP53 (Cancer Genome Project at Sanger Institute, http://www.sanger.ac.uk/) but still express p21CIP1 at protein and mRNA levels in response to mortalin depletion (Fig 1A and 1B).
Figure 1. Mortalin depletion activates proximal p21CIP1 promoter region in SK-MEL28 cells.
(A and B) Cells were infected for 3 days with lentiviral pLL3.7 expressing two different shRNAs targeting mortalin mRNA (shMort#1 and shMort#2). Uninfected (mock) or empty pLL3.7 virus-infected cells were controls. (A) Western blotting of total cell lysates. Right panel - densitometry of p21CIP1 signals normalized to β-actin signals. (B) qPCR analysis of p21CIP1 mRNA levels. Data (mean ± SEM) are fold changes relative to mock. (C) Cells were co-transfected for 3 days with pLL3.7-shMort#1 and p21CIP1-promoter-luciferase reporters (H2320, S2260, S1, S2, S3, S4 and S5) before determining luciferase activity. pGL2 is the control for luciferase vectors. Data (mean ± SEM) are fold changes relative to co-transfection with pGL2 and pLL3.7. Fold changes by shMort are numerically indicated. Experiment was performed three times with similar results. *p < 0.05.
First, we determined how a series of luciferase reporter constructs that contain different length of p21CIP1 promoter DNA respond to mortalin depletion. When SK-MEL28 cells were co-transfected with these reporters and a shRNA vector that specifically depletes mortalin (shMort), the longer reporters H2320 and S2260, containing 2320 and 2260 base pairs of p21CIP1 promoters upstream the transcription initiation site and thus two and one TP53 responsive elements respectively, robustly increased their reporter activities (Fig. 1C). Interestingly, the shorter reporters, S1, S2, S3, and S4 also displayed substantially increased luciferase activity in response to mortalin depletion, albeit lower than H2320 and S2260 (Fig. 1C). Moreover, although S2 contained the second TP53 responsive element as in S2260, it did not show any higher inducibility than S1, S3, and S4 (Fig. 1C). Of note, as compared between S4 and S5, removal of the promoter region spanning −112 to −63 base pairs completely abolished shMort-induced reporter activity (Fig. 1C). These data indicate that a regulatory element(s) located between −112 to −63 base pairs of the p21CIP1 promoter is important for mortalin depletion to induce p21CIP1 transcription.
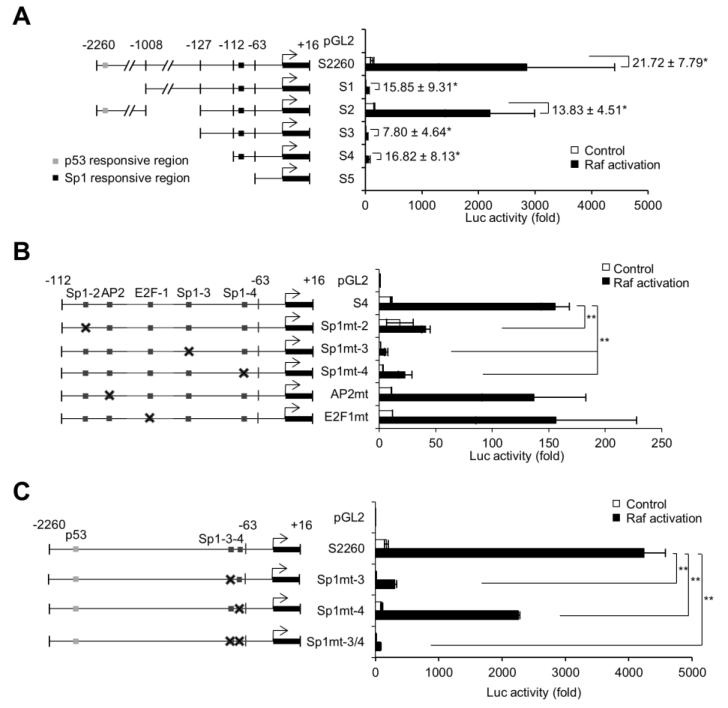
3.2. Sp1 responsive elements in the proximal p21CIP1 promoter region is required for mortalin depletion to induce p21CIP1 transcription in SK-MEL28 cells
We next examined potential transcriptional regulatory element(s) present between −112 and −63 base pairs of p21CIP1 promoter for their responsiveness to mortalin depletion by site-directed mutagenesis. Our literature review [20-22] and consensus sequence analysis using MatInspector (http://www.genomatix.de/) revealed that this p21CIP1 promoter region contains potential regulatory elements responsive to the transcription factors, AP2 and E2F1, and three potential Sp1 responsive elements, which were designated as Sp1-2, Sp1-3, and Sp1-4 (Fig. 2A). When these sequences were individually mutagenized in the S4 reporter which harbors the −112 to −63 base pair region of p21CIP1 promoter, mutations in the AP2 and E2F1 consensus sequences did not significantly reduce its responsiveness to mortalin depletion in SK-MEL28 cells (Fig. 2B). In contrast, mutation in the Sp1-3 site almost abrogated shMort-induced reporter activity of S4 while mutation in the Sp1-4 site significantly attenuated S4 activity (Fig. 2B). However, the effect of Sp1-2 site mutation was relatively weak. Mutation of the Sp1-3 site also significantly suppressed shMort-induced reporter activity of the longer reporter S2260, which contains the TP53 responsive element (Fig. 2C). Moreover, double mutation of both Sp1-3 and Sp1-4 sites completely abolished responsiveness of S2260 to mortalin depletion (Fig. 2C). These data suggest that the Sp1-like consensus sequences have roles for mortalin depletion to induce p21CIP1 transcription, wherein the Sp1-3 site has a critical role while the adjacent Sp1 sites have auxiliary roles.
Figure 2. Mortalin depletion induces p21CIP1 transcription via Sp1-like cis-elements in the proximal p21CIP1 promoter in SK-MEL28 cells.
(A) Depicted are potential Sp1, AP2, and E2F1 responsive elements in the proximal p21CIP1 promoter (−112 to −63) and their mutagenized sequences in this study. (B) Luciferase reporter activity of S4 (containing −112 to −63 base pair region of p21CIP1 promoter) and its derivatives after co-transfection with pLL3.7 or shMort#1 for 3 days. (C) Luciferase reporter activity of S2260 (containing −2260 to −63 base pair region of p21CIP1 promoter) and its derivatives after co-transfection with pLL3.7 or shMort#1 for 3 days. pGL2 is the control for luciferase reporters. Data (mean ± SEM) are fold changes relative to co-transfection with pGL2 and pLL3.7. Experiment was performed three times with similar results. *p < 0.05, **p < 0.01
3.3. Sp1 is necessary for mortalin depletion to induce p21CIP1 expression in SK-MEL28 cells
In conjunction with our previous observation that TP53 depletion did not affect p21CIP1 expression induced by mortalin depletion in SK-MEL28 cells [13] and previous studies indicating Sp1 involvement in p21CIP1 transcriptional regulation [23-26], the data above led us to examine Sp1 for its role in the TP53-independent p21CIP1 regulation in SK-MEL28 cells. We depleted Sp1 using two independent lentiviral shRNA constructs that target different Sp1 mRNA regions and examined the Sp1 knockdown effects on shMort-induced p21CIP1 expression. These two Sp1 knockdown systems substantially depleted Sp1 without significantly affecting SK-MEL28 cell viability and, indeed, abrogated shMort-induced p21CIP1 expression at both protein and mRNA levels (Fig. 3A and 3B). Consistent with this, Sp1 knockdown also abrogated shMort-induced reporter activity of S2260 in SK-MEL28 cells (Fig. 3C). Of note, Sp1 knockdown also decreased the basal reporter activity of S2260 in SK-MEL28 cells, suggesting that Sp1 may also have an effect on basal p21CIP1 transcription. These data demonstrate that Sp1 is necessary for mortalin depletion to induce p21CIP1 transcription in SK-MEL28 cells.
Figure 3. Sp1 mediates shMort-induced p21CIP1 transcription via a physical interaction with the p21CIP1 promoter in a MEK/ERK-dependent manner in SK-MEL28 cells.
(A) Western blotting of total lysates of cells co-infected for 3 days with shMort #1 and two different Sp1-targeting shRNAs (shSp1 #1 and shSp1 #2). pLKO.1 and pLL3.7 are controls for shSp1 and shMort, respectively. (B) RT-PCR analysis of p21CIP1 and Sp1 mRNA levels in cells in (A). (C) Luciferase reporter activity of S2260 after co-transfection with pLL3.7-shMort #1 and pLKO.1-shSp1 #1, or pLKO.1-shSp1 #2 for 3 days. Data (mean ± SEM) are fold changes relative to the control co-transfection (S2260, pLL3.7, and pLKO.1) which were obtained from a representative experiment performed in triplicate. *P < 0.05. (D) ChIP analysis of SP1 interaction with p21CIP1 promoter in cells infected for 3 days with pLL3.7 or shMort #1 in the presence/absence of 40 nM AZD6244. An equal volume of DMSO was used as the control for AZD6244. Data (mean ± SEM) are fold changes in Sp1-DNA interaction relative to untreated pLL3.7-infected cells. Data were obtained from three independent experiments performed in triplicate. *p < 0.05 (two-way ANOVA with Bonferroni post-tests). (E) Validation of the experimental condition for (D) by Western blotting of total cell lysates.
3.4. Mortalin depletion induces Sp1 interaction with p21CIP1 promoter in a MEK/ERK-dependent manner in SK-MEL28 cells
Knowing the necessity of Sp1 for mortalin depletion to induce p21CIP1 promoter activity in SK-MEL28 cells, we determined whether mortalin depletion induced a physical interaction between Sp1 and p21CIP1 promoter. Indeed, our ChIP analysis using an anti-Sp1 antibody and DNA oligomers amplifying the proximal p21CIP1 promoter revealed that mortalin depletion substantially increased Sp1 interaction with p21CIP1 promoter (Fig. 3D). We also determined whether this physical interaction depended upon the MEK/ERK activity because the MEK/ERK activity was necessary for mortalin depletion to induce p21CIP1 transcription in SK-MEL28 cells [13] (also shown in Fig. 3E). When mortalin was depleted in SK-MEL28 cells in the presence of the MEK1/2-specific inhibitor AZD6244, the interaction between Sp1 and the p21CIP1 promoter was significantly reduced, as determined by the ChIP analysis (Fig. 3D). Importantly, under this condition, Sp1 protein levels were not affected by mortalin depletion or AZD6244 (Fig. 3E), further suggesting the significance of physical interaction for the regulation. These data demonstrate that Sp1 can regulate p21CIP1 transcription via its interaction with p21CIP1 promoter in response to mortalin depletion in B-RafV600E cancer cells and that mortalin depletion regulates this physical interaction via the MEK/ERK pathway.
3.5. The requirement of Sp1 for Raf/MEK/ERK-induced p21CIP1 expression may be subject to TP53 status
We further determined whether the role of Sp1 was affected by TP53 status in the context of the Raf/MEK/ERK pathway-mediated p21CIP1 transcription using U251 glioma and LNCaP prostate cancer cells, which express TP53R273H and wild type TP53, respectively (Cancer Genome Project at Sanger Institute, http://www.sanger.ac.uk/). We previously demonstrated that these cell lines maintain relatively low basal MEK/ERK activity and rapidly express p21CIP1 in response to Raf/MEK/ERK activation [15]. For precise control of this response, we generated LNCaP and U251 cells stably expressing ΔRaf-1:ER, the CR3 catalytic domain of Raf-1 fused to the hormone binding domain of the estrogen receptor that can be activated by the estrogen analogue, 4-hydroxytamoxifen [27]. Activation of ΔRaf-1:ER was sufficient to induce p21CIP1 expression via MEK/ERK in these cells [15].
When Sp1 was depleted by RNA interference in U251 cells, the capacity of ΔRaf-1:ER activation to induce p21CIP1 expression at protein and mRNA levels was substantially attenuated (Fig. 4A and 4B). Consistent with this, Sp1 knockdown also strongly suppressed Raf-induced p21CIP1 promoter activity, as determined by the S2260 reporter (Fig. 4C), indicating that Sp1 is necessary for Raf/MEK/ERK to induce p21CIP1 transcription in U251 cells. Of note, similarly as in SK-MEL28 cells, Sp1 depletion also decreased the basal activity of S2260 in U251 cells (Fig. 4C), further supporting the requirement of Sp1 for p21CIP1 transcription in these TP53-mutated cells.
Figure 4. Sp1 is necessary to induce p21CIP1 transcription in U251 but not in LNCaP cells.
(A) Western blotting of total cell lysates after infection with lentiviral shSp1 #1 or shSp1 #2 for 3 days. ΔRaf-1:ER was activated by 1 μM 4-hydroxytamoxifen for 2 days. Right - densitometry of p21CIP1 signals normalized to β-actin signals. (B) RT-PCR analysis of p21CIP1 mRNA levels in cells in (A). (C) Luciferase reporter activity of S2260 after co-transfection (3 days) with pLKO.1-shSp1 #1 or #2 under ΔRaf-1:ER-activated condition (2 days). (D) Western blotting of total lysates of LNCaPRaf treated as described in (A). (E) Western blotting of total cell lysates after infection with lentiviral shTP53 #1 or #2 for 2 days. pLKO.1 is the control for shTP53. ΔRaf-1:ER was activated by 1 μM 4-hydroxytamoxifen for 2 days. Right - densitometry of p21CIP1 signals normalized to β-actin signals from (D) and (E). (F) RT-PCR analysis of p21CIP1 mRNA levels in cells in (D) and (E). (G) Luciferase reporter activity of S2260 after co-transfection (3 days) with pLKO.1-shSp1 or pLKO.1-shTP53 under ΔRaf-1:ER-activated condition (2 days). All data (mean ± SEM) are fold changes relative to untreated control cells and were obtained from a representative experiment performed in triplicate. *p < 0.05
In LNCaP cells, Sp1 knockdown also attenuated the capacity of ΔRaf-1:ER activation to induce p21CIP1 expression (Fig 4D). However, its effect was not as substantial as TP53 knockdown effects (Fig. 4E; see densitometry for comparison). Consistent with this, Sp1 knockdown did not attenuate Raf-induced p21CIP1 mRNA levels or S2260 reporter activity as effectively as TP53 knockdown (Fig. 4F and 4G). Moreover, Sp1 knockdown did not significantly augment the inhibitory effects of TP53 knockdown on Raf-induced p21CIP1 mRNA levels or S2260 reporter activity (Fig. 4F and 4G). Therefore, Sp1 is not necessary for Raf/MEK/ERK to induce p21CIP1 transcription in this TP53 wild type cell line. These data suggest that the requirement of Sp1 for Raf/MEK/ERK-mediated p21CIP1 transcription may be determined by TP53 status.
3.6. The Sp1 consensus sequences in p21CIP1 promoter are necessary for Raf/MEK/ERK to induce p21CIP1 transcription not only in TP53-mutated but also in TP53-wild type cells
Observing the insignificance of Sp1 in Raf/MEK/ERK-regulated p21CIP1 transcription in TP53 wild type cells, we determined significance of the Sp1-responsive elements identified above in TP53 wild type cells. Indeed, our evaluation of the p21CIP1 promoter truncation reporters further corroborated the significance of TP53 in LNCaP cells because the S2260 and S2 reporters, which contain TP53-responsive elements, dramatically increased luciferase activity in response to Raf activation whereas the other truncation mutants deficient of TP53-responsive element did not (Fig. 5A).
Figure 5. The Sp1-like cis-elements in the proximal p21CIP1 promoter are necessary for Raf/MEK/ERK-induced p21CIP1 transcription in LNCaP cells.
(A) Luciferase reporter activity of S2260, S1, S2, S3, S4, and S5 in cells with or without ΔRaf-1:ER activation for 2 days. (B) Luciferase reporter activity of S4 (containing −112 to −63 base pair region of p21CIP1 promoter) and its derivatives in cells with or without ΔRaf-1:ER activation. (C) Luciferase reporter activity of S2260 (containing −2260 to −63 base pair region of p21CIP1 promoter) and its derivatives in cells with or without ΔRaf-1:ER activation. Data (mean ± SEM) are fold changes relative to untreated pGL2 control. Fold changes by Raf activation are numerically indicated. All data were obtained from three independent experiments performed in triplicate. *p < 0.05
Nevertheless, although the net luciferase activity was low, the S1, S3, and S4 reporters still exhibited significant fold increases in luciferase activity in response to Raf activation (about 16, 8, and 17 folds, respectively; Fig. 5A), suggesting that the proximal p21CIP1 promoter spanning −112 to −63 base pairs still has a role in TP53 wild type cells. Indeed, site-directed mutagenesis of the consensus sequences for Sp1, but not for AP2 and E2F1, in this region significantly suppressed the reporter activity of S4 with the effect of Sp1-3 site mutation being most prominent (Fig. 5B). Moreover, mutations of these Sp1 consensus sequences could also suppress the activity of a reporter in which the TP53-responsive element is present (Fig. 5C). Consistent with the results above, mutation of the Sp1-3 site significantly suppressed Raf-induced reporter activity of S2260 while double-mutation of Sp1-3 and Sp1-4 sites completely abolished its responsiveness to Raf activation (Fig. 5C). These data strongly suggest that, although Sp1 is unnecessary, the Sp1 consensus sequences are necessary for Raf/MEK/ERK to induce p21CIP1 transcription in LNCaP cells.
4. Discussion
The present study demonstrates that Sp1 can mediate p21CIP1 transcription in response to Raf/MEK/ERK activation when functional TP53 is absent. Genetic or epigenetic alteration of CDKN1A is rare and p21CIP1 silencing in cancer has been attributed mainly to TP53 inactivation, because it occurs widely in cancer. TP53 has been established as a key regulator of p21CIP1 transcription by virtue of its regulation through the two TP53 responsive elements (−2281 to −2261 and −1393 to −1374 base pairs) present in the distal p21CIP1 promoter [3, 4]. In the context of Raf/MEK/ERK-mediated p21CIP1 transcription, TP53 is also important [16] and its inactivation has been mainly addressing p21CIP1 silencing in Raf/MEK/ERK-activated cancers, including melanoma [28]. Nevertheless, TP53-independent p21CIP1 transcription has also been reported, for which different transcription factors are employed in different biological contexts, e.g., ELK-1 in sodium arsenite-exposed human keratinocyte HaCaT cells [29], C/EBP and Ets transcription factors in MAPK-activated human hepatoma HepG2 cells [30], and STAT1 and 3 in IL-6 stimulated osteosarcoma MG63 cells [31]. Particularly in the context of B-RafV600E melanoma cells, it has been recently demonstrated that the Forkhead Box O transcription factors can mediate p21CIP1 transcription in response to Raf/MEK and JNK activation [32]. In line with these findings, the present study extends the mechanisms underlying TP53-independent p21CIP1 transcription in Raf/MEK/ERK-activated cells by identifying the roles of Sp1 and Sp1-like cis-elements in the p21CIP1 proximal promoter (a model depicted in Fig. 6).
Figure 6. A proposed role for Sp1 in p21CIP1 transcription in MEK/ERK-activated cancer cells.
We previously demonstrated that hyper activation of MEK/ERK, via mortalin depletion, induces p21CIP1 transcription not only in TP53-wild type but also in TP53-mutated B-RafV600E cancer cells [13]. The present study demonstrates that Sp1 is a transcription factor that can mediate p21CIP1 transcription in TP53-deficient cancer cells in response to MEK/ERK activation. Therefore, multiple mechanisms in addition to TP53 inactivation may be implicated in p21CIP1 transcriptional silencing in MEK/ERK-activated cancer cells.
Our studies using cells with different TP53 status suggest that the role of Sp1 in p21CIP1 transcription may be subject to TP53 status. Of note, it was previously reported that TP53 can cooperate with Sp1 to synergistically mediate p21CIP1 transcription, wherein the presence of Sp1 was important for maximal TP53 activity [20]. In contrast, TP53-independent role of Sp1 in p21CIP1 transcription was also reported [33]. Our observation appears to be consistent with the latter study, although it is possible that different cellular contexts may induce different mechanisms that determine the relationship between Sp1 and TP53. Of note, although Sp1 was dispensable for Raf/MEK/ERK-mediated p21CIP1 transcription in TP53-wild type cells, the specific Sp1-like cis-element in the p21CIP1 proximal promoter was necessary for Raf/MEK/ERK to induce p21CIP1 transcription in TP53-wild type cells, suggesting a possibility that this element may be activated by other transcription factors when Raf/MEK/ERK is activated in TP53 wild type cells. Indeed, this speculation is consistent with previous observations that this regulatory element can also be bound and activated by Kruppel-like factor 4 [34] or Sp3 [35]. It will be important in a future study to determine whether these transcription factors are involved in Raf/MEK/ERK-mediated p21CIP1 transcription in TP53-wild type cells.
Our data suggest that Sp1 may substitute for TP53 to mediate p21CIP1 transcription in response to Raf/MEK/ERK activation when functional TP53 is absent. Therefore, there should be a mechanism by which the Raf/MEK/ERK pathway activates Sp1. Sp1 activity can be regulated by various post-translational modifications, including phosphorylation, acetylation, glycosylation, and sumoylation [36-40]. Of note, it has been reported that ERK1/2 can phosphorylate Sp1 on Threonines 453 and 739 in vitro and in vivo [41]. These Sp1 phosphorylations were also detected when perifosine induced p21CIP1 transcription via MEK/ERK [24]. Nevertheless, we did not detect any significant changes in the phosphorylation status of these two threonines in our studies, whether determined by mortalin knockdown or Raf activation (data not shown). Similarly, no change in phosphorylation state of Sp1 was observed when NGF induced p21CIP1 via ERK1/2 in PC12 cells [42]. Currently, we do not know by what mechanism(s) Raf/MEK/ERK activates Sp1 to increase its interaction with the p21CIP1 promoter and how these mechanisms are affected by TP53 status.
We have proposed mortalin as a potential therapeutic target for B-RafV600E cancer because mortalin depletion effectively induced p21CIP1 expression by upregulating MEK/ERK activity in these cells [13]. Very intriguingly, this effect was TP53-indpendent in TP53-inactivated cells although TP53-dependent in TP53 wild type cells. The data in this study suggest that Sp1 addresses a mechanism by which mortalin depletion induces p21CIP1 expression in B-RafV600E cancer cells deficient of functional TP53, advancing our understanding of the mechanisms by which mortalin depletion suppresses B-RafV600E cancer. Importantly, TP53 reactivation has been recently proposed as a novel therapeutic strategy [43, 44], wherein derepression of p21CIP1 transcription is a key mechanism to induce growth arrest [28]. Given that this strategy would be limited to the tumors carrying wild type TP53 [45], our findings have potential significance in that Sp1 may be exploited for derepression of p21CIP1 transcription in TP53-inactivated tumor cells. Besides, Sp1 has been known for its role in p21CIP1 transcription induced by various drugs, including butyrate, histone deacetylase inhibitor, alkylphospholipids, phenethyl isothiocyanate, and sulforaphane, in many different cancer types [23-26, 33]. Our finding may therefore raise an interesting question whether Raf/MEK/ERK and mortalin are involved in the Sp1 activation induced by these drugs.
Highlights.
Sp1 can mediate TP53-independent p21CIP1 transcription in Raf/MEK/ERK-activated cancer cells.
The requirement of Sp1 in Raf/MEK/ERK-induced p21CIP1 transcription is subject to TP53 status.
The Sp1 consensus sequences in proximal p21CIP1 promoter are necessary for Raf/MEK/ERK-induced p21CIP1 transcription regardless of TP53 status.
Our study demonstrates a novel mechanism that may address TP53-independent p21CIP1 transcription in Raf/MEK/ERK-activated cancer cells
Acknowledgements
We thank Dr. Phyllis LuValle (Univ. of Calgary) for p21CIP1 promoter luciferase reporters H2320 and S2260; Drs. Lisa Cirillo and Akua Oduro for ChIP; and Mansi Karkhanis’s thesis committee and Park lab members for helpful discussion and technical assistance. This work was supported by the National Cancer Institute (R01CA138441) and American Cancer Society (RSGM-10-189-01-TBE) to J.I.P.
Abbreviations
- Sp1
specificity protein 1
- ERK
extracellular signal-regulated kinase
- ChIP
Chromatin immunoprecipitation
Footnotes
Publisher's Disclaimer: Thisis a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors declare no conflict of interest.
References
- [1].Warfel NA, El-Deiry WS. Curr Opin Oncol. 2013;25:52–58. doi: 10.1097/CCO.0b013e32835b639e. [DOI] [PubMed] [Google Scholar]
- [2].Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- [3].el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- [4].Waldman T, Kinzler KW, Vogelstein B. Cancer Res. 1995;55:5187–5190. [PubMed] [Google Scholar]
- [5].Abbas T, Dutta A. Nat Rev Cancer. 2009;9:400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lin S, Yang J, Elkahloun AG, Bandyopadhyay A, Wang L, Cornell JE, Yeh IT, Agyin J, Tomlinson G, Sun LZ. Mol Biol Cell. 2012;23:1569–1581. doi: 10.1091/mbc.E11-10-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Thaler S, Hahnel PS, Schad A, Dammann R, Schuler M. Cancer Res. 2009;69:1748–1757. doi: 10.1158/0008-5472.CAN-08-1377. [DOI] [PubMed] [Google Scholar]
- [8].Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- [9].Zhu J, Woods D, McMahon M, Bishop JM. Genes Dev. 1998;12:2997–3007. doi: 10.1101/gad.12.19.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lin AW, Barradas M, Stone JC, van Aelst L, Serrano M, Lowe SW. Genes Dev. 1998;12:3008–3019. doi: 10.1101/gad.12.19.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mooi WJ, Peeper DS. N Engl J Med. 2006;355:1037–1046. doi: 10.1056/NEJMra062285. [DOI] [PubMed] [Google Scholar]
- [12].Daugaard M, Rohde M, Jaattela M. FEBS Lett. 2007;581:3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- [13].Wu PK, Hong SK, Veeranki S, Karkhanis M, Starenki D, Plaza JA, Park JI. Mol Cell Biol. 2013;33:4051–4067. doi: 10.1128/MCB.00021-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Suske G. Gene. 1999;238:291–300. doi: 10.1016/s0378-1119(99)00357-1. [DOI] [PubMed] [Google Scholar]
- [15].Hong SK, Yoon S, Moelling C, Arthan D, Park JI. J Biol Chem. 2009;284:33006–33018. doi: 10.1074/jbc.M109.012591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Beier F, Taylor AC, LuValle P. J Biol Chem. 1999;274:30273–30279. doi: 10.1074/jbc.274.42.30273. [DOI] [PubMed] [Google Scholar]
- [17].Weinmann AS, Farnham PJ. Methods. 2002;26:37–47. doi: 10.1016/S1046-2023(02)00006-3. [DOI] [PubMed] [Google Scholar]
- [18].Metivier R, Penot G, Hubner MR, Reid G, Brand H, Kos M, Gannon F. Cell. 2003;115:751–763. doi: 10.1016/s0092-8674(03)00934-6. [DOI] [PubMed] [Google Scholar]
- [19].Parker JB, Palchaudhuri S, Yin H, Wei J, Chakravarti D. Mol Cell Biol. 2012;32:1654–1670. doi: 10.1128/MCB.06033-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Koutsodontis G, Tentes I, Papakosta P, Moustakas A, Kardassis D. J Biol Chem. 2001;276:29116–29125. doi: 10.1074/jbc.M104130200. [DOI] [PubMed] [Google Scholar]
- [21].Zeng YX, Somasundaram K, el-Deiry WS. Nat Genet. 1997;15:78–82. doi: 10.1038/ng0197-78. [DOI] [PubMed] [Google Scholar]
- [22].Gartel AL, Najmabadi F, Goufman E, Tyner AL. Oncogene. 2000;19:961–964. doi: 10.1038/sj.onc.1203411. [DOI] [PubMed] [Google Scholar]
- [23].Sowa Y, Orita T, Hiranabe-Minamikawa S, Nakano K, Mizuno T, Nomura H, Sakai T. Ann N Y Acad Sci. 1999;886:195–199. doi: 10.1111/j.1749-6632.1999.tb09415.x. [DOI] [PubMed] [Google Scholar]
- [24].De Siervi A, Marinissen M, Diggs J, Wang XF, Pages G, Senderowicz A. Cancer Res. 2004;64:743–750. doi: 10.1158/0008-5472.can-03-2505. [DOI] [PubMed] [Google Scholar]
- [25].Wang LG, Liu XM, Fang Y, Dai W, Chiao FB, Puccio GM, Feng J, Liu D, Chiao JW. Int J Oncol. 2008;33:375–380. [PubMed] [Google Scholar]
- [26].Chew YC, Adhikary G, Wilson GM, Xu W, Eckert RL. J Biol Chem. 2012;287:16168–16178. doi: 10.1074/jbc.M111.305292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Samuels ML, Weber MJ, Bishop JM, McMahon M. Mol Cell Biol. 1993;13:6241–6252. doi: 10.1128/mcb.13.10.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ji Z, Njauw CN, Taylor M, Neel V, Flaherty KT, Tsao H. J Invest Dermatol. 2012;132:356–364. doi: 10.1038/jid.2011.313. [DOI] [PubMed] [Google Scholar]
- [29].Shin SY, Kim CG, Lim Y, Lee YH. J Biol Chem. 2011;286:26860–26872. doi: 10.1074/jbc.M110.216721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Park JS, Qiao L, Gilfor D, Yang MY, Hylemon PB, Benz C, Darlington G, Firestone G, Fisher PB, Dent P. Mol Biol Cell. 2000;11:2915–2932. doi: 10.1091/mbc.11.9.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bellido T, O’Brien CA, Roberson PK, Manolagas SC. J Biol Chem. 1998;273:21137–21144. doi: 10.1074/jbc.273.33.21137. [DOI] [PubMed] [Google Scholar]
- [32].de Keizer PL, Packer LM, Szypowska AA, Riedl-Polderman PE, van den Broek NJ, de Bruin A, Dansen TB, Marais R, Brenkman AB, Burgering BM. Cancer Res. 2010;70:8526–8536. doi: 10.1158/0008-5472.CAN-10-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nakano K, Mizuno T, Sowa Y, Orita T, Yoshino T, Okuyama Y, Fujita T, Ohtani-Fujita N, Matsukawa Y, Tokino T, Yamagishi H, Oka T, Nomura H, Sakai T. J Biol Chem. 1997;272:22199–22206. doi: 10.1074/jbc.272.35.22199. [DOI] [PubMed] [Google Scholar]
- [34].Zhang W, Geiman DE, Shields JM, Dang DT, Mahatan CS, Kaestner KH, Biggs JR, Kraft AS, Yang VW. J Biol Chem. 2000;275:18391–18398. doi: 10.1074/jbc.C000062200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Koutsodontis G, Moustakas A, Kardassis D. Biochemistry. 2002;41:12771–12784. doi: 10.1021/bi026141q. [DOI] [PubMed] [Google Scholar]
- [36].Tan NY, Khachigian LM. Mol Cell Biol. 2009;29:2483–2488. doi: 10.1128/MCB.01828-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chu S, Ferro TJ. Gene. 2005;348:1–11. doi: 10.1016/j.gene.2005.01.013. [DOI] [PubMed] [Google Scholar]
- [38].Waby JS, Chirakkal H, Yu C, Griffiths GJ, Benson RS, Bingle CD, Corfe BM. Mol Cancer. 2010;9:275. doi: 10.1186/1476-4598-9-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Majumdar G, Harrington A, Hungerford J, Martinez-Hernandez A, Gerling IC, Raghow R, Solomon S. J Biol Chem. 2006;281:3642–3650. doi: 10.1074/jbc.M511223200. [DOI] [PubMed] [Google Scholar]
- [40].Spengler ML, Brattain MG. J Biol Chem. 2006;281:5567–5574. doi: 10.1074/jbc.M600035200. [DOI] [PubMed] [Google Scholar]
- [41].Milanini-Mongiat J, Pouyssegur J, Pages G. J Biol Chem. 2002;277:20631–20639. doi: 10.1074/jbc.M201753200. [DOI] [PubMed] [Google Scholar]
- [42].Yan GZ, Ziff EB. J Neurosci. 1997;17:6122–6132. doi: 10.1523/JNEUROSCI.17-16-06122.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, Newman J, Reczek EE, Weissleder R, Jacks T. Nature. 2007;445:661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- [44].Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sullivan KD, Gallant-Behm CL, Henry RE, Fraikin JL, Espinosa JM. Biochim Biophys Acta. 2012;1825:229–244. doi: 10.1016/j.bbcan.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]