Abstract
Context:
Appropriate risk stratification is essential in gestational diabetes (GDM) diagnosis to optimize therapeutic strategies during pregnancy. However, there are sparse data related to the newly recommended International Association of Diabetes and Pregnancy Study Groups criteria and their use in early pregnancy.
Objective:
This study sought to evaluate clinical and pathophysiological characteristics less up to gestational week (GW) 21 in women with early and late GDM onset.
Design and Setting:
This was a prospective study conducted at the Medical University of Vienna.
Patients and Interventions:
Pregnant women (n = 211) underwent an oral glucose tolerance test at 16 GW (interquartile range, 14–18 wk) with multiple measurements of glucose, insulin, and C-peptide for evaluation of insulin sensitivity and ß-cell function in addition to detailed obstetrical risk assessment. Clinical followups were performed until end of pregnancy.
Main outcome measure:
We performed a metabolic characterization of early-onset GDM.
Results:
Of 81 women, 49 (23%) showed early (GDMEarly ≤ 21 GW) and 32 (15%) later manifestation (GDMLate ≥ 24 GW) whereas 130 (62%) remained normal-glucose-tolerant (NGT). In contrast with GDMLate, GDMEarly were affected by decreased insulin sensitivity (GDMEarly vs NGT, P < .001; GDMEarlyvs GDMLate, P < .001; GDMLate vs NGT, P = .410). However, both early and late manifested subjects showed impairments in ß-cell function. GDMEarly showed highest levels of preconceptional and actual body mass index (BMI), which was related to fasting glucose (r = 0.42, P < .001) and particularly insulin sensitivity (r = −0.51, P < .001). Differences in glucose disposal between the subgroups remained constant in multivariable analysis including the strongest risk factors for GDM, ie, age, history of GDM, and BMI in our population.
Conclusions:
Early manifestation of GDM is affected by insulin resistance that is partly explained by higher degree in obesity. However, ß-cell dysfunction was also detectable in GDMLate, indicating defective compensatory mechanisms emerging already in early pregnancy.
Glucose homeostasis during normal pregnancy is characteristically affected by metabolic changes that induce a physiologic form of insulin resistance (1). Gestational diabetes (GDM) develops due to an inadequate adaptation to increasing insulin requirements and is often associated with obesity and other additional risk factors for adverse pregnancy outcome (2). However, subsequent complications such as disproportionate increased fetal growth (macrosomia) and unplanned cesarean section could be positively affected by lifestyle modification including increased physical activity, medical nutrition therapy, and if necessary, by insulin treatment of the mother (3, 4). In addition, optimal glycemic control during pregnancy and accurate risk stratification early postpartum may favorably influence the subsequent risk for diabetes and metabolic disorders in these predisposed women (5–7), and further also in the offspring by moderating the adverse effect of “fetal programming” (8, 9). Thus, early attempts of diagnosis by means of oral glucose tolerance test (OGTT) and initiation of therapy in due time are crucial, given that even minor disturbances in carbohydrate metabolism are associated with increased morbidity for both mother and child (10, 11).
The risk for the manifestation of GDM increases particularly when pregnancy progresses and insulin requirements exceed the compensatory capacities of pancreatic β cells (1, 12). Thus, guidelines traditionally recommend primary testing until the early third trimester within the scope to maximize detection rate and make cost-effective diagnosis (13, 14). Most recently, the International Association of Diabetes and Pregnancy Study Groups (IADPSG) formulated new thresholds for the diagnosis of GDM following the publication of the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) Study results and recommended standardization on a 75-g OGTT to be universally performed in gestational week (GW) 24–28 (10, 15). Depending on individual metabolic reserves GDM may manifest before these defined GWs. However, clear evidence for the benefit of earlier screening and thus therapy by randomized clinical trials in women with risk factors for GDM (eg, overweight or obesity, history of GDM, family history with type 2 diabetes, and others) are lacking until now. Actually, the pathophysiology behind the development of GDM is not fully understood but beyond this there are also sparse data on how women with early manifestation differ in their susceptibility and level of glucose impairment.
Therefore, the aim of this study was to assess clinical risk factors, particularly the effect of overweight and obesity in addition to dynamic indices of insulin sensitivity and β-cell function for a clinical and pathophysiological characterization of women with early (≤ 21 GW) and later onset of GDM according to the newly defined IADPSG criteria as a reference standard.
Materials and Methods
Study population
This prospective study was performed at the Division of Endocrinology and Metabolism, Department of Internal Medicine III, Medical University of Vienna between 2010 and 2014. Study participants were recruited among pregnant women who were referred to the diabetes outpatient clinic on or before GW 21 for the assessment of glucose tolerance. Women with known preconceptional diabetes, chronic or serious acute infections, hematological diseases or diseases of the hematopoietic system, severely impaired liver or kidney function, or if they have been tested for hepatitis C antibodies or HIV were not included. During first assessment within the framework of this study four women were classified as having overt diabetes (ie, fasting plasma glucose 126 mg/dL, glycosylated hemoglobin greater than 6.5% [47.54 mmol/mol] at the first antenatal visit) and hence were excluded from further analyses. All subjects gave written informed consent for participation in the study. The study was approved by the local ethics committee (Ethics Committee of the Medical University of Vienna) and was performed in accordance with the Declaration of Helsinki.
Laboratory measurements and clinical risk factors
All women underwent a broad risk evaluation at the initial contact including: body mass index (BMI; preconceptional and actual), age, obstetric history (history of GDM, previous abortion/stillbirth, previous birthweight > 4000 g, preconceptional hypertension), as well as high risk ethnicity (ie, Asian, African, Indian, Hispanics) and smoking status. Moreover, a 2-hour OGTT was performed up to GW 21 (median, 16 wk, interquartile range [IQR], 14–18 wk) after a 10–12-hour overnight fast. Determination of glucose, insulin, and C-peptide was performed in venous blood samples drawn at fasting as well as 30, 60, 90, and 120 minutes following a 75-g glucose load. Insulin, C-peptide, and glucose were measured according to the international standard laboratory methods at our certified Department of Medical and Chemical Laboratory Diagnostics (http://www.kimcl.at/).
In case of a negative result, clinical evaluation and diagnostic OGTT was performed during the pregnancy week 24–28 according to the IADPSG criteria. Five subjects with negative OGTT results received insulin therapy during followup due to incident macrosomia and elevated fasting glucose and thus were classified as GDM. Hence, after exclusion of four women with preexisting diabetes a total of 211 women were subdivided into three groups according to the results of the early OGTT and the clinical follow-up examinations over pregnancy: women remaining normal glucose tolerant during pregnancy (NGT), those with early impaired glucose tolerance already before or on GW 21 (GDMEarly), and women with later GDM manifestation (≥ 24 GW, GDMLate).
Parameters of insulin sensitivity and β cell function
Insulin sensitivity was described by the oral glucose insulin sensitivity index (OGIS), which represents insulin-mediated glucose clearance during an OGTT (16) and has been validated vs the glucose clamp (17). Insulin sensitivity in fasting conditions was described by the quantitative insulin-sensitivity check index (QUICKI) (18). β-cell function was estimated by the insulinogenic index (IGI), commonly a measure of first-phase insulin responses to a glucose challenge. This index is computed as IGIInsulin = Δinsulin (0–30 min)/Δglucose (0–30 min) (19). A variation of this method was devised for assessing pancreatic/prehepatic β-cell function using C-peptide instead of insulin, ie, IGIC-peptide = ΔC-peptide (0–30 min)/Δglucose (0–30 min) (20). Moreover, a modified insulinogenic index was calculated as ΔAUCInsulin/ΔAUCGlucose (μU/mL)/(mg/dL) as an index of total β cell (from posthepatic measurements) function. Late-phase insulin secretion was estimated as AUCInsulin60–120/AUCGlucose60–120. The mechanism exerted by insulin secretion to compensate for insulin resistance was described as the product of OGIS and ΔAUCInsulin/ΔAUCGlucose, sometimes defined as disposition index (DI) from OGTT. Total areas under the concentration curves (AUC) of glucose, insulin and C-peptide were calculated by using the trapezoidal method.
Statistical analysis
Continuous variables were summarized by mean ± SD and categorical variables by counts and percentages. Comparisons of continuous parameters were performed by ANOVA as well as Fisher protected least significant difference tests in NGT, GDMLate, and GDMEarly subjects (meaning that post-hoc tests are only calculated in the case of significant global tests, which is accepted as the appropriate procedure to deal with multiplicity in the case of three groups). Differences of categorical variables were assessed by using Fisher's exact test. In case of skewed distributed variables (IGIInsulin, ΔAUCInsulin/ΔAUCGlucose, DI), a square-route transformation was applied after adding 1 (ie, sqrt[x + 1]). Associations with binary outcomes (ie, GDM status) were assessed by logistic regression models. Associations between continuous variables were assessed by Pearson's product-moment correlation, and analysis of covariance for modeling multivariable associations with parameters of glucose disposal. A linear mixed effects model (random intercept) was used to describe group-specific differences in time-related changes of OGIS.
Statistical analysis was performed with R (V3.1.1) and contributing packages (21). A two-sided P ≤ .05 was considered statistically significant. There were no considerations to adjust for multiplicity in this report if not otherwise indicated.
Results
Descriptive characteristics and clinical risk factors
Descriptive analysis of the study population grouped by glucose tolerance and manifestation time of GDM are given in Table 1. GDM was diagnosed in 49 (23.2%) women ≤ 21 GW (GDMEarly), whereby 21 (42.9%) women were detected by elevated fasting glucose (ie, ≥ 92 mg/dL). Of the remaining 162 subjects 32 women (15.2%) were affected by later manifestation (GDMLate) and 130 (61.6%) remained NGT. Logistic regression indicated that GDM diagnosis was significantly related to actual BMI (odds ratio [OR], 1.09; 95% confidence interval [CI], 1.04–1.15; P = .001, per increase of 1 kg/m2) as well as prepregnancy BMI (OR, 1.07; 95% CI, 1.02–1.13; P = .004) and moreover to age (OR, 1.08; 95% CI, 1.02–1.14; P = .007, per increase of 1 y) and history of GDM (OR, 2.90; 95% CI, 1.62–5.32; P < .001).
Table 1.
Main Characteristics of the Study Population Divided by Glucose Tolerance Status During Pregnancy
| Characteristic | GDMEarly | GDMLate | NGT | P Value |
|---|---|---|---|---|
| Age, y | 33.0 ± 4.8a | 32.6 ± 4.2 | 30.8 ± 5.4 | .021 |
| BMI, kg/m2 | 31.7 ± 6.4ab | 27.7 ± 4.4 | 27.3 ± 5.6 | <.001 |
| Pregestational BMI, kg/m2 | 29.8 ± 5.7ab | 26.5 ± 4.4 | 25.9 ± 6.3 | <.001 |
| GW at initial testing | 15.6 ± 2.9 | 15.0 ± 3.3 | 16.2 ± 2.9 | .102 |
| High-risk ethnicity | 6 (12%) | 7 (22%) | 14 (11%) | .244 |
| History of GDM | 37 (76%) | 21 (66%) | 60 (47%) | .001 |
| Previous abortion/stillbirth | 13 (27%) | 10 (31%) | 49 (38%) | .377 |
| Previous birthweight > 4000 g | 9 (19%) | 6 (19%) | 16 (13%) | .428 |
| First-degree relative with T2DM | 22 (45%) | 12 (39%) | 33 (26%) | .031 |
| Preconceptional hypertension | 6 (12%) | 3 (9%) | 9 (7%) | .495 |
| Smoking | 5 (10%) | 8 (25%) | 19 (15%) | .179 |
| Insulin treatment | 34 (70%) | 24 (75%) | - | .624 |
| FPG, mg/dL | 88.6 ± 11.5ab | 80.6 ± 6.0 | 78.2 ± 5.9 | <.001 |
| G-60, mg/dL | 192.0 ± 29.3ab | 148.2 ± 22.1a | 124.7 ± 25.4 | <.001 |
| G-120, mg/dL | 137.9 ± 30.8ab | 110.2 ± 24.0a | 97.4 ± 19.2 | <.001 |
| Fasting Insulin, μg/mLd | 5.3 (1.9–9.9) | 3.8 (1.9–6.2) | 3.5 (1.9–7.2) | .239 |
| AUCGlucose, g/dL | 19.1 ± 2.6ab | 15.3 ± 1.8a | 13.4 ± 2.0 | <.001 |
| AUCInsulin, mU/mL | 8.4 ± 5.5ab | 5.5 ± 3.4 | 5.9 ± 3.3 | <.001 |
| AUCC-peptide, ng/mL | 1069 ± 334ab | 836 ± 221 | 856 ± 282 | <.001 |
| QUICKI | 0.383 ± 0.059a | 0.401 ± 0.051 | 0.407 ± 0.052 | .025 |
| OGIS, ml/min/m2 | 405 ± 64.9ab | 467 ± 57.7 | 477 ± 57.1 | <.001 |
| IGIInsulin | 0.646 ± 0.70a | 0.624 ± 0.37a | 1.028 ± 0.74 | <.001c |
| IGIC-peptide | 0.066 ± 0.03a | 0.074 ± 0.02a | 0.110 ± 0.07 | <.001 |
| Late OGTT insulin secretion | 0.549 ± 0.42 | 0.430 ± 0.26 | 0.504 ± 0.27 | .258 |
| ΔAUCInsulin/ΔAUCGlucose | 0.98 ± 0.97a | 0.90 ± 0.50a | 1.60 ± 1.25 | <.001c |
| DI | 365.0 ± 266a | 410.6 ± 219a | 753.7 ± 628 | <.001c |
Abbreviations: AUCGlucose, AUCInsulin, AUCC-peptide, area under the curve for glucose, insulin and C-peptide, respectively measured during OGTT; ΔAUCInsulin/ΔAUCGlucose, ratio of incremental area under the concentration curves of insulin and glucose; FPG, fasting plasma glucose; G-60, 1-hour glucose value of oral glucose tolerance test; G-120, 2-hour glucose value of oral glucose tolerance test; IGIInsulin, IGIC-peptide, insulin and c-peptide based calculations of insulinogenic index; T2DM, type 2 diabetes mellitus.
Data are mean and SD.
P-values were determined by one-way ANOVA.
Versus NGT.
Versus GDMLate.
P values are calculated after square root transformation.
Data are median and IQR. P values are based on the Kruskal-Wallis test.
Pathophysiological characterization
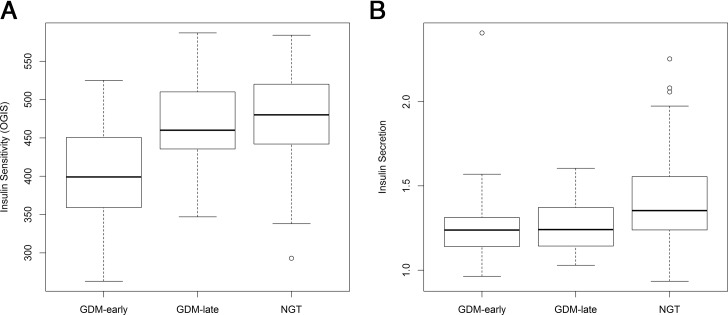
Measurements of glucose, insulin, and C-peptide as well as estimates of insulin sensitivity and β-cell function at the early antenatal visit already showed significant differences between the subgroups (GDMEarly, GDMLate, NGT): particularly, the AUC from venous concentrations of glucose (calculated from fasting to 120 min after glucose load) yielded differing results (Table 1). However, the AUCs of insulin and C-peptide showed significant elevated values in GDMEarly but comparable results between NGT and GDMLate women (AUCInsulin: GDMLate vs NGT: ß, −0.41 mU/mL; 95% CI, −2.0–1.2; P = .611; AUCC-Peptide: GDMLate vs NGT: ß, −20.0 ng/mL; 95% CI, −134.8–94.8; P = .732). As graphically presented in Figure 1, estimated insulin sensitivity (ie, OGIS) showed corroborating results as GDMEarly were particularly affected by impaired insulin sensitivity whereas GDMLate and NGT showed no differences (GDMEarly vs NGT: ß, −72.3; 95% CI, −92.4–−52.2; P < .001; GDMEarly vs GDMLate: ß, −62.4; 95% CI, −89.3–−35.5; P < .001; GDMLate vs NGT: ß, −9.9, 95% CI, −33.6–13.7; P = .410, in terms of OGIS). Also, fasting indices of insulin sensitivity indicating hepatic insulin resistance were corroboratively elevated in GDMEarly. Of note, insulin secretion was significantly lower in both GDMEarly and GDMLate vs controls suggesting impairments in early-phase β-cell function also in women with later manifestation of GDM emerging already in early pregnancy (GDMEarly vs NGT: ß, −0.14; 95% CI, −0.21–−0.06; P < .001; GDMLate vs NGT: ß, −0.14; 95% CI, −0.23–−0.05; P = .003; for square-root transformed IGIInsulin). However, this was not observed for insulin secretion from later OGTT measurements. DI (as a measurement of insulin resistance adjusted for insulin secretion) showed distinguishing results in GDMLate as well in GDMEarly compared with NGT, respectively (NGT vs GDMLate: P < .001; NGT vs GDMEarly: P < .001; GDMLate vs GDMEarly: P = .446). An evaluation of the second OGTT in the remaining nonmanifested women suggested a significant decrease of insulin sensitivity (OGIS) by 17.0 ml/min/m2 (95% CI, 7.2–26.8; P < .001) in later pregnancy (GW 24–28). However, the decrease in OGIS was more pronounced in the GDMLate compared with the NGT group (βInteraction, 38.6; 95% CI, 15.3–61.9; P = .001).
Figure 1.
Parameters of glucose disposal in pregnant women with early (GDMEarly) and later manifestation of GDM (GDMLate) compared with controls (NGT). A, Whole-body insulin sensitivity (OGIS); B, insulin secretion (sqrt transformed IGIInsulin) assessed at 16 weeks of gestation (IQR, 14–18 wk).
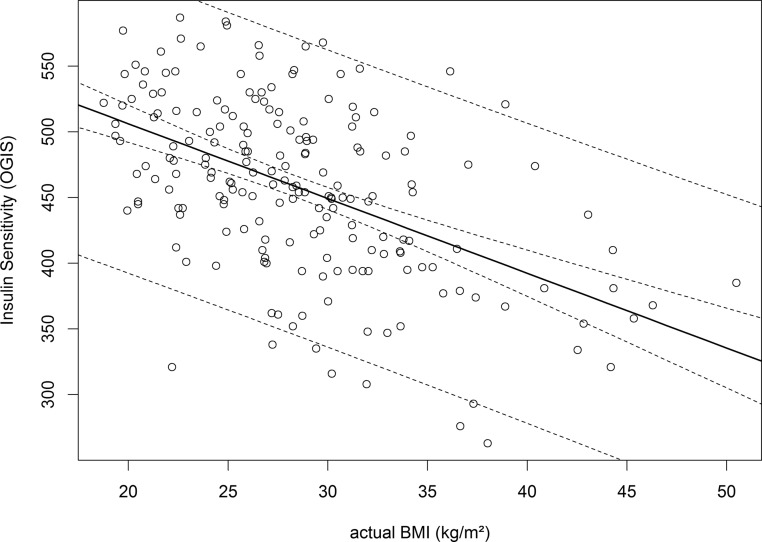
Association with BMI
As provided in Table 2 actual and prepregnancy BMI were particularly associated with fasting glucose and the level of insulin sensitivity (as visualized in Figure 2). Of note, the association with glucose levels decreased within the OGTT examination. Hence, BMI levels were only modest related to 120 minutes postload levels. Moreover, the subgroups differed particularly in BMI, whereby women with early manifestation of GDM showed significantly higher levels in actual as well as prepregnancy BMI (Table 1). Therefore, we performed analysis of covariance models for the associations with OGIS, IGIInsulin, and DI, including age, BMI, and history of GDM, in addition to time of manifestation (GDMEarly, GDMLate, NGT) as independent variables. While in each model BMI was significantly related to parameters of glucose disposal, the above-described differences of estimated insulin sensitivity and β-cell function remained significant between the subgroups with early and late manifestation or normal glucose tolerance. Actual BMI was independent of gestational age at time of initial testing.
Table 2.
Associations of BMI with Parameters of Glucose Disposal
| Parameter | Actual BMI |
Preconceptional BMI |
||||
|---|---|---|---|---|---|---|
| r | 95% CI | P Value | r | 95% CI | P Value | |
| FPG, mg/dL | 0.42 | 0.30–0.52 | <.001 | 0.39 | 0.27–0.50 | <.001 |
| G-60, mg/dL | 0.29 | 0.17–0.41 | <.001 | 0.25 | 0.12–0.38 | <.001 |
| G-120, mg/dL | 0.18 | 0.04–0.30 | .010 | 0.17 | 0.03–0.30 | .016 |
| QUICKI | −0.42 | −0.52–−0.30 | <.001 | −0.43 | −0.53-−0.31 | <.001 |
| OGIS, ml/min/m2 | −0.51 | −0.61–−0.40 | <.001 | −0.50 | −0.60-−0.38 | <.001 |
| IGIInsulina | 0.13 | −0.01–0.27 | .059 | 0.16 | 0.03–0.30 | .020 |
| ΔAUCInsulin/ΔAUCGlucosea | 0.14 | 0.00–0.28 | .050 | 0.17 | 0.02–0.30 | .022 |
| DIa | 0.03 | −0.11–0.17 | .694 | 0.05 | −0.09–0.19 | .479 |
Abbreviations: ΔAUCInsulin/ΔAUCGlucose ratio of incremental area under the concentration curves of insulin and glucose; FPG, fasting plasma glucose; G-60, 1-hour glucose value of OGTT; G-120, 2-hour glucose value of OFTT; IGIInsulin, insulin-based calculations of insulinogenic index.
Data are correlation coefficients (r) and 95% CI.
Square root transformation was performed.
Figure 2.
Scatter plot showing the association of OGTT-derived insulin sensitivity index (OGIS) to BMI in early pregnancy (median, 16 wk of gestation; IQR, 14–18).
Discussion
We aimed to perform a detailed clinical and pathophysiological characterization during early stage of pregnancy including OGTT-derived dynamic parameters for insulin sensitivity and β-cell function to stratify risk of GDM manifestation as diagnosed by the newly proposed IADPSG criteria. In this respect, we observed results indicating specific differences between women with early-onset GDM and those with diagnosis after the 24th week compared with gestations with NGT, which were already detectable at earlier period of pregnancy. Degree of overweight and obesity is stated as an important risk factor associated with insulin resistance (22); however, differences in insulin sensitivity and β-cell function remained significant related to time of GDM onset in multivariable analysis.
Our results are in line with prior pathophysiological insights in glucometabolic adaptations during pregnancy derived from studies applying the hyperinsulinemic-euglycemic clamp technique or frequently sampled iv glucose tolerance test. However the limitations of respectively small sample sizes of these studies and the high variance of estimated effects have to be concerned (12, 23, 24). In physiologic nondiabetic pregnancy insulin secretion is increased beginning from the first trimester. As pregnancy progresses, insulin sensitivity further declines, coming to a nadir in the third trimester concurrent with increase in hepatic glucose production and facilitated lipolysis (1). Consistently it is stated that although insulin requirements differ only slightly between normal and gestational diabetic pregnancies, women with GDM have defective compensatory insulin secretion in response to hyperglycemia (2). In addition, prior studies mostly suggest that women affected by severe GDM are characterized by higher insulin resistance compared with NGT (24, 25).
Our results imply that especially women with impaired (prepregnancy existing) insulin sensitivity at an early stage of gestation are predestined for earlier onset of GDM as dynamic parameters of insulin sensitivity (assessed in first or early second trimester) were significantly lower in women with early onset of GDM compared with those with later manifestation (ie, ≥ 24 GW). Moreover, insulin sensitivity during the first/early second trimester was strongly related to BMI. Hence, we suggest that overweight or obesity (or any prior existing impairment of insulin sensitivity) in addition to physiologic adaptation to pregnancy exerts a major burden on pancreatic β cells, what may promote a more severe deterioration of metabolic state and therefore cause early alterations in glucose disposal. Of note, the observed differences were only relevant in those women with GDM who had marked hyperglycemia already in early period of pregnancy (ie, the GDMEarly group). In particular, the subgroup with later onset did not show major differences in insulin sensitivity compared with NGT controls. This implies that severity and time of onset might be highly related to a preexisting background of chronic insulin resistance. Although clinical consequences are not clear, these women may represent a subgroup of high-risk among women with GDM. Thereby, elevated BMI or measurements of fasting glucose (which were shown to be closely associated) might provide useful parameters for risk stratification in early pregnancy.
Regarding insulin secretion, the data of our study suggest impairment in insulin secretion (as derived from insulin and C-peptide-based calculations of IGI) in both early and late manifestation of GDM in accordance with the suggested underlying pathophysiology of GDM. Whereas women with early-onset GDM were characterized by pre-existing insulin resistance additional to β-cell dysfunction, it is notable that women with later development of GDM in particular showed deteriorated β-cell function already in early pregnancy. Thus, compensatory mechanisms may become defective already in early pregnancy also in women with later manifestation of GDM representing first indices of further exhaustion in relation to the ascending insulin requirements during later course of pregnancy. Notably, insulin secretion during the later OGTT period was comparable between the groups, indicating that pathologic adaptation mostly relies on the early-phase insulin secretion.
To integrate OGTT-derived indices of insulin sensitivity and β-cell function in large-scale studies may be another pragmatic way in terms of convenience and cost effectiveness given that the 75-g OGTT constitutes the diagnostic criteria and is dedicated for general application (15). Although OGTT indices represent only surrogates of insulin sensitivity and β-cell function they have been validated against the gold standard clamp method in different populations [including pregnancy (26)], providing accurate results (2). Accordingly, Kirwan et al (26) could demonstrate a strong correlation of OGTT derived indices with insulin sensitivity assessed by hyperinsulinemic-euglycemic clamp during early as well as late pregnancy.
As far we know this is the first study analyzing insulin sensitivity and β-cell function with available glucose, insulin and C-peptide values during a 75-g OGTT in early pregnancy by applying the diagnostic criteria of IADPSG. Comparable data that has been published on this topic is limited. Lapolla et al (27) calculated insulin sensitivity and β cell secretion from 75-g OGTT and, in contrast with our observation, they identified a significant difference in insulin sensitivity between both subgroups compared with NGT controls, but no incipient β-cell dysfunction during early pregnancy in women with later GDM development was detected (27). However, stratification during their study period was based on the Carpenter and Coustan criteria, what may have importantly effected their results (27). The use of other diagnostic criteria might have influenced the results of another study, where diagnosis of GDM was based on the 2-hour OGTT value of greater than 7.8 mmol/l (141 mg/dL) concluding no difference in β-cell function but finding overweight as a main effect for impaired insulin sensitivity (28). Further, in a more recent study performing a 100-g OGTT, later manifestation of GDM was predicted by hyperinsulinemia in first trimester but lost significance after adjustment for BMI (29). Although traditional risk factors as age and history of GDM in previous pregnancies in addition to BMI were also associated with GDM status and glucose dynamics in our study population, the significance of our findings with regard to glucose disposal parameters and time of manifestation were not affected by these factors. Thus, diagnostic categorization based on IADPSG criteria may allow for improving differentiation of glucose disorders during early pregnancy.
Due to the observational design of our study we are not able to provide data on the implications of early diagnosis and initiation of therapy as well as effect on pregnancy outcomes in women with early manifestation. This must be accurately evaluated in future randomized studies. Another limiting aspect is that our study site represents a tertiary referral hospital including a neonatal intensive care center, thus high-risk pregnancies are more prevalent within our general patient population. As generally recommended, the referral to an early testing was based on the presence of the main risk factors associated with GDM, ie, overweight or obesity, history of GDM, family history with type 2 diabetes, and ethnicity (30). However, we must point out that the differences in glucose disposal observed in our study population might be more pronounced in a population-based, low-risk sample. Further, by the use of IADPSG criteria we could assume that disturbances of insulin and glucose dynamics may be present even in milder forms of GDM. Based on our results we suggest that women with high risk for onset of GDM must be adequately identified, whereby identification of women with later development of GDM may be improved by an appropriate risk factor analysis before performing the OGTT (31).
In summary, we performed an analysis of dynamic indices derived from OGTT measurements according to IADPSG criteria and observed significant differences in parameters of insulin sensitivity and β-cell function, which were partly explained by the degree of overweight or obesity, allowing characterization of women with early and later manifestation of GDM. Women with early-onset GDM were characterized by higher degree of insulin resistance whereas those with later manifestation were comparable to controls. This may implicate that pre-existing insulin resistance additional to emerging β-cell dysfunction during pregnancy might essentially effect time of GDM onset in women with early GDM manifestation. In contrast, women with later development of GDM show parameters of β-cell dysfunction already in early pregnancy, indicative for exhausting compensatory mechanisms during later course of pregnancy. Identification of pathophysiological peculiarities associated with time of onset of GDM could contribute to efforts in improvement of screening for metabolic disorders during pregnancy and performance of an appropriate risk categorization.
Acknowledgments
We acknowledge to Mr Johannes Scholz and Mrs Anna-Theresa Hörmayer (Department of Internal Medicine III, Division of Endocrinology and Metabolism, Medical University of Vienna) for helping in data assessment.
Contribution statement: A.K.-W. and L.B. conceived the study. Data assessment and patient recruitment was performed by L.B., C.S.G., L.P., and K.L. Statistical analysis, calculations, and data interpretation were performed by L.B., C.S.G., and G.P. L.B wrote the manuscript. C.S.G., L.P., K.L., D.B.-T., A.L., S.B.-P., G.P., and A.K.-W. reviewed and edited the manuscript. All authors have seen and approved the submission of this version of the manuscript and take full responsibility for the manuscript.
This work was supported by the Medical Scientific Fund of the Mayor of Vienna (Pr. No. 09063) to A.K.-W.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- area under the curves
- BMI
- body mass index
- CI
- confidence interval
- DI
- disposition index
- GDM
- gestational diabetes
- GW
- gestational week
- HAPO
- Hyperglycemia and Adverse Pregnancy Outcomes
- IADPSG
- International Association of Diabetes and Pregnancy Study Groups
- IGI
- insulinogenic index
- IQR
- interquartile range
- NGT
- normal glucose tolerant
- OGIS
- oral glucose insulin sensitivity index
- OGTT
- oral glucose tolerance test
- OR
- odds ratio
- QUICKI
- quantitative insulin-sensitivity check index.
References
- 1. Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, Friedman JE. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care. 2007;30(Suppl 2):S112–S119. [DOI] [PubMed] [Google Scholar]
- 2. Buchanan TA, Xiang A, Kjos SL, Watanabe R. What is gestational diabetes? Diabetes Care. 2007;30(Suppl 2):S105–S111. [DOI] [PubMed] [Google Scholar]
- 3. Coustan DR. Gestational diabetes mellitus. Clin Chem. 2013;59(9):1310–1321. [DOI] [PubMed] [Google Scholar]
- 4. Horvath K, Koch K, Jeitler K, et al. Effects of treatment in women with gestational diabetes mellitus: Systematic review and meta-analysis. BMJ. 2010;340:c13951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Göbl CS, Bozkurt L, Prikoszovich T, Tura A, Pacini G, Kautzky-Willer A. Estimating the risk after gestational diabetes mellitus: Can we improve the information from the postpartum OGTT? Am J Physiol Endocrinol Metab. 2013;304(5):E524-–E530. [DOI] [PubMed] [Google Scholar]
- 6. Göbl CS, Bozkurt L, Prikoszovich T, Winzer C, Pacini G, Kautzky-Willer A. Early possible risk factors for overt diabetes after gestational diabetes mellitus. Obstet Gynecol. 2011;118(1):71–78. [DOI] [PubMed] [Google Scholar]
- 7. Bozkurt L, Göbl CS, Tura A, et al. Fatty liver index predicts further metabolic deteriorations in women with previous gestational diabetes. PLoS One. 2012;7(2):e32710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Battista MC, Hivert MF, Duval K, Baillargeon JP. Intergenerational cycle of obesity and diabetes: How can we reduce the burdens of these conditions on the health of future generations? Exp Diabetes Res. 2011;596060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Desoye G, van Poppel M. The Feto-placental Dialogue and Diabesity. Best Pract Res Clin Obstet Gynaecol. 2014:S1521–S6934. [DOI] [PubMed] [Google Scholar]
- 10. Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. [DOI] [PubMed] [Google Scholar]
- 11. Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477–86. [DOI] [PubMed] [Google Scholar]
- 12. Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol. 1999;180(4):903–916. [DOI] [PubMed] [Google Scholar]
- 13. Waugh N, Pearson D, Royle P. Screening for hyperglycaemia in pregnancy: Consensus and controversy. Best Pract Res Clin Endocrinol Metab. 2010;24(4):553–571. [DOI] [PubMed] [Google Scholar]
- 14. Nolan CJ. Controversies in gestational diabetes. Best Pract Res Clin Obstet Gynaecol. 2011;25(1):37–49. [DOI] [PubMed] [Google Scholar]
- 15. Metzger BE, Gabbe SG, Persson B, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mari A, Pacini G, Murphy E, Ludvik B, Nolan JJ. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care. 2001;24(3):539–548. [DOI] [PubMed] [Google Scholar]
- 17. Mari A, Pacini G, Brazzale AR, Ahrén B. Comparative evaluation of simple insulin sensitivity methods based on the oral glucose tolerance test. Diabetologia. 2005;48:748–751. [DOI] [PubMed] [Google Scholar]
- 18. Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, et al. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. [DOI] [PubMed] [Google Scholar]
- 19. Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: Comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med. 1994;11:286–292. [DOI] [PubMed] [Google Scholar]
- 20. Tura A, Kautzky-Willer A, Pacini G. Insulinogenic indices from insulin and C-peptide: Comparison of beta-cell function from OGTT and IVGTT. Diabetes Res Clin Pract. 2006;72:298–301. [DOI] [PubMed] [Google Scholar]
- 21. R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing 2011, Vienna, Austria: ISBN 3–900051–07-0, URL http://www.R-project.org/. [Google Scholar]
- 22. Chu SY, Callaghan WM, Kim SY, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007;30(8):2070–2076. [DOI] [PubMed] [Google Scholar]
- 23. Buchanan TA, Metzger BE, Freinkel N, Bergman RN. Insulin sensitivity and B-cell responsiveness to glucose during late pregnancy in lean and moderately obese women with normal glucose tolerance or mild gestational diabetes. Am J Obstet Gynecol. 1990;162(4):1008–1014. [DOI] [PubMed] [Google Scholar]
- 24. Homko C, Sivan E, Chen X, Reece EA, Boden G. Insulin secretion during and after pregnancy in patients with gestational diabetes mellitus. J Clin Endocrinol Metab. 2001;86(2):568–573. [DOI] [PubMed] [Google Scholar]
- 25. Kautzky-Willer A, Prager R, Waldhausl W, et al. Pronounced insulin resistance and inadequate beta-cell secretion characterize lean gestational diabetes during and after pregnancy. Diabetes Care. 1997;20(11):1717–1723. [DOI] [PubMed] [Google Scholar]
- 26. Kirwan JP, Huston-Presley L, Kalhan SC, Catalano PM. Clinically useful estimates of insulin sensitivity during pregnancy: Validation studies in women with normal glucose tolerance and gestational diabetes mellitus. Diabetes Care. 2001;24:1602–1607. [DOI] [PubMed] [Google Scholar]
- 27. Lapolla A, Dalfrà MG, Mello G, et al. Early detection of insulin sensitivity and beta-cell function with simple tests indicates future derangements in late pregnancy. J Clin Endocrinol Metab. 2008;93(3):876–880. [DOI] [PubMed] [Google Scholar]
- 28. Qvigstad E, Voldner N, Godang K, Henriksen T, Bollerslev J. Overweight is associated with impaired beta-cell function during pregnancy: A longitudinal study of 553 normal pregnancies. Eur J Endocrinol. 2010;162(1):67–73. [DOI] [PubMed] [Google Scholar]
- 29. Grewal E, Kansara S, Kachhawa G, et al. Prediction of gestational diabetes mellitus at 24 to 28 weeks of gestation by using first-trimester insulin sensitivity indices in Asian Indian subjects. Metabolism. 2012;61(5):715–720. [DOI] [PubMed] [Google Scholar]
- 30. Kautzky-Willer A, Bancher-Todesca D, Pollak A, Repa A, Lechleitner M, Weitgasser R. Gestational diabetes mellitus. Wie Klin Wochenschr. 2012;124(Suppl 2):58–65. [DOI] [PubMed] [Google Scholar]
- 31. Göbl CS, Bozkurt L, Rivic P, et al. A two-step screening algorithm including fasting plasma glucose measurement and a risk estimation model is an accurate strategy for detecting gestational diabetes mellitus. Diabetologia. 2012;55(12):3173–3181. [DOI] [PubMed] [Google Scholar]