Abstract
Background and Aims
Little is known about how weight loss affects magnetic resonance imaging (MRI) of liver fat and volume or liver histology in patients with non-alcoholic steatohepatitis (NASH). We measured changes in liver fat and liver volume associated with weight loss using an advanced MRI method.
Methods
We analyzed data collected from a previous randomized controlled trial, in which 43 adult patients with biopsy-proven NASH underwent clinical evaluation, biochemical tests, and MRI and liver biopsy analyses at the start of the study and after 24 weeks. We compared data between patients who did and did not have at least a 5% decrease in body mass index (BMI) during the study period.
Results
Ten of 43 patients had at least a 5% decrease in BMI during the study period. These patients had a significant decrease in liver fat, based on MRI proton density fat fraction estimates (18.3% ±7.6 to 13.6% ±13.6, P=0.03)— a relative 25.5% reduction. They also had a significant decrease in liver volume (5.3%). However, no significant changes in levels of alanine aminotransferase or aspartate aminotransferase were observed with weight loss. Thirty-three patients without at least a 5% decrease in BMI had insignificant increases in estimated liver fat fraction and liver volume.
Conclusions
A reduction in BMI of at least 5% is associated with a significant decrease in liver fat and volume in patients with biopsy-proven NASH. These data should be considered in assessing effect size in studies of patients with non-alcoholic fatty liver disease or obesity that use MRI-estimated liver fat and volume as endpoints.
Keywords: Non-invasive, steatosis, biomarker, response to treatment
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) has become an increasingly common problem. It now affects approximately 30–40% of adults in the western world,[1, 2] including 60–70% of obese adults,[3] and its prevalence may continue to rise with the worldwide obesity epidemic.[2] It is well known that obesity, insulin resistance and metabolic syndrome play a central role in the development and progression of NAFLD [4–6]. Although most patients with NAFLD have a relatively benign clinical course, 10–20% have nonalcoholic steatohepatitis (NASH), which can lead to advanced fibrosis, hepatic decompensation and liver-related mortality.[7–10]
Despite the increasing clinical relevance of NAFLD, few effective therapies have been identified for this disease. Treatment of NASH with thiazolidinediones may reduce liver steatosis and inflammation, however, their use has been associated with weight gain, cardiovascular complications and bladder cancer.[11–15] In randomized controlled trials, vitamin E has also been effective in reducing steatosis and inflammation in NASH [13, 16], however, it is unclear whether this medication may be associated with an increase in all-cause mortality.[17] Other pharmacologic therapies including metformin, omega-3 fatty acids, bile acids and bile acid sequestrants have been ineffective in the treatment of NASH.[18, 19]
Weight loss remains the mainstay of treatment for NAFLD and NASH. Several studies have shown a reduction in transaminases as well as histology-determined steatosis grade and inflammation in patients with NASH who had significant weight loss.[20, 21] In retrospective and prospective cohort studies, bariatric surgery has also been effective in reducing steatosis, steatohepatitis and fibrosis in patients with NAFLD.[22, 23]
Although many studies have demonstrated that weight loss is an effective therapy for NAFLD and NASH, most have utilized intensive lifestyle or dietary interventions. It is under-appreciated if weight loss leads to a decrease in liver volume along with a parallel decrease in liver fat or whether the liver volume remains unchanged and a reduction in liver fat alone is seen. This study addresses that gap in knowledge.
In addition, many previous studies have relied on histologic findings of steatosis to determine changes in liver fat. More recently, magnetic resonance spectroscopy (MRS) has shown a quantitative reduction in liver fat with weight loss and dietary interventions.[24–26] Although MRS has been considered the gold-standard for quantitative liver fat assessment in patients with NAFLD, recent studies have utilized an advanced chemical-shift based gradient-echo MRI technique that measures the proton-density-fat-fraction (PDFF), a quantitative marker of fat content in tissue.[19, 27–29] This technique has been validated with MRS and has been shown to be more sensitive than histology-determined steatosis grade in quantifying increases and decreases in liver fat content.[30, 31] Unlike MRS, this technique creates a parametric fat map of the abdomen, which allows for assessment of changes in liver volume and fat content in other organs including the pancreas. Changes in liver volume in patients with NAFLD may be an important marker of disease progression as well as regression, as it has been linked to metabolic syndrome and reduction in steatosis and size is also noted in cirrhosis of the liver.[32, 33]
In this study, we aim to determine the quantitative effect of weight loss on MRI-PDFF estimated liver fat and liver volume in patients with biopsy-proven NASH. We hypothesized that weight loss leads to reduction in both liver fat and volume.
METHODS
Study design and patient population
This is a secondary analysis of a randomized controlled trial of 43 adult patients with biopsy-proven NASH. The primary outcome was change in MRI-estimated liver PDFF and MRI-estimated liver volume between the start (week 0) and completion (week 24) of the study. All patients were diagnosed with NASH based upon liver biopsy as well as exclusion of other causes of liver disease (detailed in the following section).
As part of the original study, all patients were randomized to receive either colesevelam, a bile acid sequestrant, or placebo over 24 weeks.[19] Patients underwent clinical evaluation, physical examination, biochemical testing and MRI at baseline and after 24 weeks. All patients provided written informed consent to participate in the study and the study was approved by the University of California at San Diego institutional review board. All patients underwent a standard history and physical exam, biochemical testing, and MRI examination at UCSD. They also all underwent an alcohol history assessment by completing the AUDIT and Skinner Lifetime Drinking questionnaires.
Two cohorts for this secondary analysis were derived according to those who had at least a 5% decrease in body-mass-index (BMI) and those who did not have at least a 5% decrease in BMI.
Inclusion and exclusion criteria, clinical and histologic evaluation are available in the supplementary text 1.
MRI protocol
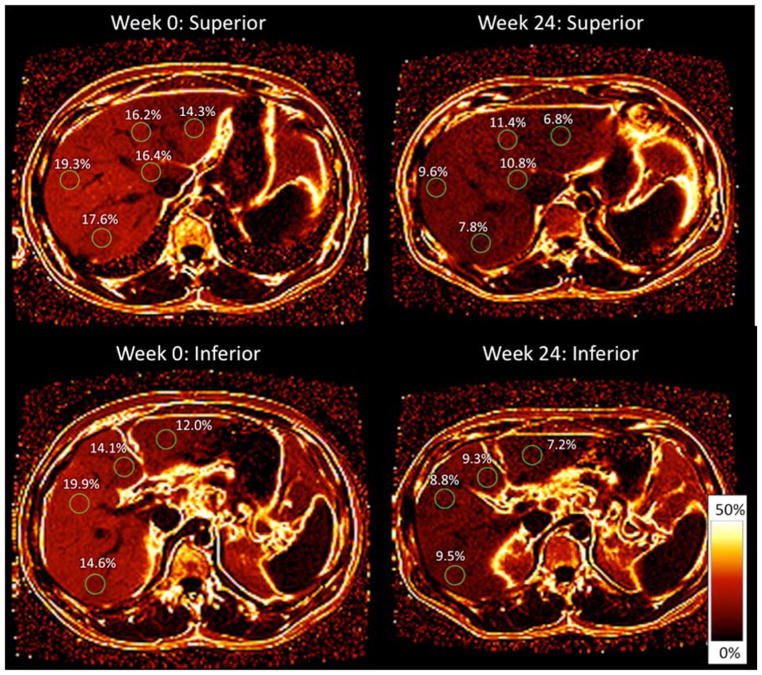
In order to quantify liver fat and volume, we used a previously described advanced chemical-shift based gradient-echo MRI technique that estimates PDFF, which is a standardized and objective measure of fat content.[27, 30, 31, 35–38] It acquires multiple echoes at different echo times with fat and water signals nominally in phase or out of phase with each other and applies an algorithm to generate a PDFF parametric map depicting fat quantity and distribution throughout the liver. This method has been shown to reliably measure liver fat content when compared to other magnetic resonance techniques and histology-determined steatosis, and it is sensitive in detecting changes in liver fat content[31, 36, 39]. In order to estimate PDFF across the entire liver, 3 regions of interest (ROIs) 300mm2 to 400mm2 in area were placed in each of the nine liver segments on the PDFF parametric maps (figure 1). In addition, fat content in the pancreas (pancreatic PDFF) was measured by placing 1 to 2 ROIs of 100mm2 in the head, body and tail of the pancreas in each slice of the PDFF parametric maps. These protocols have been described in prior studies.[27, 31] The mean of all ROIs in the liver and pancreas was calculated to determine the average PDFF in each organ. Liver volume was calculated by measuring the liver area in each slice of the original MR images and integrating this across all MRI slices.
Figure 1. Measurement of liver fat using MRI-PDFF.
A magnetic resonance image (MRI) proton density fat fraction (PDFF) parametric fat map is shown for the superior and inferior liver at week 0 and week 24 in a patient who had a 6.7% decrease in BMI during the study period. Source image was obtained with a slice thickness of 8 mm. Regions of interest (ROIs) 300 mm2 in area were obtained in each segment of the liver. The PDFF parametric fat map shows a decrease in fat fraction in each ROI from week 0 to week 24.
A single resident physician, trained in the MRI analysis, performed the measurements. The physician was blinded to clinical and histological data and was under the supervision of the radiology investigator (CS).
Statistical analysis
The two-tailed t-test was used for comparison of continuous variables across groups. The chi-squared test was used for comparisons of categorical variables. Patients were stratified into two groups according to whether a 5% or greater reduction in BMI was achieved. The mean and standard error values were calculated for demographic, biochemical, histologic and MRI-PDFF results and statistical analyses with t-tests were performed between these two groups. Paired t-tests were used to compare MRI-PDFF and liver volume changes between week 0 and week 24 of the study. All statistical analyses were performed using Excel and SPSS software packages. A P-value < 0.05 was considered statistically significant.
RESULTS
Demographic and biochemical data of patients
Forty-three patients with biopsy-confirmed NASH were included in this secondary analysis of a previously published RCT. Between the start of the study (week 0) and the completion (week 24), 10 patients had at least a 5% decrease in BMI while 33 patients either gained weight or had less than a 5% reduction in BMI. Overall, patients who had a 5% or greater decrease in BMI lost an average of 4.73 kg (±1.87) while patients who either gained weight or had less than a 5% decrease in BMI had an average weight gain of 0.61kg (±1.76). Baseline demographic and biochemical data at week 0 for these two groups were similar (table 1). Of note, patients with greater than 5% reduction in BMI had a slightly greater baseline BMI than patients without this degree of weight loss (33.7kg ±5.2 vs. 31.1 ±4.6), however, this difference was not statistically significant.
Table 1.
Baseline Demographics and Laboratory Data
| Weight loss <5% or gain (n=33) | Weight loss >5% (n=10) | p-value | |
|---|---|---|---|
| Demographics | |||
| Male (%) | 18 (54.5%) | 6 (60%) | 0.86 |
| Age (years) | 49.1 (±11.5) | 46.1 (± 13.6) | 0.49 |
| Weight (kg) | 88.5 (± 18.9) | 97.7 (± 21.6) | 0.20 |
| Height (m) | 1.67 (± 0.14) | 1.70 (± 0.13) | 0.57 |
| BMI (kg/m2) | 31.1 (± 4.6) | 33.7 (± 5.2) | 0.14 |
| Waist (cm) | 103.1 (± 13.5) | 108.9 (± 13.6) | 0.25 |
| Hip (cm) | 105.7 (± 12.0) | 109.3 (± 10.8) | 0.40 |
| Waist/Hip ratio | 0.98 (± 0.11) | 1.0 (± 0.10) | 0.63 |
| Biochemical profile | |||
| ALT (U/L) | 88.2 (± 68.0) | 62.9 (± 28.5) | 0.26 |
| AST (U/L) | 59.5 (± 46.7) | 34.5 (± 10.4) | 0.10 |
| Glucose (mg/dL) | 109.5 (± 25.7) | 111.5 (± 42.9) | 0.86 |
| Insulin (μIU/mL) | 30.6 (± 41.1) | 24.9 (± 8.8) | 0.67 |
| HOMA-IR | 9.1 (± 13.1) | 7.0 (± 4.5) | 0.63 |
| Hgb A1c (%) | 6.3 (± 0.8) | 6.3 (± 1.2) | 0.91 |
| Triglycerides (mg/dL) | 180.5 (± 135.5) | 179.5 (± 93.0) | 0.98 |
| Total cholesterol (mg/dL) | 205.0 (± 43.1) | 186.7 (± 45.2) | 0.25 |
| LDL (mg/dL) | 125.3 (± 37.3) | 102.7 (± 30.0) | 0.10 |
| HDL (mg/dL) | 48.6 (± 18.2) | 44.4 (± 6.4) | 0.48 |
| FFA (mg/dL) | 0.51 (± 0.16) | 0.52 (± 0.24) | 0.89 |
| Alk Phos (U/L) | 80.3 (± 24.5) | 71.6 (± 17.4) | 0.30 |
| GGT (U/L) | 77.7 (± 73.2) | 58.9 (± 26.3) | 0.43 |
| Total bilirubin (mg/dL) | 0.63 (± 0.44) | 0.45 (± 0.10) | 0.20 |
| Direct bilirubin (mg/dL) | 0.13 (± 0.06) | 0.11 (± 0.03) | 0.30 |
| Albumin | 4.6 (± 0.3) | 4.6 (± 0.2) | 0.99 |
Data are expressed as mean with standard deviation in parentheses unless otherwise noted. All baseline data was collected at week 0. Abbreviations for tables: BMI, body mass index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HOMA-IR, homeostatic model of insulin resistance; Hgb A1c, hemoglobin A1c; LDL, low-density lipoprotein; HDL, high-density lipoprotein; FFA, free fatty acids; Alk Phos, alkaline phosphatase; GGT, gamma glutamyl transferase. Insulin levels were measured while fasting. T-test for P-value assuming equal variance between groups.
Change in MRI-PDFF estimated liver fat and liver volume with weight loss
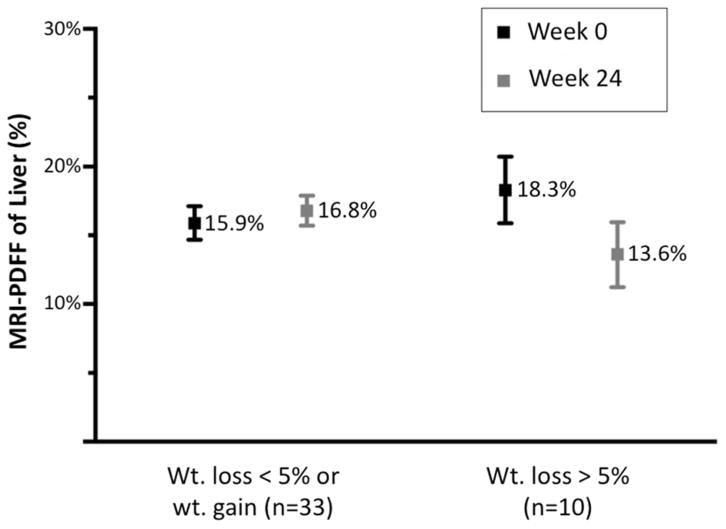
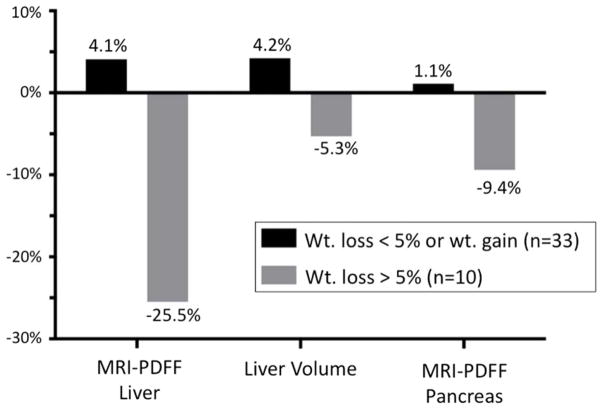
Patients with a 5% or greater decrease in BMI had slightly higher baseline MRI-estimated hepatic PDFF at week 0 than those without a 5% decrease in BMI (18.3 ±7.6% vs. 15.9 ±7.0%), however this difference was not statistically significant (p=0.36). Patients with a 5% or greater decrease in BMI had a significant decrease in MRI-estimated hepatic PDFF (18.3 ±7.6% to 13.6 ±13.6%, p=0.03), a 25.5% relative decrease (table 2, figure 2A, figure 2B). Similarly, total liver volume decreased significantly by 5.3% from 2003.8 mL (±474.4) to 1898.3 mL (±539.0), (p=0.02) in this group. A 9.4% relative decrease in MRI-estimated pancreatic PDFF was also noted in this group, however, this decrease was not significant. Patients who either gained weight or had less than 5% decrease in BMI experienced a slight increase in MRI-estimated hepatic PDFF (15.9 ±7.0% to 16.8 ±6.3%), a 4.1% relative increase. Liver volume also increased slightly by 4.2% from 1933.9 mL (±590.1) to 2015.1mL (±575.0). Neither of these changes were significant. Patients with a 5% or greater decrease in BMI also had a significant decrease in fasting glucose (111.5 ±42.9 to 95.7 ±13.2, p = 0.02) and Hgb A1c (6.3 ±1.2 to 5.9 ±0.5, p=0.02) (table 3). The mean (±SD) decrease in serum ALT between the weight loss group and no weight loss group was −17.3 (±28.9) and +12.5 (±55.5), respectively. The mean (±SD) decrease in serum AST between the weight loss group and no weight loss group was −3.6 (±16.6) and +2.3 (±44.4), respectively. Neither of these changes were statistically significant.
Table 2.
MRI-PDFF liver, liver volume and MRI-PDFF pancreas vs. weight loss
| Weight loss < 5% or weight gain (n=33) | Weight loss > 5% (n=10) | p-value** (between groups) | |||||
|---|---|---|---|---|---|---|---|
| Week 0 | Week 24 | p-value* | Week 0 | Week 24 | p-value* | ||
| MRI-PDFF liver (%) | 15.9 (7.0) | 16.8 (6.3) | 0.54 | 18.3 (7.6) | 13.6 (7.5) | 0.03 | 0.02 |
| MRI Liver Volume (mL) | 1933.9 (590.1) | 2015.1 (575.0) | 0.17 | 2003.8 (474.4) | 1898.3 (539.0) | 0.02 | 0.01 |
| MRI-PDFF pancreas (%) | 8.3 (6.7) | 8.5 (6.3) | 0.76 | 9.0 (6.4) | 8.2 (6.3) | 0.14 | 0.13 |
Data are expressed as mean with standard deviation in parentheses. Abbreviations for tables: MRI, magnetic resonance imaging; PDFF, proton density fat fraction.
P-value between week 0 and week 24 determined with paired T-test assuming equal variance between baseline and week 24 groups.
P-value between groups represents change between week 0 and week 24 between 2 groups (group 1: weight loss < 5% or weight gain, group 2: weight loss > 5%) determined with unpaired T-test assuming equal variance between groups.
Figure 2.
Figure 2A. Change in MRI-PDFF liver versus weight loss
Data are expressed as mean MRI-PDFF with standard error bars shown.
Abbreviations for tables: MRI, magnetic resonance imaging; PDFF, proton density fat fraction.
Figure 2B. Percent change in MRI-PDFF liver, liver volume and MRI-PDFF pancreas versus weight loss
Data are expressed as percent change from week 0 to week 24.
Abbreviations for tables: MRI, magnetic resonance imaging; PDFF, proton density fat fraction.
Table 3.
Biochemical changes versus weight loss
| Weight loss < 5% or weight gain | Weight loss > 5% | p-value** (between groups) | |||||
|---|---|---|---|---|---|---|---|
| Week 0 | Week 24 | p-value* | Week 0 | Week 24 | p-value* | ||
| Weight (kg) | 88.5 (18.9) | 89.1 (18.9) | 0.053 | 97.7 (21.6) | 92.9 (20.5) | <0.001 | <0.001 |
| BMI (kg/m2) | 31.1 (4.6) | 31.6 (4.6) | 0.055 | 33.7 (5.2) | 31.8 (4.7) | <0.001 | <0.001 |
| Waist (cm) | 103.1 (13.5) | 103.7 (12.1) | 0.46 | 108.9 (13.6) | 106.4 (15.6) | 0.04 | 0.04 |
| Hip (cm) | 105.7 (12.0) | 106.7 (11.3) | 0.28 | 109.3 (10.8) | 108.0 (11.7) | 0.46 | 0.24 |
| Waist/Hip ratio | 0.98 (0.11) | 0.97 (0.08) | 0.56 | 1.00 (0.10) | 0.98 (0.08) | 0.42 | 0.67 |
| ALT (U/L) | 88.2 (68.0) | 100.7 (66.9) | 0.20 | 62.9 (28.5) | 45.6 (21.9) | 0.09 | 0.22 |
| AST (U/L) | 59.5 (46.7) | 61.8 (38.5) | 0.77 | 34.5 (10.4) | 30.9 (19.3) | 0.51 | 0.68 |
| Glucose (mg/dL) | 109.5 (25.7) | 112.1 (29.8) | 0.38 | 111.5 (42.9) | 95.7 (13.2) | 0.18 | 0.02 |
| Insulin (μIU/mL) | 30.6 (41.1) | 37.8 (52.6) | 0.26 | 24.9 (8.8) | 26.7 (16.5) | 0.74 | 0.86 |
| HOMA-IR | 9.1 (13.1) | 10.8 (15.2) | 0.40 | 7.0 (4.5) | 6.1 (3.7) | 0.65 | 0.63 |
| Hgb A1c (%) | 6.3 (0.8) | 6.4 (1.0) | 0.28 | 6.3 (1.2) | 5.9 (0.5) | 0.12 | 0.02 |
Data are expressed as mean with standard deviation in parentheses. Abbreviations for tables: BMI, body mass index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HOMA-IR, homeostatic model of insulin resistance; Hgb A1c, hemoglobin A1c.
P-value between week 0 and week 24 determined with paired T-test assuming equal variance between baseline and week 24 groups.
P-value between groups represents change between week 0 and week 24 between 2 groups (group 1: weight loss < 5% or weight gain, group 2: weight loss > 5%) determined with unpaired T-test assuming equal variance between groups.
Change in liver histology with weight loss
29 of 43 patients had a repeat liver biopsy at completion of the study. This included 8 patients who had a 5% or greater decrease in BMI and 21 patients who gained weight or had less than a 5% decrease in BMI. Patients with a 5% or greater decrease in BMI had a significant decrease in steatosis grade (2.3 ±0.5 to 1.6 ±0.7, p= 0.049), however, no significant change in other components of NAS score was noted in either group in this trial (table 4).
Table 4.
Changes in liver histology versus weight loss
| Weight loss < 5% or weight gain (n=21) | Weight loss > 5% (n=8) | p-value** (between groups) | |||||
|---|---|---|---|---|---|---|---|
| Week 0 | Week 24 | p-value* | Week 0 | Week 24 | p-value* | ||
| NAS Score (0–8) | 4.6 (1.5) | 4.5 (2.0) | 0.90 | 4.8 (0.9) | 4.1 (1.4) | 0.28 | 0.43 |
| Steatosis grade (0–3) | 1.9 (0.8) | 1.5 (0.8) | 0.05 | 2.3 (0.5) | 1.6 (0.7) | 0.049 | 0.35 |
| Lobular inflammation (0–3) | 1.6 (0.7) | 1.8 (0.8) | 0.33 | 1.5 (0.5) | 1.5 (0.5) | 1.0 | 0.62 |
| Hepatocyte ballooning (0–2) | 1.1 (0.8) | 1.2 (0.7) | 0.60 | 1.0 (0.8) | 1.0 (0.5) | 1.0 | 0.77 |
Data are expressed as mean with standard deviation in parentheses. Abbreviations for tables: NAS, non-alcoholic fatty liver disease activity score.
P-value between week 0 and week 24 determined with paired T-test assuming equal variance between baseline and week 24 groups.
P-value between groups represents change between week 0 and week 24 between 2 groups (group 1: weight loss < 5% or weight gain, group 2: weight loss > 5%) determined with unpaired T-test assuming equal variance between groups.
DISCUSSION
In this secondary analysis of a randomized controlled trial using an advanced, validated MRI-method that allows non-invasive fat quantification of the liver, we demonstrate that patients with biopsy-proven NASH with at least a 5% reduction in BMI have a significant decrease in MRI-estimated liver PDFF from 18.3 ±7.6% to 13.6 ±13.6%, a relative decrease of 25.5%. This weight loss is also associated with a reduction in liver volume. We also found that weight loss leads to a decreased in pancreatic fat, however, this decrease did not reach statistical significance. Although weight loss resulted in a decrease in histology-determined steatosis grade, no decreased in NAS or reduction in transaminases was noted. In summary, these findings provide quantitative data that a 5% change in body weight is associated with a significant reduction in liver fat and volume. These data could be utilized to determine effect size and sample size estimation for future clinical trials in NASH and/or obesity. For example, if a drug/intervention is expected to lead to 5% reduction in BMI compared to placebo then a relative liver fat fraction reduction of 25% and a liver volume reduction of 5% may be expected.
As discussed previously, very few effective treatments for NASH are available. Weight-loss and lifestyle changes are considered important therapeutic strategies for reducing steatosis and inflammation in patients with NASH. In a study of 50 patients with biopsy-proven NASH treated with vitamin E, caloric restriction and or list at or placebo for 36 weeks, Harrison et al. showed that a 5% reduction in weight was associated with a significant reduction in biopsy-determined liver steatosis. A significant reduction in biopsy-determined lobular inflammation, hepatocyte ballooning and NAS was seen in patients with a 9% or greater weight reduction.[20] In a study of 31 patients with NASH, intensive diet, exercise and behavior modifications resulted in a significant reduction in NAS and an average 9.3% weight loss. In addition, degree of weight loss correlated strongly with reduction in NAS.[21]
Similar to prior studies, our study revealed a significant decrease in histology-determined liver steatosis and MRI-estimated hepatic PDFF with weight loss. Unlike prior studies by Harrison et al. and Promrat et al, weight loss in our study did not result in an improvement in histology-determined NAS.[20, 21] This may be due to sample size limitations, as not all patients had a repeat liver biopsy at completion of the study. It should be noted, however, that an improvement in NAS was seen in patients with a greater than 9% reduction in body weight and an average 9.3% reduction in body weight respectively in these prior studies. Patients in our study did not have this large of a weight loss. This suggests that a larger reduction in weight may be required to improve liver inflammation (lobular inflammation and hepatocyte ballooning) in patients with NASH. Similarly, a larger reduction in weight may be required to reduce liver transaminases, as no significant decrease in AST or ALT was seen in our study.
Early trials of weight loss in NAFLD relied on histologic assessment, however, several recent studies have also shown a decrease in MRS-determined steatosis in patients with NAFLD treated with lifestyle changes and weight loss.[24–26, 40] Overall, lifestyle modification and weight loss have reduced relative MRS-estimated liver fat by 21–80% in prior studies.[18] Our study showed a similar reduction in liver fat fraction of 25.5%, as assessed by MRI-PDFF, an advanced and standardized MRI technique. This technique has been validated with MRS and has been shown to be more sensitive than histology-determined steatosis grade in quantifying increases and decreases in liver fat content.[30, 31] Our study identified a decrease in liver volume associated with weight loss, which is likely caused by decreased liver fat and improvement in metabolic parameters associated with weight loss.[32, 33]
Pancreatic fat has been linked to liver fat content and may play a role in the development and progression of NAFLD and insulin resistance.[27, 28] Despite this association, there was only a small, insignificant reduction in MRI-estimated pancreatic PDFF with weight loss. This suggests that a greater reduction in weight may be required to result in decreased pancreatic fat content.
A prior meta-analysis of placebo patients in NASH revealed that a third of patient in the placebo arm of four studies had an improvement in a histologic parameter at the conclusion in the study.[41] Our study suggests that this may be in part due to incidental weight loss and lifestyle changes that should be accounted for when designing clinical trials for the treatment of NASH. Our study may also help in determining sample size estimation in designing future studies on NASH to target a 5% decreased in BMI with MRI-determined fat fraction as an endpoint.
Strengths and Limitations
The major strengths of this study include the use of a well-characterized patient population with biopsy-proven NASH and the use of an MRI technique that has been validated as a sensitive measure of changes in liver steatosis in patients with NAFLD. This study is also unique in assessing changes in liver volume and pancreatic fat content with weight loss. In addition, histologic assessment was available at the start and completion of our study. Despite this, we do acknowledge limitations of this study. This cohort study was derived from a prospective study of colesevelam, a bile-acid sequestrant, in the treatment of NASH. Although colesevelam did not significantly reduce liver fat or histologic parameters of NASH in this study, the effect of colesevelam may not have been entirely accounted for. In addition, there were limitations in sample size that may have reduced the ability to identify significant changes in histologic parameters associated with weight loss.
CONCLUSIONS
A reduction in BMI of at least 5% is associated with a significant decrease in MRI-estimated liver PDFF and volume in patients with biopsy-proven NASH. Although weight loss resulted in a decrease in histology-determined steatosis grade, no decreased in NAS or reduction in transaminases was noted. These data have implications in assessing effect size in NAFLD and obesity trials that use MRI-estimated liver fat and volume as endpoints.
Acknowledgments
Grant support: The study was conducted at the Clinical and Translational Research Institute, University of California at San Diego. RL is supported in part by the American Gastroenterological Association (AGA) Foundation – Sucampo – ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award; Funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, the Association of Specialty Professors, and the American Gastroenterological Association and grant K23-DK090303-02 and by the UCSD Digestive Diseases Research Development Center, US PHS grant #DK080506. This research was partially supported by the Clinical & Translational Research Institute (CTRI) at the, University of California, San Diego. The CTRI is funded from awards issued by the National Center for Advanced Translational Sciences, UL1RR031980 and Dr. Seki was supported by R01DK085252, R01AA02017204 and P42ES010337. Dr. Sirlin was supported by R01DK088925.
Role of study sponsor: The study sponsor(s) had no role in the study design, collection, analysis, interpretation of the data, and/or drafting of the manuscript.
Abbreviations
- MRI
magnetic resonance imaging
Footnotes
Potential competing interests: none
Guarantor of the article: Rohit Loomba
Writing Assistance: none
All authors report that no conflicts of interest exist.
Author contributions:
Niraj Patel - Analysis and interpretation of data, statistical analysis, drafting of the manuscript, critical revision of the manuscript, approved final submission
Iliana Doycheva - Analysis and interpretation of data, critical revision of the manuscript, approved final submission
Michael Peterson - critical revision of the manuscript, approved final submission
Jonathan Hooker – Analysis of data, critical revision of the manuscript, approved final submission
Tatiana Kisselva – critical revision of the manuscript, approved final submission
Bernd Schnabl – critical revision of the manuscript, approved final submission
Ekihiro Seki – critical revision of the manuscript, approved final submission
Claude Sirlin – development and supervision of imaging protocol, critical revision of the manuscript, approved final submission
Rohit Loomba - study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, obtained funding, study supervision, approved final submission
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 2.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–90. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 3.Bellentani S, Saccoccio G, Masutti F, et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112–7. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 4.Kotronen A, Peltonen M, Hakkarainen A, et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137:865–72. doi: 10.1053/j.gastro.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Loomba R, Abraham M, Unalp A, et al. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012;56:943–51. doi: 10.1002/hep.25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zarrinpar A, Loomba R. Review article: the emerging interplay among the gastrointestinal tract, bile acids and incretins in the pathogenesis of diabetes and non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2012;36:909–21. doi: 10.1111/apt.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–31. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 8.Bellentani S, Scaglioni F, Marino M, et al. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155–61. doi: 10.1159/000282080. [DOI] [PubMed] [Google Scholar]
- 9.Bhala N, Angulo P, van der Poorten D, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology. 2011;54:1208–16. doi: 10.1002/hep.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh S, Allen AM, Wang Z, et al. Fibrosis Progression in Nonalcoholic Fatty Liver versus Nonalcoholic Steatohepatitis: A Systematic Review and Meta-analysis of Paired-Biopsy Studies. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratziu V, Giral P, Jacqueminet S, et al. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology. 2008;135:100–10. doi: 10.1053/j.gastro.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 12.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 13.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lincoff AM, Wolski K, Nicholls SJ, et al. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA. 2007;298:1180–8. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 15.Boettcher E, Csako G, Pucino F, et al. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2012;35:66–75. doi: 10.1111/j.1365-2036.2011.04912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–68. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 18.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 19.Le TA, Chen J, Changchien C, et al. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: A randomized controlled trial. Hepatology. 2012;56:922–932. doi: 10.1002/hep.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison SA, Fecht W, Brunt EM, et al. Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology. 2009;49:80–6. doi: 10.1002/hep.22575. [DOI] [PubMed] [Google Scholar]
- 21.Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–9. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathurin P, Hollebecque A, Arnalsteen L, et al. Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology. 2009;137:532–40. doi: 10.1053/j.gastro.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 23.Mummadi RR, Kasturi KS, Chennareddygari S, et al. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2008;6:1396–402. doi: 10.1016/j.cgh.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Lazo M, Solga SF, Horska A, et al. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care. 2010;33:2156–63. doi: 10.2337/dc10-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Browning JD, Baker JA, Rogers T, et al. Short-term weight loss and hepatic triglyceride reduction: evidence of a metabolic advantage with dietary carbohydrate restriction. Am J Clin Nutr. 2011;93:1048–52. doi: 10.3945/ajcn.110.007674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong VW, Chan RS, Wong GL, et al. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2013;59:536–42. doi: 10.1016/j.jhep.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 27.Patel NS, Peterson MR, Brenner DA, et al. Association between novel MRI-estimated pancreatic fat and liver histology-determined steatosis and fibrosis in non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;37:630–9. doi: 10.1111/apt.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel NS, Peterson MR, Lin GY, et al. Insulin Resistance Increases MRI-Estimated Pancreatic Fat in Nonalcoholic Fatty Liver Disease and Normal Controls. Gastroenterol Res Pract. 2013;2013:498296. doi: 10.1155/2013/498296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reeder SB, Cruite I, Hamilton G, et al. Quantitative Assessment of Liver Fat with Magnetic Resonance Imaging and Spectroscopy. J Magn Reson Imaging. 2011;34 doi: 10.1002/jmri.22580. spcone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hines CD, Frydrychowicz A, Hamilton G, et al. T(1) independent, T(2) (*) corrected chemical shift based fat-water separation with multi-peak fat spectral modeling is an accurate and precise measure of hepatic steatosis. J Magn Reson Imaging. 2011;33:873–81. doi: 10.1002/jmri.22514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noureddin M, Lam J, Peterson MR, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology. 2013;58:1930–40. doi: 10.1002/hep.26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santini F, Giannetti M, Mazzeo S, et al. Ultrasonographic evaluation of liver volume and the metabolic syndrome in obese women. J Endocrinol Invest. 2007;30:104–10. doi: 10.1007/BF03347407. [DOI] [PubMed] [Google Scholar]
- 33.Giannetti M, Piaggi P, Ceccarini G, et al. Hepatic left lobe volume is a sensitive index of metabolic improvement in obese women after gastric banding. Int J Obes (Lond) 2012;36:336–41. doi: 10.1038/ijo.2011.243. [DOI] [PubMed] [Google Scholar]
- 34.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 35.Noureddin M, Yates KP, Vaughn IA, et al. Clinical and histological determinants of nonalcoholic steatohepatitis and advanced fibrosis in elderly patients. Hepatology. 2013 doi: 10.1002/hep.26465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuhn JP, Hernando D, Mensel B, et al. Quantitative chemical shift-encoded MRI is an accurate method to quantify hepatic steatosis. J Magn Reson Imaging. 2014;39:1494–501. doi: 10.1002/jmri.24289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hines CD, Yu H, Shimakawa A, et al. T1 independent, T2* corrected MRI with accurate spectral modeling for quantification of fat: validation in a fat-water-SPIO phantom. J Magn Reson Imaging. 2009;30:1215–22. doi: 10.1002/jmri.21957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu CY, McKenzie CA, Yu H, et al. Fat quantification with IDEAL gradient echo imaging: correction of bias from T(1) and noise. Magn Reson Med. 2007;58:354–64. doi: 10.1002/mrm.21301. [DOI] [PubMed] [Google Scholar]
- 39.Zhong X, Nickel MD, Kannengiesser SA, et al. Liver fat quantification using a multi-step adaptive fitting approach with multi-echo GRE imaging. Magn Reson Med. 2013 doi: 10.1002/mrm.25054. [DOI] [PubMed] [Google Scholar]
- 40.Johnson NA, Sachinwalla T, Walton DW, et al. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50:1105–12. doi: 10.1002/hep.23129. [DOI] [PubMed] [Google Scholar]
- 41.Loomba R, Wesley R, Pucino F, et al. Placebo in nonalcoholic steatohepatitis: insight into natural history and implications for future clinical trials. Clin Gastroenterol Hepatol. 2008;6:1243–8. doi: 10.1016/j.cgh.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]