Abstract
Context:
During puberty, reactivation of the reproductive axis occurs during sleep, with LH pulses specifically tied to deep sleep. This association suggests that deep sleep may stimulate LH secretion, but there have been no interventional studies to determine the characteristics of deep sleep required for LH pulse initiation.
Objective:
The objective of this study was to determine the effect of deep sleep fragmentation on LH secretion in pubertal children.
Design and Setting:
Studies were performed in a clinical research center.
Subjects:
Fourteen healthy pubertal children (11.3–14.1 y) participated in the study.
Interventions:
Subjects were randomized to two overnight studies with polysomnography and frequent blood sampling, with or without deep sleep disruption via auditory stimuli.
Results:
An average of 68.1 ±10.7 (± SE) auditory stimuli were delivered to interrupt deep sleep during the disruption night, limiting deep sleep to only brief episodes (average length disrupted 1.3 ± 0.2 min vs normal 7.1 ± 0.8 min, P < .001), and increasing the number of transitions between non-rapid eye movement (NREM), REM, and wake (disrupted 274.5 ± 33.4 vs normal 131.2 ± 8.1, P = .001). There were no differences in mean LH (normal: 3.2 ± 0.4 vs disrupted: 3.2 ± 0.5 IU/L), LH pulse frequency (0.6 ± 0.06 vs 0.6 ± 0.07 pulses/h), or LH pulse amplitude (2.8 ± 0.4 vs 2.8 ± 0.4 IU/L) between the two nights. Poisson process modeling demonstrated that the accumulation of deep sleep in the 20 minutes before an LH pulse, whether consolidated or fragmented, was a significant predictor of LH pulse onset (P < .001).
Conclusion:
In pubertal children, nocturnal LH augmentation and pulse patterning are resistant to deep sleep fragmentation. These data suggest that, even when fragmented, deep sleep is strongly related to activation of the GnRH pulse generator.
Sleep is intimately related to reproductive hormone secretion. One of the most striking connections occurs during puberty, when the dramatic increase in LH secretion, which marks reactivation of the GnRH pulse generator, is initially limited to sleep (1). We have now shown that LH pulses during sleep occur most frequently during slow-wave or deep sleep (known as N3) and very rarely occur during rapid eye movement (REM) or periods of wakefulness after sleep onset (2). Moreover, the consistent occurrence of deep sleep in the 5–15 minutes before an LH pulse suggests that entrance into deep sleep may stimulate pulsatile GnRH, and consequent LH, secretion during puberty (2).
Support for a clinical role of sleep in hormone secretion during puberty comes from our previous report of relatively delayed thelarche in girls with obstructive sleep apnea (OSA) who have abnormal sleep architecture (3). To investigate the hypothesis that deep sleep is an important stimulus for LH pulse onset during puberty, we conducted deep sleep disruption studies using controlled auditory stimuli in a group of healthy boys and girls and demonstrated that even when fragmented, deep sleep strongly predicts LH pulse onset.
Materials and Methods
Subjects
Subjects (n = 14) were healthy pubertal children as determined by Tanner breast staging (4) or measurement of testicular volume using an orchidometer. All girls were premenarchal. Subjects were euthyroid; were not on any medication known to interfere with sleep, linear growth, or puberty; and did not have a history of precocious puberty or premature adrenarche. Subjects with known sleep disorders or suspected to have OSA based on results of a validated sleep habits questionnaire (5) were excluded. Subjects took iron supplements for the duration of the study and for 1 month thereafter. The study was approved by the Partners Human Research Committee. Signed informed assent and consent was obtained from each subject and his/her parent.
Experimental protocol
Subjects were admitted to the Clinical Research Center of the Massachusetts General Hospital for two overnight studies spaced 2 months apart consisting of frequent blood sampling and polysomnography (PSG) with or without deep sleep disruption in randomized order. In two instances, subjects repeated the sleep disruption night (because of inadequate disruption during the first attempt) such that the normal and disrupted sleep nights were spaced 4 months apart.
PSG was performed according to standard methodology (6), and blood was sampled through an iv catheter from outside the sleeping room using a blood-sparing technique (7), as previously described (2). PSG recording began before lights out and continued until natural awakening the following morning. Lights were turned off between 9:00 and 10:30 pm, based on the subject's habitual bedtime. Blood samples (3–5 cc) were drawn every 10 minutes for 8 hours, beginning at sleep onset, defined as the first epoch of any sleep stage. Subjects were video monitored remotely by a nurse and sleep technician.
To disrupt deep sleep, auditory stimuli (3 sec, 1500 Hz tones at 40 dB, increasing in 10 dB increments to a maximum of 100 dB followed by 18 sec of noise simulating a knock on the door at 75 dB) were delivered via a speaker placed at the head of the bed (iHome iP3 stereo speaker system; SDI Technologies, Inc). Stimuli were delivered whenever at least two delta waves (≤4 Hz) appeared in a 15-second PSG recording interval. This protocol was modeled after that of Tasali et al (8) in which deep sleep was abolished in adult subjects. In the current study, tactile stimulation (shaking of the shoulder) was also used if an arousal response could not be elicited by auditory stimulation.
All blood samples were analyzed for LH using a chemiluminescent microparticle immunoassay (CMIA) (Architect; Abbott Diagnostics) with a minimum detectable concentration (MDC) of 0.07 IU/L. LH is expressed in international units per liter, as equivalents of the Pituitary Second International Reference Standard 80/552. The final morning blood sample (6:00–8:00 am) from both visits was analyzed for estradiol (E2) and progesterone (P) in girls and testosterone (T) in boys. E2, P, and T were measured using the Architect CMIA. The E2 and T assays were standardized and calibrated against liquid chromatography/tandem mass spectrometry (9, 10). The MDC for the E2 assay is 10 pg/mL, and the interassay coefficients of variation (CVs) are 9.6% and 3.9% for quality control (QC) sera containing 36 and 184 pg/mL, respectively. The MDC for the P assay is 0.1 ng/mL and the interassay CV is 5.2% for QC sera containing 0.7 ng/mL. The MDC for the T assay is 5 ng/dL and the interassay CVs are 7.1%, 5.2%, and 4.2% for QC sera containing 9, 69, and 229 ng/dL, respectively.
Hemoglobin was assessed on admission and at the completion of sampling and remained within the normal range for age (mean change of −0.1 g/dL, range ± 1.2).
Data analysis
Sleep scoring
The sleep recordings were visually scored by a registered PSG technician according to American Academy of Sleep Medicine criteria (6) in 30-second epochs as stages of non-REM (NREM) (N1, N2, or N3), REM, or wake. The arousal index was defined as the number of arousals per hour of sleep. Sleep latency (minutes) was defined as the period of time between lights out and the first epoch of sleep, and sleep efficiency was defined as the percentage of the 8-hour sampling time spent asleep. Differences in sleep architecture between the two study nights were compared using ANOVA.
Analysis of LH pulse properties
Pulsatile LH secretion was analyzed using a validated modification (11) of the Santen and Bardin method of pulse detection (12). LH pulse onset was defined as the pulse nadir. Each LH pulse was assigned to a specific sleep stage based on the PSG epoch coinciding with the time of the LH pulse nadir. LH pulse frequency as a function of sleep stage was compared using repeated-measures ANOVA with pairwise comparisons made using the Student-Newman-Keuls method. LH pulse frequency and amplitude, mean LH, and sex steroids were compared between the two study nights using two-way ANOVA with intervention and test order as factors.
LH pulse probability modeling
To determine which properties of deep sleep (eg, continuity, duration) are critical for LH pulse generation, we developed a probability model based on generalized Poisson processes. This model represents an adaptation of techniques previously used and validated to predict neural spikes (action potentials) (13), which, like GnRH neuronal firing and GnRH and LH release, are discrete events that occur in continuous time and are followed by a refractory period (14). Studies in postmenopausal women, in whom the GnRH neuronal network is not restrained by sex steroid-negative feedback, suggest that the apparent refractory period is approximately 1 hour in humans (15). In this LH pulse probability model, the apparent refractory period of the GnRH neuronal network is accounted for by including the elapsed time since the previous LH pulse (TSPP) as an independent variable.
In our initial model, we pooled the data from the normal and disrupted sleep nights and investigated the contributions of current sleep stage and TSPP to prediction of the occurrence of a given LH pulse. We included an intervention variable (normal or disrupted sleep), but this variable was dropped from subsequent models because the analysis showed it to be nonsignificant. We found that only N3 and TSPP were significant predictors of LH pulse onset (see Results).
Given the significance of N3 as a predictor of LH pulse onset, we next investigated whether this relationship reflected the co-occurrence of deep sleep and LH pulse onset or the accumulation of deep sleep over some period of time before pulse onset. We therefore added the fraction of time spent in deep sleep over time intervals ranging from 5 to 40 minutes before the current time point as an additional variable in the model. The resulting fits to the data were then analyzed to determine the following: 1) the time period over which the accumulated deep sleep was most significant; and 2) whether the goodness of fit was improved by inclusion of this variable using a χ2 test (16).
We adopted a generalized Poisson model in which the logarithm of the local Poisson intensity λk at the kth epoch (roughly, the probability of a pulse per unit time) is given by a linear combination of the independent variables, log λk = , where is a coefficient vector and represents the independent variables of sleep stage (indexed N1, N2, N3, REM), TSPP and, in the second analysis, the fraction of time spent in N3 in a fixed time window (φm, m=5 to 40 min in 5-min increments) before the current epoch. In this model, the probability of a pulse at epoch Nk is given by Pr(Nk = nk) = (1 − λk)1 − nk, where nk = 1 if there was a pulse at epoch k and nk = 0 if there was not a pulse at epoch k. We used maximum likelihood estimation to determine the value and standard errors of the coefficients, . The coefficient of variation (z statistic) was then used to determine which coefficients were significantly different from zero, and hence were significant predictors of pulse onset.
Data are expressed as mean ± SE unless otherwise indicated, and P < .05 is considered significant.
Results
Baseline characteristics
Seven premenarchal pubertal girls (Tanner II-III breasts) and seven pubertal boys (testicular volumes 4–15 cc) participated (Table 1). An additional pubertal boy completed both study visits but was excluded from the analyses because the PSG system failed to record during one of his visits. Subjects were 11.3–14.1 years old, and 29% were overweight or obese at the time of the first study visit (Table 1). There were no within-subject differences in body mass index (BMI) percentiles or pubertal stage between study visits.
Table 1.
Subject Characteristics
| Subject | Gender | Age, y | BMI, kg/m2 | BMI Percentilea | Pubertal Stageb | LH, IU/Lc | E2, pg/mLc | P, ng/mLc | T, ng/dLc |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 11.6 | 16.5 | 29 | III | 2.3 | 29 | 0.2 | |
| 2 | F | 12.3 | 19.2 | 70 | II | 4.0 | 48 | 0.2 | |
| 3 | F | 12.3 | 19.8 | 73 | III | 2.2 | 14 | 0.2 | |
| 4 | F | 12.3 | 17.9 | 44 | III | 3.4 | 38 | 0.1 | |
| 5 | F | 12.5 | 18.6 | 53 | III | 0.9 | 14 | 0.2 | |
| 6 | F | 13.2 | 25.0 | 94 | III | 3.3 | 27 | 0.1 | |
| 7 | F | 13.4 | 20.5 | 68 | III | 4.5 | 42 | 0.3 | |
| 8 | M | 11.3 | 26.1 | 97 | 5cc | 2.5 | 110 | ||
| 9 | M | 12.0 | 22.9 | 92 | 4cc | 2.7 | 362 | ||
| 10 | M | 12.2 | 29.3 | 97 | 8 cc/6 cc | 2.6 | 84 | ||
| 11 | M | 13.4 | 17.9 | 37 | 15 cc | 2.4 | 457 | ||
| 12 | M | 13.4 | 17.9 | 37 | 15 cc | 1.6 | 335 | ||
| 13 | M | 13.8 | 18.7 | 46 | 15 cc | 2.1 | 477 | ||
| 14 | M | 14.1 | 21.6 | 78 | 15 cc | 1.8 | 493 |
Abbreviations: F, female; M, male.
Age- and sex-adjusted BMI percentile greater than 85 is classified as overweight and greater than 95 is classified as obese in children (39).
Tanner breast stage or testicular volume. All girls were premenarchal.
Reproductive hormone levels (E2, P, and T) from morning (6:00–8:00 am) samples taken after the night of normal sleep.
Sleep parameters
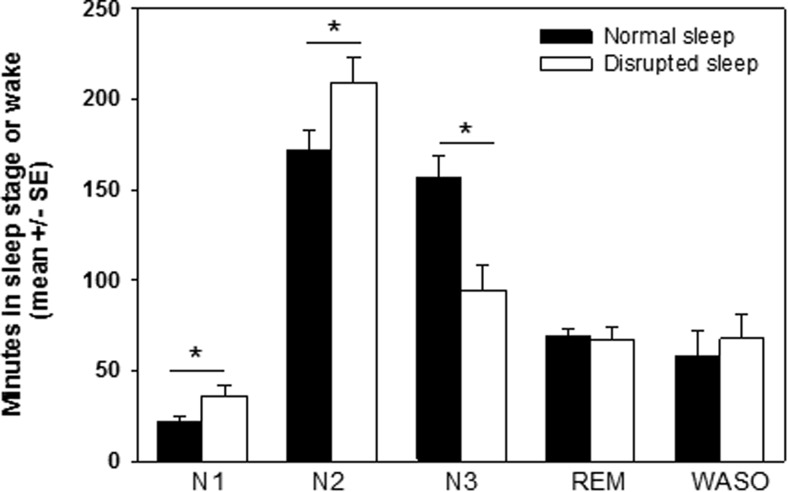
During the normal sleep night, all subjects demonstrated grossly normal sleep architecture for age. Subjects spent 32.7% ± 2.5% of total sleep time in deep sleep (Figure 1). Sleep efficiency was 87.8% ± 3.0% and sleep latency was 21.3 ± 3.5 minutes. Subjects spontaneously aroused an average of 10.3 ± 0.7 times/h, consistent with normative data in adolescents (17).
Figure 1.
Sleep disruption led to a decrease in the amount of time spent in deep sleep (N3) (P < .001) and an increase in the amount of time spent in stages N1 (P = .01) and N2 (P < .01) relative to the normal sleep night but no change in time spent in REM or time awake after sleep onset (WASO).
On average, 68.1 ± 10.7 (range 23–120) auditory or tactile stimuli were delivered to interrupt deep sleep during the sleep disruption night, resulting in a doubling of the arousal index to 22.7 ± 3.6 events/h, shorter deep sleep episodes (average length disrupted 1.3 ± 0.2 min vs normal 7.1 ± 0.8 min, P < .001; maximum length disrupted 6.1 ± 0.9 min vs normal 56.6 ± 3.8 min, P < .001), and significantly more transitions between NREM, REM, and wake relative to the normal sleep night (disrupted 274.5 ± 33.4 vs normal 131.2 ± 8.1, P = .001). There was a 40.0% ± 8.0% decrease in time spent in deep sleep and a 30.7% ± 7.4% increase in time spent in lighter sleep stages (N1+N2) relative to the normal sleep night but no change in time spent in REM (normal 14.5 ± 0.9% vs disrupted 14.1 ± 1.4%) or in the amount of wakefulness after sleep onset (normal 12.3% ± 3.0% vs disrupted 14.2% ± 3.0%, P = .4) (Figure 1).
LH pulse dynamics
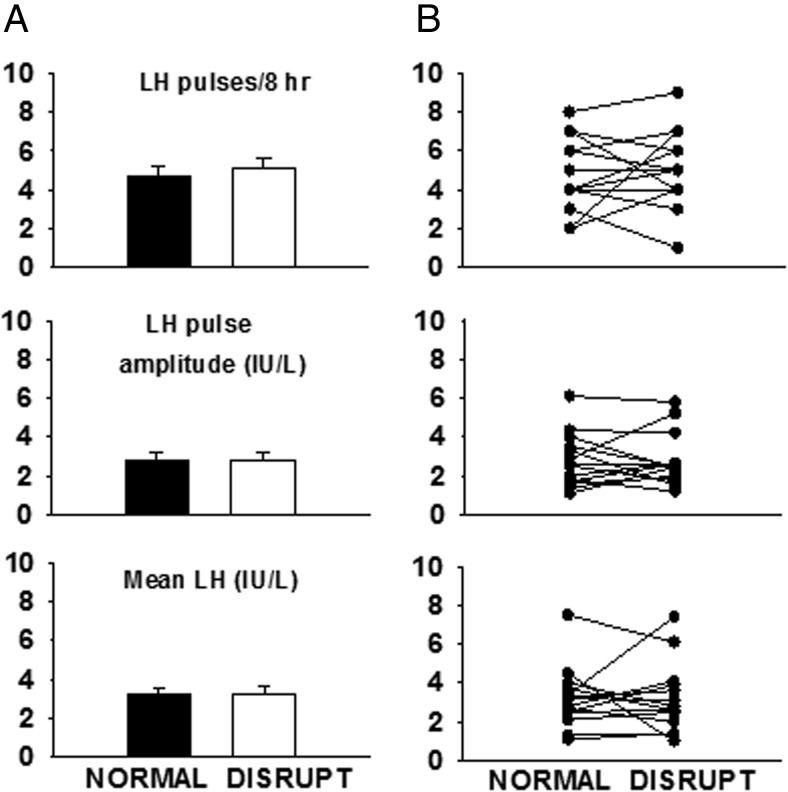
During normal sleep, subjects had an average of 4.7 ± 0.5 LH pulses during the 8-hour monitoring period, with a mean LH pulse amplitude of 2.8 ± 0.4 IU/L, resulting in mean LH levels of 3.2 ± 0.4 IU/L. LH pulse onset was most likely to coincide with an epoch of either N3 (44%, n = 29 of 66) or N2 (27%, n = 18 of 66) (P < .001). During disrupted sleep, subjects had an average of 5.1 ± 0.5 pulses with mean LH pulse amplitude of 2.8 ± 0.4 IU/L, resulting in mean LH levels of 3.2 ± 0.5 IU/L. LH pulses were most likely to initiate during N2 (38%, n = 27 of 71, P < .01)
The effect of sleep disruption on pulsatile LH secretion was inconsistent across subjects with an increase in LH pulse frequency (one to five pulses) in five subjects, a decrease in pulse frequency (one to three pulses) in seven subjects, and no change in two subjects (Figures 2 and 3). Sleep disruption was not associated with significant changes in mean LH, LH pulse amplitude (Figures 2 and 3) or sex steroid levels (normal vs disrupted: T, 331 ± 64.4 vs 341 ± 76.0 ng/dL, E2, 27.9 ± 5.0 vs 27.4 ± 3.7 pg/mL, P, 0.2 ± 0.03 vs 0.3 ± 0.05 ng/mL). Variability in measures of LH secretion could not be accounted for by gender or BMI percentile, and there was no correlation between the percent change in LH pulse frequency and the percentage change in time spent in N3.
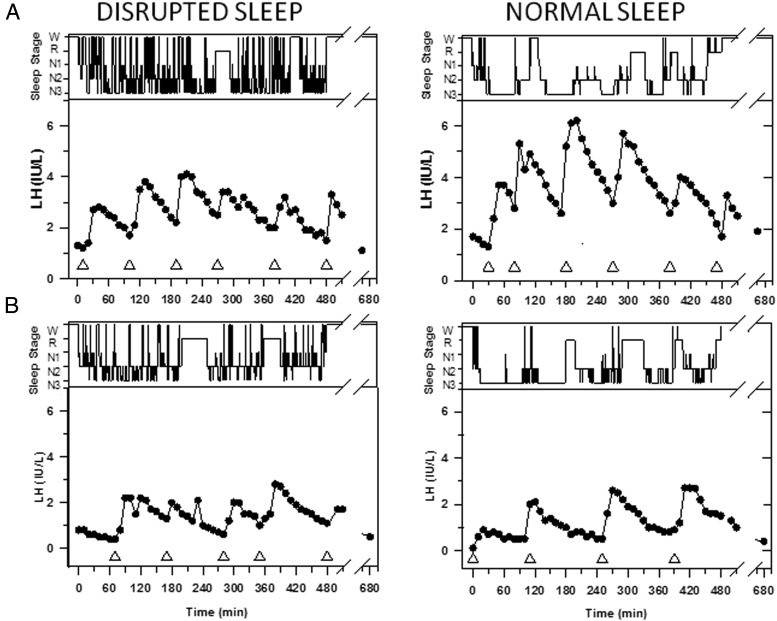
Figure 2.
Representation of sleep stages (wake, REM, N1, N2, and N3, in descending order) and LH values in two subjects studied during one night of normal (right panel) and one night of disrupted (left panel) sleep spaced 2 months apart. Note the consolidated periods of deep sleep across the night during normal sleep in contrast to the frequent sleep stage transitions during the disrupted sleep night. The nadir of each LH pulse is marked by a triangle. A, In this 11-year-old early pubertal boy (subject 8), sleep disruption was associated with a decrease in LH pulse amplitude (2.5 to 1.7 IU/L), resulting in a decrease in mean LH levels (3.7 to 2.6 IU/L). B, In this 12.5-year-old premenarcheal girl (subject 5), sleep disruption was associated with one additional pulse and a small decrease in LH pulse amplitude (1.8 to 1.2 IU/L) but no difference in mean LH levels (1.3 to 1.4 IU/L).
Figure 3.
A, Sleep disruption in pubertal children did not diminish LH pulse frequency, LH pulse amplitude, or mean LH levels (mean ± SE). B, Individual data for the 14 subjects demonstrates the heterogeneity of the LH responses to sleep disruption.
LH pulse probability modeling
The sleep disruption protocol, although eliminating consolidated deep sleep, did not prevent brief transitions into and out of deep sleep. Thus, either instantaneous deep sleep or the accumulation of deep sleep over time, albeit discontinuous, may have been sufficient to trigger GnRH and consequent LH secretion. Analysis of the contributions of the current (instantaneous) sleep stage (N1, N2, N3, REM) and TSPP in predicting LH pulse onset (model 1) indicated that only the regression coefficients associated with N3 and TSPP were statistically significant (Table 2). For sleep stages, a coefficient of 1 indicates an increased probability of an LH pulse by a factor of e1.0 ≈ 2.7. Thus, for N3, the coefficient of 0.52 in model 1 indicates that being in N3 increases the probability of an LH pulse by a factor of e0.5 ≈ 1.65. For the variable TSPP, the probability of an LH pulse increases by a factor of e(coefficient × TSPP). For example, at a TSPP of 90 minutes (a typical interpulse interval in pubertal children), the probability of another LH pulse increases by a factor of e(0.006 × 90) ≈ 1.72. Importantly, the contribution of TSPP increases exponentially with time, reflecting progressive recovery from the apparent refractory period following a pulse.
Table 2.
Generalized Poisson Model Coefficients, Their Standard Deviations, and Significance Levels for the Two Models.
| Model 1 |
Model 2 |
|||||
|---|---|---|---|---|---|---|
| SD | P Value | SD | P Value | |||
| N1 | 0.34 | 0.30 | .3 | 0.41 | 0.30 | .2 |
| N2 | 0.028 | 0.17 | .9 | 0.06 | 0.17 | .7 |
| N3 | 0.52 | 0.17 | .002 | −0.0052 | 0.22 | 1.0 |
| REM | −0.20 | 0.25 | .4 | 0.0033 | 0.26 | 1.0 |
| TSPP, min−1 | 0.0068 | 0.0018 | .002 | 0.0074 | 0.0018 | .001 |
| ϕ20 | 1.42 | 0.34 | .00001 | |||
Model 1 considers contributions only from the current sleep stage and the TSPP and indicates that both instantaneous N3 and TSPP are significant predictors of pulse onset. Model 2 includes the variable , the fraction of time spent in N3 in a 20-minute window preceding each time point.
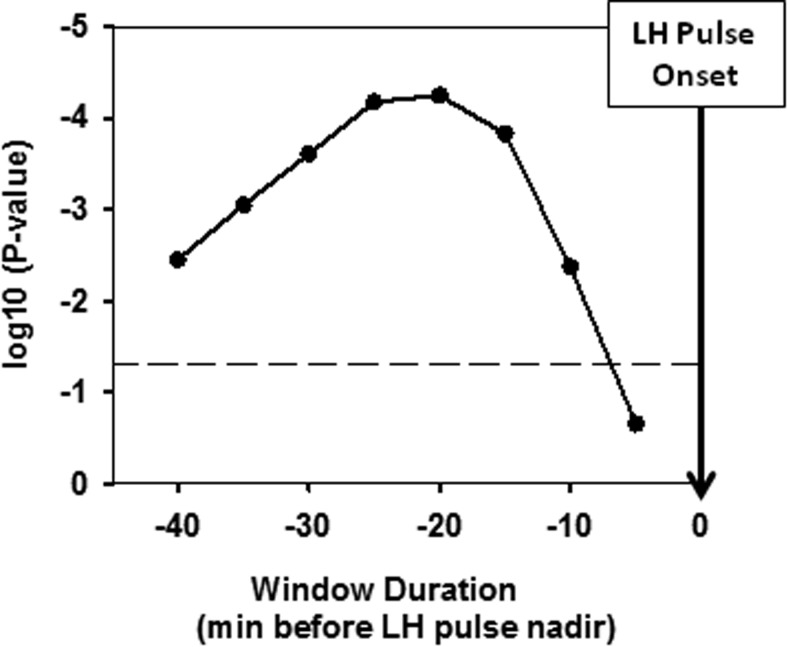
To determine whether the accumulation of N3 in the period preceding a pulse also or independently contributes to LH pulse probability, we added the fraction of time spent in N3 in a window of fixed duration before each time point. Comparison of the expanded model (model 2) with model 1 (Table 2) demonstrated that the fraction of time spent in N3 is highly significant (Figure 4), peaking at a window of 20 minutes, at which point the P value on the χ2 test is P < 10−4. Specifically, for the variable φ20, the probability of an LH pulse increases by a factor of e(coefficient × fraction of time in N3). Thus, if 80% of the preceding 20 minutes was spent in N3, the pulse probability would increase by a factor of e(1.42 × 0.8) ≈ 3.11. The coefficients for model 2 (Table 2) also demonstrate that TSPP remains a significant predictor of an LH pulse but that instantaneous N3 (at the exact time of the pulse) is no longer significant when the fraction of time spent in N3 in the preceding 20 minutes (φ20) is included in the model. These data strongly suggest that the accumulated time spent in N3 over the preceding 20 minute interval is more important in predicting LH pulse onset than being in N3 (ie, instantaneous N3) at any given time.
Figure 4.
Influence of fraction of time spent in N3, controlled for instantaneous N3, on the probability of an LH pulse in windows of increasing duration (x-axis). The figure shows the χ2 test P values (ie, the difference between models 1 and 2 described in Table 2; y-axis) for improvement in predicting an LH pulse as a function of the fraction of time spent in N3 in windows of increasing duration. The dashed line marks the 0.05 significance level. The fraction of time spent in N3 over a 20-minute window before an LH pulse is most significantly associated with pulse onset (denoted by inverted arrow at time zero).
Similar analyses of the influence of time spent in other sleep stages before an LH pulse confirmed the unique importance of N3: neither N1 nor N2 had an effect on LH pulse probability, whereas more time spent in REM was negatively correlated with pulse onset (P = .004).
In summary, the results of probability modeling demonstrate that: 1) deep sleep positively correlates with LH pulse onset; 2) the probability of an LH pulse is directly related to time spent in deep sleep over a 20-minute window; and 3) LH pulse onset is also a function of TSPP, as expected based on the apparent refractory period following each GnRH pulse.
Discussion
Reactivation of the reproductive axis during puberty is closely tied to sleep. In addition, deep sleep, rather than REM or lighter sleep stages, provides a critical stimulus for LH secretion (2), a valid surrogate marker of hypothalamic GnRH secretion. The current studies emphasize the intensity of sleep pressure in adolescents, demonstrate that pulsatile LH secretion in pubertal children is preserved in the setting of disrupted deep sleep, and suggest that GnRH and consequent LH pulse initiation are responsive to an accumulation of deep sleep, even when it is fragmented.
The sleep interruption protocol in the current study used graded auditory stimuli and was modeled on studies by Tasali et al (8), who achieved a 90% decrease in deep sleep over 3 nights in adult men. Applied to adolescents, this protocol reduced deep sleep by 40%, reflecting the intense homeostatic sleep pressure in adolescents. However, in the current studies, analysis of the number of sleep stage transitions revealed that sleep architecture was much more disorganized during the sleep disruption night than might be predicted based on sleep stage percentages alone, similar to findings in adults with OSA (18).
In the current studies, interruption of deep sleep did not diminish LH secretion in pubertal children. Nevertheless, the cumulative time spent in deep sleep remained a significant predictor of a subsequent pulse. The 40% decrease in deep sleep during the disrupted night relative to the normal sleep night represents the average decrease for all subjects and changes in deep sleep may not have been uniformly distributed across the night. Thus, sufficient N3 may have been concentrated before a pulse, even during the disrupted night, accounting for the absence of changes in LH pulse parameters. Alternatively, because the prediction model is based on combined data from both the disrupted and normal nights, it is possible that it took longer for N3 to accumulate during the disrupted night than during the normal night.
The Poisson process model used in the current studies was developed by drawing on statistical techniques that have been used and validated in vivo to predict the occurrence of neural spikes (13). With this model, the effects of both spiking history (the apparent refractory period, coded as TSPP in this analysis) and extrinsic covariates (sleep stage) on the probability of a discrete event (LH pulse) can be analyzed simultaneously. This model represents a significant improvement over previously used pure Poisson or Bernoulli models (19, 20) in which, by definition, there is no refractory period because the probability of an event is strictly independent of its history. The current model also permits discrimination of the effects of sleep stage at pulse onset from effects before pulse onset.
The results of this study, which demonstrates a role for cumulative deep sleep in LH pulse initiation, even when deep sleep is fragmented, are consistent with a causal role of deep sleep in GnRH pulse generation. Our results are also consistent with an alternative hypothesis, that there is a common upstream signal that stimulates deep sleep and LH secretion simultaneously but through independent pathways. Teasing apart the relative contributions of a serial pathway, by which deep sleep might directly trigger a GnRH pulse, and parallel pathways leading to the co-occurrence of deep sleep and GnRH pulses, must await further studies.
A causal relationship between deep sleep and GnRH pulse onset is supported by neuroanatomical evidence for a direct synaptic connection between the sleep centers of the brain and GnRH neurons. In the rodent, a cluster of γ-aminobutyric acid (GABA) and galanin-ergic neurons in the ventrolateral preoptic (VLPO) area of the hypothalamus that are responsible for generating NREM sleep (21) directly synapse on GnRH neurons (22). The absence of GnRH axonal fibers in the VLPO further suggests that VLPO neurons are presynaptic to GnRH neurons, in line with the hypothesis that sleep stimulates GnRH secretion, rather than the converse. Although the VLPO neurons are GABA-ergic and GABA has traditionally been considered to be inhibitory, our current understanding supports both inhibitory and stimulatory GABA modulation of GnRH signaling that varies with age, sex, hormonal milieu, and model organism (23).
Of relevance to the current studies, a galanin-ergic cell group homologous to the VLPO is present in the human brain in a region that has variably been named the interstitial nucleus of the anterior hypothalamus (INAH), the intermediate nucleus, or the sexually dimorphic nucleus (24). Elegant studies have demonstrated an inverse correlation between sleep fragmentation indices in older adults and postmortem cell counts in the INAH, providing strong evidence for a functional role of the INAH in sleep consolidation in humans (25). Whereas GnRH neurons are also clustered in the VLPO area of the human hypothalamus, although not specifically concentrated in the INAH (26), there have been no neuroanatomical studies to investigate the presence of connections between these two cell groups in the human.
It is possible that the presence of LH pulses during deep sleep reflects escape from an inhibitory signal that is present during REM and/or wakefulness rather than direct stimulation of antecedent GnRH secretion. Melanin-concentrating hormone (MCH) neurons in the lateral hypothalamus promote REM sleep (27), and MCH directly inhibits kisspeptin-sensitive GnRH neurons in the septal region of the mouse brain (28). Thus, GnRH secretion may be more likely to occur during NREM than REM sleep due to a decrease in the inhibitory tone set by MCH. A final possibility is that sleep-active neurons, whether the VLPO or MCH neurons, communicate with the network of kisspeptin-dynorphin-neurokinin B (KNDy) neurons in the infundibular nucleus that are known to modulate GnRH secretion in response to a variety of other internal (stress, nutrient status) and external (seasonality, circadian period) signals (reviewed in reference 29) and that serve other homeostatic functions such as thermoregulation (30).
In considering the clinical implications of this study, it is important to note that deep sleep was disrupted for only a single night. We cannot exclude the possibility that chronic deep sleep disruption may decrease LH secretion, as suggested by relatively delayed thelarche in girls with untreated OSA (3). Undiagnosed OSA may have also contributed to previous findings of decreased nocturnal LH secretion in obese pubertal girls (31, 32) because obesity increases the risk of OSA nearly 5-fold (33). In pediatric OSA, obstructive apneas tend to cluster in REM sleep (and are rare during deep sleep), and standard sleep metrics suggest that sleep architecture is preserved (34). However, both time spent in deep sleep (35) and slow-wave activity (36) increase after surgical correction of OSA, suggesting that deep sleep quality may be diminished in children with OSA as it is in adults with OSA (37). Thus, deep sleep abnormalities may in part explain the relationship between pediatric OSA and reproductive axis dysfunction, whereas hypoxia is less likely to be causal, given the absence of gas exchange abnormalities in the girls with OSA discussed above (3). Our finding that the accumulation of deep sleep over a 20-minute window was a significant predictor of LH pulse onset also raises the question of whether sleep restriction, which is rampant among teenagers (38), may be detrimental to the reproductive axis.
In conclusion, we have now shown that accumulation of deep sleep over a specific time period is strongly associated with LH pulse onset, although fragmentation of deep sleep may not interfere with pulsatile LH secretion in pubertal children. These studies suggest that the consolidation of deep sleep into discrete epochs, as occurs in normal sleep, is not critical for the nocturnal augmentation of LH secretion during puberty. At a more basic level, these studies imply that the GnRH pulse generator can keep track of the accumulation of deep sleep over relatively long stretches of time, perhaps through tracking the dissipation of the homeostatic sleep drive that occurs with increasing time spent in deep sleep. Further studies are necessary to determine whether chronic sleep disruption or sleep restriction is tolerated by an immature reproductive axis.
Acknowledgments
We thank the Clinical Research Center staff for their support in conducting these studies, Dr Doug Hayden for statistical support, Dr Amir Lahav for expertise in creating auditory stimuli, and Dr Matt Bianchi for expertise in sleep stage transition analysis.
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health.
N.D.S. received fellowship support from the National Institutes of Health (Grant K23HD073304–02), the Pediatric Endocrine Society, and Harvard Catalyst [The Harvard Clinical and Translational Science Center (Award 1UL1TR001102–01)] and financial contributions from Harvard University and its affiliated academic health care centers. A.M. is funded by the National Institutes of Health (Grant K24HL093218).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CV
- coefficient of variation
- E2
- estradiol
- INAH
- interstitial nucleus of the anterior hypothalamus
- MCH
- melanin-concentrating hormone
- MDC
- minimum detectable concentration
- NREM
- non-rapid eye movement
- OSA
- obstructive sleep apnea
- P
- progesterone
- PSG
- polysomnography
- QC
- quality control
- REM
- rapid eye movement
- TSPP
- time since the previous LH pulse
- VLPO
- ventrolateral preoptic.
References
- 1. Boyar R, Finkelstein J, Roffwarg H, Kapen S, Weitzman E, Hellman L. Synchronization of augmented luteinizing hormone secretion with sleep during puberty. N Engl J Med. 1972;287(12):582–586. [DOI] [PubMed] [Google Scholar]
- 2. Shaw ND, Butler JP, McKinney SM, Nelson SA, Ellenbogen JM, Hall JE. Insights into puberty: the relationship between sleep stages and pulsatile LH secretion. J Clin Endocrinol Metab. 2012;97(11):E2055–E2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shaw ND, Goodwin JL, Silva GE, Hall JE, Quan SF, Malhotra A. Obstructive sleep apnea (OSA) in preadolescent girls is associated with delayed breast development compared to girls without OSA. J Clin Sleep Med. 2013;9(8):813–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chervin RD, Burns JW, Subotic NS, Roussi C, Thelen B, Ruzicka DL. Method for detection of respiratory cycle-related EEG changes in sleep-disordered breathing. Sleep. 2004;27(1):110–115. [DOI] [PubMed] [Google Scholar]
- 6. Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. Darien, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 7. Adams JM, Taylor AE, Schoenfeld DA, Crowley WF, Jr, Hall JE. The midcycle gonadotropin surge in normal women occurs in the face of an unchanging gonadotropin-releasing hormone pulse frequency. J Clin Endocrinol Metab. 1994;79(3):858–864. [DOI] [PubMed] [Google Scholar]
- 8. Tasali E, Leproult R, Ehrmann DA, Van CE. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA. 2008;105(3):1044–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bui HN, Sluss PM, Blincko S, Knol DL, Blankenstein MA, Heijboer AC. Dynamics of serum testosterone during the menstrual cycle evaluated by daily measurements with an ID-LC-MS/MS method and a 2nd generation automated immunoassay. Steroids. 2013;78(1):96–101. [DOI] [PubMed] [Google Scholar]
- 10. Sluss PM, Hayes FJ, Adams JM, et al. Mass spectrometric and physiological validation of a sensitive, automated, direct immunoassay for serum estradiol using the Architect. Clin Chim Acta. 2008;388(1–2):99–105. [DOI] [PubMed] [Google Scholar]
- 11. Hayes FJ, McNicholl DJ, Schoenfeld D, Marsh EE, Hall JE. Free α-subunit is superior to luteinizing hormone as a marker of gonadotropin-releasing hormone despite desensitization at fast pulse frequencies. J Clin Endocrinol Metab. 1999;84(3):1028–1036. [DOI] [PubMed] [Google Scholar]
- 12. Santen RJ, Bardin CW. Episodic luteinizing hormone secretion in man. Pulse analysis, clinical interpretation, physiologic mechanisms. J Clin Invest. 1973;52(10):2617–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown EN, Kass RE, Mitra PP. Multiple neural spike train data analysis: state-of-the-art and future challenges. Nat Neurosci. 2004;7(5):456–461. [DOI] [PubMed] [Google Scholar]
- 14. Bourguignon JP, Gerard A, Franchimont P. Maturation of the hypothalamic control of pulsatile gonadotropin-releasing hormone secretion at onset of puberty: II. Reduced potency of an inhibitory autofeedback. Endocrinology. 1990;127(6):2884–2890. [DOI] [PubMed] [Google Scholar]
- 15. Hall JE, Lavoie HB, Marsh EE, Martin KA. Decrease in gonadotropin-releasing hormone (GnRH) pulse frequency with aging in postmenopausal women. J Clin Endocrinol Metab. 2000;85(5):1794–1800. [DOI] [PubMed] [Google Scholar]
- 16. Kim S, Putrino D, Ghosh S, Brown EN. A Granger causality measure for point process models of ensemble neural spiking activity. PLoS Comput Biol. 2011;7(3):e1001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Katz ES, D'Ambrosio CM. Pediatric obstructive sleep apnea syndrome. Clin Chest Med. 2010;31(2):221–234. [DOI] [PubMed] [Google Scholar]
- 18. Bianchi MT, Cash SS, Mietus J, Peng CK, Thomas R. Obstructive sleep apnea alters sleep stage transition dynamics. PLoS One. 2010;5(6):e11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Albers N, Bettendorf M, Herrmann H, Kaplan SL, Grumbach MM. Hormone ontogeny in the ovine fetus. XXVII. Pulsatile and copulsatile secretion of luteinizing hormone, follicle-stimulating hormone, growth hormone, and prolactin in late gestation: a new method for the analysis of copulsatility. Endocrinology. 1993;132(2):701–709. [DOI] [PubMed] [Google Scholar]
- 20. Veldhuis JD, Johnson ML, Seneta E. Analysis of the copulsatility of anterior pituitary hormones. J Clin Endocrinol Metab. 1991;73(3):569–576. [DOI] [PubMed] [Google Scholar]
- 21. Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271(5246):216–219. [DOI] [PubMed] [Google Scholar]
- 22. Boehm U, Zou Z, Buck LB. Feedback loops link odor and pheromone signaling with reproduction. Cell. 2005;123(4):683–695. [DOI] [PubMed] [Google Scholar]
- 23. Herbison AE, Moenter SM. Depolarising and hyperpolarising actions of GABA(A) receptor activation on gonadotrophin-releasing hormone neurones: towards an emerging consensus. J Neuroendocrinol. 2011;23(7):557–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gaus SE, Strecker RE, Tate BA, Parker RA, Saper CB. Ventrolateral preoptic nucleus contains sleep-active, galaninergic neurons in multiple mammalian species. Neuroscience. 2002;115(1):285–294. [DOI] [PubMed] [Google Scholar]
- 25. Lim AS, Ellison BA, Wang JL, et al. Sleep is related to neuron numbers in the ventrolateral preoptic/intermediate nucleus in older adults with and without Alzheimer's disease. Brain. 2014;137(Pt 10):2847–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rance NE, Young WS, III, McMullen NT. Topography of neurons expressing luteinizing hormone-releasing hormone gene transcripts in the human hypothalamus and basal forebrain. J Comp Neurol. 1994;339(4):573–586. [DOI] [PubMed] [Google Scholar]
- 27. Tsunematsu T, Ueno T, Tabuchi S, et al. Optogenetic manipulation of activity and temporally controlled cell-specific ablation reveal a role for MCH neurons in sleep/wake regulation. J Neurosci. 2014;34(20):6896–6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu M, Dumalska I, Morozova E, van den Pol A, Alreja M. Melanin-concentrating hormone directly inhibits GnRH neurons and blocks kisspeptin activation, linking energy balance to reproduction. Proc Natl Acad Sci USA. 2009;106(40):17217–17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, McMullen NT, Rance NE. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci USA. 2012;109(48):19846–19851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bordini B, Littlejohn E, Rosenfield RL. Blunted sleep-related luteinizing hormone rise in healthy premenarcheal pubertal girls with elevated body mass index. J Clin Endocrinol Metab. 2009;94(4):1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McCartney CR, Prendergast KA, Blank SK, Helm KD, Chhabra S, Marshall JC. Maturation of luteinizing hormone (gonadotropin-releasing hormone) secretion across puberty: evidence for altered regulation in obese peripubertal girls. J Clin Endocrinol Metab. 2009;94(1):56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1527–1532. [DOI] [PubMed] [Google Scholar]
- 34. Goh DY, Galster P, Marcus CL. Sleep architecture and respiratory disturbances in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;162(2 Pt 1):682–686. [DOI] [PubMed] [Google Scholar]
- 35. Tauman R, Gulliver TE, Krishna J, et al. Persistence of obstructive sleep apnea syndrome in children after adenotonsillectomy. J Pediatr. 2006;149(6):803–808. [DOI] [PubMed] [Google Scholar]
- 36. Ben-Israel N, Zigel Y, Tal A, Segev Y, Tarasiuk A. Adenotonsillectomy improves slow-wave activity in children with obstructive sleep apnoea. Eur Respir J. 2011;37(5):1144–1150. [DOI] [PubMed] [Google Scholar]
- 37. McArdle N, Douglas NJ. Effect of continuous positive airway pressure on sleep architecture in the sleep apnea-hypopnea syndrome: a randomized controlled trial. Am J Respir Crit Care Med. 2001;164(8 Pt 1):1459–1463. [DOI] [PubMed] [Google Scholar]
- 38. Owens J. Insufficient sleep in adolescents and young adults: an update on causes and consequences. Pediatrics. 2014;134(3):e921–e932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(suppl 4):S164–S192. [DOI] [PubMed] [Google Scholar]