INTRODUCTION
Vitamin D deficiency as measured by serum 25-hydroxyvitamin D (25(OH)D) concentration is common in the elderly U.S. population [1-2]. In community-dwelling men age 65 years or older from the MrOS Study, 71% had total serum 25(OH)D levels under 30ng/mL and 26% had levels under 20ng/mL [3]. Among older men, lower serum 25(OH)D concentrations were associated with increased hip fracture risks [4-5]. These associations were attenuated by adjustment for areal bone mineral density (aBMD). Because aBMD represents a combination of both density and structure, this evidence suggests a direct role of vitamin D not only on aBMD but on skeletal structure as well [4,6-7]. Such a role is supported by positive associations between aBMD and endogenous 25(OH)D levels as well as vitamin D supplementation [8-10]. Additionally, serum 25(OH)D levels under 20ng/mL are associated with higher average annual rates of bone loss in the total hip and trochanter in older men [11]. Finally, previous work also confirms that structural dimensions and biomechanical whole bone strength of the proximal femur are associated with hip fracture risk independent of BMD [12-13].
Although low endogenous 25(OH)D may compromise bone strength and thereby increase fracture risk, knowledge about underlying mechanisms in the proximal femur are limited. For example, it is unknown whether associations exist between serum 25(OH)D concentrations and volumetric bone mineral density (BMD) or structural dimensions of the proximal femur in elderly men. Quantitative computed tomography (QCT) facilitates investigation into skeletal structure and overall bone strength, and peripheral QCT has been used to determine associations of endogenous 25(OH)D to volumetric skeletal measures[14-20]. Specifically, in older Caucasian men, cortical volumetric BMD (vBMD), bone mineral content, and cortical thickness at the tibia and radius obtained with peripheral QCT were all positively associated with 25(OH)D [20-21]. Still, associations of QCT-derived femoral neck dimensions, vBMD and strength with endogenous 25(OH)D in older men have not been examined.
An alternative explanation for any observed associations between 25(OH)D and femoral structure and density may be the role of parathyroid hormone (PTH), which regulates 25(OH)D in circulation [23-25]. In a prospective study of men aged 19-85, low 25(OH)D and secondary hyperparathyroidism were each independently associated with cortical thinning determined by DXA scan in the femoral neck in men >55 years old, but not in younger men [26]. Other studies have found no association between 25(OH)D, PTH and tibial cortical measures [27]. To date, no studies have examined associations of serum 25(OH)D with QCT measures of the proximal femur in older men and whether the associations are attenuated after adjustment for PTH.
In this study we evaluate serum 25(OH)D levels, total intact PTH levels and volumetric BMD measures derived from QCT of the proximal femur collected by the MrOS Study. We examined whether associations exist between these serum hormone levels and specific three-dimensional variation in bone structure, volumetric bone density as well as finite element correlates previously shown to be associated with hip fracture. We hypothesized that higher serum 25(OH)D levels would be associated with greater cortical and trabecular BMD, greater cortical bone size, and smaller medullary size. We further hypothesized that any observed associations would be attenuated when adjusted for PTH concentration.
METHODS
Study Setting
The MrOS Study enrolled 5994 men from March 2000 through April 2002; details have been reported elsewhere [28-29]. In short, these men were recruited at 6 U.S. academic centers in Birmingham AL, Minneapolis MN, Palo Alto CA, Pittsburgh PA, Portland OR, and San Diego CA. Eligibility criteria were age (65 and older), ability to walk without assistance from another person and absence of bilateral hip replacement surgery. The Institutional Review Board (IRB) at each study center approved the study protocol, and men provided written informed consent when enrolled in the study.
Baseline Assessments
At the baseline visit participants completed a self-administered questionnaire including information about age, race/ethnicity, smoking and current alcohol use, medication history, and medical history. Physical activity was assessed by the Physical Activity Scale for the Elderly (PASE) score [30]. Men attended a baseline clinic visit and received skeletal, anthropometric (height, weight, etc), as well as other measures. BMI was calculated from measures of height and weight. Fasting blood samples were obtained, with one sample drawn into a foil-wrapped vial to protect from ultraviolet radiation. Serum was frozen at −80 C in a repository until funding for assays was obtained.
25(OH)D and Parathyroid Hormone Assays
In 2007, serum 25(OH)D level was obtained in a random sample of 1608 men using liquid chromatography/mass spectrometry assays. Total serum 25(OH)D has been shown to provide an accurate measure of available vitamin D stores [30]. These assays were performed on the serum stored in foil-wrapped vials [32]. All assays were highly precise, with an inter-assay coefficient of variation of 4.4% and intra-assay coefficient of variation of 4.9% [32].
In 2008, gold standard PTH immunoradiometric assays were completed among 1593 of 1608 men the original 25(OH)D random sample as described previously [28]. The smaller sample is due to a restriction on the use of the serum to men with at least 5 tubes remaining in storage. The PTH inter-assay coefficient of variation was 8.4%, with an intra-assay coefficient of variation of 5.6%.
QCT-Derived Femoral Measures
As described previously, baseline QCT scans were obtained for 3,786 men using a standardized protocol [33-34]. A hydroxyapatite standard of known density was included in all scans to allow conversion from Hounsfield units to g/cm3. Scans were reviewed centrally for quality control and processed at the University of California, San Francisco [33-34]. Additionally, in a random sample of 475 men with baseline QCT scans, femoral whole-bone strength and load-strength ratio were evaluated with finite element (FE) analysis as described previously [13]. Within this sample, the FE estimates were obtained for 356 men who also had 25(OH)D levels and were included in this analysis.
QCT femoral neck measures were the main outcomes for this analysis. Measures studied included cross-sectional area, integral, cortical and medullary volume, integral, cortical and trabecular BMD, and cortical volume as a percent of integral volume. Secondary outcomes were FE analysis of the femoral neck measures including femoral whole-bone strength and a load-strength ratio, and femoral shaft QCT measures of cross-sectional area, cortical area, medullary area, integral BMD and percent cortical area [12-13]. The femoral strength estimates the breaking force (in Newtons) for a sideways fall directly onto the trochanter, and load-strength ratio represents the ability of the proximal femur to sustain the maximum impact force in a sideways fall from standing height given the subject's height and weight, for which larger values denote a higher risk of fracture [13, 35].
Statistical Analysis
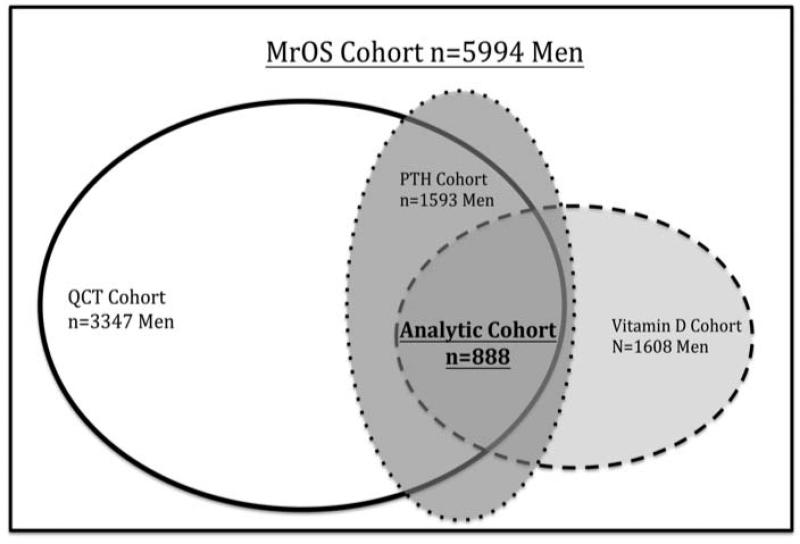
The analytic cohort consisted of the 888 MrOS participants who had complete data for the primary analysis relating the QCT-derived measures of femoral neck vBMD and structural dimensions to 25(OH)D levels (Figure 1). Within this analytic sample, 356 also had FE measures. Secondary analyses examined associations of femoral shaft vBMD and dimensions to 25(OH)D levels only, because FE analysis were not performed for this region.
Figure 1.
Analytic sample selection of 888 men with complete QCT measures of the femoral Neck, 25(OH)D, and PTH concentrations from the Osteoporosis in Men (MrOS) Study
For analysis, race/ethnicity was grouped as Caucasian/non-Caucasian due to the small number of non-Caucasian men in this sample. Cigarette smoking was classified as current, past, or never smoker. Current alcohol consumption was categorized as none, 1-7 drinks, or 7 or more drinks. Self-reported health status for age was categorized as good/excellent versus fair/poor/very poor. Self-reported medical history was categorized as presence or absence of fracture history since age 50, osteoporosis diagnosis, ever use of osteoporosis medications, and arthritis diagnosis. Season of baseline blood draw was defined as winter (January to March), spring (April to June), summer (July to September), and fall (October to December). Latitude was categorized as high, defined as over 44 degrees (Minneapolis, Pittsburgh, and Portland), and low, defined as 44 degrees and under, (Birmingham, Palo Alto, and San Diego) [3, 28].
Distributions of the baseline characteristics in the analytic sample were compared according to quartiles of 25(OH)D with one-way ANOVA for continuous variables or chi-square tests for categorical variables. Spearman rank correlation coefficients were calculated between 25(OH)D and PTH levels, and 25(OH)D and each QCT measure. Adjusted least square (LS) means of femoral neck and femoral shaft measures were estimated within the 25(OHD) quartiles using multivariable generalized linear regression models.
Variables assessed as confounders of associations between 25(OH)D and the femoral measures were age, height, weight, BMI, race, smoking status, alcohol consumption, physical activity (PASE score), self-reported health status, fracture history and non-trauma fracture history, osteoporosis medication use, season at the time of blood draw and latitude of study site. Variables were considered confounders if they altered LS means by 10% or more from baseline, and were retained in the regression model. By this variable selection method, confounding factors included in the multivariable model were age, race, BMI, height, latitude and season. In addition, we evaluated the models including the small number of men with a history of osteoporosis medication use, refit the models after excluding this group, and found the least square means were nearly identical. Therefore, we concluded that history of osteoporosis medication use did not confound any association of interest and did not exclude these men from analyses.
Tests of linear trend of LS means of each femoral neck measure across increasing quartile of 25(OH)D were also performed. To accomplish this, we replaced the quartile dummy value (1,2,3,4) with the median value of the quartile in order to better represent the 25(OH)D distribution. Last, models were fit with additional adjustment for total intact PTH (in quartiles) to explore whether this adjustment attenuated any associations between 25(OH)D and the femoral measures. All analyses were conducted using SAS 9.2 statistical software (SAS Institute Inc., Cary, NC, USA).
RESULTS
The serum 25(OH)D quartiles were similar to clinical definitions of deficiency (<20ng/mL; Q1), insufficiency (20ng/mL to <30ng/mL; Q2-Q3), and adequacy (30ng/mL or higher, Q4 [1] (Table 1). Men in our study sample in the lowest quartile of 25(OH)D (Q1, mean level 15.1 ng/mL) were younger, had a smaller BMI, reported better health, had greater physical activity, were more likely to be Caucasian and to have a clinic site at under 44 degrees latitude than those with 25(OH)D in the highest quartile (Q4, mean level 34.8 ng/mL). Those in lower vitamin D quartiles were more likely to have baseline clinic visits in the fall, winter, or spring. The characteristics of this analytic sample were similar to those of the full MrOS cohort and the larger MrOS vitamin D cohort as previously described [3].
Table 1.
Characteristics of the Analytic Cohort of 888 Men Ages 65 Years and Older by 25(OH)D Quartile
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-value | |
|---|---|---|---|---|---|
| Total 25(OH)D (ng/ml; mean ± SD) | 15.1 ± 3.7 | 22.3 ± 1.6 | 27.0 ± 1.4 | 34.8 ± 5.2 | |
| Total 25(OH)D (ng/ml, range) | (11.4-18.8) | (20.7-23.9) | (25.6-28.4) | (29.6-40) | |
| Baseline Characteristics | |||||
| Number (% cohort) | 221 | 219 | 226 | 222 | |
| Age (years; mean ± SD) | 74.5 ± 6.2 | 73.6 ± 6.0 | 73.85 ±5.8 | 72.99 ± 5.4 | 0.06 |
| Race | |||||
| Caucasian (n, %) | 188 (85) | 203 (93) | 206 (91) | 209 (94) | |
| Other (n, %) | 33 (15) | 16 (7) | 20 (9) | 13 (6) | 0.006 |
| BM (kg/m2) | 28.32 ± 4.04 | 27.41 ± 3.61 | 27.23 ± 3.47 | 26.70 ± 3.28 | <0.001 |
| Height | |||||
| Current Height in cm ( mean ± SD) | 173.14 ± 7.24 | 174.45 ± 6.94 | 173.6 ± 6.80 | 174.96 ± 6.46 | 0.02 |
| Weight | |||||
| Current Weight in kg ( mean ± SD) | 85.04 ± 13.96 | 83.71 ± 13.77 | 82.16 ± 12.09 | 81.82 ± 11.63 | 0.03 |
| Smoking status | |||||
| Current smokers (n, %) | 10 (5) | 10 (5) | 8 (4) | 2 (1) | |
| Past smokers (n, %) | 138 (62) | 127 (58) | 130 (58) | 141 (64) | |
| Never smoked (n, %) | 73 (33) | 82 (37) | 88 (39) | 79 (36) | |
| Total Pack years (mean ± SD) | 32.78 ± 27.69 | 30.15 ± 28.15 | 30.06 ± 24.85 | 29.54 ± 25.90 | 0.23 |
| Alcohol Consumption (drinks/week in the last year) | |||||
| none (n, %) | 77 (35) | 75 (34)** | 75 (33) | 61 (27) | |
| 1-<7drinks (n, %) | 80 (36) | 84 (39)** | 96 (42) | 82 (37) | |
| 7 or more (n, %) | 64 (29) | 59 (27)** | 55 (24) | 79 (36) | 0.17 |
| Physical Activity (PASE score; mean ± SD) | 134.65 ± 75.21 | 146.90 ± 65.36 | 147.32 ± 63.97 | 157.65 ± 67.44 | 0.01 |
| Reported Health Status for Age | |||||
| Good/Excellent (n, %) | 175 (79) | 190 (87) | 195 (86) | 205 (92) | |
| Very Poor/Poor/Fair (n, %) | 46 (21) | 29 (13) | 31 (14) | 17 (8) | 0.001 |
| Non-trauma Fracture since age 50 (n,%) | 43 (19) | 39 (18) | 31 (14) | 43 (19) | 0.34 |
| Inability to rise ≥ 1 time unassisted from a chair (n, %) | 4 (2) | 3 (1) | 2 (1) | 3 (1) | 0.81 b |
| Diagnosis of Osteoporosis (n, %) | 9 (4) | 8 (4) | 11 (5) | 11 (5) | 0.9 b |
| Diagnosis of Arthritis (n, %) | 102 (46) | 103 (47) | 103 (46) | 105 (47) | 0.98 |
| Ever used osteoporosis medication (n, %) | 3 (1) | 8 (4) | 8 (4) | 10 (5) | 0.25 b |
| Season of Baseline visita | |||||
| Winter (Jan-Mar; n, %) | 58 (26) | 46 (21) | 39 (17) | 20 (9) | |
| Spring (Apr-June; n, %) | 87 (39) | 82 (37) | 76 (34) | 69 (31) | |
| Summer (Jul-Sept; n, %) | 32 (14) | 49 (22) | 64 (28) | 93 (42) | |
| Fall (Oct-Dec; n, %) | 44 (20) | 42 (19) | 47 (21) | 40 (18) | <0.001 |
| Latitude of Clinic Site (n, %)a | |||||
| High (Minneapolis 44°, Pittsburgh 40° and Portland 45°; n, %) | 149 (67) | 116 (53) | 113 (50) | 96 (43) | |
| Low (Birmingham 33°, Palo Alto 37°, and San Diego 32°; n, %) | 72 (33) | 103 (47) | 113 (50) | 126 (57) | <0.001 |
Latitudes and seasons as previously described [2]
Fisher's exact method used
One participant refused to answer
Vitamin D and total intact PTH concentrations were modestly inversely correlated (r = −0.26, p<0.001) (Figure 2).
Figure 2.
Scatter plot of serum 25(OH)D and serum total intact PTH levels in 888 men aged 65 years and older in the Osteoporosis in Men Study
Adjusted mean femoral neck dimensions, before and after adjustment for PTH, are shown in Table 2 according to 25(OH)D quartile. Measures of overall femoral neck size as assessed by cross-sectional area and integral volume were not associated with 25(OH)D concentration. There was little variation of the means within quartiles and the tests of trend were not statistically significant. In contrast, femoral neck cortical volume was positively associated with 25(OH)D concentration. Further adjustment for PTH did not materially alter this association. Consistent with the observations for cortical volume, mean medullary volume was inversely associated with 25(OH)D concentration and mean cortical volume as a percentage of integral volume was positively associated with 25(OH)D concentration. The absolute differences in means across quartiles of these measures were small in magnitude.
Table 2.
Distribution of dimensions of the femoral neck within 25(OH)D quartiles with adjustments for age, race, latitude, season, height and BMI in men ages 65 and older, with and without adjustment for parathyroid hormone level (PTH).
| Outcome Measures | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p-trend |
|---|---|---|---|---|---|
| 25(OH)D (ng/ml; range) | (11.4-18.8) | (20.7-23.9) | (25.6-28.4) | (29.6-40) | |
| Femoral Neck Measures (n) | 221 | 219 | 226 | 222 | |
| Cross-Sectional Area (cm2) | |||||
| Adjusted Mean | 12.4 | 12.4 | 12.5 | 12.5 | 0.42 |
| 95% CI | (12.2, 12.6) | (12.2, 12.7) | (12.3, 12.7) | (12.2, 12.8) | |
| Further Adjusted for PTH | 12.4 | 12.4 | 12.5 | 12.5 | 0.60 |
| 95% CI | (12.2, 12.7) | (12.2, 12.7) | (12.2, 12.7) | (12.2, 12.8) | |
| Integral Volume (cm3) | |||||
| Adjusted Mean | 20.7 | 20.6 | 20.5 | 20.4 | 0.35 |
| 95% CI | (20.3, 21.0) | (20.2, 21.0) | (20.1, 20.9) | (20.0, 20.9) | |
| Further Adjusted for PTH | 20.6 | 20.6 | 20.5 | 20.4 | 0.39 |
| 95% CI | (20.2, 21.0) | (20.2, 21.0) | (20.1, 20.9) | (20.0, 20.9) | |
| Cortical Volume (cm3) | |||||
| Adjusted Mean | 9.0 | 9.1 | 9.2 | 9.4 | 0.007 |
| 95% CI | (8.8, 9.2) | (8.3, 9.3) | (9.0, 9.4) | (9.1, 9.6) | |
| Further Adjusted for PTH | 9.0 | 9.1 | 9.2 | 9.4 | 0.01 |
| 95% CI | (8.8, 9.3) | (8.8, 9.3) | (9.0, 9.4) | (9.1, 9.6) | |
| Medullary Volume (cm3) | |||||
| Adjusted Mean | 11.6 | 11.5 | 11.3 | 11.0 | 0.006 |
| 95% CI | (11.3, 12.0) | (11.2, 11.9) | (10.9, 11.6) | (10.6, 11.4) | |
| Further Adjusted for PTH | 11.6 | 11.5 | 11.3 | 11.0 | 0.01 |
| 95% CI | (11.3, 12.0) | (11.2, 11.9) | (10.9, 11.7) | (10.7, 11.4) | |
| Percent Cortical Volume | |||||
| Adjusted Mean | 44.2 | 44.4 | 45.3 | 46.5 | <0.001 |
| 95% CI | (43.2, 45.1) | (43.4, 45.4) | (44.3, 46.3) | (45.4, 47.6) | |
| Further Adjusted for PTH | 44.3 | 44.4 | 45.3 | 46.5 | <0.001 |
| 95% CI | (43.3, 45.2) | (43.4, 45.4) | (44.3, 46.3) | (45.4, 47.6) | |
Femoral neck integral vBMD was positively associated with 25(OH)D level and the test of linear trend was significant, but again, the absolute difference in means was small (Table 3). This association was not attenuated by adjustment for PTH. The weak positive association of cortical vBMD with 25(OH)D level was somewhat attenuated by adjustment for PTH and the linear trend was no longer significant after adjustment. Although trabecular vBMD was also positively associated with 25(OH)D, there was little evidence of a trend as the LS mean estimates were the same in the bottom two and in the top two quartiles regardless of adjustment for PTH.
Table 3.
Distribution of volumetric bone mineral density (vBMD) of the femoral neck within 25(OH)D quartiles adjusted for age, race, latitude, season, height and BMI in men ages 65 years and older, with and without adjustment for parathyroid hormone level (PTH).
| Outcome Measures | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p-trend |
|---|---|---|---|---|---|
| 25(OH)D (ng/ml; range) | (11.4-18.8) | (20.7-23.9) | (25.6-28.4) | (29.6-40) | |
| Femoral Neck Measures (n) | 221 | 219 | 226 | 222 | |
| Integral vBMD (g/cm3) | |||||
| Adjusted Mean | 0.289 | 0.289 | 0.300 | 0.308 | <0.001 |
| 95% CI | (0.281, 0.298) | (0.281, 0.298) | (0.292, 0.309) | (0.298, 0.318) | |
| Further Adjusted for PTH | 0.290 | 0.290 | 0.300 | 0.308 | 0.001 |
| 95% CI | (0.282, 0.299) | (0.281, 0.298) | (0.292, 0.309) | (0.299, 0.317) | |
| Cortical vBMD (g/cm3) | |||||
| Adjusted Mean | 0.528 | 0.525 | 0.534 | 0.540 | 0.03 |
| 95% CI | (0.519, 0.515) | (0.515, 0.534) | (0.525, 0.544) | (0.529, 0.550) | |
| Further Adjusted for PTH | 0.530 | 0.525 | 0.534 | 0.539 | 0.07 |
| 95% CI | (0.521, 0.539) | (0.515, 0.535) | (0.524, 0.544) | (0.529, 0.550) | |
| Trabecular vBMD (g/cm3) | |||||
| Adjusted Mean | 0.075 | 0.075 | 0.084 | 0.084 | 0.006 |
| 95% CI | (0.069, 0.081) | (0.068, 0.082) | (0.078, 0.091) | (0.077, 0.091) | |
| Further Adjusted for PTH | 0.075 | 0.075 | 0.084 | 0.084 | 0.007 |
| 95% CI | (0.068, 0.081) | (0.068, 0.082) | (0.078, 0.091) | (0.077, 0.091) | |
The 356 men in the FE sample were similar to the 532 not selected for FE (data not shown). For example, in men with and without FE respectively, mean 25(OH)D levels (25.0 ±8.0 ng/mL, 24.7 ±7.8 ng/mL, p=0.51) and PTH levels (32.7 ±13.2 pg/mL 33.3 ±15.6 pg/mL, p=0.55) were similar. Likewise, distributions of trabecular vBMD (p=0.48), cortical vBMD, (p=0.63) total femoral neck volume (p=0.19) and cortical volume (p=0.15 did not differ significantly between the two groups. The only notable difference was that men with FE data had a minimally lower BMI (27.0 ±3.4 kg/m2) than men without FE data (27.7 ±3.8 kg/m2, p=0.005), with lower weight (81.8 ±12 kg vs 84.1 ±13.4 kg, p=0.007)
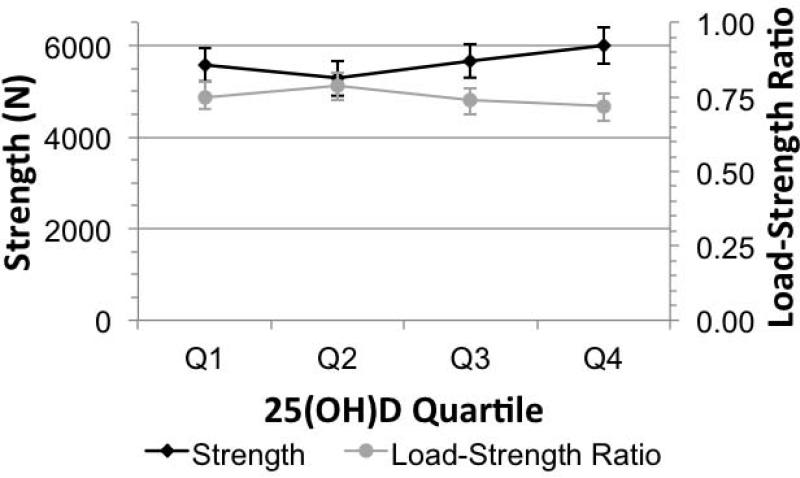
In the multivariable analysis, the adjusted mean femoral neck strength showed a non-linear association with vitamin D quartile, with average strength being lowest in quartile 2 and greatest in quartile 4 (Figure 3). The load-strength ratio followed a similar pattern, with the average ratio being higher in quartile 2 (less resistance to fracture) and lowest in quartile 4 (greater resistance to fracture). However, trends for these associations were weak (Strength, p-trend=0.05; Load-strength ratio, p-trend=0.08).
Figure 3.
Fall Strength and load:strength ratio within 25(OH)D quartiles adjusted for age, race, latitude, season, height and BMI in men ages 65 years and older
In the femoral shaft, overall bone size was not associated with 25(OH)D concentration, whereas cortical area and percent cortical area were positively associated with increasing 25(OH)D level (Supplemental Table). However, unlike in the femoral neck, medullary volume and cortical vBMD of the femoral shaft were not associated with 25(OH)D level.
DISCUSSION
In this cross-sectional study among elderly U.S. men, we observed positive associations between endogenous 25(OH)D concentrations, cortical volume, and percent cortical volume at both the femoral neck and shaft. These associations are notable because they occurred in the absence of a relation between 25(OH)D and overall bone size. In addition, 25(OH)D concentration was positively associated with femoral integral vBMD and this appeared to be mainly due to an association with trabecular vBMD. Consistent with the previously observed biomechanical contribution of cortical bone distribution and trabecular vBMD to bone strength [19, 33] we also observed a positive, albeit weak, association of 25(OH)D and FE-derived femoral strength. Contrary to our working hypothesis, the associations between 25(OH)D and skeletal dimensions at the femoral neck and shaft were independent of PTH level Thus, the sum of evidence from this study indicates that endogenous vitamin D contributes to bone strength via effects on both structural and densitometric features of the proximal femur, but the effects in absolute value were modest in magnitude.
This study represents a logical extension of work already reported in MrOS. We previously reported that men in the lowest quartile of 25(OH) were at over 2-fold increased risk of hip fracture compared to men in the highest quartile [6]. Attenuation of this association by further adjustment for aBMD suggests that a primary pathway through which 25(OH)D contributes to fracture risk has to do with bone strength. Associations of lower percent cortical volume, smaller cross-sectional area, and higher load-to-strength ratio with hip fracture risk independent of DXA aBMD [12,13] suggested the importance of examining the relation of serum vitamin D to measures of femoral strength and structure.
The role of vitamin D supplementation in bone formation and bone strength has been examined extensively. In animal models, vitamin D3 supplementation increased trabecular bone content [36]. In older men and women, randomized trials of vitamin D supplementation showed decreased medullary expansion in the femoral shaft among those receiving supplementation [9, 37]. However, these studies did not evaluate the shaft measures at different serum 25(OH)D levels and they provide no information on effects of supplementation at the femoral neck. Observational studies conflict somewhat on the relation of serum 25(OH)D with pQCT-derived tibial measures. One study showed a positive association with serum 25(OH)D and tibial cross-sectional area and vBMD among women, but not men [27]. However, a cross-sectional study within the MrOS cohort demonstrated greater cortical thickness and bone mineral content at the tibia and distal radius in men with vitamin D sufficiency (≥30ng/mL) compared to men with insufficiency (20 to <30ng/mL) [21]. We observe a similar association at the femoral neck in the MrOS cohort, with an increase in cortical volume between 25(OH)D levels averaging 15 ng/ml (Q1) and 34.8 ng/mL (Q4), a decrease in medullary volume between Q1 and Q4, and increase in cortical BMD between Q1 and Q4. Our study adds to the growing body of evidence that 25(OH)D is associated with reduced cortical thinning at multiple skeletal sites.
The question of an association between PTH and QCT-derived skeletal measures is also addressed in prior studies. An inverse association was seen with PTH concentration and cortical vBMD in the mid-femur following supplementation with calcium and vitamin D-fortified milk [37]. An inverse association between total intact PTH level and volumetric BMD determined by pQCT at the tibia was also observed in women [27]. Prior studies examining bone geometry and strength have demonstrated increased corticol porosity with recombinant PTH treatment, but an overall increase in femoral strength with an increase in trabecular bone [38-40]. In our study, adjustment for PTH did not attenuate the other associations between 25(OH)D concentration and skeletal dimensions. One potential explanation for these differences may be the measurement site at the proximal femur used in our study, which differs from prior studies.
In combination with published evidence, the pattern of greater percent cortical volume at higher 25(OH) concentration without change in overall bone size leads us to propose that vitamin D actions may reduce cortical bone loss in older men. Because adjustment for PTH did not eliminate the observed association with skeletal dimensions in this study, vitamin D may have direct action on bone structure, outside of prevention of secondary hyperparathyroidism. A potential mechanistic explanation is that higher endogenous 25(OH)D concentrations decreases endosteal resorption in the femoral neck, resulting in greater preservation of cortical bone. If this is true, this mechanism may help explain previous reports of greater hip bone loss at lower 25(OH)D concentrations [11]. Moreover, decreased endosteal resorption at higher 25(OH)D concentrations may prevent femoral cortical thinning that occurs with age, translating into reduced fracture risk with higher serum 25(OH)D [6, 34]. Further research to clarify the precise role of vitamin D supplementation on bone structure in older and younger adults is warranted.
Our study has important limitations. The cross-sectional study design precludes our ability to determine the temporal relationship between vitamin D and the femoral measures, and the observational nature of the study does not allow us to infer causality from the statistical associations. We performed multiple comparisons and cannot exclude the possibility that some of our findings were due to chance. Finally, the FE-derived measures were not available for the entire analytic cohort. The smaller sample size may explain the weak associations we observed between 25(OH)D and the measures of biomechanical strength. Due to the characteristics of the MrOS cohort and small numbers of non-Caucasian men in the vitamin D cohort our results are generalizable only to ambulatory, community-dwelling Caucasian men. Despite these limitations, the MrOS cohort is large, includes community dwelling men not selected for osteoporosis, has precise assay measurements of 25(OH)D and PTH, and has standardized QCT measures of femoral neck density, structure and strength, and the finite element analysis technique as implemented here has been validated in multiple cohorts for prediction of incident hip fractures [13,41]. The ability to quantify associations between vitamin D, PTH, and specific three-dimensional femoral measures in this cohort provides a unique opportunity to gain additional insight into reasons higher 25(OH) vitamin D has been associated with decreased fracture risk in the elderly [4-7].
In conclusion, serum 25(OH)D concentrations are positively associated with both structural dimensions and trabecular vBMD in the proximal femur among older men. Given previous evidence that femoral structure and trabecular density are associated with hip fracture risk [12, 13, 22, 41], our results provide a potential explanation for a mechanism through which low serum 25(OH)D levels may increase the risk of hip fractures in older men.
Supplementary Material
Acknowledgements
We thank the Oregon Clinical and Translation Research Institute (OCTRI) for their support and funding of this project through the OSLER TL1.
Disclosures:
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128.
This study was supported by the Oregon Students Learn and Experience Research (OSLER TL1) grant from the Oregon Clinical and Translation Research Institute (OCTRI).
Footnotes
Conflict of Interest:
Dr. Orwoll serves as a consultant for Proctor and Gamble, GlaxoSmithKline, Aventis, TAP Pharmaceuticals, and the Merck Speakers Bureau.
Dr Cauley serves as a consultant for the Merck Advisory Board, and has provided expert testimony for Fosamax litigation.
Dr Keaveny is a consultant for Amgen Inc., Merck & Co. Inc., Agnovos Healthcare LLC, and O.N.Diagnostics LLC; and is a shareholder in O.N. Diagnostics LLC.
All other authors have no conflicts of interest.
Authors’ roles: Study design: EM, LM, EH and JS. Study conduct: EM, EH, LM. Data analysis: EM, SH and LM. Data interpretation: EM, LM, EH, JC, TK and JS. Drafting manuscript: EM, LM, EH and JS. Revising manuscript content: EM, LM, EH, JS, JC, KE, JZ, TK, SH and EO. Approving final version of manuscript: EM, LM, EH, JS, JC, KE, JZ, TK, SH and EO. EM and SH take responsibility for the integrity of the data analysis.
Contributor Information
Elizabeth Martin, Oregon Health & Science University, School of Medicine.
Elizabeth Haney, Oregon Health & Science University, Department of Internal Medicine and Geriatrics.
Jackie Shannon, Oregon Health & Science University, Public Health & Preventive Medicine.
Jane Cauley, University of Pittsburgh, Epidemiology.
Kristine Ensrud, Veterans Affairs Medical Center, Department of Medicine.
Tony Keaveny, University of California, Berkeley, Departments of Mechanical Engineering and Bioengineering.
Joseph Zmuda, University of Pittsburgh, Epidemiology.
Eric Orwoll, Oregon Health & Science University, Department of Medicine, Bone & Mineral Unit.
Stephanie Litwack Harrison, California Pacific Medical Center, Research Institute.
Lynn Marshall, Oregon Health & Science University, Orthopaedics and Rehabilitation..
References
- 1.Holick MF. Vitamin D Deficiency. NEJM. 2007 Jul 19;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Zadshir A, Tareen N, Pan D, Norris K, Martins D. The prevalence of hypovitaminosis D among US adults: data from the NHANES III. Ethn Dis. 2005;15(4 Suppl 5):S5–S101. [PubMed] [Google Scholar]
- 3.Orwoll E, Nielson CM, Marshall LM, et al. Vitamin D Deficiency in Older Men. J Clin Endocrinol Metab. 2009 Apr;94(4):1214–1222. doi: 10.1210/jc.2008-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Looker AC, Mussolino ME. Serum 25-Hydroxyvitamin D and Hip Fracture Risk in Older U.S. White Adults. JBMR. 2007;23(1):143–150. doi: 10.1359/jbmr.071003. [DOI] [PubMed] [Google Scholar]
- 5.Bhattoa HP, Nagy E, More C, et al. Prevalence and seasonal variation of hypovitaminosis D and its relationship to bone metabolism in healthy Hungarian men over 50 years of age: the HunMen Study. Osteoporos Int. 2013 Jan;(1):179–86. doi: 10.1007/s00198-012-1920-2. [DOI] [PubMed] [Google Scholar]
- 6.Cauley JA, Parimi N, Ensrud KE, et al. Serum 25-Hydroxyvitamin D and the Risk of Hip and Nonspine Fractures in Older Men. J Bone Miner Res. 2010;25(3):545–553. doi: 10.1359/jbmr.090826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roddam AW, Neale R, Appleby P, Allen NE, Tipper S, Key TJ. Association between plasma 25-hydroxyvitamin D levels and fracture risk: the EPIC-Oxford study. Am J Epidemiol. 2007;166:1327–1336. doi: 10.1093/aje/kwm210. [DOI] [PubMed] [Google Scholar]
- 8.Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med. 2004;116:634. doi: 10.1016/j.amjmed.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 9.Peacock M, Liu G, Carey M, et al. Effect of Calcium or 25OH Vitamin D3 Dietary Supplementation on Bone Loss at the Hip in Men and Women over the Age of 60. J Clin Endocrinol Metab. 2000;859:3011–3019. doi: 10.1210/jcem.85.9.6836. [DOI] [PubMed] [Google Scholar]
- 10.Melin A, Wilske J, Ringertz H, Saaf M. Seasonal Variation in Serum Levels of 25-Hydroxyvitamin D and Parathyroid Hormone but no Detectable Change in Femoral Neck Bone Density in an Older Population with Regular Outdoor Exposure. JAGS. 2001;49:1190–1196. doi: 10.1046/j.1532-5415.2001.49236.x. [DOI] [PubMed] [Google Scholar]
- 11.Ensrud KE, Taylor BC, Paudel ML, et al. Serum 25-Hydroxyvitamin D Levels and Rate of Hip Bone Loss in Older Men. J Clin Endocrinol Metab. 2009;94:2773–2780. doi: 10.1210/jc.2008-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Black DM, Bouxsein ML, Marshall LM, et al. Proximal Femoral Structure and the Prediction of Hip Fracture in Men: A Large Prospective Study Using QCT. J Bone Miner Res. 2008 Aug;23(8):1326–1333. doi: 10.1359/JBMR.080316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orwoll ES, Marshall LM, Nielson CM, et al. Finite Element Analysis of the Proximal Femur and Hip Fracture Risk in Older Men. J Bone Miner Res. 2009 Dec;24(3):475–483. doi: 10.1359/JBMR.081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genant HK, Block JE, Steiger P, Glueer CC, Smith R. Quantitative computed tomography in assessment of osteoporosis. Semin Nucl Med. Oct. 1987;17(4):316–33. doi: 10.1016/s0001-2998(87)80024-7. [DOI] [PubMed] [Google Scholar]
- 15.Sabatier JP, Guaydier-Souquieres G. Noninvasive methods of bone-mass measurement. Clin Rheumatol. 1989 Jun;8(Suppl 2):41–5. doi: 10.1007/BF02207232. [DOI] [PubMed] [Google Scholar]
- 16.Genant HK, Engelke K, Fuerst T, et al. Noninvasive assessment of bone mineral and structure: state of the art. J. Bone Miner Res. 1996;11(6):707–730. doi: 10.1002/jbmr.5650110602. [DOI] [PubMed] [Google Scholar]
- 17.Grampp S, Jergas M, Lang P, et al. Quantitative CT assessment of the lumbar spine and radius in patients with osteoporosis. AJR Am J Roentgenol. 1996 Jul;167(1):133–40. doi: 10.2214/ajr.167.1.8659357. [DOI] [PubMed] [Google Scholar]
- 18.Grampp S, Genant HK, Mathur A, et al. Comparisons of noninvasive bone mineral measurements in assessing age-related loss, fracture discrimination, and diagnostic classification. J Bone Miner Res. 1997 May;12(5):697–711. doi: 10.1359/jbmr.1997.12.5.697. [DOI] [PubMed] [Google Scholar]
- 19.Bousson V, Le Bras A, Roqueplan F, et al. Volumetric quantitative computed tomography of the proximal femur: relationships linking geometric and densitometric variables to bone strength. Role for compact bone. Osteoporos. Int. 2006;17(6):855–864. doi: 10.1007/s00198-006-0074-5. [DOI] [PubMed] [Google Scholar]
- 20.Wu S, Jia HH, Hans D, et al. Assessment of volumetric bone mineral density of the femoral neck in postmenopausal women with and without vertebral fractures using quantitative multi-slice CT. J Zhejiang Univ Sci B. 2009 Jul;10(7):499–504. doi: 10.1631/jzus.B0820409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbour KE, Zmuda JM, Horwitz MJ, et al. The association of serum 25-hydroxyvitamin D with indicators of bone quality in men of Caucasian and African ancestry. Osteoporosis Int. 2011;22:2475–2485. doi: 10.1007/s00198-010-1481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheu Y, Zmuda JM, Boudreau RM, et al. Bone Strength Measured by Peripheral Quantitative Computed Tomography and the Risk of Nonvertebral Fractures: The Osteoporotic Fractures in Men (MrOS) Study. J Bone Miner Res. 2011;26(1):63–71. doi: 10.1002/jbmr.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ginde AA, Wolfe P, Camargo CA, Jr, Schwartz RS. Defining Vitamin D Status by Secondary Hyperparathyroidism in the US Population. J Endocrinol Invest. 2012 Jan;35(1):42–8. doi: 10.3275/7742. [DOI] [PubMed] [Google Scholar]
- 24.Arabi A, Baddoura R, Awada H, Salamoun M, Ayoub G, El-Hajj Fuleihan G. Hypovitaminosis D osteopathy: Is it mediated through PTH, lean mass, or is it a direct effect? Bone. 2006;39:268–275. doi: 10.1016/j.bone.2006.01.140. [DOI] [PubMed] [Google Scholar]
- 25.Vestergaard P, Jorgensen NR, Mosekilde L, Schwarz P. Effects of parathyroid hormone alone or in combination with antiresorptive therapy on bone mineral density and fracture risk – a meta-analysis. Osteoporos Int. 2007;18:45–47. doi: 10.1007/s00198-006-0204-0. [DOI] [PubMed] [Google Scholar]
- 26.Szulc P, Munoz F, Marchand F, Chapuy MC, Delmas PD. Role of Vitamin D and Parathyroid Hormone in the Regulation of Bone Turnover and Bone Mass in Men: The MINOS Study. Calc Tissue Int. 2003;73:520–530. doi: 10.1007/s00223-002-2103-5. [DOI] [PubMed] [Google Scholar]
- 27.Lauretani F, Bandinelli S, Russo CR, et al. Correlates of bone quality in older persons. Bone Oct. 2006;39(4):915–21. doi: 10.1016/j.bone.2006.03.014. Epub. 2006 May 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the Osteoporotic Fractures in Men (MrOS) study-a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the Osteoporotic Fractures in Men Study (MrOS). Contemp Clin Trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Washburn RA, Smith KW, Jette AM, Janney CA. The physical activity scale for the elderly (PASE): Development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 31.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73–8. doi: 10.1016/j.annepidem.2007.12.001. Epub 2008 Mar 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh RJ, Taylor RL, Reddy GS, Grebe SK. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab. 2006;91:3055–3061. doi: 10.1210/jc.2006-0710. [DOI] [PubMed] [Google Scholar]
- 33.Lang TF, Keyak JH, Heitz MW, et al. Volumetric quantitative computed tomography of the proximal femur: precision and relation to bone strength. Bone. 1997;21:101–118. doi: 10.1016/s8756-3282(97)00072-0. [DOI] [PubMed] [Google Scholar]
- 34.Marshall LM, Lang TF, Lambert LC, Zmuda JM, Ensrud KE, Orwoll ES. Dimensions and volumetric bone density of the proximal femur and their relation to age among older US men. J Bone Miner Res. 2006 Aug;21(8):1197–1206. doi: 10.1359/jbmr.060506. [DOI] [PubMed] [Google Scholar]
- 35.Keaveny TM, Bouxsein ML. Theoretical implications of the biomechanical fracture threshold. J Bone Miner Res. 2008;23:1541–7. doi: 10.1359/JBMR.080406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu L, Tang T, Miao Y, Hao Y, Dai K. Effect of 1,25-dihydroxy vitamin D3 on fracture healing and bone remodeling in ovariectomized rat femora. Bone. May. 2009;44(5):893–8. doi: 10.1016/j.bone.2009.01.378. Epub 2009 Feb 5. [DOI] [PubMed] [Google Scholar]
- 37.Daly RM, Bass S, Nowson C. Long-term effects of calcium-vitamin-D3-fortified milk on bone geometry and strength in older men. Bone. 2006;39:946–953. doi: 10.1016/j.bone.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Keaveny TM, Hoffmann PF, Singh M, et al. Femoral bone strength and its relation to cortical and trabecular changes after treatment with PTH, alendronate, and their combination as assessed by finite element analysis of quantitative CT scans. J Bone Miner Res. 2008;23:1974–82. doi: 10.1359/JBMR.080805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keaveny TM, McClung MR, Xiaohai W, Kopperdahl DL, Mitlak BH, Krohn K. Femoral strength in osteoporotic women treated with teriparatide or alendronate. Bone. 2012;50:165–70. doi: 10.1016/j.bone.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Kleerekoper M, Greenspan SL, Lewiecki EM, et al. Assessing the effects of teriparatide treatment on bone mineral density, bone microarchitecture, and bone strength. J Bone Jt Surg [Am] 2014;96(11):e90. doi: 10.2106/JBJS.L.01757. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kopperdahl DL, Aspelund T, Hoffmann PF, et al. Assessment of incident spine and hip fractures in women and men using finite element analysis of CT scans. J Bone Min Res. 2014;29:570–80. doi: 10.1002/jbmr.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.