Abstract
Background & Aims
Non-alcoholic fatty liver disease (NAFLD) is a risk factor for hepatocellular carcinoma (HCC). However, no systemic studies from the United States have examined temporal trends, HCC surveillance practices, and outcomes of NAFLD-related HCC.
Methods
We identified a national cohort of 1500 patients who developed HCC from 2005 through 2010 from Veterans Administration (VA) hospitals. We reviewed patients’ full VA medical records; NAFLD was diagnosed based on histologic evidence for, or the presence of, metabolic syndrome in the absence of hepatitis C virus (HCV) infection, hepatitis B, or alcoholic liver disease. We compared annual prevalence values for the main risk factors (NAFLD, alcohol abuse, HCV), as well HCC surveillance and outcomes, among HCC patients.
Results
NAFLD was the underlying risk factor for HCC in 120 patients (8.0%); the annual proportion of NAFLD-related HCC remained relatively stable (7.5%–12.0%). In contrast, the proportion of HCC cases associated with HCV increased from 61.0% in 2005 (95% confidence interval, 53.1%–68.9%) to 74.9% in 2010 (95% confidence interval, 69.0%–80.7%). The proportion of HCC cases associated with only alcohol abuse decreased from 21.9% in 2005 to 15.7% in 2010, and the annual proportion of HCC cases associated with hepatitis B remained relatively stable (1.4%–3.5%). A significantly lower proportion of patients with NAFLD-related HCC had cirrhosis (58.3%) compared to patients with alcohol- or HCV-related HCC (72.4% and 85.6%, respectively; P<.05). A significantly higher percentage of patients with NAFLD-related HCC did not receive HCC surveillance in the 3 years before their HCC diagnosis, compared to patients with alcohol- or HCV-associated HCC. A lower proportion of patients with NAFLD-related HCC received HCC-specific treatment (61.5%) than of patients with HCV-related HCC (77.5%; P<.01). However, 1-year survival did not differ among patients with HCC related to different risk factors.
Conclusions
NAFLD is the third most common risk factor for HCC in the VA. The proportion of NAFLD-related HCC was relatively stable from 2005 through 2010. Although patients with NAFLD-related HCC receive less HCC surveillance and treatment, a similar proportion survive for 1 year, compared to patients with alcohol- or HCV-related HCC.
Keywords: Liver cancer, NASH, incidence, time
INTRODUCTION
The incidence of hepatocellular carcinoma (HCC) is increasing in the United States (U.S.).1, 2 Most of this increase has been attributed to the aging of individuals infected with hepatitis C (HCV) in the 1960s and 1970s.3 However, non-alcoholic fatty liver disease (NAFLD) has become the most common cause of chronic liver disease in the U.S.(4) It is estimated that 10–46% of individuals in the U.S. have NAFLD, 3–5% may have non-alcoholic steatohepatitis (NASH).5, 6 NAFLD and NASH is also likely contributing to the burden of advanced liver disease. Indeed, most patients with cryptogenic cirrhosis are now thought to have NAFLD or NASH.7, 8 In 2010, NASH was the fourth most common cause of liver transplantation in the U.S.9 A recent systematic review showed that NAFLD/NASH related-cirrhosis is associated with higher risk of HCC, although the risk was reportedly lower than that related to HCV cirrhosis.10 Given the sheer number of patients with NAFLD/NASH, it is possible that even a small risk of HCC may translate into a large number of HCC patients. However the burden of NAFLD-related HCC is not well defined.
In addition to the unclear contribution of NAFLD to the current burden of HCC in the U.S., the clinical and prognostic features of NAFLD-related HCC are also only emerging. Available data suggest that patients with HCC due to NAFLD are older, have less aggressive tumors and are less likely to be diagnosed by surveillance compared to HCC due to viral hepatitis. However, most of this information is based on reports from referral centers and do not represent community practice.11–13
Using data obtained from the national Veterans Health Administration (VA) system, we estimated the prevalence of HCC attributable to NAFLD, alcohol abuse and HCV in a representative sample of 1,500 patients who were diagnosed with HCC during fiscal years 2005–2010. We also compared receipt of surveillance prior to HCC diagnosis, the stage of HCC at diagnosis, and subsequent outcomes (receipt of HCC treatment and overall survival) in patients with NAFLD-related HCC compared to patients with HCC from other etiologies.
METHODS
Data sources
Data were obtained from VA administrative data files and review of patient electronic medical records (EMR). Administrative data included the Medical SAS (MedSAS) Outpatient and Inpatient files, and the VA Vital Status File. The MedSAS files contain patient demographic data as well as diagnoses according to International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) and procedures according to Common Procedural Terminology (CPT) codes. We determined date of death, if any, in the Vital Status File that uses an algorithm to select the “best” date of death using the VA MedSAS Inpatient file, Beneficiary Identification & Records Locator System Death File, Medicare Vital Status file, and Social Security Administration death file.14 Patient EMR information was obtained by accessing the Compensation and Pension Records Interchange (CAPRI), which is a VA application that provides access to the EMR found in the Computerized Patient Record System (CPRS) at any VA facility nationwide. EMRs were manually reviewed using a structured data abstraction tools by trained medical record abstractors (ST and SM).
Study population
We identified a national cohort of 10,695 patients who had an HCC diagnosis in all VA hospitals during October 1, 2004 to September 30, 2011 (fiscal years 2005–2010). HCC diagnosis was identified based on the presence of ICD-9 CM code 155.0 (malignant neoplasm of liver) and in the absence of code 155.1 (intrahepatic cholangiocarcinoma).15 We subsequently selected a random computer generated sample of 2,719 patients for medical record review to confirm HCC diagnosis, and determine if eligibility criteria (below) were met. We included patients in the study cohort if they had HCC diagnosis made by histopathology or imaging criteria according to the 2005 American Association for the Study of Liver Disease or European Association for the Study of Liver Disease guidelines.16, 17 We excluded 830 patients out of 2,719 because HCC diagnosis could not be confirmed. Further, we excluded patients without recent VA healthcare utilization (at least one inpatient or outpatient encounter at any VA facility within the 1-year prior to the date of HCC diagnosis) or cases presenting with HCC recurrence and first HCC diagnosis prior to study period or recieved treatment before establishing guideline based diagnosis (n=389). Thus our final study cohort included 1,500 patients with verified HCC.
Patient characteristics and HCC management
We ascertained age, gender, race/ethnicity, clinical characteristics including Model for End Stage Liver Disease (MELD) score, indicators of advanced liver disease (ascites, encephalopathy, varices), medical comorbidities (diabetes, chronic obstructive pulmonary disease, congestive heart failure, myocardial infarction, hypertension, peripheral vascular disease and end stage renal disease) and mental health disorders (bipolar disorder, psychosis, post-traumatic stress disorder) for each patient. We ascertained Barcelona Clinic Liver Cancer (BCLC) HCC stage at diagnosis (A–D) by capturing tumor number and size from imaging report and performance status from physician notes. We classified patients as having cirrhosis if they had evidence of cirrhosis on a liver biopsy obtained any time before diagnosis of HCC, features suggestive of cirrhosis on abdominal imaging, or had abnormal values on two of three laboratory results available within 6 month prior to and 4 weeks after HCC diagnosis (albumin <3.0g/l, platelets <200,000 microliter, INR >1.1). HCC surveillance was defined as receiving abdominal ultrasound, computed tomography (CT) or magnetic resonance imaging (MRI) with an indication of screening/surveillance for HCC, or any alpha-fetoprotein (AFP) test within the 3-years prior to HCC diagnosis. All of the above information was manually abstracted from the EMR. HCC specific treatment was defined as receipt of liver transplantation, hepatic resection, ablation (alcohol, cryoablation or radiofrequency), trans-arterial chemoembolization (TACE) or chemotherapy received during the 12-months following HCC diagnosis captured by ICD-9-CM or CPT codes (Appendix).
Appendix.
ICD-9-CM and CPT Codes Used to Determine the Receipt of HCC-Specific Treatment
| Codes | |
|---|---|
| Liver transplantation | ICD-9-CM: 50.5, 50.59, 50.51, V42.7, 50.4 CPT: 47135, 47136, 47140, 47141, 47142 |
| Surgical resection | ICD-9-CM: 50.21, 50.22, 50.3 CPT: 47120, 47122, 47125, 47130 |
| Local ablation | ICD-9-CM: 50.29 CPT: 47370, 76490, 76362, 47380, 47382 |
| Trans-arterial chemoembolization |
Embolization ICD-9-CM: 38.80, 38.86 CPT: 37204, 75894 Chemotherapy within 30-days of embolization ICD-9-CM: 99.25 CPT: J9000, J9280, J9060, 96405, 96408, 96420, 96422, 96423, 96425, 96440, 96445, 96545, 96549, 0331, 0335 |
| Systemic chemotherapy | ICD-9: 99.25 CPT: J9000, J9010, J9190, J9200, J9201, J9217, J9265, J9060, J9062, J9170, J9178, J9181, J9182, J9280, J9293, J9370, J9015, J9017, J9035, J9202, J9055, 90782, 96400, 96405, 96408, 96410, 96412, 96414, 96420, 96422, 96423, 96425, 96440, 96445, 96545, 96549, 0331, 0332, 0335 |
Risk factors for HCC
Information was collected on the presence of HCV, hepatitis B (HBV) and alcohol abuse. HCV status was determined by the presence of a positive anti-HCV or HCV ribonucleic acid (RNA) tests. HBV was defined by a positive surface antigen (HBsAg). Alcohol abuse was defined as history of more than 3 drinks a day, alcoholism/alcohol abuse stated in a physician progress notes, enrollment in a substance abuse treatment program or history of alcoholic hepatitis. Less common causes of HCC such as hemochromatosis, Wilson’s disease, alpha-1 anti-trypsin deficiency or autoimmune hepatitis were captured when diagnostic laboratory tests results were positive (e.g., homozygosity for C282Y) or diagnoses were listed in the problem list or progress notes.
NAFLD-related HCC
NAFLD was defined by features of hepatic fatty infiltration on histopathology report or presence of metabolic syndrome in the absence of HCV, HBV, alcohol abuse, and no documentation of primary biliary cirrhosis, primary sclerosing cholangitis, autoimmune hepatitis, hemochromatosis or Wilson disease prior to HCC diagnosis. Metabolic syndrome was defined using National Cholesterol Education Program Adult Treatment Plan III guidelines.18 The only exception was elevated waist circumference was replaced by body mass index (BMI) > 28.8 kg/m2 in both men and women; this modification has been validated in a previous study.19 We captured hypertension, diabetes and dyslipidemia when these diagnoses were stated in physician note or problem list or when on treatment for these conditions on manual chart review.
Statistical analysis
Demographic, clinical features and receipt of HCC surveillance were compared among the three mutually exclusive groups of HCC patients: (1) NAFLD, (2) alcohol abuse only, (3) and HCV (patients having HCV irrespective of any other risk factor) using chi-square for discrete variables and t-test for continuous variables. We calculated the proportions of HCC cases attributable to each of these risk factors for the entire study period and in each fiscal year. Survival was defined as time between HCC diagnosis to death or end of study follow-up (July 30, 2012) and was examined by Kaplan Meier analyses stratified by risk factor for HCC. Differences in the cumulative survival by risk factor were evaluated using log rank tests. Cox proportional hazards models were conducted to identify the effect of NAFLD, HCV or alcohol-related HCC on time to death (mortality risk) while adjusting for several demographic and clinical characteristics. Deyo comorbidity index was calculated to adjust for non-hepatic comorbidities.20 Hazard ratios (HR) and their accompanying 95% CI were calculated. Analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
The study cohort comprised of 1,500 patients diagnosed with HCC during fiscal years 2005–2010. Their mean age at the time of diagnosis was 63.7 years (standard deviation=9.5) and the vast majority were men (99.8%). The greatest proportions of patients were white non-Hispanic (59.8%), followed by blacks (26.1%) and Hispanics (11.9%). A liver mass biopsy was available in 786 (52.4%) cases to confirm diagnosis of HCC, and the rest were diagnosed according to radiological features. HCV testing was performed in 96.1%, and HBV testing in 89.5% of the study cohort.
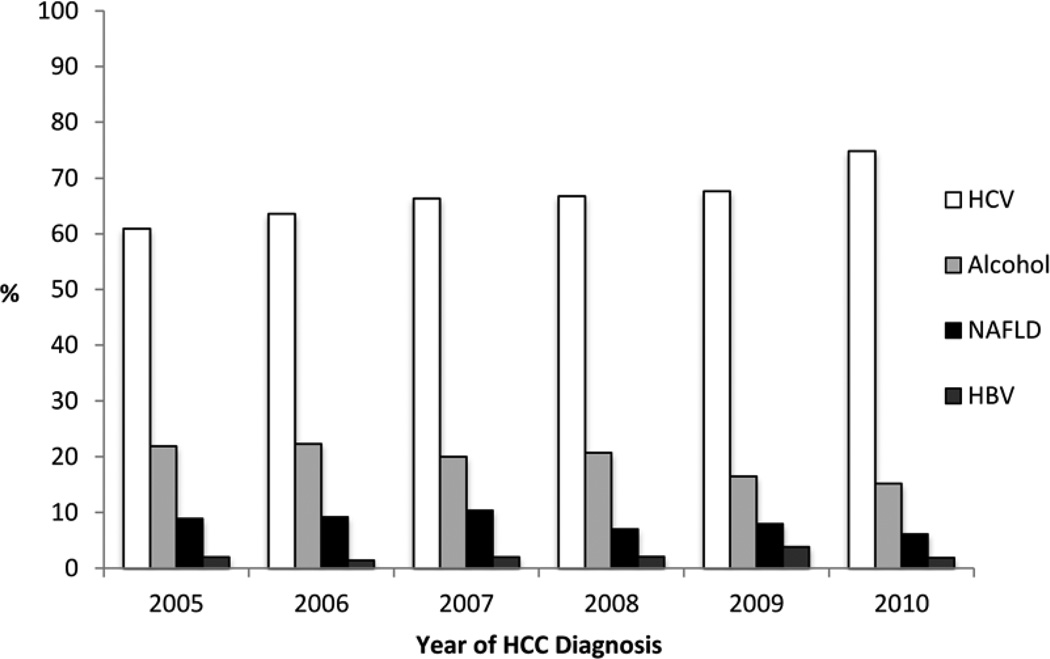
Overall, 120 (8.0%) of all HCC cases had NAFLD, 1,013 (67.5%) HCV, 69 (4.6%) HBV, 1209 (80.6%) alcohol abuse, 26 (1.7%) other risk factors (hemochromatosis, alpha-1 anti-trypsin deficiency or autoimmune hepatitis) and 927 (61.8%) had more than one risk factor. Alcohol abuse and HCV were the most common co-existent risk factors being present in 863 (57.5%) patients while in 286 (19.1%) of cases alcohol abuse was the only major risk factor. No risk factor could be identified in 39 (2.6%) patients. The annual proportions of NAFLD-related HCC were relatively stable between 7.5% and 12.0% (Figure 1). In contrast, HCV related HCC increased from 61.0% (95% CI 53.1–68.9) in 2005 to 74.9% (95% CI 69.0–80.7) in 2010. Proportion of HCC patients with alcohol abuse as the only major risk factor showed a declining trend from 21.9% (95% CI 15.2–28.6) in 2005 to 15.7% (95% CI 10.3–20.1) in 2010. The annual proportion of HBV related HCC remained relatively stable between 1.4% and 3.5%.
Figure 1.
Prevalence of HCC stratified by risk factors (NAFLD, Alcohol abuse, HCV, and HBV) over time.
NAFLD-related HCC patients were significantly older and more likely to be white compared to patients with HCV or alcohol abuse related HCC (Table 1). NAFLD-related HCC patients were less likely to have mental health comorbidities but more likely to have cardiovascular comorbidities such as hypertension or CHF compared to patients with alcohol abuse or HCV related HCC. Only 58.3% of patients with NAFLD-related HCC had underlying cirrhosis before or at time of HCC diagnosis; this proportion which was significantly lower than in patients with alcohol abuse (72.4%, P=0.02) and HCV-related HCC (85.6%, P<0.01).
Table 1.
Comparison of demographics, co-morbidities and underlying liver dysfunction among HCC patients categorized according to three major risk factors (N=1419)
| NAFLD Only n=120 |
Alcohol Abuse Only n=286 |
P- value |
HCV n=1013 |
P- value |
|
|---|---|---|---|---|---|
| Age (Mean) | 74.7 | 69.7 | <0.01 | 60.2 | <0.01 |
| Sex (%) | 0.30 | 0.29 | |||
| Male | 119 (99.2) | 286 (100.0) | 1011 (99.8) | ||
| Race (%) | <0.01 | <0.01 | |||
| White | 105 (87.5) | 198 (69.2) | 543 (53.6) | ||
| Black | 4 (3.3) | 25 (8.7) | 349 (34.5) | ||
| Hispanic | 11 (9.2) | 53 (18.5) | 107 (10.6) | ||
| Other | 0 | 10 (3.5) | 14 (1.4) | ||
| Cirrhosis (%) | 70 (58.3) | 207 (72.4) | 0.02 | 867 (85.6) | <0.01 |
| MELD Score (%) | 0.40 | 0.01 | |||
| <10 | 48 (40.0) | 97 (33.9) | 418 (41.3) | ||
| 10–19.9 | 43 (35.8) | 126 (44.1) | 458 (45.2) | ||
| 20+ | 9 (7.5) | 15 (5.2) | 46 (4.5) | ||
| Missing | 20 (16.7) | 48 (16.8) | 91 (8.9) | ||
| Mental Health Comorbidities (%) | |||||
| Bipolar disorder/ Psychosis | 6 (5.0) | 18 (6.3) | 0.61 | 129 (12.7) | 0.01 |
| Post-traumatic stress disorder | 3 (2.5) | 33 (11.5) | <0.01 | 226 (22.3) | <0.01 |
| Medical Comorbidities (%) | |||||
| Congestive Heart Failure | 25 (20.8) | 33 (11.5) | 0.01 | 62 (6.1) | <0.01 |
| Chronic Obstructive Airway | 29 (24.2) | 93 (32.5) | 0.09 | 223 (22.0) | 0.59 |
| Disease | |||||
| Diabetes | 107 (89.2) | 144 (50.4) | <0.01 | 334 (32.9) | <0.01 |
| End stage renal disease | 3 (2.5) | 2 (0.7) | 0.16 | 18 (1.8) | 0.32 |
| HIV | 1 (0.8) | 3 (1.1) | 1.00 | 42 (4.2) | 0.58 |
| Hypertension | 115 (95.8) | 242 (84.6) | <0.01 | 708 (69.9) | <0.01 |
| Myocardial infarction | 27 (22.5) | 42 (14.7) | 0.06 | 55 (5.4) | <0.01 |
| Peripheral Vascular Disease | 25 (20.8) | 48 (16.8) | 0.33 | 85 (8.4) | <0.01 |
More patients with NAFLD-related HCC (56.7%) did not receive HCC surveillance within 3-years prior to their HCC diagnosis compared to 40.2% with alcohol abuse related HCC (P<0.01) and 13.3% of those with HCV (P<0.01, Table 2). A significantly higher proportion of NAFLD-related HCC patients had AFP < 20 ng/ml measured within 6 months of HCC diagnosis as compared to alcohol abuse and HCV related HCC. At the time of HCC diagnosis, only 5.8% of NAFLD-related HCC cases were classified as early stage HCC (BCLC Stage A) compared to 5.6% and 15.7% with alcohol abuse and HCV, respectively. A higher proportion of NAFLD-related HCC tumors were well differentiated as compared to alcohol abuse and HCV related HCC although it did not reach statistical significance and many patients did not have liver biopsy (Table 2).
Table 2.
Comparison of tumor characteristics, receipt of surveillance and treatment modality among HCC patients according to risk factor (N=1419)
| NAFLD Only n=120 |
Alcohol Abuse Only n=286 |
P- value |
HCV n=1013 |
P- value |
|
|---|---|---|---|---|---|
| Surveillance (%) | <0.01 | <0.01 | |||
| Any | 52 (43.3) | 171 (59.8) | 878 (86.7) | ||
| None | 68 (56.7) | 115 (40.2) | 135 (13.3) | ||
| AFP <20ng/ml (%) | 57 (47.5) | 116 (40.6) | <0.01 | 356 (35.1) | <0.01 |
| Portal vein thrombosis (%) | 24 (20.0) | 55 (19.2) | 0.86 | 206 (20.3) | 0.93 |
| BCLC Stage (%) | 0.95 | 0.04 | |||
| Stage A | 7 (5.8) | 16 (5.6) | 159 (15.7) | ||
| Stage B | 26 (21.7) | 67 (23.4) | 246 (24.3) | ||
| Stage C | 47 (39.2) | 119 (41.6) | 354 (34.9) | ||
| Stage D | 23 (19.2) | 49 (17.1) | 163 (16.1) | ||
| Missing | 16 (13.3) | 34 (11.9) | 85 (8.4) | ||
| Tumor differentiation (%) | 0.56 | 0.23 | |||
| Well | 30 (33.3) | 45 (26.4) | 117 (24.8) | ||
| Moderate | 14 (15.6) | 34 (20) | 111 (23.3) | ||
| Poorly | 8 (8.9) | 20 (11.8) | 40 (8.4) | ||
| Missing | 38 (42.2) | 71 (41.8) | 206 (43.5) | ||
| Treatment (%) | 0.69 | <0.01 | |||
| Curative (transplantation, resection, ablation) | 13 (10.8) | 40 (13.9) | 222 (21.9) | ||
| Palliative (TACE, chemotherapy) | 14 (11.7) | 33 (11.5) | 168 (16.6) | ||
| No treatment | 93 (77.5) | 213 (74.5) | 623 (61.5) |
AFP (alpha-fetoprotein), TACE (trans-arterial chemoembolization)
The proportion of patients not receiving any HCC specific treatment was significantly higher in NAFLD-related HCC as compared to HCV-related HCC (77.5% vs. 61.5%, P<0.01, Table 2) but not different to alcohol abuse related HCC (77.5% vs. 74.5%, P=0.69). Among those who received HCC specific treatment, NAFLD-related HCC patients were significantly less likely to receive potentially curative treatment (transplantation, resection or ablation) as compared with patients with HCV related HCC (10.9% vs. 21.9%, P<0.01) but not different from alcohol abuse related HCC (10.9% vs. 13.9%, P=0.69).
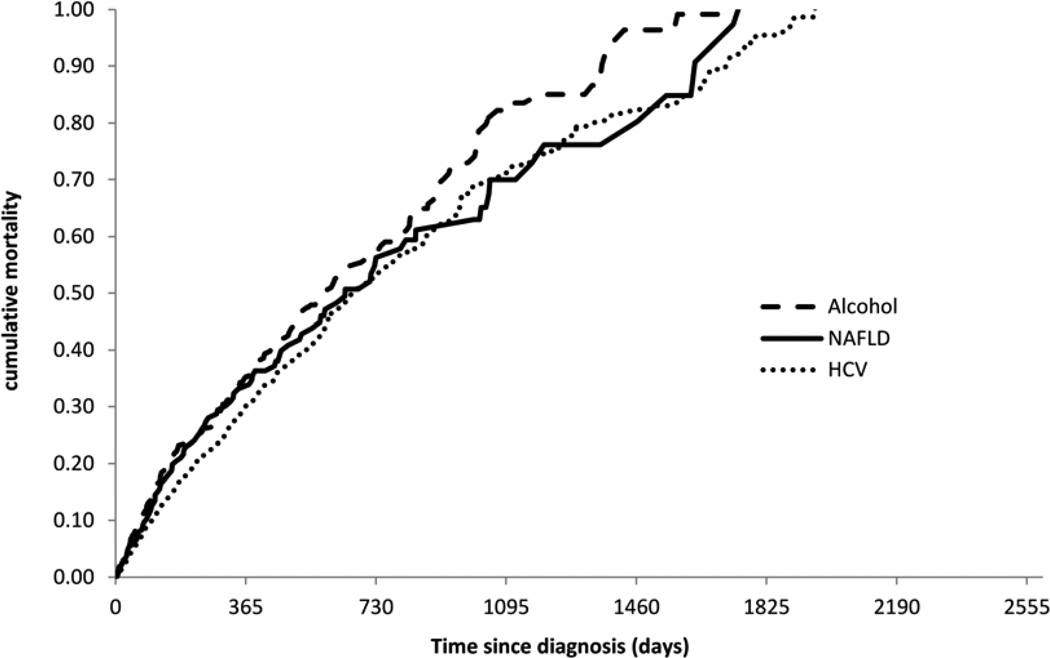
The Kaplan Meier curves (Figure 2) shows that the cumulative mortality was not significantly different across the three types of HCC risk factors (log rank=0.09). The 1-year survival following diagnosis was 46.7% in NAFLD, 44.7% in alcohol abuse and 50.0% in HCV. In unadjusted Cox proportional hazards models with alcohol abuse as the only risk factor was associated with decreased survival as compared to HCV or NAFLD-related HCC (HR 1.2, 95% CI 1.0–1.4). The fully adjusted multivariate Cox regression model is shown in Table 3. The underlying risk factors for liver disease were not significantly associated with time to death after adjusting for age, race, stage, MELD score, Deyo index, receipt of treatment and surveillance. The differences seen in the unadjusted analysis were explained almost entirely in the multivariate model by adjusting for the effects of surveillance, HCC stage and treatment received suggesting that differences in survival were either due to late detection and or non-receipt of treatment.
Figure 2.
Kaplan Meier plot showing overall mortality stratified by risk factors (NAFLD, Alcohol abuse and HCV).
Table 3.
Results from a multivariable Cox proportional hazards model examining risk of mortality among HCC patients with underlying NAFLD, alcohol abuse or HCV
| Adjusted Hazard Ratio (95% CI) | |
|---|---|
| Risk factor | |
| NAFLD | 0.8 (0.6–1.0) |
| Alcohol abuse | 0.9 (0.8–1.2) |
| HCV | 1.0 (Reference) |
| Age | 1.0 (0.9–1.0) |
| Race | |
| White | 1.0 (Reference) |
| Black | 1.1 (0.9–1.3) |
| Hispanic | 1.0 (0.8–1.2) |
| Others | 1.0 (0.6–1.5) |
| MELD score | |
| <10 | 1.0 (Reference) |
| 10–19.9 | 1.4 (1.3–1.7) |
| 20+ | 1.4 (1.1–1.9) |
| Deyo index | |
| 0 | 1.0 (Reference) |
| 1 | 1.0 (0.8–1.2) |
| 2+ | 1.0 (0.8–1.1) |
| BCLC stage | |
| A | 1.0 (Reference) |
| B | 1.7 (1.4–2.2) |
| C | 2.7 (2.2–3.3) |
| D | 4.2 (3.4–5.5) |
| Any HCC Surveillance | |
| Yes | 0.8 (0.7–0.9) |
| No | 1.0 (Reference) |
| HCC Treatment | |
| Curative (resection, transplantation, ablation) | 0.3 (0.2–0.3) |
| Palliative (TACE, chemotherapy) | 0.5 (0.5–0.6) |
| No treatment | 1.00 (Reference) |
DISCUSSION
Our study shows that in the VA population between 7.5% and 12.0% of HCC cases were related to NAFLD and this proportion remained stable over the study period. We observed that NAFLD-related HCC patients have a distinct phenotype – older age, more like to be whites and less severe liver dysfunction at diagnosis as compared to HCC from other causes. These patients received less medical attention as shown by lower rates of surveillance and treatment.
An earlier study on risk factors in HCC in VA population during 1993–1998 showed that 44.0% of HCC did not have any identifiable risk factors but NAFLD was not evaluated in that study.21 The combined proportion of NAFLD-related or cryptogenic HCC in our study is still much smaller. There are several possible reasons to explain this finding. Testing for HCV was not widespread in 1990 when the prior study was conducted so there is possibility of testing bias leading to the high number of cryptogenic HCC cases. In addition, the earlier study identified risk factors using ICD-9-CM codes which could have resulted in some potential misclassification of risk categories. In this study, we used comprehensive chart review to identify risk factors and hence increased the likelihood of accurate risk factor classification.
We observed HCV related HCC increased from 61.0% in 2005 to 74.9% in 2010. Our findings have two important implications. First, the proportion of HCV related HCC is continuing to rise thus confirming the prediction of previous modeling studies.22 Second, NAFLD-related HCC has remained stable over the study period. In the United States, two studies conducted seven years apart, both showed the proportion of NAFLD-related HCC to be stable around 13.0%.11, 23 Similar findings were reported from Europe where a multi-center study also showed the proportion of cryptogenic HCC to be stable over a period of 15 years.24 However, NAFLD and cryptogenic HCC could not be distinguished due to absence of data on body mass index and lipid profile. It is possible that given the relative recency of the obesity epidemic and lag period of several decades required for NAFLD to develop into cirrhosis and or HCC that the impact of the NAFLD-related advanced liver disease has not been realized yet.
Consistent with previous studies, we observed that NAFLD-related HCC patients were more likely to be older and white and less likely to be African-American as compared to HCV. The ethnic differences could be explained due to the higher prevalence of NAFLD among white population as well as the faster rate of disease progression in this population. There is evidence to suggest that both factors may contribute to this finding. The Dallas Heart Study showed higher prevalence of hepatic steatosis among white as compared to African-Americans despite similar prevalence of risk factors among these groups.25 Studies have also reported that African Americans are less likely to progress from fatty liver to steatohepatitis.26, 27 Another study showed that while NASH accounted for most cryptogenic cirrhosis cases among whites it was rare in African-Americans.28
NAFLD patients were less likely to receive surveillance in years prior to diagnosis of HCC. Absence of reliable serologic biomarkers to diagnose NAFLD combined with a perception that NAFLD is a benign and indolent disease could prompt less surveillance. A higher proportion of HCC with underlying NAFLD patients did not have evidence of cirrhosis as compared to patients with HCV and alcohol abuse related HCC,. This suggestsing that HCC can develop in NAFLD patients at very early stages or in the absence of cirrhosis or in the absence of cirrhosis. At present, surveillance for HCC in NAFLD without cirrhosis is not recommended.16,17 Prior studies have shown that besides NAFLD progression, obesity and diabetes mellitus are independent risk factors of HCC.29–35 It is also possible that NAFLD related cirrhosis has a lower risk of HCC than other risk factors, although the evidence for this is mixed.36,37
A high proportion of HCC cases (52%) were diagnosed by liver biopsy. This is consistent with low penetration or acceptance of society guidelines which recommend that HCC can be diagnosed radiologically without biopsy in the presence of typical imaging features.16,17 The proportion of HCC patients with BCLC stage A that may have been eligible for curative treatment was much lower in patients with underlying NAFLD as compared to HCV. In fact, NAFLD-related HCC patients were more likely not to receive any treatment. We believe this is a result of decreased surveillance leading to higher tumor burden at diagnosis. Despite this, overall survival was not different between NAFLD, HCV or alcohol abuse related HCC. This may be explained by higher proportion of AFP non-secretors suggestive of less aggressive tumor biology in these patients. Prior research has shown that increasing level of AFP is an independent predictor of mortality in HCC.38 Studies using resection specimens from HCC patients have also reported higher frequency of well-differentiated tumors in NAFLD-related HCC.39, 40 We did not observe differences in tumor differentiation among the risk categories; however tumor histopathology was available only among half of the study cohort. Thus if barriers in surveillance and treatment are overcome, NAFLD-related HCC may have better survival as compared to HCC from other risk factors. A study on outcomes of curative treatment in HCC showed that NAFLD-related HCC survived longer as compared to alcohol or HCV related HCC.13
This study has number of limitations. We used all available VA electronic medical records to identify risk factors including a search of all progress notes for any evidence of alcohol abuse prior to HCC diagnosis. However there is still chance of occult alcohol use and misclassification of risk category between alcohol use and NAFLD. We relied on biopsy or presence of metabolic syndrome in absence of other risk factors to diagnose NAFLD. Other patients may have unrecognized components of metabolic syndromes leading to underestimation of NAFLD cohort. Therefore our findings represent a conservative estimate of the burden of NAFLD-related HCC. Using only biopsy proven NAFLD will likely lead to a selection bias, as all patients with NAFLD do not undergo a biopsy to establish diagnosis. Alternate method is to use administrative data but the accuracy of diagnostic codes to capture NAFLD is not established. The positive predictive value of diagnostic codes for HCC was lower than what we have observed in our previous studies because we examined HCC irrespective of etiology (not limited to HCV+ patients) and used a strict guideline based algorithm to confirm diagnosis of HCC.15 Although not validated we used CPT codes to capture type of treatment received for HCC.
In summary, we found that in the VA population NAFLD is the third most common cause of HCC after alcohol abuse and HCV. The proportion of NAFLD-related HCC remained stable over the study period suggesting that impact of NAFLD epidemic on HCC has not been realized yet. Further studies are needed to identify effective methods to overcome barriers in practice and improve outcomes in this group of patients.
Acknowledgments
GRANT SUPPORT: This project was supported in part by the National Cancer Institute (R01 CA160738, PI: J. Davila) the facilities and resources of the Houston Veterans Affairs Health Services Research and Development Center of Excellence (HFP90-020), Michael E. DeBakey Veterans Affairs Medical Center, and the Dan Duncan Cancer Center, Houston, Texas, United States of America.
ABBREVIATIONS
- CAPRI
Compensation and Pension Records Interchange
- CPRS
Computerized Patient Record System
- CPT
Common Procedural Terminology
- EMR
Electronic medical records
- NAFLD
Non-alcoholic fatty liver disease
- HCV
Hepatitis C
- HBV
Hepatitis B
- HCC
Hepatocellular carcinoma
- ICD-9-CM
International Classification of Diseases, 9th Revision, Clinical Modification
- OPC
Outpatient Care File
- PTF
Patient Treatment File
- VA
Veterans Administration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST: No conflicts of interest exist for Drs. Mittal, Sada, El-Serag, Kanwal, Ms. Temple, Ms. May, Duan, Kramer, Richardson, and Davila.
AUTHOR CONTRIBUTIONS: Study concept and design: Davila, El-Serag, Kanwal, Mittal and Sada. Acquisition of data: May, Richardson, Sada and Temple. Analysis and interpretation of data: Duan, Davila, El-Serag and Mittal. Drafting of manuscript: Davila, El-Serag and Mittal. Critical revision of the manuscript for important intellectual content: Davila, El-Serag, Kanwal, Kramer, Sada and Mittal. Statistical Analysis: Duan and Richardson. Study supervision: Davila.
REFERENCES
- 1.El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817–823. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 2.McGlynn KA, Tarone RE, El-Serag HB. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15:1198–1203. doi: 10.1158/1055-9965.EPI-05-0811. [DOI] [PubMed] [Google Scholar]
- 3.Davila JA, Morgan RO, Shaib Y, et al. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: a population-based study. Gastroenterology. 2004;127:1372–1380. doi: 10.1053/j.gastro.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 4.Adams LA, Lindor KD. Nonalcoholic fatty liver disease. Ann Epidemiol. 2007;17:863–869. doi: 10.1016/j.annepidem.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 6.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 7.Bugianesi E, Leone N, Vanni E, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 8.Caldwell SH, Oelsner DH, Iezzoni JC, et al. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664–669. doi: 10.1002/hep.510290347. [DOI] [PubMed] [Google Scholar]
- 9.Afzali A, Berry K, Ioannou GN. Excellent posttransplant survival for patients with nonalcoholic steatohepatitis in the United States. Liver Transpl. 2012;18:29–37. doi: 10.1002/lt.22435. [DOI] [PubMed] [Google Scholar]
- 10.White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10:1342–1359. doi: 10.1016/j.cgh.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marrero JA, Fontana RJ, Su GL, et al. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349–1354. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- 12.Guzman G, Brunt EM, Petrovic LM, et al. Does nonalcoholic fatty liver disease predispose patients to hepatocellular carcinoma in the absence of cirrhosis? Arch Pathol Lab Med. 2008;132:1761–1766. doi: 10.5858/132.11.1761. [DOI] [PubMed] [Google Scholar]
- 13.Reddy SK, Steel JL, Chen HW, et al. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology. 2012;55:1809–1819. doi: 10.1002/hep.25536. [DOI] [PubMed] [Google Scholar]
- 14.VA Information Resource Center. Accessed at http://www.virec.reserach.va.gov/VSF/Overview.htm.
- 15.Davila JA, Weston A, Smalley W, et al. Utilization of screening for hepatocellular carcinoma in the United States. J Clin Gastroenterol. 2007;41:777–782. doi: 10.1097/MCG.0b013e3180381560. [DOI] [PubMed] [Google Scholar]
- 16.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 19.Ascha MS, Hanouneh IA, Lopez R, et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 20.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 21.El-Serag HB, Mason AC. Risk factors for the rising rates of primary liver cancer in the United States. Arch Intern Med. 2000;160(21):3227–3230. doi: 10.1001/archinte.160.21.3227. [DOI] [PubMed] [Google Scholar]
- 22.Thomas DL. Global control of hepatitis C: where challenge meets opportunity. Nat Med. 2013;19:850–858. doi: 10.1038/nm.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang JD, Harmsen WS, Slettedahl SW, et al. Factors that affect risk for hepatocellular carcinoma and effects of surveillance. Clin Gastroenterol Hepatol. 2011;9:617–623. doi: 10.1016/j.cgh.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 24.Giannini EG, Marabotto E, Savarino V, et al. Hepatocellular carcinoma in patients with cryptogenic cirrhosis. Clin Gastroenterol Hepatol. 2009;7:580–585. doi: 10.1016/j.cgh.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 26.Giday SA, Ashiny Z, Naab T, et al. Frequency of nonalcoholic fatty liver disease and degree of hepatic steatosis in African-American patients. J Natl Med Assoc. 2006;98:1613–1615. [PMC free article] [PubMed] [Google Scholar]
- 27.Solga SF, Clark JM, Alkhuraishi AR, et al. Race and comorbid factors predict nonalcoholic fatty liver disease histopathology in severely obese patients. Surg Obes Relat Dis. 2005;1:6–11. doi: 10.1016/j.soard.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Browning JD, Kumar KS, Saboorian MH, et al. Ethnic differences in the prevalence of cryptogenic cirrhosis. Am J Gastroenterol. 2004;99:292–298. doi: 10.1111/j.1572-0241.2004.04059.x. [DOI] [PubMed] [Google Scholar]
- 29.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 30.Moller H, Mellemgaard A, Lindvig K, et al. Obesity and cancer risk: a Danish record-linkage study. Eur J Cancer. 1994;30A:344–350. doi: 10.1016/0959-8049(94)90254-2. [DOI] [PubMed] [Google Scholar]
- 31.Oh SW, Yoon YS, Shin SA. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J Clin Oncol. 2005;23:4742–4754. doi: 10.1200/JCO.2005.11.726. [DOI] [PubMed] [Google Scholar]
- 32.Adami HO, Chow WH, Nyren O, et al. Excess risk of primary liver cancer in patients with diabetes mellitus. J Natl Cancer Inst. 1996;88:1472–1477. doi: 10.1093/jnci/88.20.1472. [DOI] [PubMed] [Google Scholar]
- 33.El-Serag HB, Richardson PA, Everhart JE. The role of diabetes in hepatocellular carcinoma: a case-control study among United States Veterans. Am J Gastroenterol. 2001;96:2462–2467. doi: 10.1111/j.1572-0241.2001.04054.x. [DOI] [PubMed] [Google Scholar]
- 34.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–468. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 35.Farrell G. Insulin resistance, obesity, and liver cancer. Clin Gastroenterol Hepatol. 2014;12:117–119. doi: 10.1016/j.cgh.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 36.Mair RD, Valenzuela A, Ha NB, et al. Incidence of hepatocellular carcinoma among US patients with cirrhosis of viral or nonviral etiologies. Clin Gastroenterol Hepatol. 2012;10:1412–1417. doi: 10.1016/j.cgh.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres DM, Williams CD, Harrison SA. Features, diagnosis, and treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2012;10(8):837–858. doi: 10.1016/j.cgh.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 38.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 39.Regimbeau JM, Colombat M, Mognol P, et al. Obesity and diabetes as a risk factor for hepatocellular carcinoma. Liver Transpl. 2004;10:S69–S73. doi: 10.1002/lt.20033. [DOI] [PubMed] [Google Scholar]
- 40.Paradis V, Zalinski S, Chelbi E, et al. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49:851–859. doi: 10.1002/hep.22734. [DOI] [PubMed] [Google Scholar]