Abstract
Context:
Increased adiposity and insulin resistance are associated with hyperglycemia and previous studies have reported that higher glucoses are associated with lower rates of weight gain. One possible mechanism is via increased energy expenditure (EE).
Objective:
To assess the relationships between changes in EE during spontaneous weight gain and concomitant changes in glucose levels.
Design and Participants:
Body composition, metabolic, and glycemic data were available from nondiabetic Native Americans who underwent two measurements of 24-h EE during eucaloric feeding in a metabolic chamber (N = 144; time between measurements: 5.0 ± 3.3 years) or resting EE by ventilated hood system during the euglycemic-hyperinsulinemic clamp (N = 261; 4.5 ± 3.2 years). Long-term follow-up data (8.3 ± 4.3 years) for weight and body composition were available in 131 and 122 subjects, respectively.
Main Outcome Measures:
Twenty four hour EE and respiratory quotient (RQ), resting (RMR), and sleeping (SMR) metabolic rates, glucose, and insulin levels, basal glucose output (BGO).
Results:
Weight gain-associated increase in fasting plasma glucose (FPG) levels was accompanied with decreased 24-h RQ (partial R = −0.24, P = .002) and increased 24-h EE, RMR, SMR, and fat oxidation after accounting for changes in body composition (partial R: 0.12 to 0.19, all P ≤ .05). Upon weight gain, BGO tended to increase (P = .07), while insulin infusion induced a decrease in EE (P = .04). Higher baseline FPG predicted lower rates of future weight gain (partial R = −0.18, P = .04).
Conclusions:
Higher FPG after weight gain was associated with greater-than-expected increase in EE. The rise in BGO and the insulin-induced EE suppression at follow-up indicate that increased hepatic gluconeogenesis may be an important mediator of EE changes associated with weight gain.
Obesity is the consequence of a chronic imbalance between energy input and output. Specifically, a sustained positive energy balance due to an increase in food intake or lower energy expenditure (EE) will result in an increased body weight with alterations in body composition (mainly increases in fat mass). Weight gain in turn induces an increase in EE generally commensurate with the greater body size. However, in longitudinal studies where EE was measured and compared before and after weight gain, it was shown that the increased EE after weight gain is greater than that expected by the increased body size (1, 2). Accordingly, the study of the adaptive mechanisms to weight gain, known as metabolic adaptation (3), may be crucial to provide insight into mechanisms of long-term body weight regulation.
The relatively greater increase in EE with weight gain presumably serves to attenuate further increases in adiposity. Weight gain also leads to decreases in insulin action and worsening glycemia, both of which have also been demonstrated to limit future weight gain. A previous study with direct assessment of insulin action showed that more insulin-resistant individuals gain less weight over time (4). This finding was corroborated by other studies using surrogate measures of insulin resistance (5, 6), whereas others report no difference (7, 8) or even increased weight gain (8, 9). Elevated glucose levels during the oral glucose tolerance test (OGTT) also predict lower rates of weight gain over time (10, 11), independent of insulin action (10). These findings indicate a role for both insulin action and glycemia in body weight regulation. Further, metabolic rate measurements during euglycemic-hyperinsulinemic clamps demonstrate that glucose oxidation, but not glucose storage, was associated with future weight gain (4), indicating that changes in glucose metabolism may have a role in the metabolic adaptation to weight gain.
The aim of this study was to assess the relationships between changes in EE during spontaneous weight gain and concomitant changes in insulin action and glucose levels in a cohort of healthy subjects who underwent repeated assessments of these physiological parameters after a long-term follow-up. We investigated this in a cohort of Native Americans recruited from the wider Phoenix Arizona area, a population with a high prevalence of obesity. We assessed whether changes in insulin action and glucose concentrations might be determinants of the metabolic adaptation to weight gain, and whether baseline values predict future changes in weight and body composition.
Materials and Methods
Volunteers participated in a longitudinal study that examined risk factors for obesity and type 2 diabetes measured on our inpatient clinical research unit at the Obesity and Diabetes Clinical Research Section of the National Institutes of Health in Phoenix Arizona.
The present study includes 144 Native Americans (96M/48F, 66% were full heritage Pima Indians) who had measure of 24-h EE in the respiratory chamber and 261 subjects (159M/102F, 78% full heritage Pima Indians) who had a measure of resting metabolic rate (RMR) prior to the glucose clamp, and a repeat measurement of 24-h EE and RMR at least one year following their baseline measurements. At each admission, subjects were between 18 and 55 years and were determined to be healthy by physical examination, medical history, and laboratory tests. Glucose tolerance was assessed by a 75 g OGTT and all subjects at each examination were free from diabetes according to the ADA diagnostic criteria (12). Body fat mass (FM) and fat free mass (FFM) were estimated at each visit by underwater weighing until August 1993 and thereafter by total body dual energy X-ray absorptiometry (DPX-1; Lunar Radiation Corp). A conversion equation was used to make measurements of body composition comparable between the two methods (13). Before participation, volunteers were fully informed of the nature and purpose of the studies, and written informed consent was obtained. The protocols were approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases.
From the cohort of 144 subjects who had repeated measures of 24-h EE at baseline, 131 had additional long-term follow-up data from an outpatient visit in a study, where Native American volunteers residing on the Gila River Indian Community were invited for examinations approximately every two years (14, 15). At these visits, height and weight were measured while fasting and in light clothing and glucose tolerance was evaluated by OGTT. Only subjects with an age <55 years, without diabetes, and women who were not pregnant at the time of follow-up were included. In a subgroup of 122 subjects, body composition data was also available.
Study protocol
On admission, subjects were given a standard weight maintaining diet (50% carbohydrates, 30% fats, and 20% proteins) to maintain body weight within 1% for three days before any tests were performed.
Respiratory chamber study
The methods for measuring 24-h EE and substrate oxidation in the respiratory chamber were previously described in detail (16). Calculation of 24-h EE components is reported in the footnote of Table 1 and in the Supplemental Appendix.
Table 1.
Demographic, Anthropometric, Glycemic, and Metabolic Characteristics of the Study Groups at Baseline and Follow-Up Visits
| Group | Baseline Visit | Follow-up Visit | Difference (% baseline) | Sig. | |
|---|---|---|---|---|---|
| Age (y) | Chamber | 25.4 ± 5.9 | 30.5 ± 6.8 | +5.1 (20.2%) | <.001a |
| Clamp | 27.7 ± 5.9 | 32.1 ± 6.2 | +4.4 (15.9%) | <.001a | |
| Body weight (kg) | Chamber | 91.7 ± 24.9 | 97.9 ± 26.8 | +6.1 (6.6%) | <.001a |
| Clamp | 95.1 ± 23.2 | 101.4 ± 25.9 | +6.2 (6.6%) | <.001a | |
| BMI (kg/m2) | Chamber | 32.8 ± 8.7 | 35.1 ± 9.2 | +2.2 (6.7%) | <.001a |
| Clamp | 34.3 ± 7.6 | 36.5 ± 8.4 | +2.1 (6.2%) | <.001a | |
| Waist circumference (cm) | Chamber | 105.1 ± 17.9 | 109.9 ± 17.7 | +4.9 (4.6%) | <.001a |
| Clamp | 109.5 ± 17.8 | 114.8 ± 19.0 | +5.6 (5.2%) | <.001a | |
| Thigh circumference (cm) | Chamber | 64.4 ± 8.9 | 64.7 ± 8.6 | 0.3 (0.4%) | .601 |
| Clamp | 65.8 ± 8.9 | 66.8 ± 8.9 | +1.0 (1.5%) | .027a | |
| Body fat (%) | Chamber | 30.8 ± 8.4 | 32.2 ± 7.4 | +1.3 (4.3%) | .002a |
| Clamp | 32.4 ± 8.7 | 34.1 ± 7.8 | +1.7 (5.3%) | <.001a | |
| Fat mass (kg) | Chamber | 29.6 ± 14.8 | 32.7 ± 15.0 | +3.1 (10.3%) | <.001a |
| Clamp | 31.9 ± 13.9 | 35.6 ± 15.3 | +3.7 (11.6%) | <.001a | |
| Fat free mass (kg) | Chamber | 62.1 ± 12.7 | 65.2 ± 14.2 | +3.0 (4.9%) | <.001a |
| Clamp | 63.2 ± 12.5 | 65.7 ± 13.5 | +2.5 (4.0%) | <.001a | |
| Fasting plasma glucose (mg/dL) | Chamber | 85.5 ± 9.8 | 88.3 ± 10.0 | +2.8 (3.3%) | .004a |
| Clamp | 89.1 ± 9.1 | 90.9 ± 9.6 | +1.8 (2.0%) | .002a | |
| 2-h plasma glucose (mg/dL) | Chamber | 111.4 ± 26.4 | 123.4 ± 29.5 | +12.0 (10.8%) | <.001a |
| Clamp | 119.8 ± 28.9 | 131.6 ± 31.8 | +11.7 (9.8%) | <.001a | |
| Glucose tolerance statusb | Chamber | ||||
| NGR | 120 (83.3%) | 92 (63.9%) | −28 (−19.4%) | <.001a | |
| IGR | 24 (16.7%) | 52 (36.1%) | +28 (19.4%) | ||
| IFG | 10 (6.9%) | 13 (9.0%) | +3 (2.1%) | .629 | |
| IGT | 22 (15.3%) | 47 (32.6%) | +25 (17.3%) | <.001a | |
| Glucose AUC (mg/dL · 180 min) | Chamber | ||||
| Total | 21 001 ± 3663 | 22 878 ± 3679 | +1877 (8.9%) | <.001a | |
| Incremental | 5605 ± 2893 | 6992 ± 3358 | +1388 (24.8%) | <.001a | |
| Fasting plasma insulin (mU/L) | Chamber | 39.9 ± 22.8 | 42.5 ± 18.4 | +2.6 (6.5%) | .134 |
| Median and IQR | 33 (25–49) | 37 (28–50) | +4 (12.1%) | .100 | |
| 2-h plasma insulin (mU/L) | Chamber | 168.7 ± 162.0 | 172.6 ± 113.4 | +3.9 (2.3%) | .805 |
| Median and IQR | 127 (72–199) | 140 (86–232) | +13 (10.2%) | .261 | |
| Insulin AUC (mU/L · 180 min) | Chamber | ||||
| Total | 31 638 ± 19 932 | 30 888 ± 14 368 | −750 (−2.4%) | .692 | |
| Incremental | 24 544 ± 17 839 | 23 325 ± 12 288 | −1219 (−5.0%) | .479 | |
| Energy intake (kcal/day) | Chamber | 2235 ± 378 | 2340 ± 395 | +105 (4.7%) | <.001a |
| 24-h EE (kcal/day) | Chamber | 2354 ± 434 | 2496 ± 447 | +142 (6.0%) | <.001a |
| 24-h energy balance (kcal/day) | Chamber | −119 ± 184 | −156 ± 187 | −37 (−30.9%) | .042a |
| SMR (kcal/day) | Chamber | 1639 ± 300 | 1726 ± 269 | +87 (5.3%) | <.001a |
| SPA (%)c | Chamber | 7.8 ± 4.4 | 7.2 ± 3.7 | −0.6 (−8.1%) | .175 |
| EE0 activity (kcal/14 · hrs)c | Chamber | 1253 ± 221 | 1327 ± 211 | +75 (6.0%) | <.001a |
| AFT (kcal/14 · hrs)c | Chamber | 293 ± 110 | 314 ± 110 | +21 (7.3%) | .090 |
| AFT (% of energy intake)c | Chamber | 13.1 ± 4.6 | 13.4 ± 4.3 | +0.3 (2.6%) | .528 |
| 24-h RQ (ratio)d | Chamber | 0.852 ± 0.024 | 0.843 ± 0.026 | −0.008 (−1.0%) | .002a |
| CARBOX (kcal/day)d | Chamber | 1098 ± 269 | 1077 ± 242 | −22 (−2.0%) | .348 |
| FATOX (kcal/day)d | Chamber | 970 ± 315 | 1120 ± 367 | +151 (15.5%) | <.001a |
| RMR (kcal/day)e | Clamp | 1779 ± 310 | 1916 ± 325 | +136 (7.8%) | <.001a |
| EE during clamp (kcal/min) | Clamp | ||||
| Basal period | 1.24 ± 0.22 | 1.33 ± 0.23 | +0.09 (7.7%) | <.001a | |
| Insulin infusion period | 1.24 ± 0.22 | 1.32 ± 0.22 | +0.08 (6.7%) | <.001a | |
| BGO (mg/kg EMBS/min) | Clamp | 1.94 ± 0.23 | 1.97 ± 0.27 | +0.04 (2.1%) | .072 |
| Glucose output suppression (%)f | Clamp | −82.4 ± 20.5 | −76.4 ± 19.4 | 6.0% | <.001a |
| M (mg/kg EMBS/min)g | Clamp | 2.69 ± 1.01 | 2.44 ± 0.81 | −0.26 (−9.8%) | <.001a |
| Median and IQR | 2.4 (2.0–2.9) | 2.2 (1.9–2.6) |
Data are reported as mean±sd. “Chamber”: study group with repeated chamber sessions (n = 144; median time between measurements: 4.2 y., IQR: 2.1–7.6 y); “Clamp”: study group with repeated glucose clamps (n = 261; median time between measurements: 3.8 yr., IQR: 2.0–5.8 y).
P < .05 vs baseline value.
Normal glucose regulation (fasting plasma glucose, FPG <100 mg/dL and 2-h plasma glucose, 2hPG <140 mg/dL); impaired glucose regulation (FPG: 100–125 mg/dL and/or 2hPG: 140–199 mg/dL); impaired fasting glucose (FPG: 100–125 mg/dL); impaired glucose tolerance (IGT, 2hPG: 140–199 mg/dL) according to American Diabetes Association diagnostic criteria (12).
Spontaneous physical activity (SPA) was detected by radar sensors and expressed as the percentage of time over the 15-min interval in which activity was detected. Only chambers with an average daily SPA <15% from 11:00 to 01:00 were included in the analysis. The EE in the inactive state (EE0 activity) was calculated as the intercept of the regression line between EE and SPA between 11:00 and 01:00. Sleeping metabolic rate (SMR) was defined as the average EE of all 15-min intervals between 01:00 and 05:00 AM during which SPA was less than 1.5%. Only chamber sessions with at least four 15-min intervals with SPA <1.5% were considered in the analysis. The awake and fed thermogenesis (AFT), as a measure of the thermic effect of food and the energy cost of being awake, was calculated as the difference between EE0 activity and SMR (27).
Carbon dioxide production (VCO2) and oxygen consumption in liters (VO2) were calculated for every 15-min interval, averaged and extrapolated to the 24-h interval. The 24-h RQ was calculated as the ratio of 24-h VCO2 to 24-h VO2 over the entire day. Carbohydrate (CARBOX) and fat (FATOX) oxidation rates were derived from the 24-h RQ after accounting for protein oxidation, estimated from measurement of 24-h urinary nitrogen excretion.
RMR was calculated as the average EE over 40 min while the subject was instructed to stay awake and motionless, and then extrapolated to 24 h.
Suppression of glucose output at the end of the low-dose insulin infusion was calculated as percent reduction of BGO.
Values of M during the final 40 min of insulin infusion were calculated taking into account variations in steady-state plasma glucose and insulin concentrations and normalized to the estimated metabolic body size (EMBS = fat free mass + 17.7 kg) (40).
Hyperinsulinemic-euglycemic clamp study
RMR was measured after an overnight fast using a respiratory hood system during the basal period of glucose clamp, as previously described (17). Basal glucose output (BGO) and insulin action (M) were assessed during a two-step hyperinsulinemic-euglycemic glucose clamp with measurement of concomitant metabolic rate, as previously described (18). The insulin-induced thermogenesis (IIT, expressed as kcal/min) was calculated as the difference between the EE measured during the final 40 minutes of insulin infusion and the EE measured during the basal period of the glucose clamp.
Statistical analysis
The Kolmogorov-Smirnov test was used to assess normality of data; insulin and M values were log-transformed to approximate a normal distribution. The safe logarithmic transformation, ie, sign(RoWC) · log10[1+abs(RoWC)], was applied to the rate of weight change (RoWC) and to the rates of FM and FFM changes to handle negative values. Changes at follow-up were analyzed by paired Student's t test when assessing differences in mean values, Wilcoxon rank test when assessing differences in skewed variables, and McNemar's test to assess differences in counts and frequency. Analysis of covariance (ANCOVA) was used to assess differences between subjects with normal (NGR) and impaired (IGR) glucose regulation after accounting for age, gender, and baseline weight.
Changes (Δ) in metabolic, anthropometric and glycemic parameters were calculated as the difference between follow-up and baseline measurements. Pearson (r) and Spearman (ρ) correlation coefficients were calculated to quantify associations between Gaussian and skewed variables, respectively. Multivariate linear regression analysis was used to identify the determinants of changes in metabolic parameters after adjustment for known physiologic determinants (19).
A P value ≤0.05 was considered statistically significant. Data are presented as mean ± SD, mean ± SE or median with interquartile range (IQR). Analyses were performed using SPSS (version 21, IBM Corp.).
Results
The characteristics of the study groups at baseline and follow-up visits are shown in Table 1. There was no difference between the two groups in the baseline anthropometric characteristics or in their changes at follow-up.
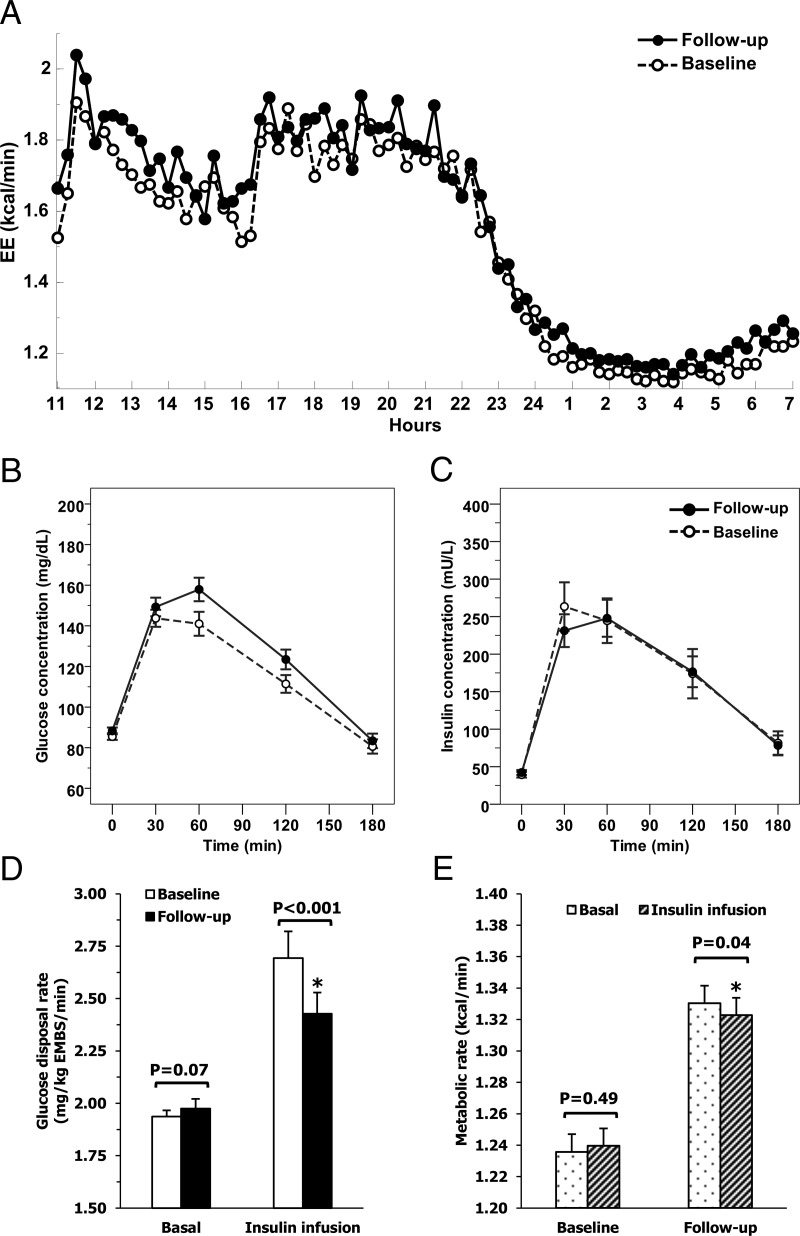
After a mean follow-up time of 5.0 ± 3.3 years (median = 4.2, IQR: 2.1–7.6) between chamber sessions (N = 144), body weight, 24-h EE and sleeping EE (SMR) were higher at follow-up (Figure 1A) whereas the “awake and fed thermogenesis” (AFT, an integrated measure of the thermic effect of food and the cost of being awake) did not vary significantly (Table 1). Fasting (FPG) and 2-h plasma glucose (2-hPG) concentrations and glucose area under the curve (AUC) during OGTT all increased at follow-up (Figure 1B), while insulin levels and AUC did not change (Figure 1C). Out of 144 subjects, 28 with NGR at baseline were classified as with IGR at follow-up, namely, 25 with impaired glucose tolerance (IGT) and 3 with impaired fasting glucose (IFG) (Table 1).
Figure 1.
Time course of energy expenditure during 24 h in the respiratory chamber (Panel A), glucose and insulin concentrations during OGTT (Panels B and C), and glucose disposal (Panel D), and metabolic rates (Panel E) during glucose clamp at baseline and follow-up visits. Time courses of energy expenditure inside the respiratory chamber (median time course of 144 subjects, follow-up time: 5.0 ± 3.3 years, weight change: +6.1 ± 11.7 kg, (Panel A), glucose (Panel B), and insulin (Panel C) concentrations during a 3-h OGTT, glucose disposal (Panel D) and metabolic rate (Panel E) during the basal phase and during insulin infusion of the glucose clamp at the baseline and follow-up visits. Error bars represent mean with 95% confidence intervals.
At the follow-up visit for the study group with repeated clamps (N = 261), subjects had similar increases in body weight, FM and FFM, and glucose concentrations as the study group with repeated chambers. Mean FPG levels increased by 2% (P = .002), and BGO tended to increase to the same extent (P = .07, Figure 1D) while insulin action (M) decreased by approximately 10% (Table 1). On average, RMR increased by approximately 8% at follow-up (P < .001) (Table 1).
Relationships between changes in metabolic, anthropometric, and glycemic parameters
At baseline, FPG (P = .009, adjusted for age, gender, FM, and FFM) but not M (P = .11), 2-hPG (P = .13) or BGO (P = .68), was an independent determinant of RMR, such that a 10 mg/dL difference in FPG was associated, on average, with a positive RMR difference of 36 kcal/d (95% CI: 9 to 62 kcal/d). Longitudinally, ΔFPG (ρ = 0.24, Figure 2C) and ΔM (ρ = −0.22, P < .001), but not ΔBGO (P = .29), were associated with ΔRMR. However, in multivariate analyses including gender, follow-up time, ΔFM and ΔFFM, only ΔFPG was an independent determinant of ΔRMR (partial R = 0.12, P = .05, Table 2), whereas ΔBGO (P = .24), Δinsulin-mediated glucose output suppression (P = .10), Δ2-hPG (P = .25) and ΔM (P = .41) were not. On average, a 10 mg/dL increase in FPG at follow-up was independently associated with an approximate 32 kcal/d increase (95% CI: 1–65 kcal/d) in follow-up RMR. Results for ΔFPG (P = .04) were still significant after adjustment for changes in fasting insulin. When including ΔFPG and ΔM in the same model, ΔFPG demonstrated a trend toward being associated with ΔRMR (partial R = 0.12, P = .06).
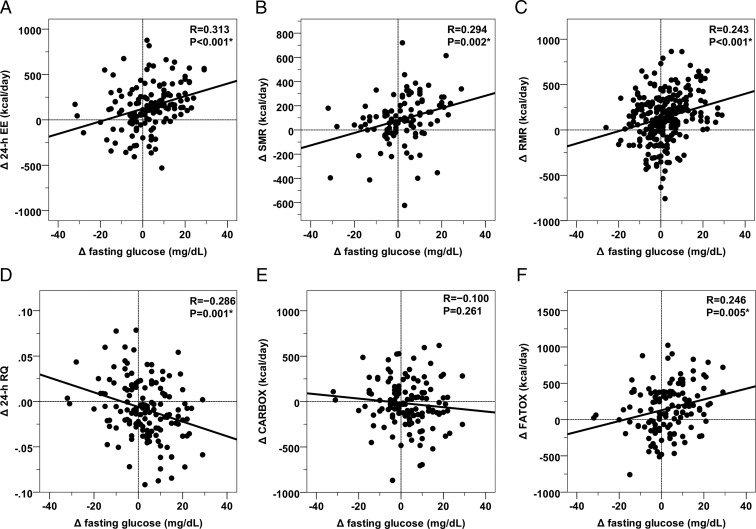
Figure 2.
Relationships between changes (Δ) in fasting glucose concentration and changes in the metabolic parameters at the follow-up visit. Direct associations between changes (Δ, calculated as follow-up minus baseline values) in fasting glucose concentration with changes in 24-h EE (Panel A), sleeping metabolic rate (SMR, Panel B), resting metabolic rate (RMR, Panel C), fat oxidation rate (FATOX, Panel E), and inverse relationship with changes in 24-h respiratory quotient (RQ, Panel D) and carbohydrate oxidation rate (CARBOX, Panel F). Associations were all significant after adjustment for changes in physiological determinants of EE measures (results reported in the main text). The best-fit line is displayed in each panel. Vertical and horizontal lines indicate points with Δ = 0 (ie, no change between baseline and follow-up visits).
Table 2.
Multivariate Models for the Determinants of EE Change at Follow-Up
| Predictors | Δ 24-h EE (kcal/day) | Δ 24-h RQ (ratio) | Δ RMR (kcal/day) |
|---|---|---|---|
| Δ Age (y) | −2.5 (5.7) | 4.0 · 10−4 (8.3 · 10−4) | 12.6 (4.8) |
| P = .659 | P = .629 | P = .009 | |
| Gender (Female) | −12.3 (36.6) | −8.2 · 10−4 (5.5 · 10−3) | −18.2 (30.0) |
| P = .738 | P = .882 | P = .545 | |
| Δ Fat mass (kg) | 9.1 (2.7) | 7.4 (2.3) | |
| P = .001 | P = .001 | ||
| Δ Fat free mass (kg) | 15.4 (3.5) | 10.3 (3.1) | |
| P < .001 | P = .001 | ||
| Δ 24-h energy balance (kcal) | 3.8 · 10−5 (1.2 · 10−5) | ||
| P = .002 | |||
| Δ Body fat (%) | 1.1 · 10−5 (5.5 · 10−4) | ||
| P = .984 | |||
| Δ Fasting glucose (mg/dL) | 3.5 (1.6) | −7.6 · 10−4 (2.4 · 10−4) | 3.2 (1.7) |
| P = .029 | P = .002 | P = .050 | |
| Intercept | 86.4 (60.0) | −5.8 · 10−3 (9.0 · 10−3) | 46.5 (51.2) |
| Explained variance | R2 = 0.393 | R2 = 0.149 | R2 = 0.224 |
β coefficients in each cell are reported as mean values with standard error (se.) and significance.
Consistent with RMR results, in univariate analyses ΔFPG was directly-related with Δ24-h EE, ΔSMR, and ΔFATOX (N = 144; ρ = 0.36, ρ = 0.33, and ρ = 0.25, respectively, P < .001, Figure 2, A, B, and F) and inversely-related to Δ24-h RQ (ρ = −0.28, P < .001, Figure 2D) but not ΔCARBOX (ρ = −0.13, P = .13, Figure 2E). In multivariate models including gender, follow-up time, ΔFM and ΔFFM, ΔFPG was an independent determinant of both Δ24-h EE (partial R = 0.17, P = .03, Table 2) and ΔSMR (partial R = 0.19, P = .02). Changes in FPG were also associated with Δ24-h RQ (partial R = −0.24, P = .002, Table 2), ΔFATOX (partial R = 0.18, P = .04), and ΔCARBOX (partial R = −0.18, P = .05) after adjustment for changes in body composition, energy balance, and follow-up time. In all the multivariate analyses, FPG was the only glycemic parameter independently associated with the EE parameters.
Insulin-induced thermogenesis (IIT)
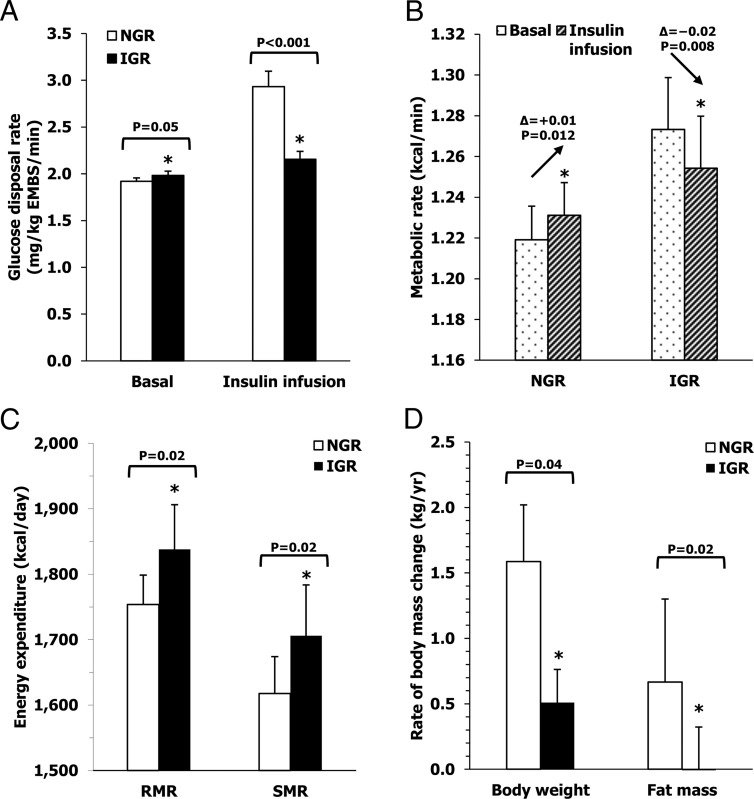
To investigate the parallel increases in FPG and EE after weight gain, we examined the effects of insulin infusion on the metabolic rate during the glucose clamp. With weight gain, both RMR and EE during insulin infusion increased by 8% and 7%, respectively (both P < .001, Table 1), in the setting of slightly higher BGO (+2%, P = .07) and markedly decreased M (−10%, P < .001). At the baseline visit, there was no difference between RMR and EE during insulin infusion (P = .49), indicating that IIT was negligible. At the follow-up visit, mean IIT was negative (P = .04, Figure 1E), reflecting decreased EE with insulin infusion as compared to basal EE. The reduction in EE with insulin infusion implicates the suppression of a high-energy process, such as gluconeogenesis, which could contribute to the higher FPG and RMR observed during fasting. To investigate this hypothesis, we compared the metabolic characteristics of subjects with normoglycemia (NGR subjects) vs those IGR subjects at the baseline visit. Compared to individuals with NGR, subjects with IGR had higher FPG (95.3 ± 8.1 vs 86.4 ± 8.3 mg/dL, P < .001 adjusted for age, gender, and body weight) and BGO (1.98 ± 0.21 vs 1.92 ± 0.24 mg/kg EMBS/min, P = .05, Figure 3A), and higher RMR (1803 ± 158 vs 1734 ± 185 kcal/d, P = .004 adjusted for age, gender, FM, and FFM) and SMR (Figure 3C). However, while individuals with NGR had an increase in EE during insulin infusion (+0.01 ± 0.06 kcal/min, P = .01), EE decreased in IGR subjects during insulin infusion (−0.02 ± 0.06 kcal/min, P = .008) resulting in a similar metabolic rate between the two groups during insulin infusion (NGR: 1.23 ± 0.02; IGR: 1.25 ± 0.03 kcal/min, P = .44). This occurred despite a higher metabolic rate in individuals with IGR during the basal phase of clamp (NGR: 1.22 ± 0.02; IGR: 1.27 ± 0.03 kcal/min, P = .04) (Figure 3B).
Figure 3.
Metabolic characteristics of subjects with NGR and IGR glucose regulation at baseline. Differences between subjects with NGR and IGR glucose regulation in glucose disposal (Panel A) and metabolic (Panel B) rates during clamp, resting, and sleeping EE (Panel C) all measured at the baseline visit and future rates of weight and fat mass changes (Panel D). Normal glucose regulation was defined as FPG <100 mg/dL and 2-h plasma glucose (2hPG) <140 mg/dL, while impaired glucose regulation was considered when FPG was in the range of 100–125 mg/dL and/or 2hPG was in the range of 140–199 mg/dL according to the American Diabetes Association diagnostic criteria (12).
Prediction of rate of future weight and body composition changes
In 131 subjects with a follow-up visit at least one year after the baseline session in the metabolic chamber (median follow-up time: 8.0 years, IQR: 5.2–11.6), on average body weight increased 11.2 ± 15.3 kg (range: −21.3 to 71.4 kg, P < .001), or 12.7 ± 16.3% (range: −20.6 to 68.9%) of initial body weight, with a mean rate of change of 1.4 ± 2.2 kg per year (1.6 ± 2.4% per year). The rate of percent weight change (RoWC%) was similar between sexes (male vs females: 1.5 ± 2.7 vs 1.8 ± 1.7%/y, P = .11), but was higher in subjects with NGR (1.8 ± 2.5%/y, N = 109) compared to those with IGR (0.6 ± 1.5%/y, N = 22, P = .03).
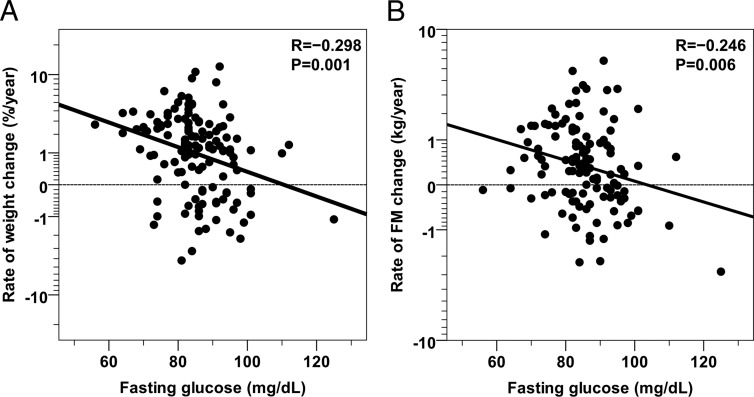
In a multivariate model including gender, baseline body weight, and age, FPG (partial R = −0.18, P = .04) was inversely-related to RoWC%, such that a 10-mg positive difference in baseline FPG corresponded to an average 0.3% decrease in body weight per year, or approximately a 0.4-kg/y decrease (Figure 4A). Together, all the baseline parameters plus gender explained 18% of the variance in RoWC%, with FPG independently explaining 3% of the total variance. Similar results for FPG were obtained when only subjects with NGR at baseline were considered (partial R = −0.19, P = .04).
Figure 4.
Relationships between baseline fasting glucose concentration and rates of future body weight and fat mass change. Inverse relationships between baseline fasting glucose concentration and the rate of future body weight change (expressed as percent of initial body weight (Panel A) and the rate of future body fat mass change (Panel B). The median follow-up time is 8.0 years (IQR: 5.2–11.6 years). Rates of weight and fat mass changes are reported on a safe-logarithmic scale. The best-fit line is displayed in each panel. Horizontal lines indicate points with Δ = 0 (ie, no change between baseline and follow-up visits). The association between fasting glucose concentration and the rate of weight change was still significant after adjustment for gender, baseline body weight and age (partial R = −0.18, P = .04) while fasting glucose was not associated with the rate of FM change after adjustment for gender, baseline body weight and age (partial R = −0.09, P = .34).
In the 122 individuals with follow-up data for body composition measures, subjects with NGR at baseline had a higher rate of FM change as compared to IGR individuals (0.7 ± 1.3 kg/y, N = 101 vs −0.1 ± 0.9 kg/y, N = 21, P = .02, Figure 3D). FPG was inversely-related to the rate of absolute FM change (ρ = −0.26, P = .003, Figure 4B) and to the rate of FM change as percent of baseline FM (ρ = −0.28, P = .002). After adjustment for gender and baseline age, FPG was still inversely-related to the rate of FM change (partial R = −0.19, P = .04), but not after further adjustment for baseline FM (partial R = −0.05, P = .55). There were no significant associations between the rate of FFM change and any baseline glycemic parameter including FPG (all P > .05).
Discussion
We examined the impact of changes in insulin action and blood glucose levels on the metabolic adaptation to weight change in an overweight population of Native Americans. Weight gain-induced hyperglycemia, rather than the decline in insulin action, was an independent predictor of the metabolic alterations. Upon weight gain, BGO tended to increase while insulin infusion induced a decrease, rather than an increase, in EE suggesting the suppression of an energy-costing process. Higher baseline FPG levels, possibly via altered BGO, predicted lower rates of future weight gain.
Obese individuals with IGT or type 2 diabetes have higher rates of EE compared to subjects with normal glucose tolerance (NGT) (20–23), while showing a decreased thermogenic response to glucose (22–26). In individuals with type 2 diabetes, hepatic glucose production is associated with 24-h and sleeping EE (21). Our longitudinal results indicate that increased FPG concentrations with weight gain were independently associated with small, but significant, increases in EE in two separate settings: 1) a metabolic chamber measuring both 24-h EE and SMR; and 2) RMR measured prior to the glucose clamp. In the metabolic chamber AFT, the daily EE component that includes the thermogenic response to food consumption (27), did not vary after weight gain, although it was negatively-related to FPG as previously reported (27). Therefore, the increase in 24-h EE (which includes both AFT and SMR) observed after weight gain was due primarily to an increased SMR.
Our findings support a role for gluconeogenesis as the main cause of higher SMR and RMR in individuals with fasting hyperglycemia (22, 28). Gluconeogenesis is an energy-costly metabolic process that generates glucose from noncarbohydrate substrates primarily overnight, is inhibited by insulin and has been hypothesized to lead to the elevated FPG levels observed with increased insulin resistance, presumably due to higher basal glucose production in the liver. Although we only observed a tendency for higher overall BGO after weight gain and did not have a direct measure of gluconeogenesis, our other findings support a role for gluconeogenesis as the link between increased ΔFPG and ΔEE. In fact, after weight gain and worsening glycemia, we observed a significant reduction in EE with insulin infusion. Similarly, a decrease in EE with insulin infusion was observed in participants with IGT, rather than the increase in EE observed in individuals with NGR due to an increase in both glucose oxidation and storage (26, 29, 30). These findings provide indirect evidence for ongoing gluconeogenesis, which is usually suppressible with insulin and, as a high-energy process, may explain a portion of the increased RMR observed after weight gain. In the IIT analysis comparing NGR vs. IGR individuals, BGO was higher in those with IGR but EE decreased with insulin infusion, indicating suppression of an energy costly process that is likely gluconeogenesis. Higher BGO also would explain the higher baseline (preinsulin infusion) metabolic rates observed in the IGR group, where energy-consuming processes caused by insulin resistance such as the Cori and the glucose-alanine futile cycles supply substrates for gluconeogenesis in the liver from muscle, thus contributing to greater BGO and RMR, as previously shown also in obese subjects with diabetes (31). Taken together, these results indicate gluconeogenesis is a major cause of the greater-than-expected increase in EE with weight gain, although future studies are warranted to establish the direct role of this process in the upregulation of EE. Other possible explanations of increased EE may include changes in glucagon levels (which were not measured in this study), increased sympathetic nervous system activity (32), or the hypothalamic nutrient sensing system in the brain (33).
Increased adiposity and insulin resistance are two physiologic phenomena that are tightly correlated. Therefore, it is difficult to disentangle their separate longitudinal effects on changes in EE and substrate oxidation. We were able to determine the changes (Δ) in these measurements in the dynamic phase of spontaneous weight change in a population of Native Americans prone to obesity. This allowed us to distinguish the effects of FM and FFM increases from changes in M and glucose concentrations, and how these affect changes in EE and substrate oxidation. The analysis of clamp data demonstrated that ΔFPG but not ΔM is a determinant of ΔRMR, suggesting that hyperglycemia rather than the reduction in insulin action causes the increased EE observed with weight gain. This finding is consistent with a previous study showing that glucose levels during the OGTT, rather than M, inversely predicted the rate of weight change in NGR subjects (10). Further, ΔFPG was inversely associated with Δ24-h RQ and directly-related to ΔFATOX independent from ΔFM and ΔFFM, indicating an increased preference for fat as a substrate for energy. Obese hyperglycemic subjects have lower rates of CARBOX and higher rates of FATOX (34). Decreased insulin action with weight gain is not only associated with reduced glycogen synthesis but also with reduced rates of glycolysis and decreased glucose transport into skeletal muscle, thereby reducing CARBOX and causing hyperglycemia. Concurrently, high rates of lipolysis in adipose tissue result in higher levels of circulating free fatty acids (FFA), which promote FATOX (35). Our finding that both increases in FM and in FPG are independent predictors of increased FATOX during weight gain may be explained by the fact that elevated plasma FFA levels are not only related to the degree of obesity [which promotes FFA turnover, (36) and FATOX (37)], but also that higher levels of FFA may inhibit CARBOX via the glucose fatty-acid cycle (38), resulting in higher circulating glucose levels.
An independent effect of ΔFPG on ΔFATOX with weight gain would lead to a higher rate of fat utilization than expected due simply to the increased fat depots, consistent with our observations. This indicates that mildly hyperglycemic individuals may be protected from future FM gain due to an increased FATOX that would limit further FM accumulation. Our longitudinal analysis supported this as IGR individuals had lower rates of both weight and FM gain, and baseline FPG inversely predicted long-term weight gain independently of age, gender, and initial body weight. Specifically, an average rise in RMR of 32 kcal/d for a 10 mg/dL increase in FPG after several years may serve as a brake on body weight in an attempt to prevent further metabolic deterioration. All these results are consistent with previous reports showing that obesity-related insulin resistance “brakes” future weight gain in nondiabetic overweight individuals albeit via a maladaptive process (4–6). We extended these observations in the present study by examining whether this relationship-specifically affects gains in FM, in FFM or both. We found that individuals with IGR at baseline had lower rates of future FM gain but no difference in changes in FFM. The higher rates of EE and FATOX associated with hyperglycemia might explain the attenuated weight and FM gains in these individuals with mildly elevated FPG levels.
One of the limitations of the present study might be that subjects with IGR may have been counseled to lose weight, thereby introducing a bias in the longitudinal analyses of spontaneous weight change. However, our results of FPG predicting weight change were confirmed in the subset of only NGR subjects. In addition, the primarily Native American ethnic composition of our cohort may limit the generalization of our findings. Nevertheless, the inverse relationship between increased insulin resistance and lower rates of weight gain in nondiabetic overweight individuals has also been observed in Hispanic (5, 6) and White (39) populations, indicating that the mechanisms of increased EE due to developing hyperglycemia are likely similar among ethnicities. Further, differences in the composition of two study groups undergoing clamp and chamber sessions (albeit overlapping) might have influenced the results to some extent.
In conclusion, by analyzing long-term longitudinal data for body composition, EE and glycemic parameters in an overweight population, we have shown that increased FPG with spontaneous weight gain induces independent changes in EE and macronutrient oxidation, specifically, higher rates of basal EE and FATOX. Hyperglycemia was accompanied by a reduction in EE during insulin infusion and a tendency for a higher BGO after weight gain. Furthermore, subjects with higher FPG at baseline had a lower rate of future weight gain due to relatively higher EE and FATOX. Hyperglycemia, rather than measured changes in insulin-mediated glucose disposal, influences future body weight changes in overweight individuals, indicating that an increased rate of gluconeogenesis may be involved in the upregulation of EE observed with weight gain.
Acknowledgments
The authors would like to thank the nursing, clinical and dietary staffs, and laboratory technicians of the clinical research center for their valuable assistance and care of the volunteers.
This work was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
ClinicalTrials.gov identifier: NCT00340132.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 2-hPG
- 2-h plasma glucose concentration during OGTT
- AFT
- awake and fed thermogenesis
- ANCOVA
- analysis of covariance
- AUC
- area under the curve
- BGO
- basal glucose output
- CARBOX
- carbohydrate oxidation rate
- EE
- energy expenditure
- EMBS
- estimated metabolic body size
- FATOX
- fat oxidation rate
- FFA
- free fatty acids
- FFM
- fat free mass
- FM
- fat mass
- FPG
- fasting plasma glucose concentration
- IFG
- impaired fasting glucose
- IGR
- impaired glucose regulation
- IGT
- impaired glucose tolerance
- IIT
- insulin-induced thermogenesis
- IQR
- interquartile range
- NGR
- normal glucose regulation
- NGT
- normal glucose tolerance
- OGTT
- oral glucose tolerance test
- RoWC
- rate of weight change
- RMR
- resting metabolic rate
- RQ
- respiratory quotient
- SMR
- sleeping metabolic rate
- SPA
- spontaneous physical activity.
References
- 1. Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. New Engl J Med. 1995;332:621–628. [DOI] [PubMed] [Google Scholar]
- 2. Weyer C, Pratley RE, Salbe AD, Bogardus C, Ravussin E, Tataranni PA. Energy expenditure, fat oxidation, and body weight regulation: a study of metabolic adaptation to long-term weight change. J Clin Endocrinol Metab. 2000;85:1087–1094. [DOI] [PubMed] [Google Scholar]
- 3. Ravussin E, Swinburn BA. Energy metabolism. In: Obesity: Theory and Therapy. New York: Raven Press, Ltd; 1993;97–123. [Google Scholar]
- 4. Swinburn BA, Nyomba BL, Saad MF, et al. Insulin resistance associated with lower rates of weight gain in Pima Indians. J Clin Invest. 1991;88:168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Valdez R, Mitchell BD, Haffner SM, et al. Predictors of weight change in a bi-ethnic population. The San Antonio Heart Study. Int J Obes Relat Metab Disord. 1994;18:85–91. [PubMed] [Google Scholar]
- 6. Hoag S, Marshall JA, Jones RH, Hamman RF. High fasting insulin levels associated with lower rates of weight gain in persons with normal glucose tolerance: the San Luis Valley Diabetes Study. Int J Obes Relat Metab Disord. 1995;19:175–180. [PubMed] [Google Scholar]
- 7. Zavaroni I, Zuccarelli A, Gasparini P, Massironi P, Barilli A, Reaven GM. Can weight gain in healthy, nonobese volunteers be predicted by differences in baseline plasma insulin concentration? J Clin Endocrinol Metab. 1998;83:3498–3500. [DOI] [PubMed] [Google Scholar]
- 8. Hodge AM, Dowse GK, Alberti KG, Tuomilehto J, Gareeboo H, Zimmet PZ. Relationship of insulin resistance to weight gain in nondiabetic Asian Indian, Creole, and Chinese Mauritians. Mauritius Non-communicable Disease Study Group. Metab Clin Exp. 1996;45:627–633. [DOI] [PubMed] [Google Scholar]
- 9. Boyko EJ, Leonetti DL, Bergstrom RW, Newell-Morris L, Fujimoto WY. Low insulin secretion and high fasting insulin and C-peptide levels predict increased visceral adiposity. 5-year follow-up among initially nondiabetic Japanese-American men. Diabetes. 1996;45:1010–1015. [DOI] [PubMed] [Google Scholar]
- 10. Pannacciulli N, Ortega E, Koska J, Salbe AD, Bunt JC, Krakoff J. Glucose response to an oral glucose tolerance test predicts weight change in non-diabetic subjects. Obesity (Silver Spring). 2007;15:632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boulé NG, Chaput JP, Doucet E, et al. Glucose homeostasis predicts weight gain: prospective and clinical evidence. Diabetes/metabolism research and reviews. 2008;24:123–129. [DOI] [PubMed] [Google Scholar]
- 12. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. In: Diabetes Care. 2003;2002/12/28 ed; S5–20. [DOI] [PubMed] [Google Scholar]
- 13. Tataranni PA, Ravussin E. Use of dual-energy X-ray absorptiometry in obese individuals. Am J Clin Nutrition. 1995;62:730–734. [DOI] [PubMed] [Google Scholar]
- 14. Knowler WC, Pettitt DJ, Saad MF, et al. Obesity in the Pima Indians: its magnitude and relationship with diabetes. Am J Clin Nutrition. 1991;53:1543S-1551S. [DOI] [PubMed] [Google Scholar]
- 15. Votruba SB, Thearle MS, Piaggi P, Knowler WC, Hanson RL, Krakoff J. Weight maintenance from young adult weight predicts better health outcomes. Obesity. 2014;22:2361–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78:1568–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bogardus C, Lillioja S, Ravussin E, et al. Familial dependence of the resting metabolic rate. New Engl J Med. 1986;315:96–100. [DOI] [PubMed] [Google Scholar]
- 18. Lillioja S, Mott DM, Howard BV, et al. Impaired glucose tolerance as a disorder of insulin action. Longitudinal and cross-sectional studies in Pima Indians. N Engl J Med. 1988;318:1217–1225. [DOI] [PubMed] [Google Scholar]
- 19. Piaggi P, Thearle MS, Bogardus C, Krakoff J. Lower energy expenditure predicts long-term increases in weight and fat mass. J Clin Endocrinol Metab. 2013;98:E703–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bogardus C, Taskinen MR, Zawadzki J, Lillioja S, Mott D, Howard BV. Increased resting metabolic rates in obese subjects with non-insulin-dependent diabetes mellitus and the effect of sulfonylurea therapy. Diabetes. 1986;35:1–5. [DOI] [PubMed] [Google Scholar]
- 21. Fontvieille AM, Lillioja S, Ferraro RT, Schulz LO, Rising R, Ravussin E. Twenty-four-hour energy expenditure in Pima Indians with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992;35:753–759. [DOI] [PubMed] [Google Scholar]
- 22. Weyer C, Bogardus C, Pratley RE. Metabolic factors contributing to increased resting metabolic rate and decreased insulin-induced thermogenesis during the development of type 2 diabetes. Diabetes. 1999;48:1607–1614. [DOI] [PubMed] [Google Scholar]
- 23. Nair KS, Webster J, Garrow JS. Effect of impaired glucose tolerance and type II diabetes on resting metabolic rate and thermic response to a glucose meal in obese women. Metabolism. 1986;35:640–644. [DOI] [PubMed] [Google Scholar]
- 24. Ravussin E, Acheson KJ, Vernet O, Danforth E, Jéquier E. Evidence that insulin resistance is responsible for the decreased thermic effect of glucose in human obesity. J Clin Invest. 1985;76:1268–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Golay A, Schutz Y, Meyer HU, et al. Glucose-induced thermogenesis in nondiabetic and diabetic obese subjects. Diabetes. 1982;31:1023–1028. [DOI] [PubMed] [Google Scholar]
- 26. Ravussin E, Bogardus C, Schwartz RS, et al. Thermic effect of infused glucose and insulin in man. Decreased response with increased insulin resistance in obesity and noninsulin-dependent diabetes mellitus. J Clin Invest. 1983;72:893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Piaggi P, Krakoff J, Bogardus C, Thearle MS. Lower “awake and fed thermogenesis” predicts future weight gain in subjects with abdominal adiposity. Diabetes. 2013;62:4043–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Consoli A, Nurjhan N, Capani F, Gerich J. Predominant role of gluconeogenesis in increased hepatic glucose production in NIDDM. Diabetes. 1989;38:550–557. [DOI] [PubMed] [Google Scholar]
- 29. Bogardus C, Lillioja S, Mott D, Zawadzki J, Young A, Abbott W. Evidence for reduced thermic effect of insulin and glucose infusions in Pima Indians. J Clin Invest. 1985;75:1264–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lillioja S, Mott DM, Zawadzki JK, Young AA, Abbott WG, Bogardus C. Glucose storage is a major determinant of in vivo “insulin resistance” in subjects with normal glucose tolerance. J Clin Endocrinol Metab. 1986;62:922–927. [DOI] [PubMed] [Google Scholar]
- 31. Zawadzki JK, Wolfe RR, Mott DM, Lillioja S, Howard BV, Bogardus C. Increased rate of Cori cycle in obese subjects with NIDDM and effect of weight reduction. Diabetes. 1988;37:154–159. [DOI] [PubMed] [Google Scholar]
- 32. Esler M, Rumantir M, Wiesner G, Kaye D, Hastings J, Lambert G. Sympathetic nervous system and insulin resistance: from obesity to diabetes. Am J Hypertension. 2001;14:304S-309S. [DOI] [PubMed] [Google Scholar]
- 33. Lam TK, Schwartz GJ, Rossetti L. Hypothalamic sensing of fatty acids. Nature Neurosci. 2005;8:579–584. [DOI] [PubMed] [Google Scholar]
- 34. Felber JP, Ferrannini E, Golay A, et al. Role of lipid oxidation in pathogenesis of insulin resistance of obesity and type II diabetes. Diabetes. 1987;36:1341–1350. [DOI] [PubMed] [Google Scholar]
- 35. Golay A, Felber JP, Meyer HU, Curchod B, Maeder E, Jéquier E. Study on lipid metabolism in obesity diabetes. Metabolism. 1984;33:111–116. [DOI] [PubMed] [Google Scholar]
- 36. Nestel PJ, Whyte HM. Plasma free fatty acid and triglyceride turnover in obesity. Metabolism. 1968;17:1122–1128. [DOI] [PubMed] [Google Scholar]
- 37. Lillioja S, Bogardus C, Mott DM, Kennedy AL, Knowler WC, Howard BV. Relationship between insulin-mediated glucose disposal and lipid metabolism in man. J Clin Invest. 1985;75:1106–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. [DOI] [PubMed] [Google Scholar]
- 39. Wedick NM, Mayer-Davis EJ, Wingard DL, Addy CL, Barrett-Connor E. Insulin resistance precedes weight loss in adults without diabetes : the Rancho Bernardo Study. Am J Epidemiol. 2001;153:1199–1205. [DOI] [PubMed] [Google Scholar]
- 40. Lillioja S, Bogardus C. Obesity and insulin resistance: lessons learned from the Pima Indians. Diabetes/metabolism reviews. 1988;4:517–540. [DOI] [PubMed] [Google Scholar]