Abstract
The outcomes of children with congenital hemolytic anemia (CHA) undergoing total splenectomy (TS) or partial splenectomy (PS) remain unclear. In this study, we collected data from 100 children with CHA who underwent TS or PS from 2005–2013 at 16 sites in the Splenectomy in Congenital Hemolytic Anemia (SICHA) consortium using a patient registry. We analyzed demographics and baseline clinical status, operative details, and outcomes at 4, 24, and 52 weeks after surgery. Results were summarized as hematologic outcomes, short-term adverse events (AEs) (≤ 30 days after surgery), and long-term AEs (31–365 days after surgery). For children with hereditary spherocytosis, after surgery there was an increase in hemoglobin (baseline 10.1 ± 1.8 gm/dl, 52 week 12.8 ± 1.6 gm/dl; mean ± SD), decrease in reticulocyte and bilirubin as well as control of symptoms. Children with sickle cell disease had control of clinical symptoms after surgery, but had no change in hematologic parameters. There was an 11% rate of short-term AEs and 11% rate of long-term AEs. As we accumulate more subjects and longer follow-up, use of a patient registry should enhance our capacity for clinical trials and engage all stakeholders in the decision-making process.
Keywords: Congenital hemolytic anemia, hereditary spherocytosis, sickle cell disease, partial splenectomy, splenectomy
Introduction
For severely affected children with congenital hemolytic anemia (CHA), a splenectomy can control hemolysis and limit sequestration crises [1]. However, the risks associated with total splenectomy (TS) such as sepsis, thrombosis, or pulmonary hypertension have led to increasing interest in partial splenectomy (PS). A PS is designed to remove enough spleen to gain the desired hematological outcome while preserving splenic function [1–4]. Although PS has been increasingly used for children with CHA, a prospective clinical trial comparing surgical therapies or surgical versus medical therapies has not been done [3].
Recent health care initiatives have demonstrated the value of evidence-based practices based on large clinical datasets [5, 6]. However, the use of standard datasets for the study of surgical procedures and less common conditions is difficult, as these sets often do not record outcomes considered most important by clinicians and families [3, 7, 8]. In addition, research on spleen surgery in CHA is challenged by the heterogeneity of diseases, small number of subjects, variations in technique, and inconsistent use of standardized data [3]. In these settings, a patient registry is a powerful tool to collect data to guide clinical therapies.
To improve the care of children with CHA, we formed SICHA (Splenectomy in Congenital Hemolytic Anemia), a research consortium of pediatric surgeons and hematologists at 16 sites across North America. To help families and clinicians understand the outcomes of spleen surgery for CHA and to support the development of clinical trials, our consortium developed a web-based patient registry to collect standardized data for children with CHA undergoing TS or PS. The goal of this report is to describe the outcomes for first 100 children in this registry over one year of follow-up. The secondary goal of this report is to assess data quality in this registry.
Methods
Registry design
To collect outcomes of children undergoing PS or TS, we created a web-based registry using the Research Electronic Data Capture (REDCap) platform, which is an open-source application for clinical research used by over 1000 partners in the National Institutes of Health Clinical and Translational Science Awards consortium [9]. For registry design, all site investigators first identified important issues for a family and clinician considering spleen surgery, based on our consortium’s previous comparative effectiveness review.[3] These concerns include the need use of standardized data and definitions of all outcomes, analyses of disease type, and need for multicenter study.
Given these goals, we constructed a data dictionary to record important variables, which were agreed upon by all site investigators. Our registry incorporated principles as described by the Agency for Health Care Research and Quality, including the a priori identification of clinical questions, a web-based interface for data entry, and use of established data standards.[10] All site research staff underwent training via a webinar, and we developed a Manual of Procedures (MOP) to ensure consistent practices across sites.
Subject enrollment
Children 2–17 years of age were eligible if they had a diagnosis of a CHA and underwent TS or PS at a SICHA site between 2005 and 2013 (See Appendix for site roster). Diagnoses included hereditary spherocytosis (HS), sickle cell disease (SCD, including hemoglobin SS, SC, S-beta thalassemia), or other type of CHA. All diseases were defined using standard clinical criteria, including family history, laboratory analysis, or genetic analysis as appropriate [3]. Other indications for splenectomy, such as trauma or idiopathic thrombocytopenia purpura were excluded.
Each site’s institutional Review Board (IRB) approved this study, and each site retained all identifying information. If a family chose to participate, staff obtained written informed consent from the parents or guardians. As only de-identified data were collected centrally into the registry, the registry was designated as exempt from IRB review. A Data Use Agreement was executed between each site and the central coordinating center to enhance data exchange.
Data were collected prospectively from 76 children from 2008–2014 and retrospectively from 24 children from 2005–2008. There were 8 eligible children reported to the central coordinating center whose parents declined enrollment, although the total number of eligible children who were not enrolled was not collected. To test feasibility of this registry, we pilot tested it at a single site and previously reported these findings in 24 children with SCD [11]. Based on this pilot experience, we eliminated extraneous variables, refined technical aspects of the data entry, and expanded inclusion to multiple types of CHA prior to rollout as a multicenter registry.
As an observational registry, this study did not dictate any clinical care, including the choice between TS and PS, use of laparoscopy or laparotomy, specific laboratory testing, or use of concurrent surgical procedures, although all of these outcomes were collected. For children undergoing partial splenectomy, we encouraged maintenance of 10%–20% of normal splenic volume, based on previous clinical and laboratory studies [3, 12–14].
Data abstraction
We collected demographics and clinical status at baseline, operative data, and outcomes at 4, 24 and 52 weeks after surgery. Data were collected from medical records and entered into a web based case report form (CRF) at each site by a research assistant. Baseline data was obtained from values within 4 weeks prior to surgery; four-week outcomes within 2–8 weeks after surgery, 24-week outcomes within 16–32 weeks, and 52-week outcomes within 48–56 weeks. In cases of multiple data within these time frames, the data closest to the time point were recorded. Laboratory values prior to preoperative transfusions were used as baseline data.
We recorded 68 variables, including demographics, disease, indications for surgery, clinical, laboratory and imaging data, and use of laparoscopy (See Supplemental Data 1 for variable list). We used established clinical and data standards for outcome definitions (See Supplemental Data 2).
Adverse events (AEs) included infection (including type), acute chest syndrome (ACS, for SCD patients only), thrombotic events, reoperation, and death (see Appendix 2 for definition of AEs). For children undergoing PS, we collected baseline and postoperative measurements of the spleen size using ultrasound, and calculated splenic volume (expressed as percentage of baseline volume) using the method of De Odorico [15].
Data quality
We audited the first 50 subjects (including both retrospective and prospective subjects) to determine the rate of erroneous data. Erroneous data were identified by a second review of all primary source data, and comparing with final data in the registry. Erroneous data were defined as either an error in data coding (i.e. the wrong value was recorded on the written CRF or transcribed onto the electronic CRF), or failure to use the data from the paper CRF.
We audited all 100 subjects to measure the rate of missing data, including the rate of submission of CRFs as well as individual variables. We defined a CRF as submitted if a CRF had at least one completed variable submitted for that time point, and an individual variable as submitted if there were any data recorded for that variable at that time point.
Data analysis
We summarized results into hematologic outcomes, short-term AEs (≤ 30 days after surgery), and long-term AEs (31–365 days after surgery). This structure was chosen to communicate important outcomes to all stakeholders, including families [3]. We analyzed hematologic outcomes and AEs based on type of disease (HS versus SCD) as well as by type of procedure (PS versus TS). Due to small number of children with other types of CHA, detailed analysis of outcomes was limited to children with HS or SCD.
We determined differences between cohorts using Pearson’s Chi squared or Fisher’s exact test for categorical data, and Mann–Whitney U test, Kuskal-Wallis rank sum test or Wilcoxon signed ranks test for continuous data, using a two-tailed significance of 5%. Comparisons of preoperative and postoperative symptoms were made using McNemar’s test for matched data. Categorical variables were expressed as percentage, and continuous variables by median and interquartile range (IQR) or mean ± standard deviation (SD). For hematologic data, we graphically described trends of mean ± standard error (SE), and confirmed differences between baseline and each time point by matched analysis of non-parametric continuous data using the Wilcoxon signed ranks test, with analysis limited to children who had data at baseline and at that time point. All analyses were performed using R version 3.0.2.
Results
Demographics
As of November 2014, 134 children were enrolled in the registry from 16 sites (mean 8.9 subjects/site, median 3 subjects/site), although this report is limited to the first 100 children who reached at least one year after surgery (Table 1). There was an equal distribution of boys and girls, with expected racial and ethnic disparity by disease type. Use of preoperative vaccines and postoperative antibiotics was high. Most children had HS (n=40) or SCD (n=50), with subtypes of SCD including hemoglobin SS (n=30) or SC (n=10). Ten children had another type of CHA, including pyruvate kinase deficiency (n=1), alpha thalassemia/hemoglobin E-Constant Spring (n=3), beta thalassemia intermedia (n=1), beta thalassemia major (n=1), hemoglobin CC (n=1), hemoglobin Koln (n=1), or CHA, unspecified (n=2).
Table 1.
Demographics of children with congenital hemolytic anemia undergoing total splenectomy or partial splenectomy
| Overall | Hereditary spherocytosis |
Sickle cell disease |
Other | p-value | |
|---|---|---|---|---|---|
| N | 100 | 40 (40%) | 50 (50%) | 10 (10%) | |
| Demographics | |||||
| Gender | 0.453 | ||||
| Male | 49 (49%) | 19 (47.5%) | 27 (54%) | 3 (30%) | |
| Female | 51 (51%) | 21 (52.5%) | 23 (46%) | 7 (70%) | |
| Race/Ethnicity | <0.001 | ||||
| White | 35 (35%) | 30 (75%) | 0 (0%) | 5 (50%) | |
| Black | 53 (53%) | 6 (15%) | 46 (92%) | 1 (10%) | |
| Hispanic | 7 (7%) | 3 (7.5%) | 4 (8%) | 0 (0%) | |
| Other | 5 (5%) | 1 (2.5%) | 0 (0%) | 4 (40%) | |
| Preoperative Vaccinations | |||||
| Pneumovax | 0.001 | ||||
| Yes/No or unknown | 87/13 (87%) | 34/6 (85%) | 48/2 (96%) | 5/5 (50%) | |
| Meningovax or Menactra | 0.001 | ||||
| Yes/No or unknown | 80/20 (80%) | 30/10 (75%) | 46/4 (92%) | 4/6 (40%) | |
| Postoperative Antibiotics | 0.348 | ||||
| Yes/No or unknown | 98/1 (99%) | 38/1 (97%) | 50/0 (100%) | 10/0 (100%) |
Numbers represent counts (%) for each variable in the overall cohort and grouped by diagnosis. P-values represent Pearson’s Chi-square or Fisher’s exact test as appropriate for a difference between the diagnostic groups.
The type of procedure differed by disease. For children with HS, 19/40 (48%) received a PS and 21/40 (52%) received TS. For children with SCD, 34/50 (68%) received TS and 16/50 (32%) received a PS. The majority of children undergoing TS had an initial laparoscopic approach (47/54, 87%). In contrast, only 12/46 (26%) of children with PS had a laparoscopic approach, and 6/12 (50%) were converted to laparotomy.
Hematologic outcomes
Hereditary Spherocytosis
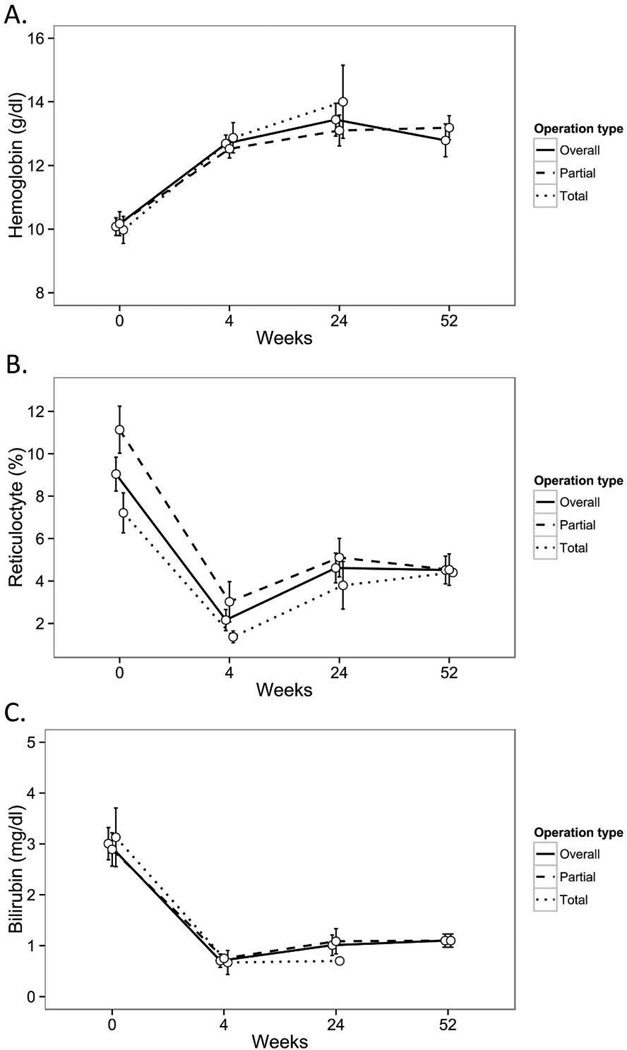
Most children with HS had resolution of their clinical symptoms following TS or PS (Table 2). For children with HS, we found an increase in hemoglobin (baseline 10.1 ± 1.8 gm/dl, 4 week 12.7 ± 1.5 gm/dl, 24 week 13.4 ± 2.1 gm/dl, 52 week 12.8 ± 1.6 gm/dl; mean ± SD), decrease in reticulocyte count (baseline 9.0 ± 4.4%, 4 week 2.2 ± 2.5%, 24 week 4.6 ± 2.8%, 52 week 4.5 ± 2.0%), and decrease in bilirubin (baseline 3.0 ± 1.4 mg/dl, 4 week 0.7 ± 0.6 mg/dl, 24 week 1.0 ± 0.6 mg/dl, 52 week 1.1 ± 0.3 mg/dl) (Fig 1A–C). By matched analysis, the increase in hemoglobin was significant (p<0.05) at each time point compared to baseline. Both TS and PS led to an increase in hemoglobin at all time points compared to baseline (Fig 1A–C), although there was not adequate data to compare outcomes between TS and PS.
Table 2.
Clinical symptoms at baseline and after total splenectomy or partial splenectomy for children with hereditary spherocytosis or sickle cell disease
| Hereditary spherocytosis | Sickle cell disease | |||||
|---|---|---|---|---|---|---|
| Baseline | Postop | p-value | Baseline | Postop | p-value | |
| Splenic sequestration | 8 (20%) | 1 (2.6%) | 0.034 | 45 (90%) | 1 (2.1%) | <0.001 |
| Transfusions | 8 (20%) | 0 (0%) | 0.005 | 13 (26%) | 1 (2%) | <0.001 |
| Aplastic or anemic crisis | 5 (12.5%) | NR | NR | NR | ||
| Hypersplenism | 0 (0%) | NR | 4 (8%) | NR | ||
| Splenomegaly | 11 (27.5%) | 0 (0%) | <0.001 | 5 (10%) | 0 (0%) | 0.025 |
Numbers represent counts (%) for symptoms at baseline and postoperatively for children with hereditary spherocytosis or sickle cell disease following total splenectomy or partial splenectomy. Transfusions-History of transfusions or participation in regular transfusion program. p-values represent McNemar’s for matched pair differences between baseline and postoperative symptoms. Any symptom reported during 1 year follow-up was counted as having a postoperative symptom, with multiple episodes during follow-up counted only once. NR-Not recorded
Figure 1.
(A–C)-Hematologic outcomes following partial splenectomy or total splenectomy in 40 children with hereditary spherocytosis. Data represent hemoglobin (Panel A), reticulocyte count (Panel B) or serum bilirubin (Panel C) at baseline, and at 4, 24, or 52 weeks after partial splenectomy (n=19) or total splenectomy (n=21). All data expressed as mean, with error bars representing standard error. Data on hemoglobin and bilirubin in TS group at 52 weeks had too few data entries to include in the analysis,
Sickle Cell Disease
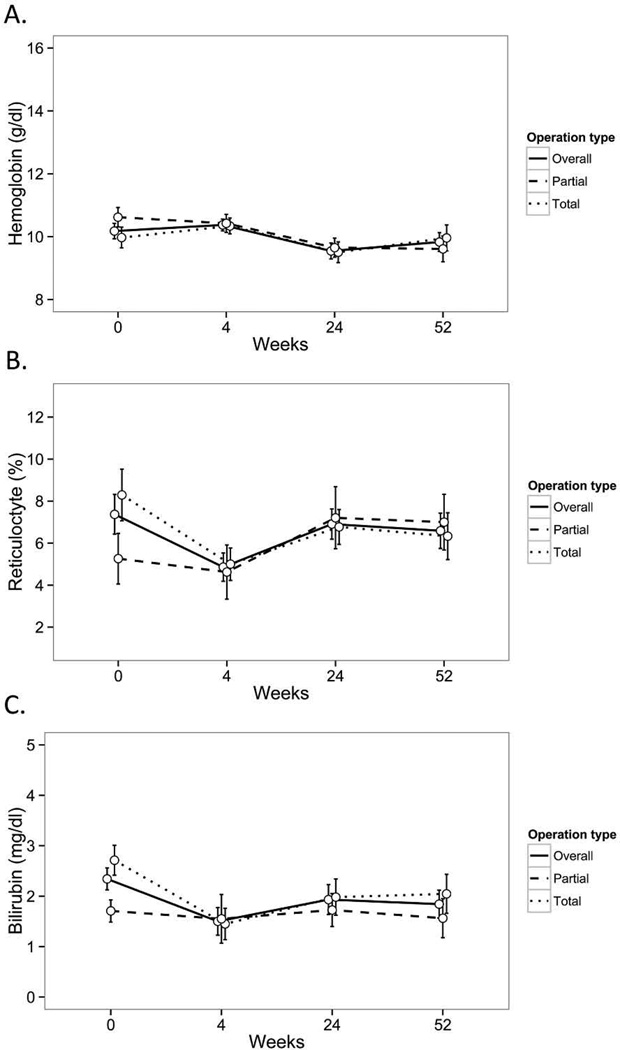
Children with SCD had resolution of their clinical symptoms following TS or PS, including splenic sequestration or participation in a regular transfusion program (Table 2). In contrast to children with HS, children with SCD had similar hemoglobin, reticulocyte count, and serum bilirubin postoperatively compared to baseline (Fig 2A–C). Both TS and PS resulted in similar hemoglobin after surgery compared to baseline. The small number of children with SCD precluded comparison of outcomes between TS and PS as well as comparison of outcomes between hemoglobin SS and SC disease.
Figure 2.
(A–C)-Hematologic outcomes following partial splenectomy or total splenectomy in 50 children with sickle cell disease. Data represent hemoglobin (Panel A), reticulocyte count (Panel B) or serum bilirubin (Panel C) at baseline, and at 4, 24, or 52 weeks surgery after partial splenectomy (n=16) or total splenectomy (n=34). All data expressed as mean, with error bars representing standard error.
Splenic Growth and Function following Partial Splenectomy
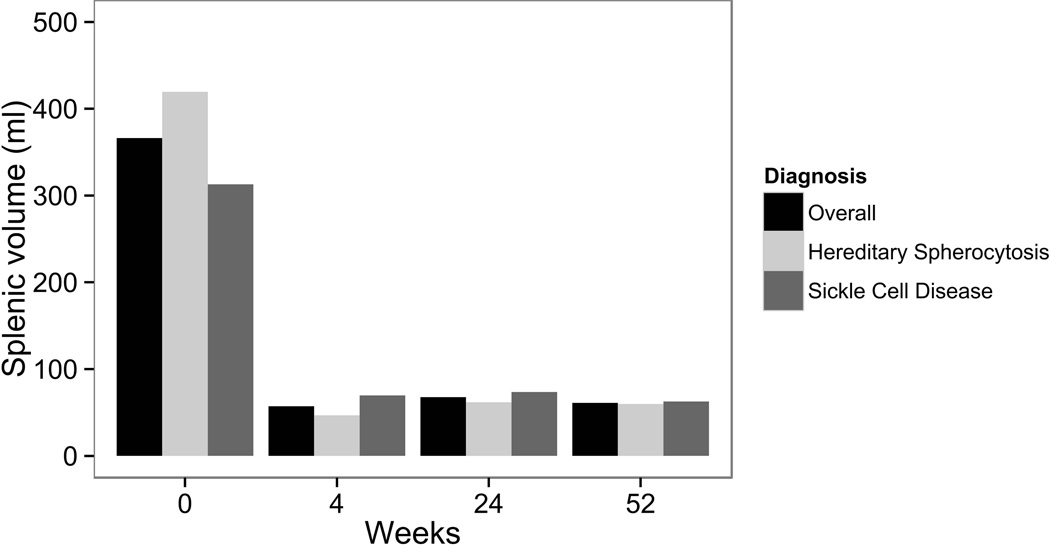
For all 35 children undergoing PS, the measured splenic volume was stable over 1 year: (Fig 3; baseline volume 413 ± 328 ml (100%); 4 week 58 ± 66 ml (14%); 24 week 45 ± 35 ml (10%); 52 week 63 ± 42 ml (12%); expressed as mean ± standard deviation (SD) and percentage of baseline volume). No child required a completion splenectomy during 1 year of follow-up. We were not able to analyze preservation of splenic function following PS (i.e. nuclear medicine liver spleen scans, Doppler splenic ultrasound, notations of Howell-Jolly bodies, etc.), as these studies were not performed consistently across sites.
Figure 3.
Splenic volume in 35 children following partial splenectomy, including 19 children with hereditary spherocytosis and 16 children with sickle cell disease. Data represent mean splenic volume at each time point measured by ultrasound as calculated using the method of De Odorico [15].
Adverse events
The overall rate of short-term AEs was 11%, with no death recorded. For children with HS, there was a 2.5% rate of infection. In children with SCD, there was a 10.0% rate of infection and 12.0% rate of acute chest syndrome (ACS).
The overall rate of long-term AEs was also 11%, with no deaths recorded. For children with HS, there were no infections or thrombotic events, and one reoperation (3.1%) over one year of follow-up. Children with SCD had a 14.3% rate of infection, including two with sepsis, one with upper respiratory tract infection (URI), one with parainfluenza, one with influenza B, and one unspecified. Children with SCD had a 9% rate of ACS and a 2% rate of blood transfusion. There was one child (2%) with a thrombotic event, who had a transient ischemic attack 24 weeks after TS.
Data quality
Audit of the first 50 patients (24 retrospective, 26 prospective) demonstrated 5 erroneous variables out of 4,873 entries (0.1%). All of these were with data coding, i.e. the wrong value was recorded on the CRF or transcribed onto the electronic CRF, with no failures to use the data from the paper CRF. Of note, there were 8 children with HS who were reported to have splenic sequestration, which likely reflects an error in data coding.
Analysis of missing data showed that 92% (369 out of 400) of all CRFs were completed. The rate of CRF completion was highest at early time points: 100% at baseline, 96% at 4 weeks, 91% at 24 weeks, and 82% at 52 weeks. Analysis of individual variables showed that 1 2,189 out of 16,400 (74.3%) were completed.
Discussion
For children with CHA, the outcomes following TS or PS are poorly defined, leaving families and clinicians in a quandary when deciding between surgical therapies or surgical vs medical therapies. Most existing studies in this area are either single center or small multicenter reports, and are limited by use of non-standardized data (see review [3]). Furthermore, many of the studies in this area were performed before the widespread use of Prevnar-13 [17], and the current rate of infectious events following splenectomy is unknown. In our current report from a patient registry of 100 children with CHA undergoing TS or PS, we found that both procedures result in favorable clinical outcomes and have a low rate of AEs over one year of follow-up. This observational study is the first multicenter report using standardized data in this area, and should be of great value to families and clinicians considering splenectomy.
The clinical outcomes of TS and PS differ between diseases. For HS, all types of splenectomy resulted in control of clinical symptoms as well as improvement in hematologic laboratory parameters. Although our registry did not compare outcomes between PS and TS, both procedures had improved hematologic outcomes after surgery, a finding consistent with most previous literature [3, 4, 14, 18, 19]. A prospective randomized clinical trial will be required to definitively compare surgical procedures or surgical vs medical therapies. Finally, although control of clinical symptoms of primary importance to families, we suggest that future studies of splenectomy examine the specific goals of families and clinicians as they chose between surgical or medical therapies, as well as assessment of satisfaction with the outcomes.
For children with SCD, all types of splenectomy control clinical symptoms, particularly the need for transfusions, although there is no effect on hemoglobin or other hematologic parameters. This finding is consistent with most previous literature [20–22], although some single center case series have shown improvement in hemoglobin levels [23, 24]. Given the heterogeneity of different types of SCD, a clinical trial will require a large number of enrolled children to compare partial or total splenectomy, as well as to define the optimal candidates for each procedure in terms of use of hydroxyurea, age, and functional splenic status.
There is a low overall rate of AEs after PS or TS, although children with SCD have continued risks of infection and ACS, which is likely related to their underlying disease. In contrast to reports describing a high rate of vascular thrombosis after splenectomy [25, 26], we recorded only one child with a thrombotic event, and this event likely is attributable to SCD as it occurred 24 weeks following surgery. However, as we collected only symptomatic thrombotic events over one year, the long-term risk of vascular complications may be greater than those clinically evident. We also found that no children required a completion splenectomy during the one year observation period, in contrast to the 10–40% rate reported in small case series over several years of follow-up [4, 14, 27, 28].
We learned several lessons about a registry that are helpful for the study of CHA. First, we recognized that early definition of important issues for all stakeholders, including families, is essential. During pilot testing we collected 110 variables and later realized that many did not address important concerns and/or were not routinely tested. Using a smaller data dictionary allowed for less site burden and improved data quality. Second, optimal registry design using standardized data is critical to maximize data quality. Our missing data rate is similar to other large observational registries [29, 30]. Although the use of a protocol with required tests would minimize missing data, we caution against mandating these practices for an observational pediatric registry, as this may decrease the rate of enrollment [31].
We observed several findings in regards to variability of care. Although this observational study was not designed to compare outcomes between procedures or sites, we found a low rate of laparoscopy in children undergoing PS, which may be due to the complex nature of laparoscopy for PS or reflect clinical care patterns which are currently evolving towards increased use of laparoscopy. In contrast to PS, TS is generally performed laparoscopically, which is attractive to families as laparoscopic splenectomy results in a shorter length of stay and less morbidity than an open procedure [3]. However, if given a choice between a laparoscopic TS and an open PS, it is unclear which families would prefer, as both options offer a different set of benefits. Furthermore, even if family preference would drive the choice towards a given procedure, is this optimal for a child? Although these issues are beyond the scope of this current report, we suggest that future study of splenectomy in CHA should address how these issues affect the family decision-making process.
There are several limitations to our study. First, this report has only one year of follow-up. However these findings should be of great value to families and clinicians, as the rate of sepsis following splenectomy is highest during the first year after surgery [32]. We do recognize that understanding the long-term outcomes (i.e. decades) following TS or PS is critical to understand the role of surgery, and plan to continue follow-up for several years after surgery. Secondly, as an observational study, a registry does not allow for comparison of surgical procedures or surgical versus medical therapies. Third, some important outcomes such as splenic function following partial splenectomy are difficult to assess though an observational study, as these tests are not performed consistently across sites. Finally, our registry used an informed consent process that may have skewed the results, as the consent process may change enrollment from a population based cohort to a selective cohort [33]. Although informed consent traditionally has been fundamental to clinical research, some experts suggest that the consent process for registries should be reassessed, as the public health value of this information may outweigh the risks to personal identity [33].
In conclusion, we found favorable clinical outcomes and a low rate of AEs in children with CHA following PS or TS using a multicenter patient registry. These findings demonstrate the great value as well as the inherent limitations of a patient registry, and define expected outcomes of these procedures. As we accumulate a greater number of children in this registry, extend long-term follow-up, we should enhance our understanding of these procedures, facilitate the design of clinical trials, and engage all stakeholders in the decision-making process when considering surgery.
Supplementary Material
Acknowledgments
In addition to the authors, the SICHA consortium collaborators include: Brittany Herzberg, Jeffrey M. Ferranti, Sofia Mouttalib, Meredith Nahm, Rachel Richesson, Denise C. Snyder. We thank Terri Ainsworth, Mark Bettger, Ceci Chamorro, Phyllis Kennel, Justin Levens, Joan Wilson and the Duke Office of Clinical Research (DOCR) their assistance. The DOCR is supported by the Duke School of Medicine, made possible through CTSA Grant Number UL1TR001117 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not represent the official view of NCATS or NIH.
This work was supported in part by the Duke University Office of Clinical Research and the Duke School of Medicine, made possible through Clinical and Translational Science Awards Grant Number UL1TR001117 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research.
Abbreviations
- CHA
congenital hemolytic anemia
- HS
hereditary spherocytosis
- REDCap
Research Electronic Data Capture
- SCD
sickle cell disease
- SICHA
Splenectomy in Congenital Hemolytic Anemia consortium
- PS
partial splenectomy
- TS
total splenectomy
Appendix
SICHA Site Roster
Alberta Children’s Hospital, Calgary, AB
Children’s Hospital of Boston, Boston, MA
Children’s Mercy Hospital, Kansas City, MO
Cincinnati Children’s Medical Center, Cincinnati, OH
Duke University Medical Center, Durham, NC
Hospital for Sick Children, Toronto, ON
Medical College of Wisconsin, Milwaukee, WI
St. Jude Children’s Research Hospital, Memphis, TN
St. Louis Children’s Hospital, St. Louis, MO
Stanford University, Palo Alto, CA
University of Arkansas, Little Rock, AR
University of Florida, Gainsville, FL
University of Indiana, Indianapolis, IN
University of Michigan, Ann Arbor, MI
University of Texas/MD Anderson Cancer Center, Houston, TX
Vanderbilt University, Nashville, TN
Footnotes
A complete list of the members of the Splenectomy in Congenital Hemolytic Anemia (SICHA) consortium appears in the Acknowledgments.
Authorship Contributions
Contribution: H.E.R, B.R.E., J.R., S.L., A.R., C.T., M.B., N.W., M.W.H, C.S., R.L.B., T.K., J.C.L., M.C., K.T.O., J.P.S., S.S., M.S., A.M.D., K.N., K.B., D.P.W., S.D., B.G., S.E.C., S.D., L.D., S.I., M.K., F.R., S.B., A.C., M.A., R.S., M.L.B. all contributed to experimental design, data collection, analysis, and revision of manuscript. H.E.R. wrote the first draft of the paper.
Conflict-of-interest disclosure: All authors have no financial relationships or conflicts of interest relative to this article to disclose.
References
- 1.Gallagher PG. Red cell membrane disorders. Hematol. 2005;13:13–18. doi: 10.1182/asheducation-2005.1.13. [DOI] [PubMed] [Google Scholar]
- 2.Bader-Meunier B, Gauthier F, Archambaud F, et al. Long-term evaluation of the beneficial effect of subtotal splenectomy for management of hereditary spherocytosis. Blood. 2001;97:399–403. doi: 10.1182/blood.v97.2.399. [DOI] [PubMed] [Google Scholar]
- 3.Rice HE, Crary SE, Langer JC, et al. Comparative effectiveness of different types of splenectomy for children with congenital hemolytic anemias. J Pediatr. 2012;60:684–689. doi: 10.1016/j.jpeds.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 4.Buesing KL, Tracy ET, Kiernan C, et al. Partial splenectomy for hereditary spherocytosis: A multi-institutional review. J Pediatr Surg. 2011;46:178–183. doi: 10.1016/j.jpedsurg.2010.09.090. [DOI] [PubMed] [Google Scholar]
- 5.Steinbrook R. Health care and the American Recovery and Reinvestment Act. N Engl J Med. 2009;360:1057–1060. doi: 10.1056/NEJMp0900665. [DOI] [PubMed] [Google Scholar]
- 6.Institute of Medicine. Initial national priorities for comparative effectiveness research. Washington, DC: The National Academies Press; 2009. [Google Scholar]
- 7.Riley GF. Administrative and claims records as sources of health care cost data. Med Care. 2009;47:S51–S55. doi: 10.1097/MLR.0b013e31819c95aa. [DOI] [PubMed] [Google Scholar]
- 8.Grosse SD, Boulet SL, Amendah DD, et al. Administrative data sets and health services research on hemoglobinopathies: A review of the literature. Am J Prev Med. 2010;38:S557–S567. doi: 10.1016/j.amepre.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Harris PA, Thielke R, Taylor R, et al. Research Electronic Data Capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2008;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gliklich RE, Dreyer NA. Registries for Evaluating Patient Outcomes: A User's Guide. Rockville, MD: Agency for Healthcare Research and Quality; 2010. [PubMed] [Google Scholar]
- 11.Mouttalib S, Rice HE, Snyder D, et al. Evaluation of partial and total splenectomy in children with sickle cell disease using an internet-based registry. Pediatr Blood Cancer. 2012;59:101–104. doi: 10.1002/pbc.24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baykal A, Aydin C, G H, et al. Experimental study of the effects of splenectomy and partial splenectomy on bacterial translocation. J Trauma Injury Infec Crit Care. 1999;46:1096–1099. doi: 10.1097/00005373-199906000-00024. [DOI] [PubMed] [Google Scholar]
- 13.Rice HE, Oldham KT, Hillery CA, et al. Clinical and hematological benefits of partial splenectomy for congenital hemolytic anemias in children. Ann Surg. 2003;237:281–288. doi: 10.1097/01.SLA.0000048453.61168.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tchernia G, Gauthier F, Mielot F, et al. Initial assessment of the beneficial effect of partial splenectomy in hereditary spherocytosis. Blood. 1993;81:2014–2020. [PubMed] [Google Scholar]
- 15.De Odorico I, Spaulding KA, Pretorius DH, et al. Normal splenic volumes estimated using three-dimensional ultrasonography. J Ultra Med. 1999;18:231–236. doi: 10.7863/jum.1999.18.3.231. [DOI] [PubMed] [Google Scholar]
- 16.Ballas SK, Lieff S, Benjamin LJ, et al. Definitions of the phenotypic manifestations of sickle cell disease. Am J Hematol. 2010;85:6–13. doi: 10.1002/ajh.21550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubin LG, Schaffner W. Clinical practice. Care of the asplenic patient. N Engl J Med. 2014;371:349–356. doi: 10.1056/NEJMcp1314291. [DOI] [PubMed] [Google Scholar]
- 18.Crary SE, Troendle S, Ahmad N, et al. Traditional laboratory measures of cardiovascular risk in hereditary spherocytosis. Pediatr Blood Cancer. 2010;55:684–689. doi: 10.1002/pbc.22640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morinis J, Dutta S, Blanchette V, et al. Laparoscopic partial vs total splenectomy in children with hereditary spherocytosis. J Pediatr Surg. 2008;43:1649–1652. doi: 10.1016/j.jpedsurg.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Kalpatthi R, Kane ID, Shatat IF, et al. Clinical events after surgical splenectomy in children with sickle cell anemia. Pediatr Surg Int. 2010;26:495–500. doi: 10.1007/s00383-010-2587-4. [DOI] [PubMed] [Google Scholar]
- 21.Haricharan RN, Roberts JM, Morgan TL, et al. Splenectomy reduces packed red cell transfusion requirement in children with sickle cell disease. J Pediatr Surg. 2008;43:1052–1056. doi: 10.1016/j.jpedsurg.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 22.Svarch E, Nordet I, Valdes J, et al. Partial splenectomy in children with sickle cell disease. Haematologica. 2003;88:222–223. [PubMed] [Google Scholar]
- 23.Al-Salem AH. Indications and complications of splenectomy for children with sickle cell disease. J Pediatr Surg. 2006;41:1909–1915. doi: 10.1016/j.jpedsurg.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 24.Nouri A, de Montalembert M, Revillon Y, et al. Partial splenectomy in sickle cell syndromes. Arch Dis Child. 1991;66:1070–1072. doi: 10.1136/adc.66.9.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnett C, Hsue P, Machado R. Pulmonary hypertension: an increasingly recognized complication of hereditary hemolytic anemias and HIV infection. J Am Med Assoc. 2008;299:324–331. doi: 10.1001/jama.299.3.324. [DOI] [PubMed] [Google Scholar]
- 26.Crary SE, Buchanan GR. Vascular complications after splenectomy for hematologic disorders. Blood. 2009;114:2861–2868. doi: 10.1182/blood-2009-04-210112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gill FM, Sleeper LA, Weiner SJ, et al. Clinical events in the first decade in a cohort of infants with sickle cell disease. Blood. 1995;86 [PubMed] [Google Scholar]
- 28.Slater BJ, Chan FP, Davis K, et al. Institutional experience with laparoscopic partial splenectomy for hereditary spherocytosis. J Pediatr Surg. 2010;45:1682–1686. doi: 10.1016/j.jpedsurg.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 29.Østgård LSG, Nørgaard JM, Severinsen MT, et al. Data quality in the Danish National Acute Leukemia Registry: a hematological data resource. Clin Epidemiol. 2013;5:335–344. doi: 10.2147/CLEP.S48411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Reilly GM, Cameron PA, Jolley DJ. Which patients have missing data? An analysis of missingness in a trauma registry. Injury. 2012;43:1917–1923. doi: 10.1016/j.injury.2012.07.185. [DOI] [PubMed] [Google Scholar]
- 31.Hoberman A, Shaikh N, Bhatnagar S, et al. Factors that influence parental decisions to participate in clinical research: consenters vs nonconsenters. JAMA Pediatr. 2013;167:561–566. doi: 10.1001/jamapediatrics.2013.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leonard AS, Giebink GS, Baesl TJ, et al. The overwhelming postsplenectomy sepsis problem. World J Surg. 1980;4:423–432. doi: 10.1007/BF02393164. [DOI] [PubMed] [Google Scholar]
- 33.Kulynych J, Korn D. The effect of the new federal medical-privacy rule on research. N Engl J Med. 2002;346:201–204. doi: 10.1056/NEJM200201173460312. [DOI] [PubMed] [Google Scholar]
- 34.Perrin EC, Cole CH, Frank DA, et al. Criteria for Determining Disability in Infants and Children: Failure to Thrive. Rockville, MD: Agency for Healthcare Research and Quality; 2003. [PMC free article] [PubMed] [Google Scholar]
- 35.Young NS, Brown KE. Parvovirus B19. N Engl J Med. 2004;350:586–597. doi: 10.1056/NEJMra030840. [DOI] [PubMed] [Google Scholar]
- 36.Platt OS. Acute chest syndrome of sickle cell disease. N Engl J Med. 2000;342:1904–1907. doi: 10.1056/NEJM200006223422510. [DOI] [PubMed] [Google Scholar]
- 37.Gaynes RP, Horan TC. Hospital Epidemiology and Infection Control. Baltimore: Williams & Wilkins; 1996. Surveillance of nosocomial infections; pp. 1–14. [Google Scholar]
- 38.Lynch AM, Kapila R. Overwhelming postsplenectomy infection. Infec Dis Clin North Am. 1996;10:693–707. doi: 10.1016/s0891-5520(05)70322-6. [DOI] [PubMed] [Google Scholar]
- 39.Vaccine Assessment and Monitoring team of the Department of Vaccines and Biologicals. WHO–recommended standards for surveillance of selected vaccine-preventable diseases. Geneva 27, Switzerland: World Health Organization; 2003. [Google Scholar]
- 40.National Insitutes of Health. Coagulation, platelets and thromobsis in sickle cell disease pathophysiology. NIH GUIDE. 1994 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.