Summary
Repetitive suberythemal UVA and/or UVB exposures were used to generate comparable UV-induced tans in human skin over the course of 2 weeks. In order to evaluate the potential photoprotective values of those UVA- and/or UVB- induced tans and to avoid the confounding issue of residual UV-induced DNA damage, we waited 1 week before challenging those areas with a 1.5 MED dose of UVA+UVB after which we measure DNA damage. The results show that the type of UV used to induce skin pigmentation affects the redistribution of melanin in the skin and/or de novo melanin synthesis. The UVA-induced tans failed to even provide a minimal SPF of 1.5, which suggests that producing a tan with UVA-rich sunlamps prior to a holiday or vacation is completely counterproductive.
Keywords: ultraviolet, skin, pigmentation, DNA damage, photoprotection
Humans have long marveled at the sun, their skin and the interplay between them. In addition to visible and infrared light, the sun produces ultraviolet radiation (UV) that is classically defined into 3 fractions: UVA (320–400 nm), UVB (280–320 nm) and UVC (100–280 nm) (CIE Technical Committee 2–17, 1989). The critical role of UV, either from the sun and/or from artificial UV sources used commercially, has been detailed extensively with respect to skin cancer and skin photoaging. Not surprisingly, an entire industry has been built around topical photoprotection as an approach to prevent skin cancer and reduce photoaging. The organized transfer and spatial distribution in the epidermis of melanosomes containing differing amounts of eumelanin and pheomelanin serve as the body’s natural sunscreen with a personalized sun protection factor (SPF) gradient as diverse as the human population. This study shows that suberythemal UVA-induced tans fail to even provide a minimal SPF of 1.5, which suggests that producing a tan with UVA-rich sunlamps prior to a holiday or vacation is a completely counterproductive DNA damage photoprotection strategy.
The field of pigment cell biology has focused primarily on understanding the shorter wavelengths within UVB because of their well-known carcinogenic effects. UVB is known to cause erythema or sunburn, blistering of the skin and visual impairment; however, with intense or repeated UVB exposures, it can cause immune suppression (Clydesdale et al., 2001; Whitmore et al., 2001) and permanent eye damage (Young and Sands, 1998) can develop. UV signature DNA lesions are primarily caused by UVB irradiation and to a lesser degree by UVA (Mouret et al., 2006). The longer wavelengths of UVA penetrate much deeper into the skin, leading to photoaging (Kligman and Kligman, 1986; Krutmann, 2000). UVA has a predominant role in forming reactive oxygen species (ROS), i.e. oxidant intermediates such H2O2 or OH, immediately after UV exposure. Oxidative stress occurs when skin cells cannot neutralize increased levels of ROS catalyzed by NADPH oxidase or lipid peroxidation (Thiele et al., 1998). The deleterious effects of UVA on the skin are often overlooked due to the more dramatic carcinogenic effects caused by UVB in terms of the significant amounts of cyclobutane pyrimidine dimers (CPDs) formed in DNA after UVB exposure (Hart et al., 1977; Setlow, 1966; Tan et al., 1970). However, both UVA and UVB have been implicated in skin cancer formation and, therefore, neither component should be overlooked when interpreting their UV exposure contribution to risk versus benefit analyses (Noonan et al., 2001; Noonan et al., 2012; Setlow et al., 1993; Wood et al., 2006). Over time, the potential development of squamous cell carcinoma (Karagas et al., 2002), basal cell carcinoma (Mabruk et al., 2009) and even malignant melanoma (Gallagher et al., 2005) after UVA and/or UVB exposure becomes a concern (International Agency for Research on Cancer, 2007). Of particular concern in recent years has been melanoma, which has become the predominant cancer in fair-skinned young women 25–29 years old (Bleyer et al., 2006; Fast Stats: 2009; Purdue et al., 2008). In addition, there is an increased melanoma incidence rate in women 20–49 years old over the last 15 years compared to males of the same age range (Fast Stats: 2009).
Melanosomes, specialized organelles produced only in melanocytes, acquire the machinery necessary to produce both eumelanin and pheomelanin in a stepwise process prior to their transfer to keratinocytes. Different percentages of DHI- and DHICA-melanins copolymerize to form a very insoluble brown-black eumelanin. In contrast, the addition of cysteine to the dopaquinone is required to produce the more soluble photolabile reddish-brown pheomelanin. It has been suggested that the pheomelanin degradation leads to more photolytic products that in turn might affect the photoprotective ability of eumelanin (Simon and Peles, 2010). Red hair with higher pheomelanin composition produces increased amounts of ROS after UVA-rich exposure as detected by electron spin resonance spectroscopy (ESR) (Fernandez et al., 2012), which was previously suggested as early as 1978 (Chedekel et al., 1978). Using ESR, another group has also reported that it is not the melanin type but rather the low melanin concentration in fair-skinned individuals that makes them more susceptible to ROS production and at risk for UVA carcinogenesis (Haywood et al., 2008). Therefore the amount and ratio of eumelanin to pheomelanin may be a critical point with regard to UV-induced cancers given the reduced absorption characteristics (by up to 25%) of increased pheomelanin content in the context of the overall melanin composition models proposed (Ito and Wakamatsu, 2008; Simon & Peles, 2010). In addition, melanosomes containing melanin are strong light scatterers with a broad UV-visible absorption spectrum above 320 nm (Anderson, 1993; Wolbarsht et al., 1981), which provides a shielding mechanism for keratinocytes, melanocytes and underlying dermal components against UV radiation (Kobayashi et al., 1998; Pathak, 1995). Melanin absorbs a portion of the UV energy, transforming it into heat that is dissipated into the body. This underscores the importance of melanosome distribution to keratinocytes within the epidermis as melanosome localization can account for a SPF of up to 12 for moderately pigmented individuals (Anderson, 1993; Nielsen et al., 2006).
Previously, we reported a modest photoprotective effect from repetitive suberythemal doses of UVB, but no photoprotection or increase in melanin synthesis from repetitive suberythemal doses of UVA (Miyamura et al., 2011). However, in that study, the confounding issue of residual DNA damage immediately after production of the UV-induced tan posed a problem in assessing photoprotection. In this follow-up clinical study, 2 weeks of repetitive suberythemal exposure to UVA and/or UVB generated comparable UV-induced tans upon which a UV challenge dose was applied after a time lag of 1 week to allow repair of the residual DNA damage.
In order to mimic solar exposure in its emission spectrum while at the same time matching the ozone effect of attenuating UVC and short UVB wavelengths, a solar simulator (Oriel 1600 W Solar Simulator, Newport Corporation, Stratford, CT, USA) was used as previously detailed (Miyamura et al., 2011; Wolber et al., 2008). Following determination of the minimal erythema dose (MED) of each subject enrolled (see Supplementary Materials and Methods, Table S1), a series of 3 areas on the lower back of 12 subjects (5 females, 7 males) were suberythemally irradiated 5 times with 0.4 MED in week 1 and then 5 times with 0.5 MED in week 2 (Mondays through Fridays) with UVA, UVB and UVA+UVB doses indicated in Table S1. Those regimens elicited equivalent UV-induced tans amongst those 3 types of UV treatments (Figure 1A). A 4th skin area was not exposed to the repetitive UV as a control. One week later, all 4 areas (including the unexposed control area) received a 1.5 MED UVA+UVB challenge dose (Figure 1A). Biopsies were acquired 1 week after the repetitive UV treatment (prior to the UV challenge), immediately after the UV challenge, and 4 days after the UV challenge. One group was used for histology and another group was used for gene expression analysis as indicated in Table S1. No erythema was detected at the time points measured, except when comparing the UVB-treatment area before UV to the 4 day post-UV challenge timepoint (P<0.05) (Figure 1B). This suggested that the suberythemal repetitive UV-treatments did not produce significant increases in erythema, which was part of the design of the study. Clearly, the level of pigmentation increased significantly with the different UV treatments compared to the unexposed control that received only the UV challenge dose (Figure 1C). UVA and/or UVB treatments prior to the UV challenge lead to increased pigmentation measured by diffuse reflectance. The increased pigmentation was further underscored by the level of melanin staining found in skin sections using the silver stain Fontana-Masson method (Bancroft and Stevens, 1982) (Figure 2A). The relationship between melanin content and the distribution caused by the different UV treatments has already been exhaustively evaluated previously (Miyamura et al., 2011; Wolber et al., 2008) and will not be discussed here.
Figure 1.
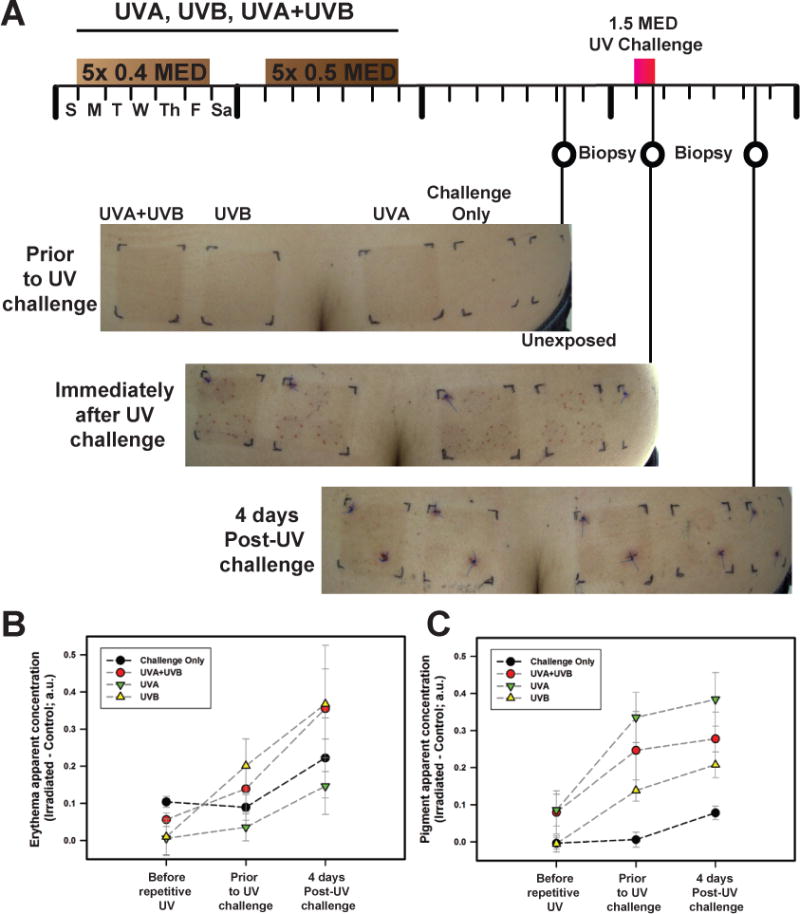
Protocol for UVA- and/or UVB- tanning and challenge, and effects on erythema and pigmentation of the skin. (A) Timeline of suberythemal repetitive irradiations with UVA and/or UVB for 2 weeks. In week 1, a 0.4 MED dose of UV was applied daily (Monday through Friday) and 0.5 MED UV was applied daily in week 2. One week later, biopsies were taken from each of the 4 skin areas on each subject. The following week, a 1.5 MED UVA+UVB challenge was applied to each skin area and biopsies were taken immediately after the challenge and also 4 days later. Images of skin at the different biopsy time points are depicted; shown for subject #16531. (B) Diffuse reflectance spectroscopy indicates minimal to no erythema developed prior to and immediately after the UV challenge. There were no statistically significant differences between any of the UV treatment areas or time points with the exception of the UVB-induced tan area that showed a statistically significant difference in erythema (P<0.05) prior to the UV challenge compared to the 4 days post-UV challenge. (C) Pigmentation levels increased after UVA, UVB and UVA+UVB (P<0.01, P<0.002 and P<0.05, respectively) compared to control area prior to UV challenge, corroborating the visual tan development and remained elevated at 4 days post-UV challenge (P<0.05). In addition, pigmentation levels increased significantly with UVA only (P<0.05) and UVB only (P<0.005) 4 days after the UV challenge compared to prior to the repeated UV regimen. The UV challenge induced significant pigmentation increases in the unexposed control area (P<0.01). Data points correspond to mean values calculated from apparent concentration diffuse reflectance spectroscopy values acquired from 5 subjects ± SE; a.u. = arbitrary units.
Figure 2.
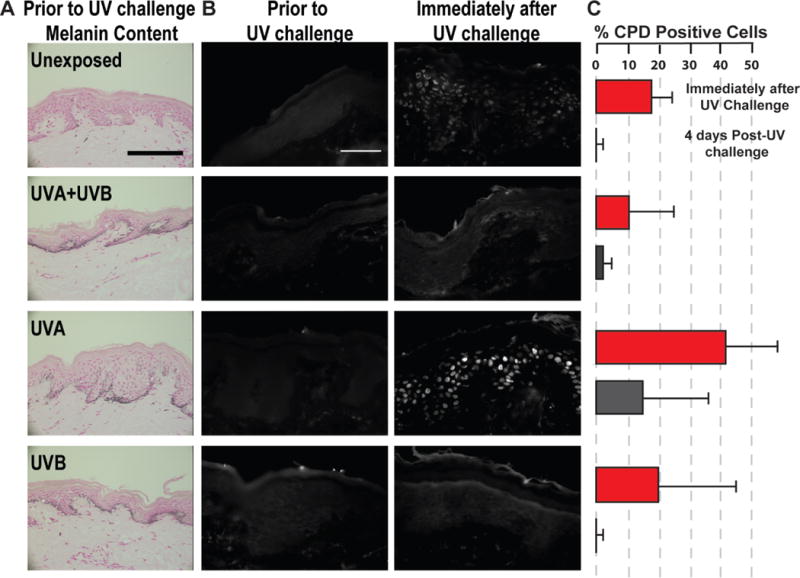
UVA-induced pigmentation is not photoprotective. (A) Melanin content of human skin sections detected by Fontana-Masson silver staining show clear visual increases in melanin with the 3 UV treatments compared to the unexposed control; representative micrographs are shown for subject #18910; scale bar = 50 μm. The brightfield images were taken with an AxioCam MRc Rev 3 color camera (Carl Zeiss, Inc., Oberkochen, Germany) attached to a DMRB microscope (Leica Microsystems, Bannockburn, IL, USA). (B) Micrographs (subject #18910) depict no CPD-positive cells 7 days after 2 weeks of suberythemal repetitive UV treatments prior to UV challenge. Following the 1.5 MED UV challenge, the UVA-induced pigmentation provided no photoprotection immediately after the UV challenge. Immunofluorescence images were taken with a ORCA-ER Black and White CCD digital camera (Hamamatsu Photonics K.K., Hamamatsu, Japan) attached to the Axiovert S100 inverted microscope (Carl Zeiss, Inc., Oberkochen, Germany); scale bar = 50 μm. (C) The percentages of CPD-positive cells (means ± SEM, n=5) were significantly increased compared to unexposed areas for UVA-induced and unexposed areas receiving the 1.5 MED UV challenge (red bars; P<0.05).
The primary focus of this follow-up study was to evaluate whether photoprotection is afforded by UV-induced tans produced from different UV spectral bands. DNA photoproducts, such as CPDs, were not detected prior to the UV challenge for any of the UV treatments (Figure 2B) even with a monoclonal antibody able to detect DNA lesions with UV doses as low as 0.5 J/m2 in formalin fixed paraffin-embedded tissue sections (Mori et al., 1991). This showed that detectable levels of CPDs caused by the repetitive UV exposures had been repaired by that time. Following the 1.5 MED UV challenge, UVA-induced tans provided no measureable photoprotection (Figure 2B). CPD-positive cells (white) were numerous and clear in the UVA-induced tans and the unexposed control area receiving the UV challenge but hardly detected in the UVB-induced tans with or without UVA (Figure 2B and 2C). UVA-induced tans provided no photoprotection under the conditions tested even though there was comparable skin tanning among the different UV treatments. Due to the increased production and distribution of melanin, there is a very modest photoprotective benefit from UVB-induced tans at the suberythemal doses tested. The 4 days post-UV challenge time point showed adequate repair rates with no statistical significant differences between the UV treatments (Figure 2C).
Previously, we reported the gene expression patterns of melanocyte-specific genes and paracrine factor signaling involved in skin responses and melanogenesis after UVA and/or UVB exposure (Choi et al., 2010). Here, the follow-up study microarray group is reported (see Supplementary Materials and Methods) and has been deposited at www.ncbi.nlm.nih.gov/geo, #GSE56754. There were 13,776 probes with a P<0.05 indicating they are differentially expressed over time. Furthermore, there were 688 probes with a P<0.05 where the genes have an altered pattern with different UV-treatments over time. Heatmaps for UVA (Figure 3A), UVB (Figure 3B) and UVA+UVB (Figure 3C) show two-dimensional clustering of the common differentially expressed genes which have a significant raw P<0.05, in one of the paired comparisons: UVA vs. Control, UVB vs. Control, and UVA+UVB vs. Control, at all 3 time points. It is obvious that those differential genes have different expression patterns between the UV-treatments and unexposed controls, which were represented by the different heat map patterns. The top 20 genes identified by UV-treatment effect with a P<0.05 highlight many of the known pigment genes, including TYR, DCT, TYRP1, SILV, MLANA and EDNRB (Figure 3D). These results corroborate the melanocyte-specific genes detected previously after different UV treatments (Choi et al., 2010).
Figure 3.
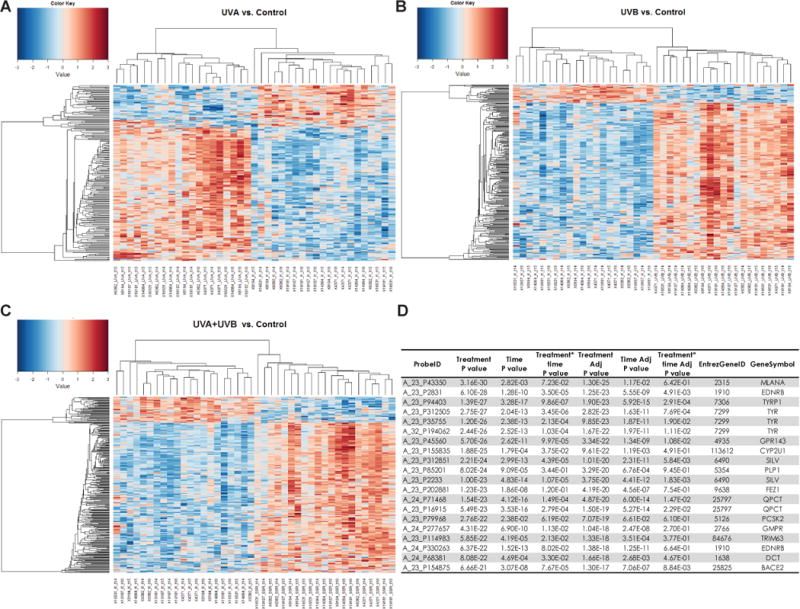
Heatmaps of UV-induced genes show UV spectral expression changes for genes according to the types of UV treatment and times. Microarray analysis is shown prior to UV challenge, immediately after the UV Challenge, and 4 days after the UV Challenge for the UV-irradiated sites compared to unexposed control skin. Heatmap clustering was generated for UVA (A), UVB (B) and UVA+UVB (C) vs the unexposed control (red (max = +3) to blue (min = −3) color gradient). (D) Top 20 gene probes identified by treatment effect P value determined by the Repeated Measure Analysis of Variance model.
Given the significance of DNA damage (from photoproducts and oxidation) in relation to melanomagenesis, altered gene expression patterns in relevant repair or stress response pathways were evaluated. To determine if differentially expressed genes in Nucleotide Excision Repair (NER) or Nuclear Factor-erythroid 2-Related factor 2 (NRF2)- mediated oxidative stress response pathways were overrepresented, the Ingenuity Pathway Analysis software platform (IPA, 2013 Ingenuity Systems, Redwood City, CA; www.ingenuity.com) was used (see Supplementary Materials and Methods). There were no statistically significant differences in NER across all UV treatments at each of time points which corroborates the results of the CPD repair (Figure 2C). The response to oxidative stress is mediated in part by the role of NRF2 to modulate the activation of antioxidants. The NRF2-mediated oxidative stress response pathway showed a significant reduction in NRF2 immediately after the UV challenge in both UVA-induced (Figure S1) and UVB-induced (Figure S2) tans. This response was also repeated in the UVA+UVB-induced tans and the unexposed skin that received the UV challenge dose. Not surprisingly, there were more antioxidant genes upregulated in the NRF2-mediated oxidative stress response pathway in the UVA-induced tan receiving the UV challenge (which had elevated CPD levels). Specific to only the UVA-induced tan, there was a statistically significant (P<0.05) 2.8 fold increase in mitochondrial superoxide dismutase 2 (SOD2) immediately after the UV challenge (Figure S1). Interestingly, this gene did not show any increases before the challenge dose after receiving 2 weeks of repetitive UVA, although it is important to keep in mind that this time point was one week after the last UV irradiation. The antioxidant enzyme, SOD2, has already been reported to show increased gene expression after solar simulated irradiation in human fibroblasts (Leccia et al., 2001), and has been indicated to be protective against UV when SOD2 levels are elevated (Matsui et al., 2003). Since SOD2 gene expression was not increased in the unexposed area that received the UV challenge, it is possible that the repetitive UVA irradiation sensitized SOD2 to respond to the excess UV-induced oxidative stress immediately after the challenge dose. As a result, the in vivo response kinetics to oxidative stress after UVA and/or UVB merits further inquiry.
For the first time, these results overcome the previous clinical study limitations of erythema production, the use of high UVA doses, the lack in achieving full pigmentation potential after the last UV exposure (which can take at least 5 days) and the presence of residual UV-induced DNA damage to properly evaluate the putative photoprotection of repetitive UVA- and/or UVB- induced tans produced in human skin. It is quite clear that UVA-induced tans fail entirely to provide any photoprotective benefit, even at the level of a SPF of 1.5. This coupled with the fact that UVA-induced tans do not involve increased melanin production (Wolber et al., 2008) but rather its redistribution along with other aspects previously discussed elsewhere (Choi et al., 2010; Miyamura et al., 2011), underscores the misleading concept of a perceived benefit from tans produced by UVA-rich sunlamps.
Supplementary Material
Significance.
Evidence for the role of UVA in melanoma formation and the increased risk of carcinogenesis has become compelling, especially in light of the commercial use of UVA-rich sunlamps. This in turn affects the perceived photoprotective benefit of developing a UV-induced tan prior to a holiday or vacation in the sun. This follow-up study confirms the lack of photoprotection from UVA-induced tans without the confounding issue of residual DNA damage. Suberythemal UVA-induced tans exacerbate the amount of preventable UV exposure by producing a deceptive visual pigmentation of the skin that provides no sunscreen effect.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Cancer Institute at NIH.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Anderson RR. Optics of the Skin. In: Lim HW, Soter NA, editors. Clinical Photomedicine. New York: Marcel Dekker Inc; 1993. pp. 19–36. [Google Scholar]
- Bancroft JD, Stevens A. Theory and Practice of Histological Techniques. New York: Churchill Livingstone; 1982. [Google Scholar]
- Bleyer A, Viny A, Barr R. Cancer in 15- to 29-year-olds by primary site. Oncologist. 2006;11:590–601. doi: 10.1634/theoncologist.11-6-590. [DOI] [PubMed] [Google Scholar]
- Chedekel MR, Smith SK, Post PW, Pokora A, Vessell DL. Photodestruction of pheomelanin: role of oxygen. Proc Natl Acad Sci U S A. 1978;75:5395–5399. doi: 10.1073/pnas.75.11.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W, Miyamura Y, Wolber R, Smuda C, Reinhold W, Liu H, Kolbe L, Hearing VJ. Regulation of human skin pigmentation in situ by repetitive UV exposure: molecular characterization of responses to UVA and/or UVB. J Invest Dermatol. 2010;130:1685–1696. doi: 10.1038/jid.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIE Technical Committee 2–17. CIE Technical Report N° CIE 85, Solar Spectral Irradiance. Commision Internationale de l’Eclairage (CIE) Central Bureau; Vienna, Austria. 1989. [Google Scholar]
- Clydesdale GJ, Dandie GW, Muller HK. Ultraviolet light induced injury: immunological and inflammatory effects. Immunol Cell Biol. 2001;79:547–568. doi: 10.1046/j.1440-1711.2001.01047.x. [DOI] [PubMed] [Google Scholar]
- Fast Stats. An interactive tool for access to SEER cancer statistics. Surveillance Research Program, National Cancer Institute; Oct 26, 2009. http://seer.cancer.gov/faststats. [Google Scholar]
- Fernandez E, Barba C, Alonso C, Marti M, Parra JL, Coderch L. Photodamage determination of human hair. J Photochem Photobiol B. 2012;106:101–106. doi: 10.1016/j.jphotobiol.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Gallagher RP, Spinelli JJ, Lee TK. Tanning beds, sunlamps, and risk of cutaneous malignant melanoma. Cancer Epidemiol Biomark Prevent. 2005;14:562–566. doi: 10.1158/1055-9965.EPI-04-0564. [DOI] [PubMed] [Google Scholar]
- Hart RW, Setlow RB, Woodhead AD. Evidence that pyrimidine dimers in DNA can give rise to tumors. Proc Natl Acad Sci U S A. 1977;74:5574–5578. doi: 10.1073/pnas.74.12.5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood RM, Lee M, Andrady C. Comparable photoreactivity of hair melanosomes, eu- and pheomelanins at low concentrations: low melanin a risk factor for UVA damage and melanoma? Photochem Photobiol. 2008;84:572–581. doi: 10.1111/j.1751-1097.2008.00343.x. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: A systematic review. Int J Cancer. 2007;120:1116–1122. doi: 10.1002/ijc.22453. [DOI] [PubMed] [Google Scholar]
- Ito S, Wakamatsu K. Chemistry of mixed melanogenesis–pivotal roles of dopaquinone. Photochem Photobiol. 2008;84:582–592. doi: 10.1111/j.1751-1097.2007.00238.x. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Stannard VA, Mott LA, Slattery MJ, Spencer SK, Weinstock MA. Use of tanning devices and risk of basal cell and squamous cell skin cancers. J Natl Cancer Inst. 2002;94:224–226. doi: 10.1093/jnci/94.3.224. [DOI] [PubMed] [Google Scholar]
- Kligman LH, Kligman AM. The nature of photoaging: its prevention and repair. Photodermatology. 1986;3:215–227. [PubMed] [Google Scholar]
- Kobayashi N, Nakagawa A, Muramatsu T, Yamashina Y, Shirai T, Hashimoto MW, Ishigaki Y, Ohnishi T, Mori T. Supranuclear melanin caps reduce ultraviolet induced DNA photoproducts in human epidermis. J Invest Dermatol. 1998;110:806–810. doi: 10.1046/j.1523-1747.1998.00178.x. [DOI] [PubMed] [Google Scholar]
- Krutmann J. Ultraviolet A radiation-induced biological effects in human skin: relevance for photoaging and photodermatosis. J Dermatol Sci. 2000;23(Suppl 1):S22–S26. doi: 10.1016/s0923-1811(99)00077-8. [DOI] [PubMed] [Google Scholar]
- Leccia MT, Yaar M, Allen N, Gleason M, Gilchrest BA. Solar simulated irradiation modulates gene expression and activity of antioxidant enzymes in cultured human dermal fibroblasts. Exp Dermatol. 2001;10:272–279. doi: 10.1034/j.1600-0625.2001.100407.x. [DOI] [PubMed] [Google Scholar]
- Mabruk MJ, Toh LK, Murphy M, Leader M, Kay E, Murphy GM. Investigation of the effect of UV irradiation on DNA damage: comparison between skin cancer patients and normal volunteers. J Cutan Pathol. 2009;36:760–765. doi: 10.1111/j.1600-0560.2008.01164.x. [DOI] [PubMed] [Google Scholar]
- Matsui H, Lin LR, Ho YS, Reddy VN. The effect of up- and downregulation of MnSOD enzyme on oxidative stress in human lens epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:3467–3475. doi: 10.1167/iovs.02-0830. [DOI] [PubMed] [Google Scholar]
- Miyamura Y, Coelho SG, Schlenz K, Batzer J, Smuda C, Choi W, Brenner M, Passeron T, Zhang G, Kolbe L, Wolber R, Hearing VJ. The deceptive nature of UVA tanning versus the modest protective effects of UVB tanning on human skin. Pigment Cell Melanoma Res. 2011;24:136–147. doi: 10.1111/j.1755-148X.2010.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Nakane M, Hattori T, Matsunaga T, Ihara M, Nikaido O. Simultaneous establishment of monoclonal antibodies specific for either cyclobutane pyrimidine dimer or (6-4)photoproduct from the same mouse immunized with ultraviolet-irradiated DNA. Photochem Photobiol. 1991;54:225–232. doi: 10.1111/j.1751-1097.1991.tb02010.x. [DOI] [PubMed] [Google Scholar]
- Mouret S, Baudouin C, Charveron M, Favier A, Cadet J, Douki T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc Natl Acad Sci USA. 2006;103:13765–13770. doi: 10.1073/pnas.0604213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen KP, Zhao L, Stamnes JJ, Stamnes K, Moan J. The importance of the depth distribution of melanin in skin for DNA protection and other photobiological processes. J Photochem Photobiol. 2006;82:194–198. doi: 10.1016/j.jphotobiol.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Noonan FP, Recio JA, Takayama H, Duray P, Anver MR, Rush WL, DeFabo EC, Merlino G. Neonatal sunburn and melanoma in mice. Nature. 2001;413:271–272. doi: 10.1038/35095108. [DOI] [PubMed] [Google Scholar]
- Noonan FP, Zaidi MR, Wolnicka-Glubisz A, Anver MR, Bahn J, Wielgus A, Cadet J, Douki T, Mouret S, Tucker MA, Popratiloff A, Merlino G, De Fabo EC. Melanoma induction by ultraviolet A but not ultraviolet B radiation requires melanin pigment. Nat Commun. 2012;3:884. doi: 10.1038/ncomms1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak MA. Functions of melanin and protection by melanin. In: Zeise L, Chedekel MR, Fitzpatrick TB, editors. Melanin: Its Role in Human Photoprotection. Overland Park: Valdenmar Publ; 1995. pp. 125–134. [Google Scholar]
- Purdue MP, Freeman LE, Anderson WF, Tucker MA. Recent trends in incidence of cutaneous melanoma among US Caucasian young adults. J Invest Dermatol. 2008;128:2905–2908. doi: 10.1038/jid.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow RB. Cyclobutane-type pyrimidine dimers in polynucleotides. Science. 1966;153:379–386. doi: 10.1126/science.153.3734.379. [DOI] [PubMed] [Google Scholar]
- Setlow RB, Grist E, Thompson K, Woodhead AD. Wavelengths effective in induction of malignant melanoma. Proc Natl Acad Sci USA. 1993;90:6666–6670. doi: 10.1073/pnas.90.14.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JD, Peles DN. The red and the black. Acc Chem Res. 2010;43:1452–1460. doi: 10.1021/ar100079y. [DOI] [PubMed] [Google Scholar]
- Tan EM, Freeman RG, Stoughton RB. Action spectrum of ultraviolet light-induced damage to nuclear DNA in vivo. J Invest Dermatol. 1970;55:439–443. doi: 10.1111/1523-1747.ep12260585. [DOI] [PubMed] [Google Scholar]
- Thiele JJ, Traber MG, Packer L. Depletion of human stratum corneum vitamin E: an early and sensitive in vivo marker of UV induced photo-oxidation. J Invest Dermatol. 1998;110:756–761. doi: 10.1046/j.1523-1747.1998.00169.x. [DOI] [PubMed] [Google Scholar]
- Whitmore SE, Morison WL, Potten CS, Chadwick CA. Tanning salon exposure and molecular alterations. J Amer Acad Dermatol. 2001;44:775–780. doi: 10.1067/mjd.2001.112581. [DOI] [PubMed] [Google Scholar]
- Wolbarsht ML, Landers MB, III, Stefansson E. Vasodilation and the etiology of diabetic retinopathy: a new model. Ophthalmic Surg. 1981;12:104–107. [PubMed] [Google Scholar]
- Wolber R, Schlenz K, Wakamatsu K, Smuda C, Nakanishi Y, Hearing VJ, Ito S. Pigmentation effects of solar-simulated radiation as compared with UVA and UVB radiation. Pigment Cell Melanoma Res. 2008;21:487–491. doi: 10.1111/j.1755-148X.2008.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SR, Berwick M, Ley RD, Walter RB, Setlow RB, Timmins GS. UV causation of melanoma in Xiphophorus is dominated by melanin photosensitized oxidant production. Proc Natl Acad Sci U S A. 2006;103:4111–4115. doi: 10.1073/pnas.0511248103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S, Sands J. Sun and the eye: prevention and detection of light-induced disease. Clin Dermatol. 1998;16:477–485. doi: 10.1016/s0738-081x(98)00021-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


