Abstract
Context:
Inflammation in the pancreas can cause β-cell stress, leading to diabetes development. Access to human pancreas tissues via the Network for Pancreatic Organ Donors with Diabetes (nPOD) has allowed characterization of pathways leading to this inflammation.
Objective:
12-Lipoxygenase (12-LO) induces inflammation and has been implicated in diabetes development. Our goal was to determine expression of 12-LO in human islets from control, autoantibody-positive, type 1 diabetic, and type 2 diabetic nPOD pancreas donors.
Design:
Pancreas tissues from nPOD donors were examined by immunohistochemistry and immunofluorescence for islet expression of 12-LO in different subsets of islet cells.
Participants:
Donor pancreas samples were obtained from nPOD based on disease status (control, n = 7; autoantibody-positive, n = 8; type 1 diabetic, n = 17; or type 2 diabetic donors, n = 15).
Main Outcome Measure:
Determination of 12-LO expression within human islets served as the main outcome measure, including distinguishing which types of islet cells expressed 12-LO.
Results:
Islets from control participants (nondiabetic) lacked islet expression of 12-LO. Of donors in the other groups, 25% to 37% expressed islet 12-LO with a clear inverse relation between the numbers of β-cells and 12-LO+ cells within islets of 12-LO+ cases. 12-LO expression was not seen within macrophages, endothelial cells, α-cells, or β-cells, but only within cells expressing low levels of pancreatic polypeptide (PP) and increased levels of vimentin.
Conclusions:
12-LO expression colocalizes within a specific type of islet PP+ cell under prediabetic and diabetic conditions. The costaining of PP and vimentin suggests that 12-LO participates in the process leading to β-cell dedifferentiation in the islet.
Type 1 diabetes (T1D) develops after inflammation and autoimmunity against pancreatic insulin-producing β-cells. Although this is recognized as a T cell–mediated disease, recent advancements in our understanding of inflammation in the islet have brought to light other key factors in T1D pathogenesis.
One such key factor is 12-lipoxygenase (12-LO) (an ALOX-12 gene product). 12-LO is a member of the lipoxygenase family of enzymes, which catalyze the oxygenation of polyunsaturated fatty acids, resulting in the synthesis of eicosanoids. The production of lipid-mediator eicosanoids contributes substantially to the regulation of inflammation and immunity (1–3). Although there are 5 lipoxygenase genes (ALOX-5, ALOX-12S, ALOX-12R, ALOX-15–1, and ALOX-15–2) in humans, only ALOX-12S mRNA is expressed in human islets and plays a role in islet health (4, 5).
12-LO has been implicated in the early stages of autoimmune diabetes development. 12/15-LO (functional equivalent for human 12-LO)-null mice are fully protected from low-dose streptozotocin-induced and spontaneous diabetes development on the nonobese diabetic (NOD) background (6, 7). In addition, 12-LO may play a role in islet inflammation associated with type 2 diabetes (T2D) based on studies of diet-induced obese mice and human islets from T2D donors (8, 9). The mechanisms of diabetes protection by 12-LO deletion is actively being studied; however, a reduction in signal transducer and activator of transcription 4 activation (10) and reduced expression of proinflammatory cytokines in both macrophages and islets (11) appear to be prominent effects. Furthermore, treatment with proinflammatory cytokines has increased expression of 12-LO in human islets and decreased β-cell function after treatment with the downstream product of 12-LO activation, 12-S-hydroxyeicosatetraenoic acid (5). Inhibition of 12-LO in human islets decreased 12-S-hydroxyeicosatetraenoic acid levels and improved islet health (12). 12-LO–induced damage can occur by activation of endoplasmic reticulum stress pathways (13), as well as by increased oxidative stress (8).
Insulin-producing β-cells have been shown to undergo dedifferentiation into other pancreatic endocrine cell lineages in the islets (α-cells, δ-cells, or pancreatic polypeptide [PP]–producing cells) (14) under times of stress in murine models of T2D (15). Given the evidence that β-cells are metabolically stressed under T1D-like conditions (16), it is relevant to examine this concept in human pancreas tissues by comparing normal, autoantibody-positive (AAb+), T1D, and T2D patients.
Vimentin, which plays a key role in the epithelial-to-mesenchyme transition in the development of cancers (reviewed in Ref. 17), is increased under stressful conditions. Dedifferentiated islet cells in T2D express higher levels of vimentin (14), as do tubular complexes in the context of islet neogenesis in the BioBreeding rat model of T1D (18).
For the first time in this study, we used samples from the Network for Pancreatic Organ Donors with Diabetes (nPOD) (www.jdrfnpod.org) consortium (19) to study 12-LO expression under inflammatory conditions in the pancreas. We found that 12-LO is up-regulated under the inflammatory conditions seen in AAb+ and T1D donors with insulin-positive islets or in T2D patients. α-Cells and β-cells were not the source of islet 12-LO expression, but rather 12-LO is seen in cells producing low levels of PP. In many cases, PP expression is paired with increasing levels of vimentin expression in 12-LO+ cells in the islets. These data suggest that activation of 12-LO in the islet contributes to inflammation and stress-induced dedifferentiation of insulin-producing β-cells in prediabetic and diabetic conditions.
Materials and Methods
Human pancreatic sections
Paraffin-embedded sections were obtained from nPOD except for 1 T2D sample provided by Beta-Pro with the appropriate approvals from the institutional review board at Eastern Virginia Medical School. A total of 7 nondiabetic donors, 17 donors with T1D, 15 donors with T2D, and 8 AAb+ donors were included in this study (Supplemental Table 1). Information regarding donors' demography, histology, and systemic disease status were provided by nPOD and Beta-Pro. The status of autoantibodies was determined by nPOD using methods published earlier (20).
Immunofluorescent staining
Sections (7 μm thick) were baked at 50°C overnight or at 56°C for 60 minutes, deparaffinized, and rehydrated in descending ethanol concentrations. Next, sections were subjected to heat-based antigen retrieval in citrate buffer (Vector Laboratories) and incubated overnight at 4°C with the first primary antibody (Supplemental Table 2). After being washed in PBS, sections were incubated with species-specific Cy2 (or Cy3)-conjugated secondary antibodies (1:200 dilution; Jackson ImmunoResearch) for 90 minutes at room temperature. After being washed with PBS and blocked with blocking solutions (Dako), sections were again incubated overnight at 4°C with the second primary antibody followed by incubation with species-specific secondary antibody labeled with an appropriate fluorochrome to visualize a second antigen. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (Sigma-Aldrich), and sections were mounted using Vectashield (Vector Laboratories) and evaluated using an epifluorescent microscope (Axiophot; Zeiss). Additional sections were evaluated by using a confocal microscope (LSM510; Zeiss) equipped with the appropriate software.
Morphometry
For morphometric comparison of human islets from 12-LO-positive and -negative cases, a composite image of 50 visual fields at ×200 magnification (area of 50 000 μm2) was captured using the mosaic function of AxioVision analysis software (v4.7; Zeiss). Five composite images of the pancreas were obtained per case, and at least 3 cases per group were studied. Areas positive for insulin or 12-LO were defined by setting a threshold signal for each antigen, and the size of the positive area was quantified automatically based on this threshold. Hematoxylin and eosin (HE)-stained images on the nPOD website from serial sections of the blocks we analyzed were used to determine the islet area for each donor. To quantitate the PP staining intensity, images of 30 islets from the uncinate and nonuncinate areas of pancreas sections immunostained for PP were analyzed for mean densitometric values of PP+ areas using AxioVision v4.7. To normalize staining, mean densitometric background staining with nonimmune serum was subtracted from values obtain for the PP sections. Observers blinded to the clinical information of donors performed all measurements
Statistics
Data are presented as means ± SEM and were analyzed using either one-way ANOVA with a Dunnett posttest in comparison with nondiabetics or the Student t test (Prism; GraphPad Software). A value of P < .05 was considered significant.
Results
12-LO islet expression in AAb+, T1D, and T2D donor pancreata
12-LO was not detected within islets of any of the 7 nondiabetic specimens we studied (Figure 1A). Nondiabetic specimens exhibited the expected proportion of α-, β-, F-, and δ-cells as described previously (21).
Figure 1.
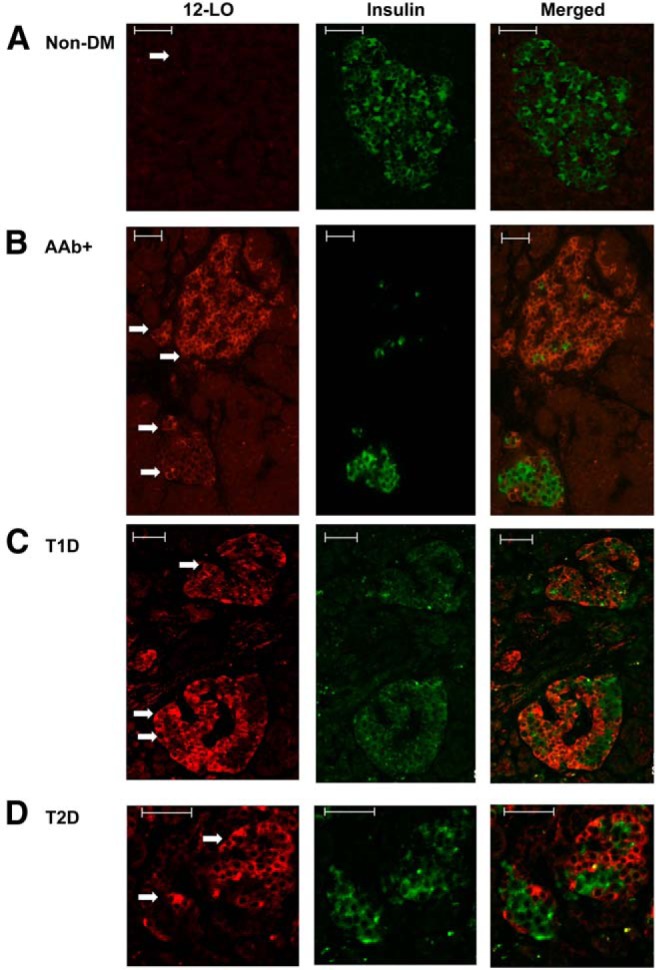
12-LO protein expression in human pancreatic islets. Immunofluorescence was used to determine the expression of 12-LO (red) and insulin (green) in nondiabetic nPOD6112 (A), AAb+ nPOD6023 (B), TID nPOD6038 (C), and T2D nPOD6273 (D) pancreatic sections. Arrows indicate islets. Scale bars correspond to 50 μm.
AAb+ individuals are at higher risk for future development of T1D and include those at the preclinical stage of T1D (22). Of 8 AAb+ samples tested, 3 pancreatic donors (37%) expressed 12-LO within their islets (Supplemental Table 1) with variability in 12-LO positivity both between donors and within islets from the same donor. In nPOD6090, the signal could only be identified in a few islets (<5%), with a small number of cells showing a positive signal within the islets (Supplemental Figure 1A). 12-LO protein was readily identified in multiple islets of nPOD6023, with the percentage of cells exhibiting positive staining within an islet varying within the same donor (Figure 1B, an islet with 1 arrow vs 2 arrows). The third specimen (nPOD6267) exhibited a significantly lower number, but not intensity in staining, of 12-LO+ islets and fewer 12-LO+ cells within an islet than nPOD6023. Despite the variation in 12-LO expression across these cases, a specific pattern of 12-LO staining could be identified: insulin and 12-LO signals were mutually exclusive, and it appeared that 12-LO positivity increased when there were fewer insulin-positive β-cells within the islet (Figure 1B).
Of 17 T1D cases tested, 3 (nPOD6113, nPOD6046, and nPOD6038) expressed 12-LO protein (Supplemental Table 1 and Figure 1C). Interestingly, these 3 cases all had a substantial number of remaining insulin-positive β-cells present in most islets, although insulin levels were lower than those in the nondiabetic samples (Figure 2A). 12-LO expression patterns in these cases were remarkably similar to those observed in AAb+ patients with respect to the inverse relationship between insulin-positive cells and 12-LO+ cells. A small population of islets containing large numbers of 12-LO+ cells with marked reduction of β-cells could be identified, as was seen in AAb+ cases (Supplemental Figure 1, B and C). No 12-LO protein was detected in samples from T1D donors that lacked islet insulin staining. 12-LO staining in the positive cases was limited to islets with insulin-positive β-cells but was not detectable in islets that are totally negative for insulin staining.
Figure 2.
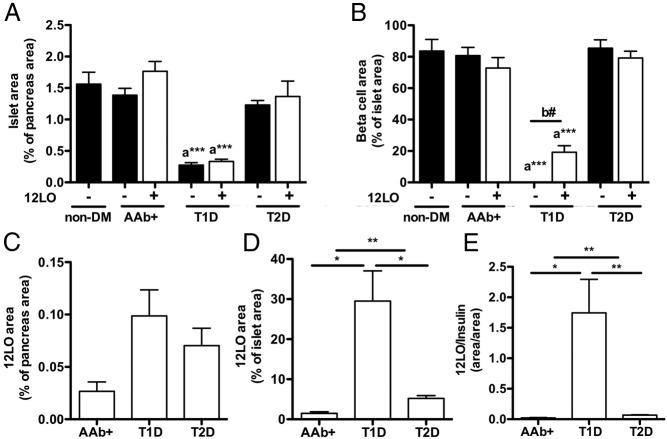
Morphometric analyses of islets in 12-LO+ and 12-LO− cases. Islet area, β-cell area, and 12-LO+ area were determined as in Materials and Methods in 12-LO+ and 12-LO− cases from nondiabetic (non-DM), AAb+, T1D, and T2D subjects. 12-LO expression was not seen in any nondiabetic donors. Data are means ± SEM (n = 3–4). A and B, islet area (A) and β-cell area (B) were compared using 2 statistics: a, one-way ANOVA with Dunnett posttest in comparison with nondiabetic, ***, P < .001; b, Student t tests between 12-LO+ and 12-LO− donors of each diabetic status, #, P < .05. C–E, 12-LO+ areas were expressed in relation to pancreatic area (C), islet area (D), and β-cell area (E) in 12-LO+ subjects. A Student t test was use for comparison between the indicated groups: *, P < .05; **, P < .01.
Of 15 T2D donors tested, 6 (40% of cases) stained positive for 12-LO (Supplemental Table 1 and Figure 1D), with a general expression pattern similar to that of the AAb+ and T1D cases described above (Figure 1, B–D). For the T2D specimens, both the inverse relationship between insulin and 12-LO, as well as the variability in the intensity of the 12-LO staining and the number of positive cells within an islet, were evident. There was no clear correlation between 12-LO positivity and either disease duration or reported therapy.
To gain further insight into the relationship between 12-LO expression and general islet parameters, we evaluated the total islet area as defined by HE staining, β-cell area as defined by the insulin-positive area, and 12-LO+ area by morphometry (Figure 2, A and B). Islet area was markedly reduced in T1D regardless of 12-LO positivity, and islet area was mildly reduced in T2D donors that were negative for 12-LO compared with that in nondiabetic donors (Figure 2A). Furthermore, reductions in β-cell area were evident based on decreased insulin staining of β-cells from both T1D and T2D donors. Interestingly, expression of 12-LO in T1D donors correlated with greater numbers of insulin-positive β-cells compared with those in 12-LO-negative T1D donors (Figure 2B, P < .05), confirming our observation that 12-LO staining was limited to islets with residual insulin-positive β-cells (Figure 1C). Because insulin-negative islets presumably represent later stages of T1D pathogenesis, this observation indicates that 12-LO expression is restricted to islets at the early stages of T1D, consistent with the preclinical studies indicating a role for this enzyme as a contributor to early β-cell dysfunction (11).
When values were corrected for islet area and insulin staining, 12-LO was most prominent in the T1D group followed by the T2D and AAb+ groups (Figure 2, C–E). The comparisons of clinical characteristics between donors with different diabetic status and 12-LO positivity showed the expected increase in body mass index in T2D, reduced serum C-peptide levels in T1D, and increased hemoglobin A1c levels in T1D (Supplemental Table 3). Although the sample size is limited, hemoglobin A1c was lower in T2D donors with 12-LO staining in islets compared with that in donors without 12-LO staining. The other parameters, such as the presence of systemic disease with an impact on inflammation, insulitis and pancreatic inflammation determined by HE staining, or autoantibodies, were not associated with 12-LO positivity (Supplemental Tables 1 and 4).
Analysis of cell type(s) expressing 12-LO
Next, we characterized the population of 12-LO+ islet cells using the AAb+ case (nPOD6023). Neither insulin- nor glucagon-positive cells colocalized with 12-LO+ cells (Figure 3, A and B), which was similar to the pattern seen in T1D cases (Supplemental Figure 2). Because 12-LO expression has been reported in macrophages and endothelial cells (23, 24), we tested whether 12-LO expression colocalized in these cells. CD31+ endothelial cells were readily identified in large numbers within islets (Figure 3C); however, very few macrophages positive for MAC-2 were visualized within islets (Figure 3D). Neither CD31 nor MAC-2 expression colocalized with 12-LO staining (Figure 3, C and D), indicating that these cells do not contribute to the observed pattern of 12-LO expression within pancreatic islets. In addition, we performed immunostaining for somatostatin, ghrelin, and PP with 12-LO in the pancreas of nPOD6023. Neither somatostatin nor ghrelin colocalized with 12-LO-expressing cells (data not shown). In striking contrast, 12-LO+ islets also contained a high proportion of PP-positive cells, and there was a significant colocalization of 12-LO and PP (Figure 4, A and B). Further evaluation by confocal microscopy confirmed the high rate at >90% colocalization of 12-LO and PP within the same cell (Figure 4C).
Figure 3.
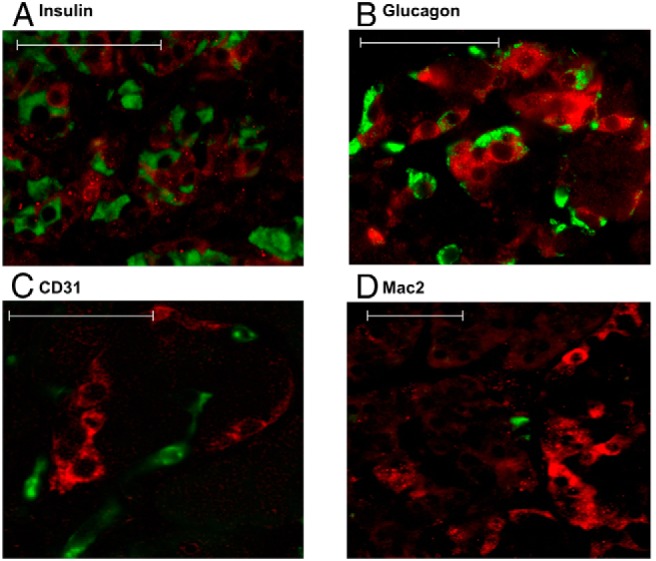
12-LO+ cells in islets do not colocalize with insulin, glucagon, CD31, or MAC-2 in an AAb+ case. Immunofluorescence was used to analyze the expression of 12-LO (red) and insulin (green, A), glucagon (green, B), CD31 (green, C), and Mac2 (green, D) in AAb+ nPOD6023. Scale bars correspond to 50 μm.
Figure 4.
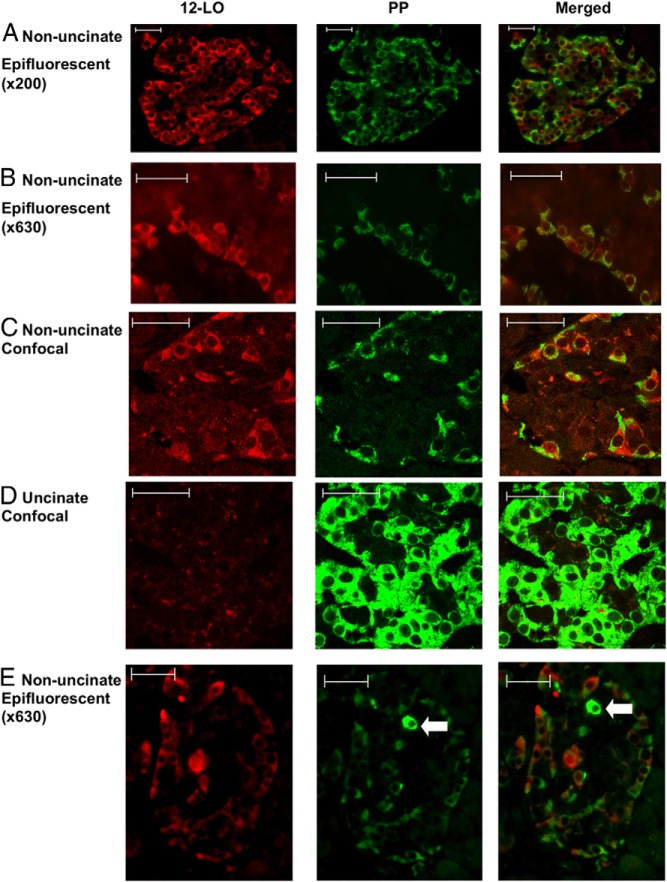
Islets with 12-LO+ cells are enriched with PP staining. Immunofluorescent analysis of AAb+ nPOD6023 for 12-LO (red) and PP (green) is shown. An Axiophot epifluorescent microscope (A and B) or an LSM510 confocal microscope (C–E) was used to capture images in the nonuncinate (A, B, C, and E) and uncinate (D) areas of the pancreas. The arrows indicate cells strongly positive for PP staining in E. Scale bars correspond to 50 μm.
To further explore the nature of PP cells in 12-LO+ islets, islets from the uncinate region of the pancreas that are predominantly composed of typical PP cells (21, 25) were analyzed for the expression of 12-LO and intensity of PP expression. Interestingly, the prototypical, fully differentiated PP-positive cells from the uncinate region were negative for 12-LO, regardless of the diabetes status of the donors (Figure 4D). PP cells from the uncinate region stained much more intensely for PP than the PP and 12-LO double-positive (PP/12-LO+) cells identified in islets of the nonuncinate areas (Figure 4C). Moreover, we identified highly positive PP cells that were 12-LO−, adjacent to PP/12-LO+ cells in the same islet (Figure 4E, arrow). These results suggested that PP/12-LO+ cells are distinct from typical differentiated PP cells. The levels of PP intensity in typical islets of the uncinate region were comparable in all groups, except for minor changes noted in the AAb+ and T1D groups (Figure 5A), and much higher than in the nonuncinate areas (Figure 5B). Importantly, 12-LO+ cases show a clear increase in PP intensity in the nonuncinate area compared with that in islets from the nondiabetic groups and 12-LO− donors in the AAb+, T1D, and T2D groups (Figure 5B and Supplemental Figure 3, A and B). The close association of PP intensity and 12-LO positivity in islets was also demonstrated as a significant correlation between the two in Figure 5C. In 12-LO− donors, PP+ cells, if present, invariably exhibited staining intensities comparable to those from the uncinate region and were fewer in number. This observation is consistent with the well-established patterns of differentiated PP cells in human islets (21, 25).
Figure 5.
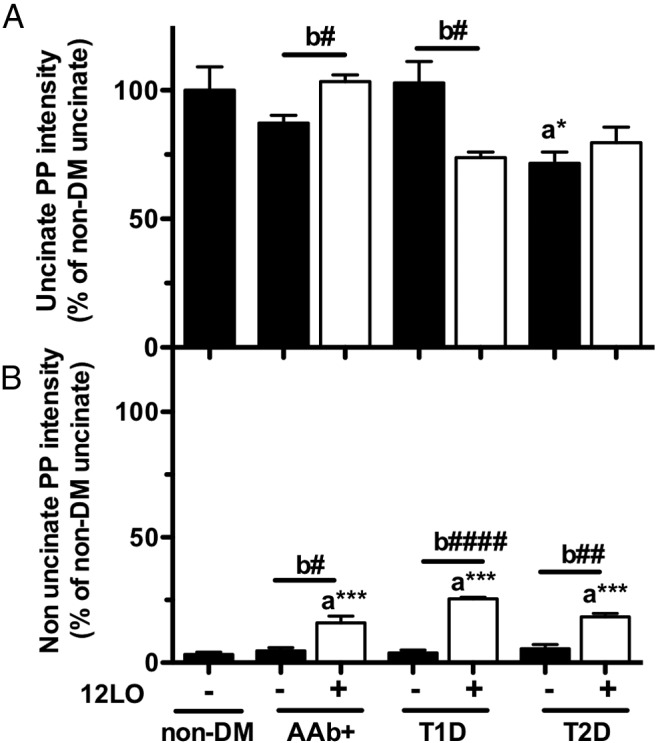
Quantification of PP intensity in islets from 12-LO+ and 12-LO− donors. The intensity of PP immunostaining within islets was quantified as in Materials and Methods in the uncinate (A) and nonuncinate (B) regions of the pancreas. Donors with AAb+, T1D, and T2D were separated based on the positivity of 12-LO in islets. We did not identify subjects with 12-LO+ cells in nondiabetic (non-DM) donors. Values are expressed as taking the average intensity of PP staining in the uncinate region of nondiabetic donors as 1. Data are means ± SEM (n = 3–4). Two statistical comparisons were performed: a, one-way ANOVA with Dunnett posttest in comparison with nondiabetic, *, P < .05; ***, P < .001; b, Student t tests between 12-LO+ and 12-LO− donors of each diabetic status, #, P < .05; ##, P < .001; ####, P < .0001.
Presence of 12-LO and vimentin within islets
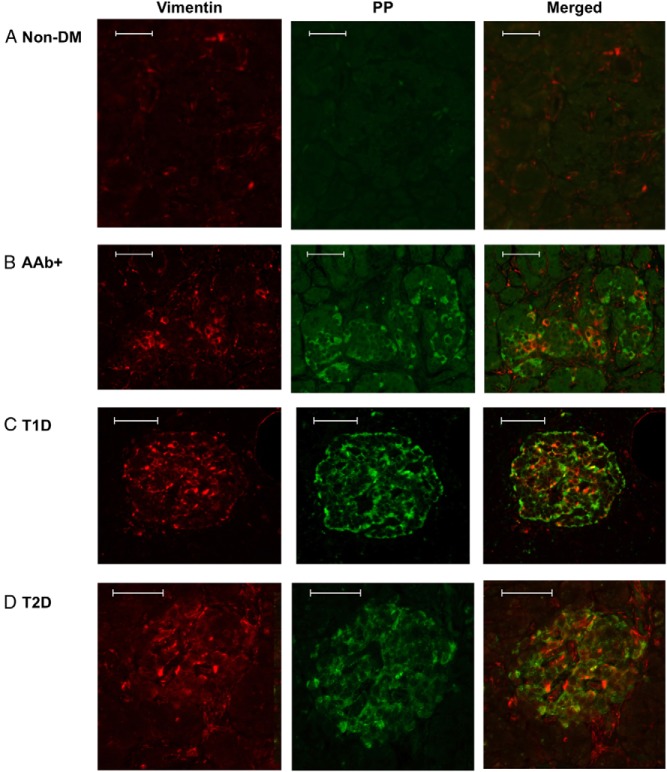
Recently, the appearance of PP-expressing cells in atypical areas of islets has been linked to β-cell dedifferentiation in an experimental mouse model (26). Furthermore, vimentin has been associated with dedifferentiated β-cells in a lineage-tracing study of mouse models of diabetes (14). Therefore, we tested vimentin expression in 12-LO+ cases. In nondiabetic specimens, vimentin staining exhibited the expected, restricted pattern limited to blood vessel cells (Figure 6A). In contrast, AAb+ and diabetic specimens that were highly enriched with PP/12-LO+ cells showed an increase in vimentin+ cells in islets. A subset of these vimentin+ cells also expressed low levels of PP (Figure 6, B–D), whereas PPhigh uncinate cells were invariably vimentin−, indicating that they are well-differentiated cells (Supplemental Figure 4, A and B). Vimentin expression, as well as that of several classes of 12-LO enzymes, has been associated with leukocytes that are frequently enriched in diabetic islets (9). Therefore, we analyzed the staining patterns of CD45 (a pan-leukocyte marker) with relation to vimentin. Whereas there was an increase in CD45 cells in diabetic specimens, most CD45+ cells were present outside of the islets (Supplemental Figure 5). Only a small percentage of these CD45 cells contributed to the increased numbers of vimentin+ cells within islets (Supplemental Figure 5).
Figure 6.
Vimentin expression in islets of 12-LO+ donors. Immunofluorescence was used to determine the expression of vimentin (red) and PP (green) in nondiabetic nPOD6013 (A), AAb+ nPOD6023 (B), T1D nPOD6038 (C), and T2D nPOD6157 (D) pancreas sections. Scale bars correspond to 50 μm.
We previously reported that ALOX12 (human genes for 12-LO), not ALOX15, was the primary lipoxygenase mRNA expressed in human islets (5). In this study, we performed immunostaining for 15LO-1, the protein product of ALOX15. 15LO-1 was not detectable in human islets, regardless of the diabetes status of the donor (Supplemental Figure 6). Positive staining was observed within acinar tissue, without clear differences in diabetic and nondiabetic samples.
Discussion
12-LO gene and protein expression levels are increased in islets of animal models of diabetes (11, 27). Moreover, targeted deletion of 12-LO in animal models of T1D (NOD) and T2D (diet-induced) leads to reduced β-cell inflammation and prevention of diabetes development (7, 8). The current results provide evidence that 12-LO activation is relevant in human diabetes based on the close association between islet 12-LO expression and diabetic phenotypes. The results indicate that significant expression of 12-LO within human islets appears to be restricted to subjects at risk for T1D (AAb+) and those affected by TID and T2D, whereas 15LO-1 is not expressed in islets under any conditions. Furthermore, islet 12-LO immunostaining appears to be limited to the early stages of T1D, and 12-LO expression in diabetic islets may be linked to a specific and unique cellular subpopulation.
The expression of 12-LO in pancreatic islets in situ has been reported previously in nondiabetic rodents (28, 29). However, this is the first study to evaluate staining and localization of 12-LO in human prediabetic and diabetic pancreas tissues. 12-LO was not detected in typical endocrine cells, endothelial cells, or immune cells within islets, but expression was detected in human islets under presumed stress conditions, such as subjects with AAb expression or diabetes. The lack of 12-LO immunostaining in nondiabetic human islets from nPOD is consistent with studies of isolated human islets showing that both mRNA and protein levels of 12-LO were very low in nondiabetic human islets (5). Previously, we observed that ALOX12 mRNA expression in human islets from T2D donors varies widely and may be 20-fold higher than in nondiabetic islets (9). Thus, the 12-LO protein expression detected in diabetic nPOD pancreas sections may represent cells with substantially increased 12-LO levels.
Within the T1D cases examined, all cases with residual insulin-positive cells also expressed 12-LO; however, those lacking residual insulin-positive cells did not express 12-LO. Combined with a significant fraction of AAb+ subjects that are 12-LO+, 12-LO expression seems to be highly up-regulated in islets at early stages of T1D development or when there is on-going inflammation involving insulin-producing β-cells in islets. When the temporal development of autoimmune diabetes was followed in NOD mice, 12/15-LO gene (a murine homolog of human 12-LO) expression is prominent in islets before development of insulitis or pronounced β-cell loss (11). In addition, a study of Zucker diabetic fatty rats (model of T2D) showed that 12-LO was one of the earliest genes up-regulated during the prediabetic stage and declined as β-cells were lost (27). Furthermore, moderately functional islets, from human T2D donors that were able to secrete insulin in response to glucose, exhibited up-regulated ALOX12 expression, but dysfunctional T2D islets or nondiabetic islets did not (9). Collectively, data from 12-LO expression patterns in diabetic specimens suggest that 12-LO is a key molecule activated at an early stage of β-cell demise in both forms of diabetes and may play a role in accelerating the loss of functional β-cell mass.
Interestingly, most 12-LO-expressing cells we identified also coexpress PP. These cells express PP at levels lower than those observed in PP cells from the uncinate region, which is known to be enriched with differentiated PP cells in nondiabetic humans (21). In contrast, authentic PP cells, which express high levels of the peptide in the uncinate and nonuncinate areas, did not express 12-LO in any cases. These observations suggest the presence of 2 different populations of PP-positive cells in the pancreas (“high” and “low”), of which the low PP-expressing cells seem to be uniquely associated with 12-LO expression in islets during the earlier stages of diabetes and absent in nondiseased islets. Others have implied that there is an association between increased PP cells and islet dysfunction. Hanley et al (30) reported that PP levels in circulation and PP mRNA levels in pancreatic islets are increased in T2D patients. The enrichment of PP cells in the islets of Zucker diabetic fatty rats was reported (31), which is the same model showing increased 12-LO mRNA expression in islets (27). Notably, we did not detect PP/12-LO+ cells in T1D cases with long-standing disease lacking insulin-positive β-cells. Because PP/12-LO+ cells are restricted to islets at the early stage of diabetes, this population of cells may correspond to dedifferentiated β-cells identified through lineage tracing in mouse models of diabetes (14, 26). Direct and indirect evidence in mice indicates that β-cells under stress or pathological conditions may lose insulin positivity and express PP, a peptide expressed by cells developmentally proximal to β-cells (32). Collombat et al (33) reported that misexpression of the arx gene in differentiated β-cells is sufficient to abrogate insulin production and convert them to cells that express PP and/or glucagon. Talchai et al (14) have shown that β-cells lose insulin positivity and acquire other endocrine phenotype(s) during the development of hyperglycemia in FOXO1-deficient mice. More recently, the conversion of β-cells to insulin-negative, PP-positive cells was noted in mice after pancreatectomy (26). Thus, the PP/12-LO+ cells observed in islets of human AAb+, T1D, and T2D donors may represent β-cells that have lost the ability to secrete insulin. A subpopulation of low PP/12-LO+ cells also expressed vimentin, which is a conditional marker of the less differentiated precursors of β-cells (14), providing another indication that 12-LO expression may coincide with the enrichment of cells exhibiting markers linked to dedifferentiated β-cells. Future studies will explore whether PP/12-LO+ cells represent dedifferentiated β-cells that can be restored to functionality by blocking of 12-LO activity or expression.
12-LO+ staining in T2D pancreata confirms previous data demonstrating high ALOX12 expression in a subset of human islets from T2D donors (9). Considering the proinflammatory nature of 12-LO products (5), this result provides support for the contribution of inflammation in islet dysfunction in T2D (34), along with reported up-regulation of inflammatory mediators including IL-1β, IL-12, CCL2, CCL13, and CXCL10 (35–38). Further studies addressing the relationship between 12-LO expression and the expression of other inflammatory mediators will probably delineate a process that leads to progressive β-cell loss in T2D.
In summary, we have demonstrated that islet 12-LO expression is linked to the early stages of diabetes progression in humans. In addition, 12-LO expression coincides with the appearance of a specific subpopulation of human islet cells that exhibit some features of dedifferentiated β-cells. These findings support the idea that 12-LO is an attractive therapeutic target to prevent loss of functional β-cell mass in diabetes.
Acknowledgments
We thank Dr Shivatra Noi Talchai (King Mongkut's University Technology at Thonburi) for advice about vimentin staining.
This work was supported by the Network for Pancreatic Organ Donors with Diabetes (nPOD), a collaborative type 1 diabetes research project sponsored by the Juvenile Diabetes Research Foundation. Organ Procurement Organizations partnering with nPOD to provide research resources are listed at http://www.jdrfnpod.org/for-partners/npod-partners. This research was funded in part by NIH 1 UC4 DK104166-01 (JLN, MAM). J.L.N. and M.A.M. were supported by the Juvenile Diabetes Research Foundation. J.L.N. and Y.I. were supported by a nonembryonic stem cell research fund and research enhancement grant from Eastern Virginia Medical School.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AAb+
- autoantibody-positive
- HE
- hematoxylin and eosin
- 12-LO
- 12-lipoxygenase
- NOD
- nonobese diabetic
- nPOD
- Network for Pancreatic Organ Donors with Diabetes
- PP
- pancreatic polypeptide
- T1D
- type 1 diabetes
- T2D
- type 2 diabetes.
References
- 1. Dobrian AD, Lieb DC, Cole BK, Taylor-Fishwick DA, Chakrabarti SK, Nadler JL. Functional and pathological roles of the 12- and 15-lipoxygenases. Prog Lipid Res. 2011;50:115–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuhn H, O'Donnell VB. Inflammation and immune regulation by 12/15-lipoxygenases. Prog Lipids Res. 2006;45:334–356. [DOI] [PubMed] [Google Scholar]
- 3. Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 2001;294:1871–1875. [DOI] [PubMed] [Google Scholar]
- 4. Weaver JR, Holman TR, Imai Y, et al. Integration of pro-inflammatory cytokine, 12-lipoxygenase and NOX-1 in pancreatic islet β cell dysfunction. Mol Cell Endocrinol. 2012;358:88–95. [DOI] [PubMed] [Google Scholar]
- 5. Ma K, Nunemaker CS, Wu R, Chakrabarti SK, Taylor-Fishwick DA, Nadler JL. 12-Lipoxygenase products reduce insulin secretion and β-cell viability in human islets. J. Clin. Endocrinol. Metab. 2010;95:887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bleich D, Chen S, Zipser B, Sun D, Funk CD, Nadler JL. Resistance to type 1 diabetes induction in 12-lipoxygenase knockout mice. J Clin Invest. 1999;103:1431–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McDuffie M, Maybee NA, Keller SR, et al. Nonobese diabetic (NOD) mice congenic for a targeted deletion of 12/15-lipoxygenase are protected from autoimmune diabetes. Diabetes. 2008;57:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tersey SA, Maier B, Nishiki Y, Maganti AV, Nadler JL, Mirmira RG. 12-Lipoxygenase promotes obesity-induced oxidative stress in pancreatic islets. Mol Cell Biol. 2014;34:3735–3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Butcher MJ, Hallinger D, Garcia E, et al. Association of proinflammatory cytokines and islet resident leucocytes with islet dysfunction in type 2 diabetes. Diabetologia. 2014;57:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang Z, Chen M, Ellett JD, et al. Autoimmune diabetes is blocked in Stat4-deficient mice. J Autoimmun. 2004;22:191–200. [DOI] [PubMed] [Google Scholar]
- 11. Green-Mitchell SM, Tersey SA, Cole BK, et al. Deletion of 12/15-lipoxygenase alters macrophage and islet function in NOD-Alox15null mice, leading to protection against type 1 diabetes development. PLoS One. 2013;8:e56763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luci DK, Jameson JB, 2nd, Yasgar A, et al. Synthesis and structure-activity relationship studies of 4-((2-hydroxy-3-methoxybenzyl)amino)benzenesulfonamide derivatives as potent and selective inhibitors of 12-lipoxygenase. J Med Chem. 2014;57:495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cole BK, Kuhn NS, Green-Mitchell SM, et al. 12/15-Lipoxygenase signaling in the endoplasmic reticulum stress response. Am J Physiol Endocrinol Metab. 2012;302:E654–E665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150:1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lombardi A, Ulianich L, Treglia AS, et al. Increased hexosamine biosynthetic pathway flux dedifferentiates INS-1E cells and murine islets by an extracellular signal-regulated kinase (ERK)1/2-mediated signal transmission pathway. Diabetologia. 2012;55:141–153. [DOI] [PubMed] [Google Scholar]
- 16. Tersey SA, Nishiki Y, Templin AT, et al. Islet β-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the non-obese diabetic mouse model. Diabetes. 2012;61:818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tania M, Khan MA, Fu J. Epithelial to mesenchymal transition inducing transcription factors and metastatic cancer. Tumour Biol. 2014;35:7335–7342. [DOI] [PubMed] [Google Scholar]
- 18. Wang GS, Rosenberg L, Scott FW. Tubular complexes as a source for islet neogenesis in the pancreas of diabetes-prone BB rats. Lab Invest. 2005;85:675–688. [DOI] [PubMed] [Google Scholar]
- 19. Pugliese A, Yang M, Kusmarteva I, et al. The Juvenile Diabetes Research Foundation Network for Pancreatic Organ Donors with Diabetes (nPOD) Program: goals, operational model and emerging findings. Pediatr Diabetes. 2014;15:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Campbell-Thompson M, Wasserfall C, Kaddis J, et al. Network for Pancreatic Organ Donors with Diabetes (nPOD): developing a tissue biobank for type 1 diabetes. Diabetes Metab Res Rev. 2012;28:608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang X, Zielinski MC, Misawa R, et al. Quantitative analysis of pancreatic polypeptide cell distribution in the human pancreas. PLoS One. 2013;8:e55501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Achenbach P, Bonifacio E, Koczwara K, Ziegler AG. Natural history of type 1 diabetes. Diabetes. 2005;54(suppl 2):S25–S31. [DOI] [PubMed] [Google Scholar]
- 23. Wuest SJ, Crucet M, Gemperle C, Loretz C, Hersberger M. Expression and regulation of 12/15-lipoxygenases in human primary macrophages. Atherosclerosis. 2012;225:121–127. [DOI] [PubMed] [Google Scholar]
- 24. Nie D, Tang K, Diglio C, Honn KV. Eicosanoid regulation of angiogenesis: role of endothelial arachidonate 12-lipoxygenase. Blood. 2000;95:2304–2311. [PubMed] [Google Scholar]
- 25. Gersell DJ, Gingerich RL, Greider MH. Regional distribution and concentration of pancreatic polypeptide in the human and canine pancreas. Diabetes. 1979;28:11–15. [PubMed] [Google Scholar]
- 26. El-Gohary Y, Tulachan S, Wiersch J, et al. A smad signaling network regulates islet cell proliferation. Diabetes. 2014;63:224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tokuyama Y, Sturis J, DePaoli AM, et al. Evolution of β-cell dysfunction in the male Zucker diabetic fatty rat. Diabetes. 1995;44:1447–1457. [DOI] [PubMed] [Google Scholar]
- 28. Shannon VR, Ramanadham S, Turk J, Holtzman MJ. Selective expression of an arachidonate 12-lipoxygenase by pancreatic islet β-cells. Am J Physiol. 1992;263:E828–E836. [DOI] [PubMed] [Google Scholar]
- 29. Kawajiri H, Zhuang D, Qiao N, et al. Expression of arachidonate 12-lipoxygenase in rat tissues: a possible role in glucagon secretion. J Histochem Cytochem. 2000;48:1411–1419. [DOI] [PubMed] [Google Scholar]
- 30. Hanley SC, Austin E, Assouline-Thomas B, et al. β-Cell mass dynamics and islet cell plasticity in human type 2 diabetes. Endocrinology. 2010;151:1462–1472. [DOI] [PubMed] [Google Scholar]
- 31. Howarth FC, Al Kitbi MK, Hameed RS, Adeghate E. Pancreatic peptides in young and elderly Zucker type 2 diabetic fatty rats. JOP. 2011;12:567–573. [PubMed] [Google Scholar]
- 32. Herrera PL, Nepote V, Delacour A. Pancreatic cell lineage analyses in mice. Endocrine. 2002;19:267–278. [DOI] [PubMed] [Google Scholar]
- 33. Collombat P, Hecksher-Sorensen J, Krull J, et al. Embryonic endocrine pancreas and mature β cells acquire α and PP cell phenotypes upon Arx misexpression. J Clin Invest. 2007;117:961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Imai Y, Dobrian AD, Morris MA, Nadler JL. Islet inflammation: a unifying target for diabetes treatment? Trends Endocrinol Metab. 2013;24:351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Donath MY, Dalmas E, Sauter NS, Boni-Schnetzler M. Inflammation in obesity and diabetes: islet dysfunction and therapeutic opportunity. Cell Metab. 2013;17:860–872. [DOI] [PubMed] [Google Scholar]
- 36. Taylor-Fishwick DA, Weaver JR, Grzesik W, et al. Production and function of IL-12 in islets and β cells. Diabetologia. 2013;56:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Igoillo-Esteve M, Marselli L, Cunha DA, et al. Palmitate induces a pro-inflammatory response in human pancreatic islets that mimics CCL2 expression by beta cells in type 2 diabetes. Diabetologia. 2010;53:1395–1405. [DOI] [PubMed] [Google Scholar]
- 38. Schulthess FT, Paroni F, Sauter NS, et al. CXCL10 impairs beta cell function and viability in diabetes through TLR4 signaling. Cell Metab. 2009;9:125–139. [DOI] [PubMed] [Google Scholar]