Abstract
Juvenile hormone III (JH) is synthesized by the corpora allata (CA) and plays a key role in mosquito development and reproduction. A decrease in JH titer during the last instar larvae allows pupation and metamorphosis to proceed. As the anti-metamorphic role of JH comes to an end, the CA of the late pupa once again synthesizes JH, which plays an essential role in orchestrating reproductive maturation. In spite of the importance of Aedes aegypti as a vector, a detailed study of the changes of JH hemolymph titers during the gonotrophic cycle has never been performed. In the present studies, using a High Performance Liquid Chromatography coupled to a Fluorescent Detector (HPLC-FD) method, we measured changes in JH levels in the hemolymph of female mosquitoes during the pupal and adult stages. Our results revealed tightly concomitant changes in JH biosynthesis and JH hemolymph titers during the gonotrophic cycle of female mosquito. Feeding high sugar diets resulted in an increase of JH titers, and mating also modified JH titers in hemolymph. In addition these studies confirmed that JH titer in mosquitoes is fundamentally determined by the rate of biosynthesis in the CA.
Keywords: Juvenile hormone, mosquito, hemolymph, biosynthesis, corpora allata
1. Introduction
Aedes aegypti is an important vector of viral diseases such as yellow fever, dengue fever and Chikungunya (Chen and Vasilakis, 2011; Burt et al., 2012). This cosmopolitan mosquito is also an excellent model organism for the study of the endocrine regulation of reproduction (Klowden, 1997; Raikhel et al., 2002). Juvenile hormones (JHs) play a key role in insect development and reproduction (Riddiford, 2012; Goodman and Cusson, 2012). JH delays metamorphosis of immature insects until they have reached an appropriate stage and size. Then, during the final larval instar, a decline in JH secretion permits a metamorphic molt (Smykal et al., 2014). JHs are synthesized by the corpora allata (CA), a pair of endocrine glands with neural connections to the brain (Tobe and Stay, 1985). In A. aegypti the CA is inactive for most of the duration of the pupal stage (Nouzova et al., 2011; Rivera-Perez et al, 2014). As the anti-metamorphic role of JH comes to an end, the CA of the late pupa (or pharate adult) is reactivated and starts synthesizing JH, which would now play an essential role orchestrating reproductive maturation (Klowden, 1997).
After adult eclosion, female mosquitoes must mature before they can produce eggs in response to a blood meal. Adult female A. aegypti shows dynamic changes in JH biosynthesis that are linked to the three major stages of ovary development: previtellogenesis, the ovarian resting stage and the vitellogenesis period (Klowden, 1997; Noriega, 2004; Rivera-Perez et al., 2014). JH-dependent ovarian previtellogenic maturation involves changes in primary follicles, nurse cells and follicular epithelium (Gwadz and Spielman, 1973; Raikhel and Lea, 1983, 1991). Almost 40 years ago, JH levels were measured by coupled gas chromatography-mass spectrometry in whole body extracts of female A. aegypti (Shapiro et al., 1986). The amount of JH in whole body extracts rose over the first 2 days after emergence from 0.7 to 7.5 ng/g, and then slowly fell over the next 5 days in females not given a blood meal. In females fed blood, whole body JH levels fell during the first 3 h to 2.3 ng/g. The rate of decline then slowed so that levels had reached their lowest point (0.4 ng/g) by 24 h after the blood meal. By 48 h, levels started to rise again until 96 h when they were equivalent to pre-blood meal levels. In spite of the importance of A. aegypti as a vector and a research model, a detailed analysis of JH hemolymph titers during the gonotrophic cycle has never been performed.
In the present studies, using a High Performance Liquid Chromatography coupled to a Fluorescent Detector (HPLC-FD) method, we measured changes in JH levels in the hemolymph of female mosquitoes during the pupal and adult stages. Our results revealed tightly concomitant changes in JH biosynthesis and JH hemolymph titers during the gonotrophic cycle of female mosquito. Feeding high sugar diets resulted in an increase of JH titers, and mating also modified JH titers in hemolymph. These studies also confirmed that JH titer in mosquitoes is fundamentally determined by the rate of biosynthesis in the CA.
2. Materials and methods
2.1. Insects
A. aegypti of the Rockefeller strain were reared at 28 °C and 80% humidity as previously described (Nouzova et al., 2011). Female pupae were isolated, and unmated females were kept separately from males since adult emergence. Mated females were obtained by mixing them with males immediately after emergence in a 1 female: 2 male ratio. Adult mosquitos were offered a cotton pad soaked in a 3% or 20% sucrose solution. Four-day-old female mosquitoes were fed pig blood equilibrated to 37 °C, and ATP was added to the blood meal to a final concentration of 1mM immediately before use as previously described (Nouzova et al., 2011).
2.2. Hemolymph collection
Hemolymph of adult mosquitoes was obtained by perfusion (Hernandez et al., 1999). Fine needles were made from 100-μl micro-glass capillary tubes using a pipette puller P-30 (Sutter Instrument, Novato, CA) and mounted in a pipette pump (Drummond, Broomall, PA). Needles were inserted manually through the neck membrane into the thoracic cavity, and insects were perfused with 20 μl of a “bleeding solution” of phosphate-buffered saline (PBS) (100 mM NaCl, 25 mM NaHCO3, pH 7.2) containing a protease inhibitor cocktail (1 mM phenylmethylsulfonyl fluoride, 1 mM ethylenediaminetetraacetic acid, 0.2 mM Na-p-tosyl-L-lysine chloromethyl ketone and,1 mM leupeptine). The hemolymph was obtained from a small tear made laterally on the intersegmentary membrane of the last abdominal segment (Supplemental figure 1). The first drop of perfused hemolymph was collected directly on a glass silanized tube (Thermo Scientific) placed on ice. Larvae and pupae were briefly washed with 70% ethanol and air-dried, and hemolymph was obtained by capillary action using a 10 μl micropipette tip containing 2 μl of bleeding solution that was introduced into a small tear made laterally on the intersegmentary membrane between the 4th and 5th abdominal segments.
Immediately after hemolymph was collected 100 μl of PBS containing the protease inhibitor cocktail were added to the tube. For each data point at least four independent samples of hemolymph were collected from pools of five insects each.
2.3. JH and methyl farnesoate quantification
JH and methyl farnesoate (MF) quantifications by high performance liquid chromatography coupled to a fluorescent detector (HPLC-FD) were done as previously described by Rivera-Perez et al. (2012; 2014).
2.4. Dissections of corpora allata complexes and JH biosynthesis assay
Adult female mosquitos were cold-anesthetized and brain-corpora allata–corpora cardiaca complexes (BR-CA-CC) were dissected and incubated at 32 °C for 4 h in 150 μl of tissue culture media M-199 (Gibco, Grand Island, NY, USA) containing 2% Ficoll, 25 mM HEPES (pH 6.5) and methionine (50 μM) (Supplemental fig. 2). Biosynthesized JH was labelled with a fluorescent tag and analyzed by reverse phase high performance liquid chromatography coupled to a fluorescent detector (HPLC-FD) as previously described (Rivera-Perez et al., 2012).
2.5. Statistical analysis
Statistical analyses were performed using the GraphPad Prism Software (San Diego, CA, USA). The results are expressed as means ± S.E.M. Significant differences (p< 0.01) were determined with a one tailed students t-test performed in a pair wise manner or by one-way ANOVA followed by Tukey’s test or Fischer’s Least significant difference test.
3. Results
3.1 Efficiency of the hemolymph’s collection protocol
We developed a simple protocol to collect adult mosquito hemolymph in reproducible conditions. The injection of 20 μl of bleeding solution generated always a pendant drop of the same volume that detached itself by gravity and fell into the collecting tube. This protocol assured the reproducible recovery of single drops of similar volumes. Nevertheless, the efficiency of our hemolymph collecting protocol was investigated by doing two consecutive identical bleedings of the same insects; followed by the analysis of the JH III recovered in each of the bleedings. Recovery in the first bleeding was over 85% when JH titers were high, and close to 100% when JH titers were low (Supplemental figure 3).
3.2 JH hemolymph titers in pupae and adult female A. aegypti
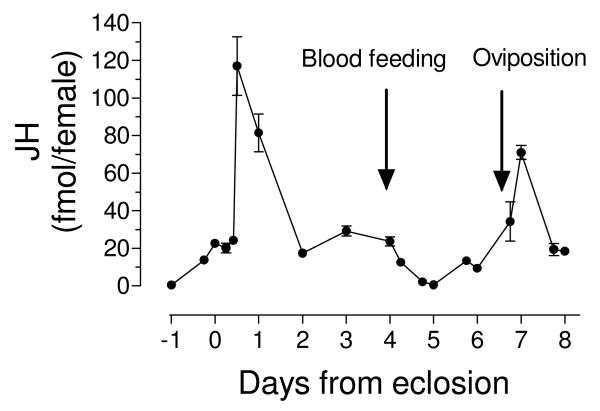
The changes in the titers of JH detected in hemolymph extracts of female A. aegypti before and after adult emergence, as well as following a blood meal are presented in Fig. 1 and Supplemental table 1. Titers were undetectable in early pupae and significantly increased by 6 h before adult eclosion. They remained relatively constant with titers around 20 fmol/female during the first 10 h in newly eclosed adults, but had a quick five-fold increase in the period between 10 to 12 h after adult eclosion (117 fmol/female). Titers were back at a 20 fmol/female level by 2 days after adult eclosion, and remained constant until females were blood fed 4 days after adult eclosion. JH titers decreased rapidly after blood feeding, and reached its lowest levels (0.6 fmol/female) 24 h after a blood meal. Titers increased by 48 h and peaked again three days after a blood meal to the highest titer of over 70 fmol/female, decreasing again to a 20 fmol/female level by 4 days after a blood meal.
Fig. 1. JH hemolymph titers in pupae and adult mated female A. aegypti.
X axis: Days represent times before (pupa) and after adult eclosion. Females were blood fed 4 days after eclosion (Blood feeding). Y axis: JH titers expressed as fmol/female. Each data point represents the mean ± SEM of at least four independent replicates of groups of 5 females.
3.3. Coordinated changes in JH biosynthesis and JH hemolymph titers
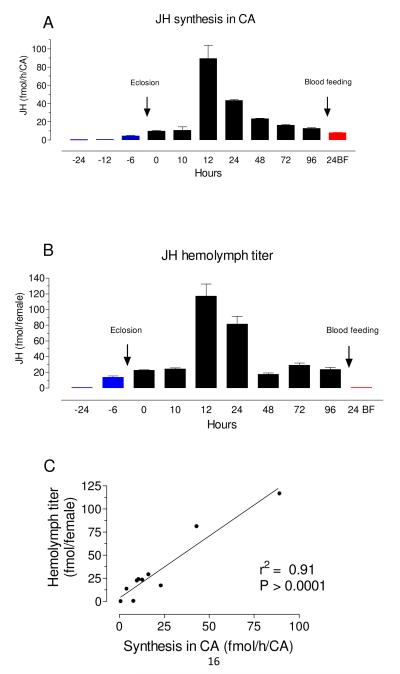
We observed a highly significant positive correlation (r2 = 0.91, P> 0.0001) when changes in JH hemolymph titers were compared with previously measured changes in JH biosynthesis rates (Rivera-Perez et al., 2014) (Fig. 2). This correlation was confirmed by measuring JH titers and JH synthesis rates from the same insects; hemolymph was collected from 3% sugar-fed females with high titers (12 h after emergence) and lower titers (72 h after emergence), and JH biosynthesis was evaluated from CA dissected from the same insects (Supplemental figure 4). Hemolymph JH titers and JH biosynthesis rates were both about 10 fold higher in insects analyzed 12 h after adult eclosion when compared with insects analyzed 72 h after eclosion, underscoring again the correlation between the biosynthetic activity of the CA and the titers of JH in hemolymph.
Fig. 2. Coordinated changes in JH biosynthesis and JH hemolymph titers.
A) JH biosynthesis by CA dissected from pupa, sugar-fed and blood-fed adult females are from Rivera-Perez et al., 2014. X axis: Hours represent times before (blue = pupa) and after adult eclosion (black = sugar-fed), or after blood feeding (red = BF). Y axis: JH biosynthesis expressed as fmol/h/CA. Bars represent the means ± SEM of three independent replicates of three groups of 3 CA. B) JH hemolymph titers from pupa, sugar-fed and blood-fed adult females. X axis is as in A. Y axis: JH titers expressed as fmol/insect. Bars represent the means ± SEM of at least four independent replicates of groups of 5 females. C) Relationship between JH biosynthesis and JH hemolymph titers from pupa, sugar-fed and blood-fed adult females.
3.4. Sugar feeding and mating affect JH hemolymph titers
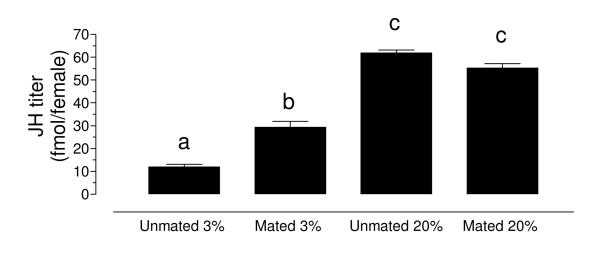
To test the effect of a high and low sugar diet, as well as the effect of mating on JH titers, unmated and mated females were fed with a 3% or a 20% sucrose solution, and JH hemolymph titers were evaluated 3 days after emergence (Fig. 3). Feeding high sugar diets resulted in a very significant increase in JH titers in both virgin and mated females (2-3 fold). Females raised on a high sugar diet (20%) also synthesized more JH that females fed a low sugar diet (3%) (Supplemental figure 5). Mating also increased JH titers in hemolymph of nutritionally stressed females (3%), but had no significant effect on the titers of well-nourished females (20%).
Fig. 3. Sugar feeding and mating affect JH hemolymph titers.
Unmated and mated females were fed a 3% or a 20% sucrose solution, and JH hemolymph titers were evaluated 3 days after emergence. JH titers are expressed as fmol/female. Bars represent the means ± SEM of at least four independent replicates of groups of 5 females. Significant differences (p<0.01) were determined with one-way ANOVA followed by Tukey’s test.
3.5. Methyl farnesoate is abundant in hemolymph of larvae, but undetectable in hemolymph of adults
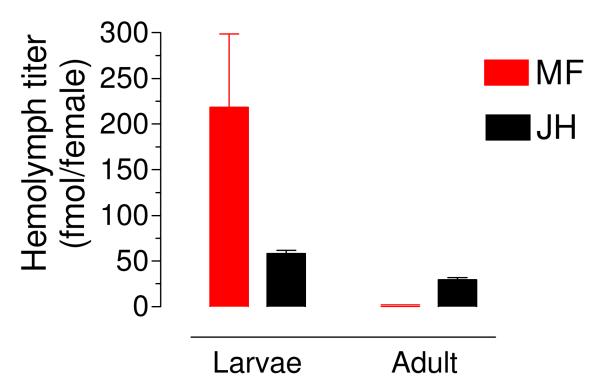
MF has been previously described as a circulating hormone in several insect species. The hemolymph of female fourth instar larvae had over 200 fmol of methyl farnesoate (MF) per insect; on the other hand MF was undetectable in the hemolymph of adult females (Fig. 4). On the contrary, JH III was readily detectable in the hemolymph of larvae and adult female mosquitoes.
Fig. 4. Methyl farnesoate is abundant in hemolymph of larvae, but undetectable in hemolymph of adult.
Larvae: hemolymph from female fourth instar larvae. Adult: hemolymph from a pool of sugar-fed mated females. MF: methyl farnesoate. JH: juvenile hormone. Values are expressed as fmol per insect. Bars represent the means ± SEM of at least four independent replicates of groups of 5 females.
4. Discussion
4.1. Coordinated changes in JH biosynthesis and JH hemolymph titers in female mosquitoes
JH titer is regulated by the balance between biosynthesis and release of the hormone from the CA and its degradation and clearance from the hemolymph by tissue uptake and excretion (Feyereisen, 1985; Goodman and Couson, 2012). Numerous studies indicate that JH biosynthesis is a major regulator of JH titer; it is also widely accepted that JH is not stored in the CA and therefore the amount of JH “released” to the incubation medium or hemolymph represents the amount of JH synthesized (Feyereisen, 1985). JH biosynthesis has been comprehensively studied in A. aegypti (reviewed in Noriega, 2014); conversely, there is a single “classical” study that measured JH titers in whole body extracts of female mosquito by coupled gas chromatography-mass spectrometry (Shapiro et al., 1986). The changes of JH in whole body extracts have an overall match with the profile of changes in JH biosynthesis in sugar and blood fed females (Li et al., 2003; Rivera-Perez et al., 2014). Titers in whole body are low in pupae, increase after adult emergence and peak during the first two days of adult life, to slowly decrease after that in sugar-fed insects. Blood feeding results in a significant decrease in JH biosynthesis (Li et al., 2003; Rivera-Perez et al., 2014) and JH whole body titers (Shapiro et al., 1986).
Our current studies revealed in more detail the concordance between JH biosynthesis and hemolymph JH titers. They also underscore the dynamic character of circulating titers. During a gonotrophic cycle, JH hemolymph levels sharply increased or decreased; fluctuating between 120 fmol/female and 0.5 fmol/female. The overall profile of changes in the first gonotrophic cycle (from adult eclosion to day 4) was similar to that observed in the second gonotrophic cycle (from day 6 to day 8); although the “peak” of JH titer seems to be larger in the first cycle; something similar was observed when JH synthesis was compared in the first two gonotrophic cycles (Li et al., 2003; Nouzova et al., 2011); emphasizing again the correlation between the biosynthetic activity of the CA and the titers of JH in hemolymph. Positive correlations between hemolymph JH titers and the in vitro rates of JH biosynthesis have been previously reported in numerous insects, such as the grasshopper Locusta migratoria (Couillaud et al., 1985), the crickets Gryllus binaculatus (Klein et al., 1993) and G. firmus (Zhao and Zera, 2004), and the cockroaches Nauphoeta cinerea (Lanzrein et al., 1975) and Blatella germanica (Treiblmayr et al., 2006).
MF is the only sesquiterpenoid identified in the hemolymph of crustaceans (Laufer and Biggers, 2001), where it might play the role of a JH. The potential role of MF as a “JH” in insect preimaginal stages is a controversial issue that is just starting to be addressed. MF has JH activity in the Drosophila white puparial bioassays (Harshman et al., 2010; Jones et al., 2010), and it is abundant in the hemolymph of several insects (Teal et al., 2014). If MF is a true JH hormone in insects still remains to be proved. A direct effect of MF activating the JH receptor has been shown in Bombyx mori (Kayukawa et al (2012). MF is the immediate biosynthetic precursor of JH III in mosquitoes, and therefore is very abundant in CA extracts (Rivera-Perez et al., 2014). On the other hand we have never detected the release of MF by the CA of adult A. aegypti. Coincidently, while MF was very abundant in the hemolymph of mosquito larvae, it was undetectable in the hemolymph of adult female mosquito.
4.2. Changes in hemolymph titers reveal the diverse roles of JH orchestrating reproductive maturation
Three major periods can be defined in the development of the ovaries during a gonotrophic cycle in A. aegypti mosquitoes: previtellogenesis, resting stage and vitellogenesis (Klowden, 1997). Females emerge with 40 μm immature follicles that grow into 100 μm mature previtellogenic oocytes in the next 24-48 h. Oocytes remain in a dynamic “state of arrest”, and will enter vitellogenesis only after a blood meal (Hagedorn et al., 1977; Klowden, 1997). JH directly controls nutrient allocation into the ovaries in the previtellogenic phases, and indirectly influences the fate of vitellogenic follicles after a blood meal (Clifton and Noriega, 2011; Clifton and Noriega, 2012).
We detected a significant increase in JH titers in newly eclosed females, which was followed by a major peak 12 h later. The activation of JH synthesis and the rise of hemolymph titers after adult eclosion occurs in 2 steps (Rivera-Perez et al., 2014). First there is a developmentally-regulated 2-fold increase in JH synthesis at eclosion that brings JH synthetic rates to a value of 10-15 fmol/h and hemolymph titers to levels of 20-25 fmol/female. A major increase in synthesis and titers follows around 12 h post-ecdysis, but only if teneral nutrients are above a particular threshold (Caroci et al., 2004; Rivera et al., 2014). Decapitation during these first 12 h of imaginal life precludes this nutritionally-dependent second increase of JH synthesis, suggesting that the brain plays a key role sensing the nutritional status and stimulating CA to its maximum activity (Hernandez-Martinez et al., 2007). Only when reserves are appropriate the brain directs the CA to synthesize enough JH to activate previtellogenic reproductive maturation (Caroci et al., 2004; Hagedorn et al., 1977). The two-step activation guarantees that a proper rise of JH titers concurs with adult eclosion to induce expression of a number of JH-dependent genes, without allocating resources to reproduction until the nutritional status is properly assessed.
When mosquitoes were fed a low sugar diet (3%) for three days, JH hemolymph titers were significantly reduced when compared with females raised on a high sugar meal (20%). It has been previously described that during the ovarian resting stage, female mosquitoes are capable of a fine-tuning of JH synthesis at rates that are proportional to the supply of acetyl units acquired with a sugar-meal (Perez-Hedo et al., 2014). Starvation reduces JH synthesis via a decrease in insulin signaling in the CA (Perez-Hedo et al., 2013). Besides nutrients, mating also causes profound changes to the female mosquito’s physiology and behavior. Mating enhances egg development (Klowden and Chambers, 1991), and part of this effect is mediated through the transfer of JH III during copulation (Clifton et al., 2014). In our studies, mating also increased JH titers in hemolymph of nutritionally stressed females, but had no significant effect on the titers of well-nourished females. Understanding the complex interactions between modulatory factors that control JH biosynthesis, such as diet and mating, is a topic of future research.
The lowest JH hemolymph titers in adult female mosquitoes were detected 24 h after ingesting a blood-meal. Blood feeding results in an active suppression of JH synthesis with significant decreases in enzyme transcripts and activities (Li et al., 2003; Nouzova et al., 2011; Rivera-Perez et al., 2014). A reduction of JH titers during the vitellogenic stage is critical for the appropriate completion of oogenesis in mosquitoes (Patterson, 1974; Shapiro et al., 1986). After blood feeding a major reduction in JH synthesis, together with the previously described remarkable increase in JH esterase activity in hemolymph (Shapiro et al., 1986), assured a quick and effective decrease in JH hemolymph titers to almost undetectable levels.
In summary, our results revealed tightly correlated changes in JH biosynthesis and JH hemolymph titers during the gonotrophic cycle of female mosquito. Our studies revealed that changes in the physiological state of the mosquitoes caused by sugar-feeding and mating modified JH titers in hemolymph. In addition, our analysis confirmed that the JH titer in mosquitoes is primarily determined by the rate of biosynthesis in the CA.
Supplementary Material
JH III levels were measured in the hemolymph of pupae and adult female mosquitoes.
There were coordinated changes in JH biosynthesis and JH hemolymph titers
Feeding high sugar diets resulted in an increase of JH titers.
Mating also modified JH titers in hemolymph.
MF was detected in the hemolymph of larvae but not in adult female mosquito.
ACKNOWLEDGMENTS
We thank Professor Matthew Degennaro for critical reading of the manuscript. This work was supported by NIH Grant No AI 45545 to F.G.N.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. Chikungunya: a re-emerging virus. Lancet. 2012;379:662–671. doi: 10.1016/S0140-6736(11)60281-X. [DOI] [PubMed] [Google Scholar]
- Caroci A, Li Y, Noriega FG. Reduced juvenile hormone synthesis in mosquitoes with low teneral reserves prevents ovarian previtellogenic development in Aedes aegypti. Journal of Experimental Biology. 2004;207:2685–2690. doi: 10.1242/jeb.01093. [DOI] [PubMed] [Google Scholar]
- Couillaud F, Mauchamp B, Girardie A. Regulation of juvenile hormone titer in the African locust. Experientia. 1985;41:1165–1167. [Google Scholar]
- Chen R, Vasilakis N. Dengue — Quo tu et quo vadis? Viruses. 2011;3:1562–1608. doi: 10.3390/v3091562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton ME, Noriega FG. Nutrient limitation results in juvenile hormone-mediated resorption of previtellogenic ovarian follicles in mosquitoes. Journal of Insect Physiology. 2011;57:1274–1281. doi: 10.1016/j.jinsphys.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton ME, Noriega FG. The fate of follicles after a blood meal is dependent on previtellogenic nutrition and juvenile hormone in Aedes aegypti. Journal of Insect Physiology. 2012;58:1007–1019. doi: 10.1016/j.jinsphys.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton ME, Correa S, Rivera-Perez C, Nouzova M, Noriega FG. Male Aedes aegypti mosquitoes use JH III transferred during copulation to influence previtellogenic ovary physiology and affect the reproductive output of female mosquitoes. Journal of Insect Physiology. 2014;64:40–47. doi: 10.1016/j.jinsphys.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyereisen R. Regulation of juvenile hormone titer: synthesis. In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology Biochemistry and Pharmacology. Vol. 7. Pergamon Press; Oxford: 1985. pp. 391–430. [Google Scholar]
- Goodman WG, Cusson M. The Juvenile Hormones. In: Gilbert LI, editor. Insect Endocrinology. Academic Press; San Diego: 2012. pp. 310–365. [Google Scholar]
- Gwadz RW, Spielman A. Corpus allatum control of ovarian development in Aedes aegypti. Journal of Insect Physiology. 1973;19:1441–1448. doi: 10.1016/0022-1910(73)90174-1. [DOI] [PubMed] [Google Scholar]
- Hagedorn HH, Turner S, Hagedorn EA, Pontecorvo D, Greenbaum P, Pfeiffer D, Flanagan TR. Post-emergence growth of the ovarian follicles of Aedes aegypti. Journal of Insect Physiology. 1977;23:203–206. doi: 10.1016/0022-1910(77)90030-0. [DOI] [PubMed] [Google Scholar]
- Harshman LG, Song KD, Casas J, Schuurmans A, Kuwano E, Kachmane SD, Riddiford LM, Hammock BD. Bioassays of compounds with potential juvenoid activity on Drosophila melanogaster: Juvenile hormone III, bisepoxide juvenile hormone III and methyl farnesoates. J Insect Physiol. 2010;56:1465–1470. doi: 10.1016/j.jinsphys.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez S, Lanz H, Rodriguez MH, Torres JA, Martinez-Palomo A, Tsutsumi V. Morphological and cytochemical characterization of female Anopheles albimanus (Diptera: Culicidae) hemocytes. Journal of Medical Entomology. 1999;36:426–434. doi: 10.1093/jmedent/36.4.426. [DOI] [PubMed] [Google Scholar]
- Hernandez-Martinez S, Mayoral JG, Li Y, Noriega FG. Role of juvenile hormone and allatotropin on nutrient allocation, ovarian development and survivorship in mosquitoes. Journal of Insect Physiology. 2007;53:230–234. doi: 10.1016/j.jinsphys.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G, Jones D, Li X, Tang L, Ye L, Teal P, Riddifordd L, Sandifera C, Borovsky D, Marting J-R. Activities of natural methyl farnesoids on pupariation and metamorphosis of Drosophila melanogaster. J Insect Physiol. 2010;56:1456–1464. doi: 10.1016/j.jinsphys.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Kayukawa T, Minakuchi C, Namiki T, Togawa T, Yoshiyama M, Kamimuraa M, Mitaa K, Imanishia S, Kiuchia M, Ishikawab Y, Shinoda T. Transcriptional regulation of juvenile hormone-mediated induction of Krüppel homolog 1, a repressor of insect metamorphosis. Proc Natl Acad Sci USA. 2012;109:11729–11734. doi: 10.1073/pnas.1204951109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein PM, Lorenz MW, Donglin H, Hoffmann K. Age dependency and regulatory properties of juvenile hormone III biosynthesis in adult male crickets, Gryllus bimaculatus. Journal of Insect Physiology. 1993;39:315–324. [Google Scholar]
- Klowden MJ, Chambers GM. Male accessory gland substances activate egg development in nutritionally stressed Aedes aegypti mosquitoes. Journal of Insect Physiology. 1991;37:721–726. [Google Scholar]
- Klowden MJ. Endocrine aspects of mosquito reproduction. Archives of Insect Biochemistry and Physiology. 1997;35:491–512. [Google Scholar]
- Lanzrein B, Hasimoto M, Parmakovich V, Nakanishi K, Wilhelm R, Luscher M. Identification and quantification of juvenile hormones from different developmental stages of the cockroach Nauphoeta cinerea. Life Sciences. 1975;16:1271–1284. doi: 10.1016/0024-3205(75)90312-4. [DOI] [PubMed] [Google Scholar]
- Laufer H, Biggers WJ. Unifying concepts learned from methyl farnesoate for invertebrate reproduction and post-embryonic development. Integrative Comparative Biology. 2001;41:442–457. [Google Scholar]
- Li Y, Hernandez-Martinez S, Unnithan GC, Feyereisen R, Noriega FG. Activity of the corpora allata of adult female Aedes aegypti: effects of mating and feeding. Insect Biochemistry and Molecular Biology. 2003;33:1307–1315. doi: 10.1016/j.ibmb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Noriega FG. Nutritional regulation of JH synthesis: a mechanism to control reproductive maturation in mosquitoes? Insect Biochemistry and Molecular Biology. 2004;34:687–693. doi: 10.1016/j.ibmb.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Noriega FG. Juvenile hormone biosynthesis in insects: What is new, what do we know, what questions remain? ISRN. 2014 doi: 10.1155/2014/967361. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouzova M, Edwards MJ, Mayoral JG, Noriega FG. A coordinated expression of biosynthetic enzymes controls the flux of juvenile hormone precursors in the corpora allata of mosquitoes. Insect Biochemistry and Molecular Biology. 2011;41:660–669. doi: 10.1016/j.ibmb.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JW. A comparison of the morphogenetic and sterilizing activities of juvenile hormone mimics on Aedes aegypti. Journal of Insect Physiology. 1974;20:2095–2106. doi: 10.1016/0022-1910(74)90036-5. [DOI] [PubMed] [Google Scholar]
- Pérez-Hedo M, Rivera-Perez C, Noriega FG. The insulin/TOR signal transduction pathway is involved in the nutritional regulation of juvenile hormone synthesis in Aedes aegypti. Insect Biochemistry Molecular Biology. 2013;43:495–500. doi: 10.1016/j.ibmb.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Hedo M, Rivera-Perez C, Noriega FG. Starvation increases insulin sensitivity and reduces juvenile hormone synthesis in mosquitoes. PLoS ONE. 2014;9:e86183. doi: 10.1371/journal.pone.0086183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raikhel AS, Lea AO. Previtellogenic development and vitellogenin synthesis in the fat body of a mosquito: An ultrastructural and immunocytochemical study. Tissue Cell. 1983;15:281–299. doi: 10.1016/0040-8166(83)90023-x. [DOI] [PubMed] [Google Scholar]
- Raikhel AS, Lea AO. Control of follicular epithelium development and vitelline envelope formation in the mosquito; role of juvenile hormone and 20-hydroxyecdysone. Tissue and Cell. 1991;23:577–591. doi: 10.1016/0040-8166(91)90015-l. [DOI] [PubMed] [Google Scholar]
- Raikhel AS, Kokoza VA, Zhu J, Martin D, Wang SF, Li C, Sun G, Ahmed A, Dittmer N, Attardo G. Molecular biology of mosquito vitellogenesis: from basic studies to genetic engineering of antipathogen immunity. Insect Biochemistry and Molecular Biology. 2002;32:1275–1286. doi: 10.1016/s0965-1748(02)00090-5. [DOI] [PubMed] [Google Scholar]
- Riddiford LM. How does juvenile hormone control insect metamorphosis and reproduction? General and Comparative Endocrinology. 2012;179:477–484. doi: 10.1016/j.ygcen.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Rivera-Perez C, Nouzova M, Noriega FG. A quantitative assay for the juvenile hormones and their precursors using fluorescent tags. PLoS ONE. 2012;7(8):e43784. doi: 10.1371/journal.pone.0043784. doi:10.1371/journal.pone.0043784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Perez C, Nouzova M, Lamboglia I, Noriega FG. Metabolic analysis reveals changes in the mevalonate and juvenile hormone synthesis pathways linked to the mosquito reproductive physiology. Insect Biochemistry and Molecular Biology. 2014;51:1–9. doi: 10.1016/j.ibmb.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AB, Wheelock GD, Hagedorn HH, Baker FC, Tsai LW, Schooley DA. Juvenile hormone and juvenile hormone esterase in adult females of the mosquito Aedes aegypti. Journal of Insect Physiology. 1986;32:867–877. [Google Scholar]
- Smykal V, Daimon T, Kayukawa T, Takaki K, Shinoda T, Jindra M. Importance of juvenile hormone signaling arises with competence of insect larvae to metamorphosis. Developmental Biology. 2014;390:221–230. doi: 10.1016/j.ydbio.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Teal PE, Jones D, Jones G, Torto B, Nyasembe V, Borgemeister C, Alborn HT, Kaplan F, Boucias D, Lietze VU. Identification of methyl farnesoate from the hemolymph of insects. Journal of Natural Products. 2014;77:402–405. doi: 10.1021/np400807v. [DOI] [PubMed] [Google Scholar]
- Tobe SS, Stay B. Structure and regulation of the corpus allatum. Advances in Insect Physiology. 1985;18:305–431. [Google Scholar]
- Treiblmayr K, Pascual N, Piulachs M-D, Keller T, Belles X. Juvenile hormone titer versus juvenile hormone synthesis in female nymph and adults of the German cockroach, Blatella germanica. Journal of Insect Science. 2006;6:1–7. doi: 10.1673/031.006.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Zera AJ. A morph-specific daily cycle in the rate of JH biosynthesis underlies a morph-specific daily cycle in the hemolymph JH titer in a wing-polymorphic cricket. Journal of Insect Physiology. 2004;50:965–973. doi: 10.1016/j.jinsphys.2004.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.