Abstract
Background & Aims
Little is known about geographic variations in health care for patients with cirrhosis. We studied geographic and hospital-level variations in care of patients with cirrhosis in the United States (US), using inpatient mortality as an outcome for comparing hospitals. We also aimed to identify features of patients and hospitals associated with lower mortality.
Methods
We used the 2009 US Nationwide Inpatient Sample to identify patients with cirrhosis, based on ICD-9-CM diagnosis codes for cirrhosis or 1 of its complications (ascites, hepatorenal syndrome, upper gastrointestinal bleeding, portal hypertension, or hepatic encephalopathy). Multi-level modeling was performed to measure variance among hospitals.
Results
There were 102,155 admissions for cirrhosis in 2009, compared to 74,417 in 2003. Overall inpatient mortality was 6.6%. On multivariable-adjusted logistic regression, patients hospitalized in the Midwest had the lowest odds ratio (OR) of inpatient mortality (OR, 0.54; P<.001). Patients who were transferred from other hospitals (OR, 1.49; P<.001) or had hepatic encephalopathy (OR, 1.28; P<.001), upper gastrointestinal bleeding (OR, 1.74; P<.001), or alcoholic liver disease (OR, 1.23; P=.03) had higher odds of inpatient mortality than patients without these features. Those who received liver transplants had substantially lower odds of inpatient mortality (OR, 0.21; P<.001). Multi-level modeling showed that 4% of the variation in mortality could be accounted for at the hospital level (P<.001). Adjusted mortality among hospitals ranged from 1.2% to 14.2%.
Conclusions
Inpatient cirrhosis mortality varies considerably among US hospitals. Further research is needed to identify hospital- and provider-level practices that could be modified to improve outcomes.
Keywords: NIS, chronic liver disease, regional variation, survival
Introduction
Geographic variation in health care has been demonstrated across a wide variety of conditions, including surgeries as well as acute and chronic diseases such as heart attack and heart failure.1–4 A recent report by the Institute on Medicine documented broad variation in resource utilization amongst hospital referral regions (HRRs), and this variation was most pronounced for inpatient and sub-acute care.3,5 While some areas of gastroenterology have been studied from a geographic perspective, little research has been done evaluating geographic or hospital variation in resource utilization or outcomes for chronic liver disease.6
The end stage of most chronic liver diseases, cirrhosis is a high-cost, highly morbid condition, whose health utilization and economic burden has been increasing.7 Furthermore, inpatient costs for chronic liver disease are high. While liver disease ranked as the 17th most common outpatient GI diagnosis, chronic liver disease and viral hepatitis ranked as the 8th most common principal diagnose, more than C diff infections.8 Mortality is likewise high, with chronic liver disease the 4th leading cause of death for persons aged 45 to 54 years old – and this may actually be underestimated.9 Therefore, understanding variation in cirrhosis care would have important implications for health policy.
The purpose of this study was to evaluate geographic and hospital-level variation in cirrhosis care in the United States. For the reasons stated above, we focused on inpatient care, and chose inpatient mortality as a concrete outcome by which to compare hospitals. Our primary hypothesis was that inpatient mortality would vary by hospitals, despite adjustment for severity of illness and other confounding variables. We then aimed to identify patient and hospital characteristics associated with lower mortality.
Methods
Data Acquisition
Data for this paper were drawn from the 2009 Nationwide Inpatient Sample (NIS). The NIS is the largest all-payer publicly available inpatient care database which is maintained by the Agency for Healthcare Research and Quality as part of the Healthcare Cost and Utilization Project. It consists of a 20% stratified, two-stage clustered sample of approximately 1050 US community hospitals (for the 2009 sample), including long-term acute care hospitals (LTACs). Federal hospitals (including Veteran’s Administrations, Department of Defense and Indian Health Services hospitals) and sub-acute rehabilitation hospitals were excluded. Strata for sampling are based on 5 hospital-level characteristics: US Census region, hospital control, urban/rural location, teaching status, and bed size. After stratification, all discharges for that hospital for that year are included in the dataset which, for the 2009 sample, included a total of 7.8 million discharges. For the 2009 NIS sample, 44 states were included (states not included were Alabama, Alaska, Delaware, District of Columbia, Idaho, and North Dakota). Each record in the NIS includes information for a specific discharge including multiple patient-level and hospital-level characteristics. Each discharge also includes ICD-9 codes for up to 15 discharge diagnoses and procedure codes. The reliability of the NIS has been established by rigorous comparison with the National Hospital Discharge Survey.10
Inclusion Criteria
All inpatient discharges for 2009 for cirrhosis or portal hypertensive complications were included and were defined by ICD-9-CM diagnosis codes as previously published.7 Inclusion criteria included a primary diagnosis of cirrhosis (571.2, 571.5, 571.6) or portal hypertensive complication (portal hypertension [572.3], ascites [789,59], hepatic encephalopathy [572.2], upper GI bleed [456.0, 456.2, 578.0, 578.1, 578.9], and hepatorenal syndrome [572.4]). If the primary diagnosis of cirrhosis was found, a secondary diagnosis of one of the portal hypertensive complications was required and vice versa. Discharges were limited to patients over the age of 18. All discharges with missing mortality data were excluded (n=15).
Outcomes and Predictors Definitions
The primary outcome was inpatient mortality, defined as death prior to hospital discharge. Predictors were defined as patient-level predictors and included age, gender, race, primary insurance payer, patient’s home location (defined by metropolitan and micropolitan population estimates), income quartile by zip code, presence of hepatitis C (HCV) (ICD-9-CM codes 070.41, 070.44, 070.51, 070.54, 070.70, 070.71), alcoholic liver disease (ALD) (571.0–571.3), ascites (789.59), upper GI bleeding including variceal bleeding (456.0, 456.2, 578.0, 578.1, 578.9), hepatic encephalopathy (572.2), hepatorenal syndrome (HRS) (572.4), hepatocellular carcinoma (HCC) (155.0), transfer status, admission type, and receipt of liver transplant. Additionally, diabetes (250.xx), acute kidney injury (584.5–584.9) and infection were included as covariates given their potential influence on individual mortality and their prior inclusion in multivariable modeling within the NIS.7,11 Infection was defined as a composite of pneumonia (481, 482.xx, 483.0, 483.1, 483.8, 484.3, 484.5, 484.8, 485.x, 486.x), sepsis (995.91, 995.92, 785.52, 0380, 0381, 03811, 03812, 03819, 0382–0384, 03840–44, 03849, 0388, 0389), urinary tract infection (599.0, 590.10, 590.11, 590.80), cellulitis (682.0–682.9), bacteremia (790.7), cholangitis (576.1), Clostridium difficile infection (008.45), and spontaneous bacterial peritonitis (567.23). Case-mix severity was controlled for using the APR-DRG Risk Mortality score, a proprietary mortality risk score developed by 3M and included with the Nationwide Inpatient Sample for purpose of adjusting mortality risk. The APR-DRG mortality risk score has been validated as the most discriminative and predictive mortality risk score for cirrhotic patients in the NIS.11 Hospital-level predictors, including hospital region, bed size, urban/rural location, and teaching status, were included given their use as strata for sampling within the NIS as well as due to prior studies showing improved care for cirrhotic patients admitted to teaching hospitals.12 High volume hospitals were defined as hospitals with 30 cirrhosis admissions or more over the one-year study period. Thirty admissions was chosen as this represented the bottom quartile of admission volume for the full cirrhosis cohort. Transplant hospital was defined as any hospital that had performed at least one transplant over the study period.
Statistical Analysis
Descriptive statistics were performed with mortality as a dichotomous outcome using logit regression with conversion to odds ratios in survey-weighted data. Three broad groups were analyzed in univariate analysis: cirrhosis discharges from all hospitals, discharges from hospitals with less than thirty admissions (low-volume hospitals) and discharges from hospitals with greater than thirty cirrhosis admissions (high-volume hospitals) with comparison made between low- and high-volume hospital discharges using Pearson’s chi-squared for dichotomous or categorical variables and t-testing for continuous variables as appropriate in survey-weighted data. Because the data are hierarchically structured (patients within hospitals), mixed-effect hierarchical logistic regression was used on the non-survey-adjusted high-volume hospital cohort to estimate the extent to which variation in mortality by hospital exceeded that expected due to random chance (STATA command: xtmelogit). Hierarchical regression was also used to determine whether there remained any statistically significant variation in mortality between hospitals after adjustment for patient characteristics. However, these analyses were conducted on non-survey adjusted data due to absence of appropriate weights for hierarchical modeling in complex survey data analysis.
In order to accurately make national and regional-level inferences, analyses of patient and hospital characteristics associated with mortality were conducted using non-hierarchical logistic regression with attention to complex survey sampling methods, utilizing svyset commands in STATA. All hierarchical and multivariate logistic regression analyses were performed on the high-volume hospital cohort to improve reliability. Multivariable logistic regression models were created by including all variables with p <0.10 on unadjusted analysis. Age, gender, liver transplant status and presence of hepatocellular carcinoma were included despite non-significant p values based upon conceptual hypotheses that these may have an impact on mortality when controlling for other predictors. Cirrhosis complications (eg. ascites, variceal bleeding, hepatorenal syndrome, encephalopathy) as well as infection, diabetes, and kidney injury were included given significant p-values as well as prior studies showing an effect on mortality with inclusion of these variables.7,11 Race, patient location, and patient income reported as quartiles by zip code were non-significant across bivariate and multivariate analyses and thus were excluded from the final multivariable regression. Discrimination and goodness-of-fit statistics were calculated on non-survey adjusted models to estimate survey-adjusted model characteristics. For our final model, there were 20 total variables (including sampling strata variables) with a C-statistic of 0.87. On the basis of visual examination of Hosmer-Lemeshow tables, our model was well-calibrated across all deciles of mortality. In hierarchical models, patient characteristics as well as hospital region, hospital location (urban/rural), teaching status, and hospital bed-size (small, medium, or large) were included in order to adjust for NIS sampling strata. All statistics were performed using STATA version 12.1. P values were two-sided with alpha set at 0.05.
Results
Patient Characteristics
After survey adjustment, the national estimate of total cirrhosis inpatient admissions at all hospitals was 102,155 for the year 2009 (0.3% of total inpatient admissions at all hospitals). (see table 1) There were 78,083 cirrhosis admissions to high-volume hospitals, leaving approximately one-quarter (n=24,072) admitted to low-volume hospitals. In the full cohort, the average age was 58 years old with 63% male. 57% were white, 17% were Hispanic and 7% were black. 59% resided in or near metropolitan areas with 1 million or greater population. Most patients (91.8%) were not transferred in from another hospital. 21% carried a diagnosis of hepatitis C, 60% carried a diagnosis of alcohol-related liver disease. 8.6% had upper GI bleeding while 2.7% specifically were diagnosed with a variceal bleed. 43% had hepatic encephalopathy. 64% had ascites while only 7.7% had hepatorenal syndrome. 3.2% had hepatocellular carcinoma. 1.7% had a liver transplant with 43% receiving a paracentesis and 15% receiving an EGD during their hospital stay. 30% had diabetes, 24% had an infection, and 20% had acute kidney injury in the full cohort. With respect to insurance status, nearly two-thirds were primarily covered by either Medicare or Medicaid (38% and 23%, respectively).
Table 1.
Characteristics of Inpatient Cirrhosis Admissions in all hospitals
| Full Cirrhosis Cohort N = 102,155 |
High Volume Hospital Cohort N= 78,083 |
Low Volume Hospital Cohort N= 24,072 |
P value# |
|
|---|---|---|---|---|
| In-hospital mortality | 6946 (6.8%) | 5153 (6.6%) | 1803 (7.5%) | 0.08 |
| Age | 57.7 | 57.1 | 59.5 | 0.82 |
| Male | 63.2% | 64% | 60% | <0.001 |
| Race | <0.001 | |||
| • White | 65.6% | 62.5% | 76.5% | |
| • Black | 8.0% | 8.2% | 7.4% | |
| • Hispanic | 19.6% | 22.2% | 10.8% | |
| • Asian/PI | 1.9% | 2.1% | 1.2% | |
| • Native | 1.4% | 1.3% | 1.8% | |
| American | 3.4% | 3.8% | 2.3% | |
| • Other | ||||
| Mean length of stay (days) | 6.1 | 6.3 | 5.3 | 0.85 |
| Insurance status | <0.001 | |||
| Medicare | 38.3% | 35.9% | 46.2% | |
| Medicaid | 23.2% | 24.3% | 19.5% | |
| Private/HMO | 23.3% | 23.7% | 22.1% | |
| Self Pay | 10.2% | 10.8% | 8.3% | |
| No Charge | 0.89% | 1.0% | 0.44% | |
| Other | 3.90% | 4.0% | 3.4% | |
| Hospital bed size | <0.001 | |||
| Small | 10.2% | 5.4% | 25.8% | |
| Medium | 24.8% | 22.1% | 33.6% | |
| Large | 65.0% | 72.5% | 40.6% | |
| % Cirrhosis admits by hospital region | 0.004 | |||
| Northeast | 18.4% | 19.2% | 15.7% | |
| Midwest | 19.9% | 17.7% | 27.4% | |
| South | 37.9% | 37.4% | 39.4% | |
| West | 23.8% | 25.7% | 17.6% | |
| Hospital location | <0.001 | |||
| Urban | 91.3% | 98.1% | 69.3% | |
| Rural | 8.7% | 1.9% | 30.7% | |
| Teaching status | <0.001 | |||
| Teaching | 49.4% | 60.8% | 12.2% | |
| Non-teaching | 50.6% | 39.2% | 87.8% | |
| Admission type | 0.009 | |||
| Emergency | 74.4% | 75.8% | 70.1% | |
| Urgent | 18.3% | 18.0% | 19.1% | |
| Elective | 7.2% | 6.1% | 10.7% | |
| Patient location | <0.001 | |||
| • Metro 1mil | 35.4% | 40.6% | 18.7% | |
| • Fringe 1mil | 22.0% | 22.2% | 21.3% | |
| • 250K–999K | 19.5% | 21.2% | 14.0% | |
| • 50K–249K | 7.6% | 6.5% | 11.2% | |
| • Micropolitan | 9.6% | 5.6% | 22.5% | |
| • Neither | 5.8% | 3.8% | 12.3% | |
| Transfer status | <0.001 | |||
| Not a transfer | 91.8% | 90.7% | 95.3% | |
| Transfer | 8.2% | 9.3% | 4.7% | |
| Income by quartile | 0.22 | |||
| • 1–39K | 31.6% | 31.1% | 33.5% | |
| • 39K–48K | 26.9% | 26.3% | 28.8% | |
| • 48K–63K | 23.6% | 24.1% | 22.2% | |
| • 63K+ | 17.8% | 18.5% | 15.5% | |
| Hepatitis C | 21.5% | 23.1% | 16.4% | <0.001 |
| Alcoholic liver disease | 59.7% | 59.9% | 58.9% | 0.49 |
| Ascites | 64% | 64.6% | 62% | 0.04 |
| GI bleed | 8.6% | 8.6% | 8.6% | 0.95 |
| Hepatic encephalopathy | 43.4% | 42.4% | 46.5% | 0.002 |
| Hepatorenal syndrome | 7.7% | 8.1% | 6.3% | 0.006 |
| HCC | 3.2% | 3.5% | 2.2% | 0.002 |
| Diabetes | 30.3% | 30.0% | 31.5% | 0.16 |
| Infection& | 24.1% | 24.4% | 23.1% | 0.14 |
| Acute Kidney Injury | 20.1% | 23.1% | 15.1% | <0.001 |
| APR-DRG Mortality | ||||
| Risk Score | <0.001 | |||
| • Minor | 7.3% | 6.7% | 9.3% | |
| • Moderate | 37.7% | 37.1% | 39.6% | |
| • Major | 37.1% | 37.2% | 36.8% | |
| • Extreme | 18.0% | 19.0% | 14.4% | |
| Procedures | ||||
| Liver Transplant | 1.7% | 2.2% | 0 | 0.02 |
| Paracentesis | 42.6% | 44% | 37.5% | <0.001 |
| EGD | 15.3% | 16.2% | 12.5% | <0.001 |
Note: some percentages do not add up to 100% due to missing values
Comparison made between high-volume and low-volume cohort
Composite of spontaneous bacterial peritonitis, pneumonia, sepsis, Clostridium difficile infection, cholangitis, bacteremia, urinary tract infection, and cellulitis.
In comparing the high- to low-volume cohorts, there was a trend towards significance for mortality with patients admitted to low-volume hospitals having slightly higher mortality (7.5% vs 6.6%, P=0.08). In general, patient’s at high-volume hospitals were more likely to be male and Hispanic, to have HCV (23.1% vs 16.4%, p<0.001), ascites (64% vs 62%, p=0.04), HRS (8.1% vs 6.3%, p=0.006), acute kidney injury (21.7% vs 15.1%, p<0.001) and HCC (3.5% vs 2.2%, p=0.002). See Table 1. Patients in higher-volume hospitals also tended to be at higher mortality risk (19% in extreme category vs 14.4%, p<0.001) and were more likely to undergo liver transplant (2.2% vs 0, p=0.02), paracentesis (44% vs 37.5%, p<0.001), and EGD (16.2% vs 12.5%, p<0.001). There was no difference between these groups with respect to age, mean length of stay, income, alcoholic liver disease, or GI bleed. More patients were transferred in at high-volume hospitals than at low-volume hospitals (9.3% vs 4.7%, p<0.001)
Hospital characteristics
For the full cohort, 90% of all admissions occurred in urban hospitals, 49% of hospitals were teaching hospitals and 64% were categorized as “large.” See Table 1. 17.6% of patients in the full cohort were admitted to a transplant hospital compared to 23% of the high-volume cohort. 235 hospitals had thirty or more cirrhosis admissions over the study period with a range from 30 to 417. Mean number of cirrhosis admissions was 103 (SD 83.7) with a median of 73. High-volume hospitals were more likely to be urban (98.1% vs 69.3%, p<0.001), teaching (60.8% vs 12.2%, p<0.001), and have large bedsize (72.5% vs 40.6%, p<0.001).
Patient outcomes
Of the 102,155 cirrhosis admissions in 2009, 6,946 (6.8%) died while in-hospital. The mean total length of stay was 6 days. For the high-volume cohort, 6.6% died while in-hospital (6.6% vs 7.5%, p=0.08) with a mean total length of stay of 6.3 days compared to 5.3 days in low-volume hospitals (p=0.85).
Geographic variation in mortality
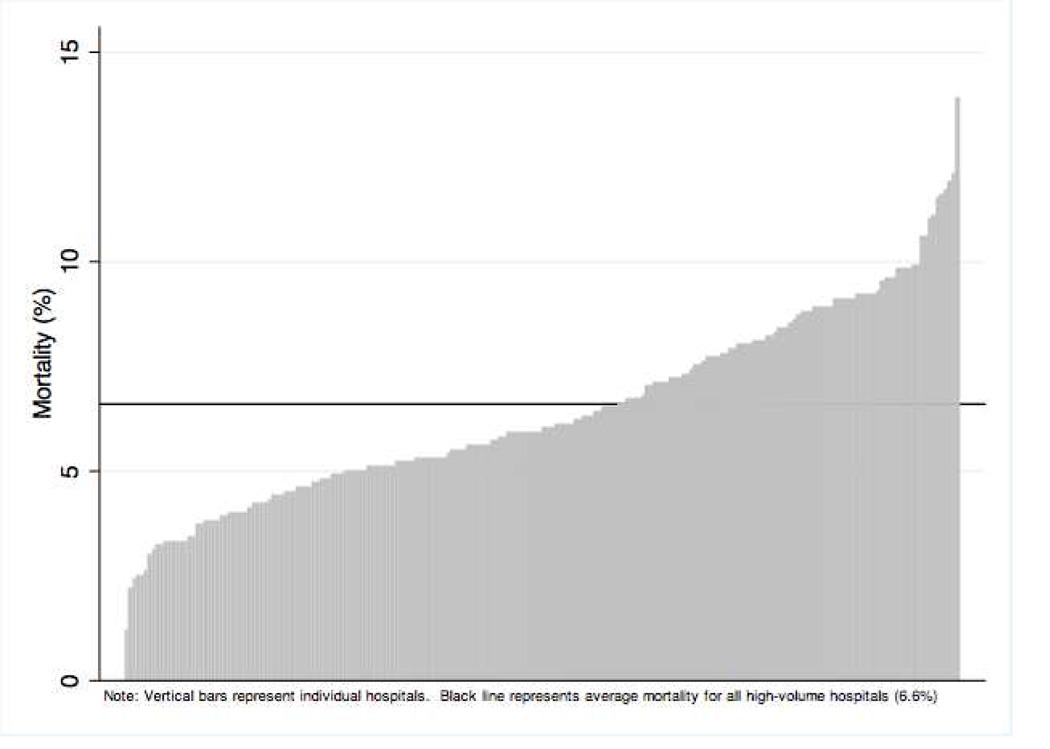
Unadjusted logistic regression showed no difference in mortality between census regions (see Tables 2 and 3). After adjusting for patient-level characteristics, significant variation in odds of inpatient mortality was evident across regions (Midwest OR 0.54, P<0.001). This regional variation persisted in sensitivity analysis with removal of transfer patients and those undergoing liver transplantation (data not shown). Multilevel modeling performed on the unadjusted survey sample to account for hierarchical data structure showed that 3% of the variation in mortality could be accounted for at the hospital-level (SD 1.6–5.3%, p<0.001). After adjusting for patient characteristics which were significant on multivariable logistic regression (age, insurance status, ascites, ALD, hepatic encephalopathy, upper GI bleeding, liver transplant, APR-DRG score and transfer status, diabetes, infection, and acute kidney injury) and survey strata (hospital region, urban/rural location, teaching status, and bed-size) this changed to 4% (SD 2.1–7.2%, p=<0.001). The adjusted mortality rate by hospital varied from 1.2 to 14.2% as shown in Figure 1. A sensitivity analysis performed on hospitals with more than 100 cirrhosis admissions revealed hospital mortality ranging from 3.3% to 13.3%. A second sensitivity analysis performed only on transplant hospitals (defined as hospitals which performed at least one transplant) produced a mortality range of 5.1% to 13.2%.
Table 2.
Unadjusted and Adjusted Odds Ratios for Inpatient Cirrhosis Mortality (High Volume Hospital Cohort)
| N=78,083 | Unadjusted | Adjusted for Patient Characteristics |
||
|---|---|---|---|---|
| Hospital Region | Odds Ratio | P value | Odds Ratios | P value |
| Northeast | 1 | 1 | ||
| Midwest | 0.88 | 0.36 | 0.54 | <0.001 |
| South | 1.04 | 0.70 | 0.92 | 0.53 |
| West | 1.12 | 0.34 | 0.78 | 0.12 |
| Patient Traits | ||||
| Age | 1.01 | 0.006 | 1.01 | 0.05 |
| Gender | 1.04 | 0.62 | 1.08 | 0.38 |
| Insurance Status | ||||
| • Private/HMO | 1 | 1 | ||
| • Medicare | 0.83 | 0.03 | 0.80 | 0.05 |
| • Medicaid | 0.86 | 0.12 | 0.98 | 0.87 |
| • Self-pay | 1.00 | 0.96 | 1.26 | 0.08 |
| Transferred In | 2.40 | <0.001 | 1.49 | 0.001 |
| Ascites | 1.12 | 0.08 | 0.82 | 0.01 |
| Hepatic encephalopathy | 1.78 | <0.001 | 1.28 | 0.001 |
| Hepatorenal Syndrome | 7.6 | <0.001 | 1.09 | 0.43 |
| Upper GI bleed | 2.6 | <0.001 | 1.74 | <0.001 |
| Hepatitis C | 0.77 | 0.003 | 0.83 | 0.32 |
| Alcoholic Liver Disease | 1.40 | <0.001 | 1.23 | 0.03 |
| Liver transplant | 0.73 | 0.25 | 0.21 | <0.001 |
| Hepatocellular carcinoma | 1.18 | 0.32 | 0.83 | 0.32 |
| Diabetes | 0.40 | <0.001 | 0.49 | <0.001 |
| Infection | 3.0 | <0.001 | 1.41 | <0.001 |
| Acute Kidney Injury | 6.77 | <0.001 | 1.53 | <0.001 |
| APRDRG Mortality Risk Score | ||||
| • Minor | 1 | 1 | ||
| • Moderate | 1.11 | 0.78 | 1.26 | 0.56 |
| • Major | 5.27 | <0.001 | 4.97 | <0.001 |
| • Extreme | 54.7 | <0.001 | 39.5 | <0.001 |
| Hospital Traits | ||||
| Hospital Bedsize | 1.09 | 0.24 | 1.05 | 0.60 |
| Rural/Urban | 1.28 | 0.02 | 1.27 | 0.19 |
| Teaching Status | 1.23 | 0.01 | 1.11 | 0.27 |
Table 3.
Probability of Mortality by US Census Region (High-Volume Cohort).
| Hospital Region |
Unadjusted Model | Adjusted for Patient Characteristics |
P-value |
|---|---|---|---|
| Northeast | 6.5% | 7.5% | Reference |
| Midwest | 5.8% | 4.6%* | <0.001 |
| South | 6.7% | 7.0% | 0.534 |
| West | 7.3% | 6.2% | 0.124 |
P<0.001 for between group comparison: Northeast vs Midwest
Figure 1.
Hospital level variation in inpatient mortality for cirrhotic patients.
Patient and hospital predictors of mortality
In multivariable regression, as shown in Table 2, being transferred resulted in increased odds of mortality (1.49, p=0.001). Individual cirrhosis comorbidities that carried increased risk of inpatient mortality were hepatic encephalopathy (OR 1.28, p=0.001), upper GIB (OR 1.74, p<0.001), and alcoholic liver disease (OR 1.23, p=0.03). Presence of hepatitis C and hepatocellular carcinoma were not associated with increased mortality (OR 0.83, p>0.05). Diabetes and ascites were associated with a lower odds ratio of death (OR 0.49 and 0.82, with p=<0.001 and p=0.01, respectively). Liver transplantation was associated with a marked decrease in inpatient mortality (OR 0.21, P<0.001) while increasing APR-DRG mortality risk score was associated with dramatically increased inpatient mortality odds (OR 39.5 p<0.001 for “extreme” category).
With respect to hospital-level characteristics, rural/urban location (OR 1.27, p>0.05), bed-size (OR 1.05, p>0.05) and teaching status (OR 1.11, p>0.05) were not associated with increased mortality in the survey-adjusted analysis (see Table 2).
Discussion
In our study of variation in inpatient cirrhosis mortality using the Nationwide Inpatient Sample, significant variation was found between regions, with the Midwest having the lowest overall probability of inpatient mortality. Even more significantly, however, a large degree of variation in inpatient mortality was noted between hospitals on multilevel modeling, with 4% of mortality attributable to the hospital level. Surprisingly, the adjusted probability of mortality varied over ten-fold between hospitals. To our knowledge, this study represents the first demonstration of significant hospital-to-hospital variation in mortality for cirrhotic patients.
Based upon this data, which used the same inclusion criteria as prior studies using the NIS, inpatient cirrhosis admissions have increased 30% within the last six years.7 Rising inpatient admissions appear to reflect the rising burden of liver disease overall in the US, and, in spite of already high overall mortality, there is reason to believe that mortality has been underestimated in recent years.9 Given that variation in health care outcomes and utilization is most pronounced in the inpatient and subacute settings3 and given that the burden of cirrhosis in the inpatient setting appears to be rising based upon our data, understanding predictors for such variation becomes important. Studies performed in other disciplines, including surgery and cardiology, have likewise found variation in inpatient mortality.1,13 Our study shows significant between-hospital variation in mortality, but reasons for this large variation are only partially explained by our models.
Variation in disease outcomes and resource utilization has been widely studied in other acute and chronic diseases but little research has been done applying this concept to chronic liver disease.1–3,6,14,15 Previous studies of inpatient cirrhosis hospitalizations using NIS data failed to show significant regional variation and did not include an analysis of hospital-level variation, though these studies utilized different disease severity adjustments.7 An advantage to our study was our use of the APR-DRG risk mortality score which, as the most accurate risk adjustment methodology for cirrhotic mortality in the NIS, would more reliably control for mortality risk in calculating predicted probabilities of mortality.11,16 As expected, certain patient-level traits, such as upper GI bleeding, alcoholic liver disease and hepatic encephalopathy, were associated with higher mortality, even when adjusting for overall disease severity. The lower mortality seen with diabetes is most likely to due to coding bias, where chronic conditions are often left off of the discharge diagnosis list for sicker patients, resulting in the appearance of improved mortality for patients with these listed conditions.11,17 Additionally, there may be unmeasured hospital and provider-level variables which more fully explain the variation in mortality, such as timely recognition and management of cirrhosis complications, receipt of appropriate medications, and procedural complications, among others.
Among these unmeasured variables, outpatient variables, such as access to subspecialty care and differences in care coordination, could also be behind inpatient mortality differences. Previous studies have shown that access to subspecialty care in both the inpatient and outpatient settings improves quality of care and some outcomes.12,18–21 However, in Medicare patients hospitalized for cirrhosis, only 42% received an Evaluation & Management (E&M) code submitted by a gastroenterologist during their hospitalization and, of those who survived to one year post-discharge, only 45% had an E&M code submitted by a gastroenterologist in the outpatient setting.22 NIS data does not permit evaluation for subspecialty inpatient treatment, so it is unclear if subspecialty access in the inpatient setting played a role in mortality differences. Additionally, readmission rates have been shown to be high for cirrhotics and are associated with higher inpatient mortality.23 While it is unclear what portion of such readmissions are preventable, they present a possible target for improving outcomes in cirrhotic inpatient care. Access to liver transplantation, which is incompletely measured within the NIS, may also explain some of the mortality variation, as receipt of liver transplant is associated with dramatically lower inpatient mortality in our models.
There are several limitations to our study. As an administrative database, the NIS itself does not allow for patient-specific identification, therefore we are unable to link inpatient hospital admission with outpatient medical records to determine if outpatient factors, such as adherence to medications, implementation of quality of care guidelines, access to subspecialty care, or readmissions contributed to mortality. Additionally liver-specific disease severity measures, such as MELD scores, are not included in the NIS data. This gap is somewhat ameliorated by our use of the APR-DRG mortality risk score which specifically categorizes patients based upon their overall risk of mortality rather than simply on aggregate number of comorbidities, as with the Charlson-Deyo or Elixhauser risk adjustment methodologies. The NIS also does not include Veteran’s Administration hospitals, an exclusion which likely results in underestimation of overall inpatient mortality for liver disease given the high burden of HCV and alcohol-related liver disease with the VA population.24,25 While transplantation was the most significant factor in our analysis, many details with respect to transplantation are unavailable in this database. We also elected to exclude all hospitals with 30 or fewer cirrhotic admissions. While this improved reliability while maintaining sample size, the loss of lower-volume hospitals leaves a sizeable portion of the hospital universe unanalyzed. Given that there was a trend towards greater mortality in lower-volume hospitals, which did not reach statistical significance, the exclusion of low-volume hospitals may underestimate mortality. Additionally, our study covered only one year of discharges, making it difficult to determine if hospital mortality levels were consistent across several years. Despite these limitations, our finding of wide variation in mortality between hospitals remained robust to multiple covariate adjustments and sensitivity analyses.
With the increasing burden of inpatient cirrhosis admissions, attention to prevention of cirrhosis as well as outpatient management with a goal of decreasing admissions and readmissions represent necessary next steps in research. Our findings indicate that there are substantial differences in inpatient cirrhosis mortality between different regions of the US and between hospitals, which could have national policy implications given the high percentage of patients insured by Medicare and Medicaid. Further research into both inpatient and outpatient factors leading to inpatient mortality variation are needed to shed light on the causes of this variation and determine ways to improve survival.
Acknowledgments
JLM is supported by a T32DK062708 educational grant. MLV is support by NIH grant K23DK085204.
The authors would like to thank Rodney Hayward, MD for assistance with statistical analysis and the data partners of the Healthcare Cost and Utilization Project (www.hcup-us.ahrq.gov/hcupdatapartners.jsp).
Abbreviations
- ALD
alcoholic liver disease
- APR-DRG
All Patient Refined Diagnosis Related Groups
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HRS
hepatorenal syndrome
- IC9-CM
Clinical Modification of the International Classification of Diseases, 9th Edition
- LOS
length of stay
- NIS
Nationwide Inpatient Sample
- OR
odds ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
JLM- none
CRR-none
AKM-none
MLV-none
Author Contributions:
JLM: study design, statistical analysis, data analysis and interpretation, manuscript drafting, review and finalization
CRR: data analysis and interpretation
AKM: data analysis and interpretation, manuscript review and finalization
MLV: study design, data analysis and interpretation, manuscript review and finalization
References
- 1.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N. Engl. J. Med. 2009;361:1368–1375. doi: 10.1056/NEJMsa0903048. [DOI] [PubMed] [Google Scholar]
- 2.Song Y, Skinner J, Bynum J, et al. Regional variations in diagnostic practices. N. Engl. J. Med. 2010;363:45–53. doi: 10.1056/NEJMsa0910881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newhouse JP, Garber AM. Geographic variation in Medicare services. N. Engl. J. Med. 2013;368:1465–1468. doi: 10.1056/NEJMp1302981. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Baik SH, Fendrick AM, et al. Comparing local and regional variation in health care spending. N. Engl. J. Med. 2012;367:1724–1731. doi: 10.1056/NEJMsa1203980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newhouse JP, Garber A, Graham RP. Interim Report of the Committee on Geographic Variation in Health Care Spending and Promotion of High-Value Health Care: Preliminary Committee. Washington, DC: National Academies Press; 2013. [PubMed] [Google Scholar]
- 6.Dominitz JA, Baldwin L-M, Green P, et al. Regional variation in anesthesia assistance during outpatient colonoscopy is not associated with differences in polyp detection or complication rates. Gastroenterology. 2013;144:298–306. doi: 10.1053/j.gastro.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen GC, Segev DL, Thuluvath PJ. Nationwide increase in hospitalizations and hepatitis C among inpatients with cirrhosis and sequelae of portal hypertension. Clin. Gastroenterol. Hepatol. 2007;5:1092–1099. doi: 10.1016/j.cgh.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 8.Peery AF, Dellon ES, Lund J, et al. Burden of Gastrointestinal Disease in the United States: 2012 Update. Gastroenterology. 2012;143:1179–1187. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asrani SK, Larson JJ, Yawn B, et al. Underestimation of liver-related mortality in the United States. Gastroenterology. 2013;145:375–382. doi: 10.1053/j.gastro.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whalen D, Houchens R, Elixhauser R. US Agency for Healthcare Research and Quality; 2014. [Online February 22, 2008]. 2005 HCUP Nationwide Inpatient Sample (NIS) Comparison Report, HCUP Methods Series Report #2008-01; pp. 1–40. Available at http://www.hcup-us.ahrg.gov/reports/methods.jsp. [Google Scholar]
- 11.Myers RP, Quan H, Hubbard JN, et al. Predicting in-hospital mortality in patients with cirrhosis: results differ across risk adjustment methods. Hepatology. 2009;49:568–577. doi: 10.1002/hep.22676. [DOI] [PubMed] [Google Scholar]
- 12.Kanwal F, Kramer JR, Buchanan P, et al. The quality of care provided to patients with cirrhosis and ascites in the Department of Veterans Affairs. Gastroenterology. 2012;143:70–77. doi: 10.1053/j.gastro.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 13.Bradley EH, Herrin J, Curry L, et al. Variation in hospital mortality rates for patients with acute myocardial infarction. Am. J. Cardiol. 2010;106:1108–1112. doi: 10.1016/j.amjcard.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Fisher ES, Wennberg DE, Stukel TA, et al. The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care. Annals of internal medicine. 2003;138:273–287. doi: 10.7326/0003-4819-138-4-200302180-00006. [DOI] [PubMed] [Google Scholar]
- 15.Fisher ES, Wennberg DE, Stukel TA, et al. The implications of regional variations in Medicare spending. Part 2: health outcomes and satisfaction with care. Annals of internal medicine. 2003;138:288–298. doi: 10.7326/0003-4819-138-4-200302180-00007. [DOI] [PubMed] [Google Scholar]
- 16.Myers RP, Hubbard JN, Shaheen AAM, et al. Hospital performance reports based on severity adjusted mortality rates in patients with cirrhosis depend on the method of risk adjustment. Ann Hepatol. 2012;11:526–535. [PubMed] [Google Scholar]
- 17.Jencks SF, Williams DK, Kay TL. Assessing hospital-associated deaths from discharge data. The role of length of stay and comorbidities. JAMA: The Journal of the American Medical Association. 1988;260:2240–2246. [PubMed] [Google Scholar]
- 18.Kanwal F, Kramer J, Asch SM, et al. An Explicit Quality Indicator Set for Measurement of Quality of Care in Patients With Cirrhosis. YJCGH. 2010;8:709–717. doi: 10.1016/j.cgh.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 19.Ayanian JZ, Landrum MB, Guadagnoli E, et al. Specialty of ambulatory care physicians and mortality among elderly patients after myocardial infarction. N. Engl. J. Med. 2002;347:1678–1686. doi: 10.1056/NEJMsa020080. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen GC, Saibil F, Steinhart AH, et al. Postoperative Health-Care Utilization in Crohn's Disease: The Impact of Specialist Care. Am. J. Gastroenterol. 2012;107:1522–1529. doi: 10.1038/ajg.2012.235. [DOI] [PubMed] [Google Scholar]
- 21.Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089–1095. doi: 10.1053/jhep.2001.29204. [DOI] [PubMed] [Google Scholar]
- 22.Mellinger JL, Volk ML. Multidisciplinary management of patients with cirrhosis: a need for care coordination. Clin. Gastroenterol. Hepatol. 2013;11:217–223. doi: 10.1016/j.cgh.2012.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volk ML, Tocco RS, Bazick J, et al. Hospital readmissions among patients with decompensated cirrhosis. Am. J. Gastroenterol. 2011;107:247–252. doi: 10.1038/ajg.2011.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mansell D, Penk W, Hankin CS, et al. The illness burden of alcohol-related disorders among VA patients: the veterans health study. J Ambul Care Manage. 2006;29:61–70. doi: 10.1097/00004479-200601000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Sloan KL, Straits-Tröster KA, Dominitz JA, et al. Hepatitis C tested prevalence and comorbidities among veterans in the US Northwest. J Clin Gastroenterol. 2004;38:279–284. doi: 10.1097/00004836-200403000-00016. [DOI] [PubMed] [Google Scholar]