Abstract
Tumors actively suppress antitumor immunity creating formidable barriers to successful cancer immunotherapy. The molecular mechanisms underlying tumor-induced immune tolerance are largely unknown. In the present study, we show that dendritic cells (DCs) in the tumor microenvironment (TME) acquire the ability to metabolize vitamin A to produce retinoic acid (RA), which drives T regulatory responses and immune tolerance. Tolerogenic responses were dependent on induction of vitamin A-metabolizing enzymes via the β-catenin/T Cell Factor (TCF) pathway in DCs. Consistent with this observation, DC-specific deletion of β-catenin in mice markedly reduced T regulatory responses and delayed melanoma growth. Pharmacological inhibition of either vitamin A metabolizing enzymes or the β-catenin/TCF4 pathway in vivo had similar effects on tumor growth and T regulatory responses. Hence, β-catenin/TCF4 signaling induces local regulatory DC and regulatory T cell phenotypes via the RA pathway, identifying this pathway as an important target for anticancer immunotherapy.
Introduction
Tumors promote immune tolerance, and DCs play an important role in this. Although the molecular mechanisms of immune suppression by DCs are not fully understood, the ability of DCs to induce and activate regulatory T cells (Treg) is involved (1-5). Retinoic acid (RA), an active metabolite of vitamin A, regulates a broad array of immune responses (6,7). Accumulated evidence suggests an important role for RA in immune tolerance in the gut by regulating the functions of antigen presenting cells (APCs), and by promoting the induction and activation of Treg (6,7). A recent study has shown that TME contains high levels of RA, and APCs are major producers of RA (8). However, its role in Treg induction and activation in response to tumor-induced immune tolerance is not known. Moreover, molecular mechanisms whereby tumors induce APCs to produce RA remain poorly understood. So, we hypothesized that tumors, through DCs, exploit the RA pathway as a mechanism of immune evasion.
β-catenin is a transcriptional co-factor which, upon activation, interacts with several family transcription factors (TFs) such as TCFs (9), PPARγ (10,11), Foxo (12,13), VDR (12,14), IRF3 (15) etc. Upon interaction with TFs, β-catenin can either promote or suppress the expression of target genes (9,13,14). Aberrant β-catenin signaling is associated with cancer development, progression and even metastasis (9,16,17). A recent study has demonstrated that tumors activate β-catenin in APCs including DCs (18). However, its role in the induction and activation of Treg responses to tumor is not known. In addition, the downstream mediator of β-catenin signaling in DCs that drives immune tolerance to tumors remains poorly understood. In our previous study with intestinal DCs, we have shown that the β-catenin pathway programs DCs to a regulatory state and promotes immune tolerance to commensal microbiota (19). So, we hypothesize that tumors promote immune tolerance by activating the β-catenin/TCF pathway in DCs to induce RA, which drives T regulatory responses.
In this study, using murine tumor models, we show that tumors program DCs to produce RA, which promotes immune suppression by inducing T regulatory responses. This is mediated through the induction of vitamin A-metabolizing enzymes via the β-catenin/TCF pathway in DCs, which in turn drives T regulatory responses and suppresses T cell effector response.
Materials and Methods
Mice
C57BL/6 male mice 6–12 wk of age were purchased from The Jackson Laboratory (Bar Harbor, ME). OT-II (Rag 2−/−) mice were purchased from Taconic. TCF/LEF-reporter mice (20), β-catenin floxed mice (21) and CD11c-cre (22) mice were originally obtained from Jackson Laboratories and bred on-site. β-catenin floxed mice were crossed with transgenic mice expressing Cre recombinase under the control of a CD11c promoter, to generate mice lacking β-catenin in DCs (β-catΔDC). Successful cre-mediated deletion was confirmed by polymerase chain reaction (PCR) and protein expression analyses as previously described (19). TCF4 floxed mice (23) were crossed to CD11c-cre mice to generate mice with DCs deficient in TCF4 (TCF4ΔDC) and successful cre-mediated deletion was confirmed by PCR (19). All the mice were housed under specific pathogen-free conditions in the Laboratory Animal Services of Georgia Regents University. Animal care protocols were approved by the Institutional Animal Care and Use Committee of Georgia Regents University.
Antibodies and reagents
Antibodies against mouse CD4 (GK1.5), CD8a (53-6.7), CD45 (30-F11), Foxp3 (clone FJK-16s), IL-10 (JES5-16E3), CD11c (clone N418), I-Ab (clone 25-9-17), CD90.1 (HIS51), V alpha 2 TCR (B20.1), V beta 5.1/5.2 TCR (MR9-4), IFN-γ (XMG1.2), CD80 (16-10A1), CD86 (GL1), CD274 (MH5) and CD273 (TY25) were purchased from eBioscience. Non-phospho active β-catenin, β-catenin and TCF4 antibodies were obtained from Cell signaling technology. β-galactosidase (β-gal) antibody was purchased from Abcam. Retinol, all-trans retinoic acid (ATRA) (Sigma-Aldrich), retinoid acid receptor antagonists LE135 (Tacoris), LE540 (Wako, Japan), Citral (Sigma-Aldrich), JW55 (Tocris) and XAV 939 (Tocris) were dissolved in DMSO (1 mM). OVA323–339 (ISQVHAAHAEINEAGR) peptide was purchased from Anaspec.
Plasmids, cell culture, transient transfection and reporter assay
Raldh2 promoter luciferase construct was kindly provided by Dr. Ofelia M. Martínez-Estrada (24). Wild type β-catenin, dominant negative β-catenin and active β-catenin plasmids were provided by Dr. Zuoming Sun (City of Hope). Wild type (WT)-TCF4 and dominant negative TCF4 plasmids were from Addgene. 293T cells were cultured in DMEM supplemented with 10% FBS, 2 mM glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin. Cells (2–3×105 in each well of a 24 well-plate) were transfected with the Raldh2 promoter reporter plasmid (100 ng) and expression vectors (500 ng) by Lipofectamine method (Invitrogen). The total amount of transfected DNA was kept constant by adjusting the amount of the empty vector. Cells were collected after 24 h and lysed in 200 μl reporter lysis buffer and luciferase activities were measured by the Luciferase system, according to the manufacturer’s instructions (Promega), and normalized against Renilla luciferase activities. “Fold Induction” represents normalized luciferase activity divided by the result of reporter-only groups.
Tumor model and in-vivo treatment
Age matched littermate controls were used for tumor studies. B16 melanoma expressing ovalbumin, clone MO4, was obtained from Dr. David Munn (Georgia Regents University, Augusta, GA, USA) (25) and EL4 lymphoma expressing ovalbumin, clone E.G7-OVA (EG7) was obtained from the American Type Culture Collection. To establish tumors, 5×105 MO4 and EG7 cells were injected subcutaneously (s.c.) into mice in the shaved flank region. The tumor growth was monitored every 3 days by measuring the perpendicular diameters. The experiments were terminated when tumor size reached area greater than 2 cm2. The tumors were excised and tumor-draining lymph nodes (TDLNs) were collected for various analyses. To study the effect of disulfiram, ATRA, JW55 and XAV 939 in vivo, tumor transplanted C57BL/6 or β-cateninΔDC or respective littermate control mice were injected intraperitoneally (i.p.) with disulphiram (5 mg/kg) or ATRA (2.5 mg/kg), JW55 (2mg/kg) or XAV 939 (2 mg/kg) every 3 days on day 5, 8, 11 and 14 after tumor implantation. Alternatively, mice were treated from day 10 followed by day 13, 16 and 19 post tumor transplantation with XAV939 or with citral (2mg/ml) in water from day 5 after tumor implantation until day 17 post tumor inoculation. In some experiments, tumor-bearing mice were administered with these treatments and on day 9, 4×106 sorted CD4+CD25− T cells from Rag−/− OT-II TCR transgenic mice were injected by intravenous (i.v.) route. Five days later, tumors and TDLNs were removed and stained with antibodies and analyzed by flow cytometry.
Cell cultures
CD45+CD11c+ cells from TDLN or tumor were isolated by FACS sorting and co-cultured with naive OT-II (CD4+CD25−) T cells in the presence of OVA peptide (2 μg/ml), transforming growth factor β (TGF-β) (1 ng/ml), and IL-2 (5 ng/ml). After 5 days, cells were stained and analyzed for CD4 and Foxp3 expression. In some experiments, purified CD11c+ DCs (2×104) cells were treated with disulfiram (Sigma-Aldrich) (100 nM), retinol (500 nM), ATRA (50 nM) or LE135/LE540 (1 μM) as in our previous study(26) and followed by co-culture with naive CD25−CD4+ OT-II T cells (1×105) in 200 μl RPMI complete medium containing OVA peptide (2 μg/ml) and TGFβ (1 ng/ml) in 96-well round-bottomed plates. After 5 days, cells were collected and analyzed by flow cytometry.
Flow cytometry analysis
To measure cytokines, single-cell suspensions from spleen, TDLN or tumor were ex vivo stimulated with phorbol 12-myristate 13-acetate (PMA)/Ionomycin and BrefedinA/monesin (eBioscience) for 6 hours at 37 °C, intracellular staining of IFN-γ and IL-10 was performed. To measure Treg or β-catenin or β-gal expression, corresponding Ab were added after permeabilization and fixation of cells(27). To detect aldehyde dehydrogenase (ALDH) activity, we used ALDEFLUOR kit (Stemcell) following the manufacturer’s recommendations(27). Flow cytometric analysis were performed using a FACS LSRII system (BD Biosciences) and the data were analyzed using FlowJo software (Ashland, OR).
Real-time PCR
Total mRNA was isolated from purified splenic, lymph node, and tumor CD11c+ DCs using the Omega Total RNA Kit according to the manufacturer’s protocol. cDNA was generated using the RNA to cDNA Ecodry Premix Kit (Clontech) according to the manufacturer’s protocol. cDNA was used as a template for quantitative real-time PCR using SYBR Green Master Mix (Roche), and gene-specific primers (19,26). PCR analysis was performed using a MyiQ5 ICycler (BioRad). Gene expression was calculated relative to Gapdh.
Statistical analyses
Statistical analyses were performed using GraphPad Prism software. An unpaired one - tailed Student’s t test was used to determine statistical significance for tumor areas, mRNA expression levels, Treg percentages, and cytokines released by various cell types between different groups. A P value less than 0.05 (*) was considered to be significant, a P value less than 0.01 (**) was considered to be very significant, and a P value less than 0.001 (***) was considered to be extremely significant.
Results
Tumors induce expression of RA-synthesizing enzymes in DCs
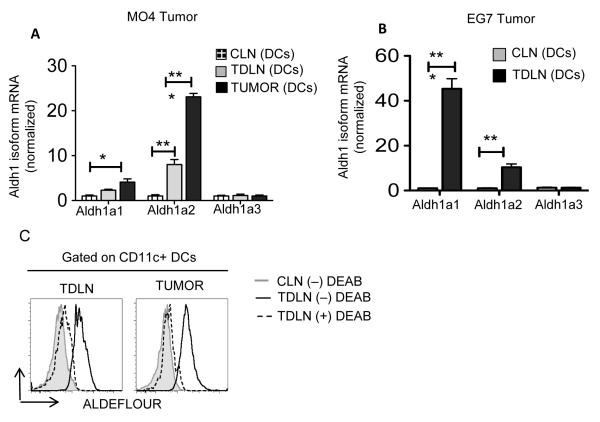
TME and tumor-draining lymph nodes (TDLNs) have elevated levels of RA and tumor APCs act as a major producer/source of RA (8). Within cells, RA is produced from vitamin A (retinol) via a two-step enzymatic pathway where retinol is first oxidized to retinaldehyde (retinal) followed by conversion to RA (7). First, we investigated whether DCs from TME and TDLNs express enzymes that are critical for RA biosynthesis. RTPCR analysis showed that control lymph node (CLN) DCs and TDLN DCs expressed similar levels of alcohol dehydrogenase (Adh)1 and Adh5, suggesting Adh expression in DCs is not regulated in response to tumor (Data not shown). Interestingly, however, TDLN DCs and tumor DCs showed a significant increase in the expression of Aldh1a1 and Aldh1a2 isoforms compared to the CLN DCs (Fig 1A, 1B). This was unexpected because unlike intestinal DCs, peripheral DCs do not express these genes under homeostatic conditions (7). To further validate these findings, we performed Aldh activity assay. TDLN DCs and TME DCs showed markedly increased Aldh activity compared to CLN DCs or DEAB (Aldh1 specific inhibitor)-treated DCs (Fig 1C). With further analysis of DC subsets, we observed high levels of Aldh1 activity in TDLN CD11c+CD11b− DCs and plasmacytoid DCs (pDCs) (data not shown). Taken together, these data indicate that DCs express high levels of Aldh1, a key enzyme in RA synthesis, in response to tumors.
Figure 1. DCs express vitamin A-metabolizing enzymes in response to tumor.
Real-time PCR analysis of mRNA levels of Aldh1 isoforms (Aldh1a1, Aldh1a2, Aldh1a3) expression in CD11c+ DCs enriched from TDLNs, CLNs and tumors pooled from WT mice on day 9 post-tumor inoculation (A) MO4 and (B) EG7 (n= values are represented in triplicates from two independent experiment with pooled DCs obtained from 5 mice per experiment). (C) Ex vivo analysis of ALDH activity in CD11c+ DCs cells enriched from TDLNs, CLNs and tumors from WT mice (n= cells were pooled from 5-6 tumor bearing mice). DEAB, a specific inhibitor of ALDH, was used as control for background fluorescence. Data are representative of at least two independent experiments. Error bars show mean values ± SD. Statistical levels of significance were analyzed by the Student t test (unpaired). *, P<0.05; **, P<0.01; ***, P<0.001.
TDLN DCs induce T regulatory cell differentiation via a RA-dependent pathway
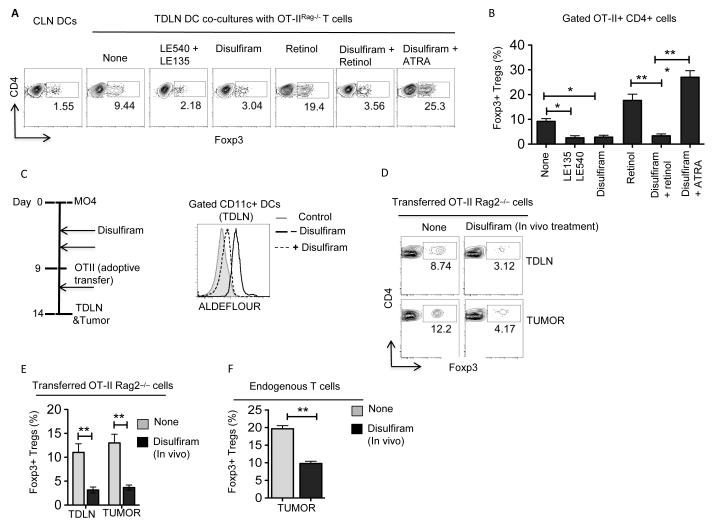
TME conditions DCs to acquire regulatory properties that mostly induce T regulatory responses (1,3,4,28). However, it is not known whether Treg activation and induction by DCs in the TME is dependent on RA. We hypothesized that Treg activation and induction by DC is depenendent on RA. To test this, we evaluated the ability of TDLN DCs to promote differentiation of naive CD4+ T cells into Foxp3+ Tregs in the presence of RA inhibitors. As shown in Figure 2A, blocking Aldh1 activity with disulfiram (Aldh1 inhibitor) (26) significantly reduced the ability of TDLN DCs to induce the differentiation of Treg cells. Since TDLN DCs express Aldh1, we next determined whether these DCs could metabolize vitamin A (retinol) to RA, and promote Treg differentiation. Accordingly, addition of exogenous vitamin A (retinol) enhanced the conversion of CD4 T cells to Foxp3+ Tregs, indicating that TDLN DCs can actively metabolize vitamin A to RA and drive Treg differentiation (Fig 2A, B). Furthermore, addition of pan-RAR inhibitors (LE540/LE135) to the co-cultures markedly reduced the proportion of Tregs induced by TDLN DCs (Fig 2A, B), suggesting that Treg induction is dependent on RA and the RAR/RXR-pathway. Taken together, these data demonstrate that TDLN DCs can metabolize vitamin A to RA and can drive Treg differentiation.
Figure 2. RA in TME induces Foxp3+ Treg cells.
(A and B) CD11c+ DCs were sorted from TDLNs and CLNs of MO4 tumor bearing WT mice on day 9-11 post-tumor inoculation and were treated with or without disulfiram (1 μM). After 3 h, DCs were washed and co-cultured with CD4+CD25− cells from Rag−/−OTII mice in RPMI1640 medium containing OVA peptide (2 μg/ml) and TGFβ (1 ng/ml), in the presence or absence of LE540+LE135 (1 μM) or retinol (1 μM) or ATRA (all-trans retinoic acid) (5 nM) combination for 5 days. Representative FACS plots (A) and frequencies (B) of Foxp3+CD4+ T (n=3; DCs were pooled from 5 to 6 mice and experiment was repeated at least two times). (C) Ex vivo analysis of ALDH activity in CD11c+ DCs enriched from TDLNs, CLNs and tumors from WT mice treated with or without disulfiram on day 14 post-tumor inoculation. DEAB, a specific inhibitor of ALDH, was used as control for background fluorescence. Data shown are representative histograms gated on CD45+ CD11c+ cells from one experiment of two independent experiments. DCs were pooled from 5 to 6 tumor bearing mice for each experiment. (D and E) MO4 bearing mice treated with or without disulfiram were adoptively transferred with OT-II T cells from Rag−/−OTII mice on day 9 after tumor inoculation. After 5 days, FACS analysis was performed on CD45+Vα2+Vβ5.1/5.2+ CD4+ T cells from TDLNs and tumor tissues. (D) Data shown are percentage and representative dot plots of OVA-specific Foxp3+CD4+ T cells. (n=3 to 4 mice per experiment. Experiment was repeated two times). (E) Data are from experiment (D) showing cumulative frequencies of adoptively OVA-specific Foxp3+CD4+ T cells and show mean values ± SEM (n 6 to 8 mice). Error bars show mean values ± SEM. Statistical levels of significance were analyzed by the Student t test (unpaired) * P<0.05; ** P<0.01; *** P<0.001.
To investigate the in vivo role of RA in Treg induction in response to tumor, we examined the effects of pharmacological inhibition of Aldh1 activity with disulfiram as performed in our previous study (26). WT mice implanted with MO4 tumor cells were treated with or without disulfiram and followed by adoptive transfer of naïve OT-II CD4+ T cells (Fig 2C). Five days post-adoptive transfer, the frequency of OVA-specific Foxp3+CD4+ Treg cells in the tumor and TDLN were analyzed. First, we assessed whether treatment with disulfiram inhibited Aldh1 activity in TDLN DCs. Treatment of tumor-bearing mice with disulfiram significantly decreased the Aldh1 activity in TDLN DCs as compared to untreated control group (Fig 2C). In addition, disulfiram treatment of tumor-bearing mice significantly delayed tumor growth in mice compared to control mice (Supplementary Figure 1A). Next, we analyzed the differences in frequency of Foxp3+ OT-II T cells treated with or without disulfiram. Blocking Aldh1 activity markedly reduced the frequency of induced Tregs both in TDLN and in tumor tissue compared to untreated mice (Fig 2D, E). Moreover, disulfiram treatment also affected the frequency of endogenous Tregs within the tumor (Fig 2F). These findings were further confirmed with citral, another Aldh1 inhibitor (Supplementary Figure 1B, C)(29). These observations demonstrate that pharmacological blocking of vitamin A-metabolizing enzyme suppresses Treg differentiation and enhances antitumor immunity. Thus, RA is necessary for in vivo induction of Treg cells in response to tumors.
β-catenin/TCF4 is critical for the expression of vitamin A-metabolizing enzymes in DCs
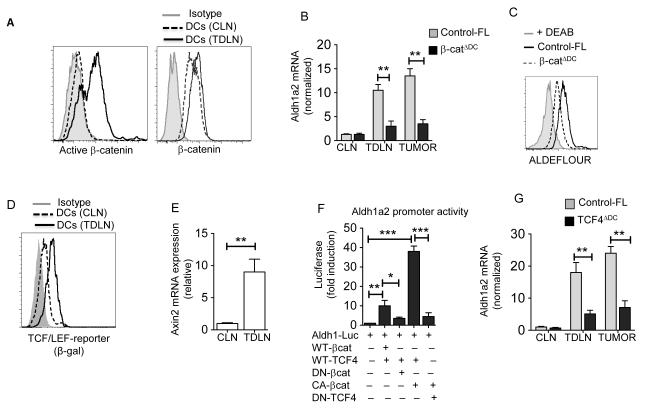
Recent studies have shown that tumors activate β-catenin in immune cells including DCs (18,30). Our previous study on intestinal DCs has shown that Aldh1a1 and Aldh1a2 are β-catenin target genes (19). We hypothesized that tumor-induced activation of the β-catenin pathway in DCs is critical for up-regulation of RA synthesizing enzymes. So, we assessed whether tumors activate β-catenin in DCs using an antibody that recognizes the active form of β-catenin. Consistent with a previous study (18), TDLN DCs showed increased levels of active β-catenin compared to CLN DCs or isotype control (Fig 3A, Right panel). However, both CLN DCs and TDLN DCs expressed a similar level of total β-catenin (Fig 3A, Left Panel), suggesting that tumors induce the activation of β-catenin in DCs but do not affect its expression. Next, we examined whether induction of Aldh1 in TDLN DCs is dependent on the activation of β-catenin. In contrast to TDLN DCs from control-FL mice, DCs deficient in β-catenin (19) expressed significantly lower levels of Aldh1 in response to MO4 and EG7 tumors (Fig. 3B; Supplementary Figure 2). Consistent with this observation, β-catenin-deficient TDLN DCs showed markedly reduced Aldh1 activity compared to respective control-FL TDLN DCs (Fig. 3C). Collectively, these data demonstrate that TME program DCs to express Aldh1a2 in a β-catenin-dependent manner.
Figure 3. β-catenin/TCF4 pathway induces vitamin A-metabolizing enzymes in DCs.
(A) Representative histograms showing active-β-catenin (Left) and β-catenin (Right) expression in CD11c+ cells from MO4 tumor TDLN and CLN of tumor-free C57BL/6 (WT) mice on day 9 post tumor inoculation. (B) Real-time PCR analysis of Aldh1a2 expression from TDLNs, CLNs and tumors (n=3) and (C) Ex vivo analysis of ALDH activity in CD11c+ DCs enriched from TDLNs from β-catΔDC and control-FL mice. (D) Representative histograms showing β-gal expression in CD11c+ cells from TDLNs and CLNs of TCF/LEF-LacZ reporter mice. (E) Axin2 mRNA expression analyzed by qRTPCR in TDLNs and CLNs on day 9 post inoculation (n=). The result represents fold increase over the CLNs. (F) Aldh1a2 promoter activity in 293T cell after 24 h transfection reporter plasmid alone or co-transfected with WT-β-catenin (WT-βcat), dominant negative β-catenin (DN-βcat), Active β-catenin (CA-βcat), WT-TCF4 or dominant negative TCF4 (DN-TCF4) expression vectors. The results are represented as fold activation relative to Aldh1 promoter vector transfection control alone (G) Real-time PCR analysis of Aldh1a2 expression in CD11c+ DCs enriched from TDLNs, CLNs and tumors from TCF4ΔDC and control-FL mice. Iso: isotype control. DCs were enriched and pooled from 5 to 6 tumor bearing mice (A, C and D). Each sample was done in triplicate; the average and SD are shown. The experiment was repeated atleast two (B, E and G) or three (F) times with similar results. Error bars show mean values ± SEM. Statistical levels of significance were analyzed by the Student t test (unpaired) * P<0.05; ** P<0.01; *** P<0.001.
We next investigated the downstream signaling pathways through which β-catenin can induce Aldh1 expression in DCs. The TCF family of transcription factors is one of the main downstream mediators of β-catenin signaling (9) and DCs highly express the TCF4 isoform (31). So, we tested whether tumor-induced activation of β-catenin in DCs promotes TCF/LEF-dependent gene transcription using TCF/LEF β-gal reporter mice (19). TDLN DCs from the reporter mice showed strong β-gal expression compared to CLN DCs (Fig 3D). Consistent with this, we also observed increased expression of the β-catenin/TCF4 target gene, Axin2 in TDLN DCs compared to CLN DCs (Fig 3E). Taken together, our data demonstrates that activation of β-catenin induces the expression of TCF4 target genes in DCs in response to tumor.
Based on the above observation, we reasoned that TCF4 is the downstream mediator of β-catenin signaling in DCs that drives the expression of RA-synthesizing enzymes in response to tumor. Promoter analysis using consensus TCF binding sites showed several potential TCF sites in the Aldh1a2 promoter region with some near the transcription initiation regions. To further investigate transcriptional regulation of Aldh1a2 by β-catenin/TCF4, 293T cells were stably transfected with a 830-bp Aldh1a2-promoter luciferase reporter plasmid (24) either alone or in combination with wild type or mutant β-catenin or TCF4 expression plasmids. Co-transfection with WT β-catenin and WT-TCF4 plasmids resulted in 10-12-fold increase of Aldh1a2 promoter activity (Fig. 3F). Likewise, expression of active β-catenin further increased promoter activity. In contrast, expression of a dominant negative β-catenin or TCF4 markedly decreased its activity (Fig. 3F). Consistent with these observations, deletion of TCF4 specifically in DCs resulted in decreased expression of Aldh1a2 gene in response to tumor (Fig. 3G). Taken together, these results clearly demonstrate that TME induces expression of activated β-catenin in DCs, which interacts with TCF4 and promotes transcriptional activation of Aldh1 gene expression.
DC-specific deletion of β-catenin limits T regulatory cell induction and tumor growth
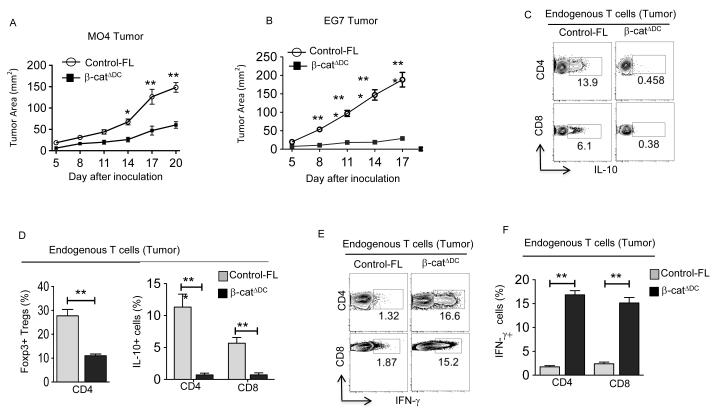
The preceding experiments showed that tumor-associated DCs produce RA and drive T regulatory cell differentiation. So, we hypothesized that tumor-induced activation of the β-catenin/TCF pathway in DCs induces T regulatory responses and promotes immune tolerance. To test this, we monitored MO4 and EG7 tumor growth over time in mice that specifically lack β-catenin in DCs (19). Deletion of β-catenin in DCs (β-catΔDC) significantly reduced tumor growth compared to that seen with WT mice (Fig. 4A, B). Next, we examined the effector phenotype of tumor-infiltrating lymphocytes (TILs) isolated from both groups of mice. Interestingly, we observed a significant decrease in the frequency of Foxp3+CD4+ Treg cells, IL-10+CD4+ Tr1 cells and IL-10+CD8+ T cells in tumors of β-catΔDC mice compared to controls (Fig. 4C, D). In contrast, melanomas isolated from β-catΔDC mice contained a higher frequency of IFN-γ+ CD4+ and IFN-γ+ CD8+ T cells compared to melanomas of control mice (Fig 4E, F). These findings were further confirmed with EG7 tumor (Supplementary Figure 3A). Collectively, these results show that tumor-mediated activation of β-catenin in DCs promotes T regulatory responses and limits antitumor immunity.
Figure 4. DC-specific deletion of β-catenin enhances antitumor immunity in mice.
(A) MO4 melanoma progression in β-catΔDC and Control-FL mice (n=10-12). (B) EG-7 tumor progression in β-catΔDC and Control-FL mice (n=6-8). (C-F) Representative dot plots and percentages of IL-10+CD4+, Foxp3+CD4+, IL-10+CD8+, IFN-γ+CD4+ and IFN-γ+CD8+ T cells isolated from MO4 melanoma in Control-FL and β-catΔDC mice on day 14 post inoculation (n=5 to 6 mice). Data are representative of two independent experiments and show mean values ± SEM. Statistical levels of significance were analyzed by the Student t test (unpaired). *, P<0.05; **, P<0.01; *** P<0.001.
Next, we wished to test whether enhanced anti-tumor immunity and reduced T regulatory responses observed in β-catΔDC mice are due to changes in DC maturation or activation. Phenotypic characterization of TDLN CD11c+ DCs from β-catΔDC mice expressed greatly enhanced levels of the activation markers CD80 and CD86, and reduced levels of co-inhibitory molecules such as PDL1 and PDL2. These observations suggest that the β-catenin pathway also regulates the activation status of DCs. However, we observed similar levels of MHC class II expression in TDLN CD11c+ DCs isolated from WT and β-catΔDC mice (Supplementary Figure 3B). This observation is consistent with previous studies suggesting that the β-catenin pathway is not critical for DC maturation (27,32,33). Taken together, these results show that tumor-mediated activation of β-catenin in DCs limits antitumor immunity at least in part by regulating the expression levels of co-inhibitory and co-stimulatory molecules.
Tumor promotes regulatory DCs via a β-catenin/RA axis
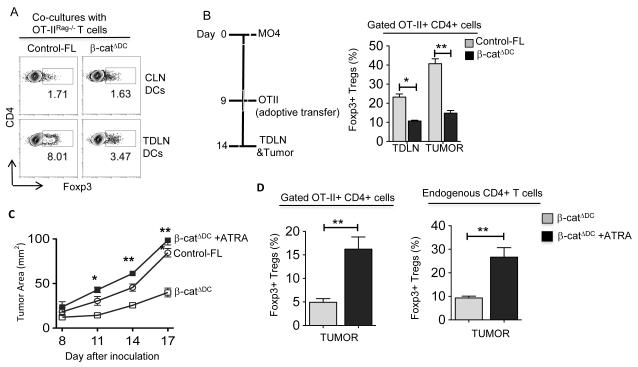
The preceding results demonstrated that activation of β-catenin in DCs promotes T regulatory response and limits T cell effector response. So, we reasoned that tumor-induced activation of the β-catenin pathway imparts regulatory phenotype on DCs and promotes T regulatory response. Therefore, we tested the ability of TDLN DCs isolated from control-FL and β-catΔDC mice to promote differentiation of naive CD4+ T cells into Foxp3+ Tregs. TDLN DCs from control-FL mice induced a higher frequency of Foxp3+ Treg cells compared to CLN DCs (Fig 5A). In contrast, TDLN DCs from β-catΔDC mice induced a lower frequency of Foxp3+ Tregs compared to control TDLN DCs (Fig 5A).
Figure 5. Loss of β-catenin impairs DCs ability to induce Treg cells differentiation and ATRA treatment can restore it.
(A) CD11c+ cells were sorted from CLNs (Top Panels) and TDLNs (Bottom Panels) of MO4 tumor bearing β-catΔDC and control-FL mice and were co-cultured with CD4+CD25− naïve T cells as described in Fig 2A. Data are shown as percentage and representative dot plots of Foxp3+CD4+ T cells of one experiment of two independent experiments (DCs were pooled and enriched from 5 to 6 mice for each experiment). Each sample was done in triplicate. (B) In vivo OT-II Treg differentiation in β-catΔDC and control-FL mice was performed as described in Fig 2D. Data are represents cumulative percentage of OVA-specific Foxp3+CD4+ T cells in TDLNs and tumors isolated from β-catΔDC and control-FL mice. (n=5-6). (C) The MO4 melanoma progression in β-catΔDC mice treated with or without ATRA (2.5 mg/kg) every 3 days from day 1 after tumor inoculation. Data are mean tumor size and are cumulative representative of two independent experiments β-catΔDC versus β-catΔDC +ATRA. (n=6-8 mice; Combined results of two independent experiments are shown). (D) In vivo OT-II Treg differentiation in β-catΔDC treated with or without ATRA was performed as describe in Fig 2D. Percentage of OVA-specific Foxp3+CD4+ Treg cells and endogenous Foxp3+CD4+ Treg cells in TDLNs and tumors isolated from β-catΔDC and Control-FL mice treated with or without ATRA (n= 6 to 8 mice). Data represent two independent experiments and show mean values ± SEM. Statistical levels of significance were analyzed by the Student t test (unpaired). *, P<0.05; **, P<0.01; ***, P<0.001.
To extend these observations in vivo, we implanted MO4 melanoma cells in control-FL and β-catΔDC mice and on day 9, naïve OT-II CD4+ T cells were adoptively transferred as shown in Fig 5B. Consistent with in vitro results, the frequency of induced OVA-specific Foxp3+CD4+ Treg cells was markedly reduced both in TDLN and in tumors of β-catΔDC mice compared to control mice in response to tumor burden (Fig 5B). Altogether, these data demonstrate that tumor-induced activation of β-catenin in DCs imparts regulatory phenotype and drives Treg cell differentiation.
The above results show that deletion of β-catenin in DCs results in loss of regulatory phenotype and limits DCs ability to drive Treg induction. Moreover, these DCs express low levels of RA-synthesizing enzymes. Therefore, we hypothesized that exogenous delivery of RA would promote T regulatory cell differentiation and restore the tumor growth in β-catΔDC mice. To test this, we challenged these mice with MO4 cells and then treated them with RA. As expected, tumor growth in the β-catΔDC mice was delayed and lagged behind tumor growth seen with control mice (Fig 5C). In contrast, RA treatment of β-catΔDC mice accelerated tumor growth compared to the untreated mice (Fig 5C). We then asked whether this accelerated tumor growth is associated with an increase in endogenous as well as antigen-specific Tregs. Injection of OT-II CD4+ T cells into RA-treated β-catΔDC tumor-bearing mice resulted in increased frequency of OVA-specific Foxp3+ CD4+ Tregs within tumors (Fig. 5D). We also observed marked increase in the frequency of endogenous Foxp3+ CD4+ Tregs within tumors (Fig. 5D). These data suggest that RA can induce Tregs and promote tumor growth, further emphasizing the contribution of the β-catenin/TCF4-RA axis in immune tolerance to tumors.
Pharmacological inhibition of the β-catenin/TCF pathway impairs T regulatory cell induction and enhances anti-tumor immunity
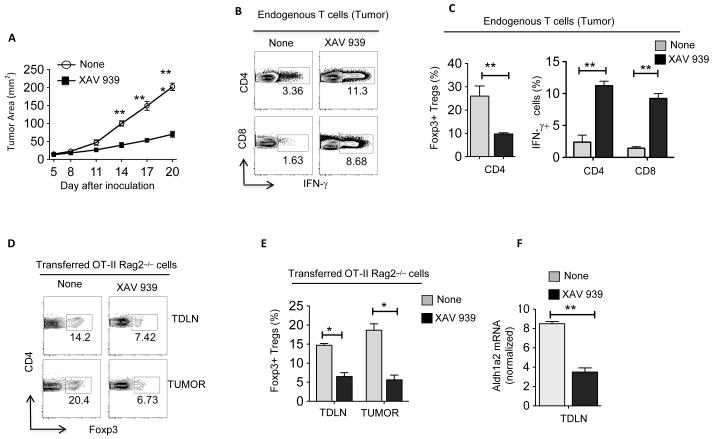
To explore the above findings in a clinically relevant model, we examined the effects of pharmacological inhibition of the β-catenin/TCF pathway with the tankyrase inhibitor, XAV 939 (34). Accordingly, treatment of melanoma or EG7 tumor-bearing WT mice with XAV 939 delayed tumor growth compared to untreated mice (Fig 6A, Supplementary Figure 4A). In addition, therapeutic treatment of mice with XAV 939 at day 10 post tumor transplantation, when the tumor volume is greater, also delayed the tumor growth (Supplementary Figure 4B). Consistent with the reduced tumor growth, XAV 939 treatment resulted in significant decrease in numbers of Treg cells and an increase in the frequencies of IFN-γ+CD4+ and IFN-γ+CD8+ effector T cells infiltrating the tumor (Fig 6B, C, Supplementary Figure 4C). Furthermore, the ratio of effector T cells to Treg cells was markedly increased within the tumor of treated mice compared to the control mice (data not shown). Consistent with this observation, XAV 939 treatment of tumor-bearing mice that had received OT-II CD4+ T cells by adoptive transfer resulted in the decrease in frequency and number of OVA-specific Treg cells within tumors (Fig 6D,E). Consistent with the reduced Treg induction, XAV 939 treatment markedly reduced the expression of vitamin A-metabolizing enzymes (Fig 6F, Supplementary Figure 4D). We confirmed these findings in using another β-catenin/TCF inhibitor, JW55 (35,36) (Supplementary Figure 5). Collectively, these data demonstrate that blocking the β-catenin/TCF4 pathway suppresses Treg differentiation and enhances antitumor immunity.
Figure 6. Therapeutic effect of blocking the β-catenin pathway against established tumors.
The MO4 melanoma progression in WT mice treated with or without XAV 939 (2 mg/kg) every 3 days from day 5 after tumor inoculation. (A) Data are mean tumor size and are cumulative representative of two independent experiments (n= 10-12 mice; Data show combined results of two independent experiments with 5 to 6 mice per experiment). (B and C) Representative dot plots and percentage of IFN-γ+CD4+, IFN-γ+CD8+ and Foxp3+CD4+ T cells isolated from MO4 on day 20 post inoculation. (D, E) In vivo OT-II Treg differentiation in WT mice treated with or without XAV 939 was performed as describe in Fig 2D. Data are representative dot plots (D) and cumulative percentage (E) of induced OVA-specific Foxp3+CD4+ Treg cells isolated from TDLN and tumor (n= 5 to 6 per sample). Data are representative of two independent experiments and show mean values ± SEM. Combined results of two separate experiments are shown with 5-6 mice/group in each experiment. (F) Real-time PCR analysis of Aldh1a2 expression in CD11c+ DCs enriched from TDLNs from WT mice treated with or without XAV 939. Each sample was done in triplicate; the average and SD are shown. The experiment was repeated two times with similar results. Statistical levels of significance were analyzed by the Student t test (unpaired) *, P<0.05; **, P<0.01; ***, P<0.001.
Discussion
Tumors actively suppress immune responses and this represents a fundamental barrier to cancer immunotherapy. DCs are important in this process but the molecular mechanisms of immune suppression by DCs are not fully understood. The current study demonstrates for the first time that RA in the TME directly drives immune suppression by inducing T regulatory responses. A key mechanism of this is through induction of vitamin A-metabolizing enzymes in DC via the β-catenin/TCF pathway, which in turn drives T regulatory responses and suppresses T effector cell response. Pharmacological intervention of vitamin A-metabolizing enzyme activity or the β-catenin/TCF pathway limits T regulatory responses and enhances antitumor immunity. Collectively, these findings support the hypothesis that tumors induce DCs to produce RA by activating the β-catenin/TCF4 pathway, thereby promoting immune tolerance. Hence, this pathway constitutes a new target for cancer immunotherapy. Several aspects of these findings deserve further comment.
First, the role of RA in the induction of Treg cells in the intestine and gut tolerance has been extensively studied (6,7). However, its role in tumor-induced immune tolerance is poorly understood. TME has high levels of RA, and APCs are major producers of RA (8). Also, the signaling pathways that promote DCs to metabolize vitamin A into RA are not known. Our studies demonstrate that DCs in the TME acquire the ability to metabolize vitamin A via the activation of the β-catenin/TCF4 pathway, which programs them to promote T regulatory responses. RA acts directly on CD4 cells inducing T regulatory responses and suppresses T effector cell response. Deletion of β-catenin or TCF-4 in DCs resulted in significant decrease in Aldh1 expression, and these DCs were less potent in inducing Tregs in response to melanoma. Consistent with this observation, pharmacological inhibition of Aldh1 activity significantly delayed tumor growth and enhanced the anti-tumor immunity. In addition to inducing Tregs, recent studies indicate that RA can also act directly on DCs imparting regulatory phenotype via induction of SOCS3 (26,37).
Second, aberration in the β-catenin/TCF signaling pathway is associated with poor prognosis in many human tumors but the focus of most research has been on the effects of this pathway on the tumor cells themselves (16,38,39). In models of gut tolerance, our previous studies have shown that β-catenin signaling in DCs programs them into a regulatory state and promotes T regulatory responses (19,32,40); however whether this pathway promotes immune suppression in tumors was not known. The present study clearly demonstrates that tumor-induced activation of the β-catenin/TCF4 pathway programs DCs into an immunosuppressive state and promotes immune tolerance. TME can also modulate DC function by regulating DC activation and maturation, by regulating the expression of co-stimulatory and co-inhibitory molecules. Our study shows that deletion of β-catenin resulted in increased expression of costimulatory molecules and decreased expression co-inhibitory molecules. This phenotype is associated with DC in an immunogenic state, resulting in enhanced anti-tumor immunity. In addition to inducing Tregs, β-catenin activation in DCs can also affect cross priming of CD8 T cell responses against tumors (18). Although β-catenin is known to suppress DC activation (7), it remains to be determined whether β-catenin directly regulates DC activation or does so indirectly via RA.
The receptor(s) on DCs or factor(s) in TME that activate β-catenin in DCs are currently not known. Emerging studies show that TME contains high levels of Wnt ligands (16,38,39), TGF-β (41) and TLR ligands (42). These molecules can also initiate signaling within DCs to activate the β-catenin pathway, to promote immune suppression. The role of these molecules if any in the activation of β-catenin pathway in DCs in TME is currently unknown. In addition, the downstream mediator of β-catenin signaling in DCs was not known. The present study shows that TCF4 is one of the key mediators of β-catenin signaling in DCs in TME. Upon activation, β-catenin is known to interact with other transcription factors such as PPARγ (10,11), Foxo (12,13), VDR (12,14) and IRF3 (15), which are known regulators of immune responses, but additional studies will be required to determine role of β-catenin in the regulation of these transcription factors and their target genes in response to anti-tumor immunity.
Other regulatory factors such as Indoleamine 2,3-dioxygenase (IDO), IL-10 and TGF-β, though not analyzed in present study, may play a role in tumor tolerance through the β-catenin/TCF pathway (3,26,40,41). The present study was focused on RA because blocking RA essentially recapitulated the effects of a conditional knockout of β-catenin in mice. Furthermore, exogenous addition of RA fully restored tumor growth and immune suppression in β-catenin conditional knockout mice. Thus, our data indicate that signaling initiated by the β-catenin /TCF4 pathway in DCs promotes both tumor growth and immune suppression.
In summary, our study reveals a novel mechanistic link between the β-catenin/TCF pathway in DCs, RA and Tregs by which tumors promote immune tolerance. The present data demonstrate that pharmacological disruption of this pathway altered DC function, resulting in enhanced immune response against established tumors. These findings have important implications for tumor immunotherapy, suggesting that blocking the β-catenin pathway in DCs helps the goal of breaking tumor-induced immune suppression by enhancing antitumor immunity against established tumors.
Supplementary Material
Acknowledgments
We thank Jeanene Pihkala and William King for technical help with FACS sorting and analysis; Dr. Madhav Sharma for assistance with mouse tumor models; Janice Randall with mouse husbandry; Dr. Martinez-Estrada for Raldh2 promoter luciferase construct.
This work was supported by National Institutes of Health awards DK097271; AI04875 (SM) and GRU Cancer Center Seed Grant.
Footnotes
Conflict of Interest: Authors disclose no conflict of interest.
References
- 1.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annual review of immunology. 2007;25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nature reviews Immunology. 2010;10(8):554–67. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. The Journal of clinical investigation. 2007;117(5):1147–54. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nature reviews Cancer. 2012;12(4):265–77. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall JA, Grainger JR, Spencer SP, Belkaid Y. The role of retinoic acid in tolerance and immunity. Immunity. 2011;35(1):13–22. doi: 10.1016/j.immuni.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manicassamy S, Pulendran B. Retinoic acid-dependent regulation of immune responses by dendritic cells and macrophages. Seminars in immunology. 2009;21(1):22–7. doi: 10.1016/j.smim.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo Y, Pino-Lagos K, Ahonen CA, Bennett KA, Wang J, Napoli JL, et al. A retinoic acid--rich tumor microenvironment provides clonal survival cues for tumor-specific CD8(+) T cells. Cancer research. 2012;72(20):5230–9. doi: 10.1158/0008-5472.CAN-12-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434(7035):843–50. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 10.Alastalo TP, Li M, Perez Vde J, Pham D, Sawada H, Wang JK, et al. Disruption of PPARgamma/beta-catenin-mediated regulation of apelin impairs BMP-induced mouse and human pulmonary arterial EC survival. The Journal of clinical investigation. 2011;121(9):3735–46. doi: 10.1172/JCI43382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Wang H, Zuo Y, Farmer SR. Functional interaction between peroxisome proliferator-activated receptor gamma and beta-catenin. Molecular and cellular biology. 2006;26(15):5827–37. doi: 10.1128/MCB.00441-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoogeboom D, Essers MA, Polderman PE, Voets E, Smits LM, Burgering BM. Interaction of FOXO with beta-catenin inhibits beta-catenin/T cell factor activity. The Journal of biological chemistry. 2008;283(14):9224–30. doi: 10.1074/jbc.M706638200. [DOI] [PubMed] [Google Scholar]
- 13.Yan Y, Lackner MR. FOXO3a and beta-catenin co-localization: double trouble in colon cancer? Nature medicine. 2012;18(6):854–6. doi: 10.1038/nm.2799. [DOI] [PubMed] [Google Scholar]
- 14.Shah S, Islam MN, Dakshanamurthy S, Rizvi I, Rao M, Herrell R, et al. The molecular basis of vitamin D receptor and beta-catenin crossregulation. Molecular cell. 2006;21(6):799–809. doi: 10.1016/j.molcel.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 15.Yang P, An H, Liu X, Wen M, Zheng Y, Rui Y, et al. The cytosolic nucleic acid sensor LRRFIP1 mediates the production of type I interferon via a beta-catenin-dependent pathway. Nature immunology. 2010;11(6):487–94. doi: 10.1038/ni.1876. [DOI] [PubMed] [Google Scholar]
- 16.Macheda ML, Stacker SA. Importance of Wnt signaling in the tumor stroma microenvironment. Current cancer drug targets. 2008;8(6):454–65. doi: 10.2174/156800908785699324. [DOI] [PubMed] [Google Scholar]
- 17.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nature reviews Cancer. 2008;8(5):387–98. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 18.Liang X, Fu C, Cui W, Ober-Blobaum JL, Zahner SP, Shrikant PA, et al. beta-catenin mediates tumor-induced immunosuppression by inhibiting cross-priming of CD8(+) T cells. Journal of leukocyte biology. 2014;95(1):179–90. doi: 10.1189/jlb.0613330. [DOI] [PubMed] [Google Scholar]
- 19.Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, et al. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329(5993):849–53. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, et al. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(6):3299–304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, et al. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128(8):1253–64. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 22.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. The Journal of experimental medicine. 2007;204(7):1653–64. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angus-Hill ML, Elbert KM, Hidalgo J, Capecchi MR. T-cell factor 4 functions as a tumor suppressor whose disruption modulates colon cell proliferation and tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(12):4914–9. doi: 10.1073/pnas.1102300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guadix JA, Ruiz-Villalba A, Lettice L, Velecela V, Munoz-Chapuli R, Hastie ND, et al. Wt1 controls retinoic acid signalling in embryonic epicardium through transcriptional activation of Raldh2. Development. 2011;138(6):1093–7. doi: 10.1242/dev.044594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. The Journal of clinical investigation. 2007;117(9):2570–82. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manicassamy S, Ravindran R, Deng J, Oluoch H, Denning TL, Kasturi SP, et al. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nature medicine. 2009;15(4):401–9. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manoharan I, Hong Y, Suryawanshi A, Angus-Hill ML, Sun Z, Mellor AL, et al. TLR2-Dependent Activation of beta-Catenin Pathway in Dendritic Cells Induces Regulatory Responses and Attenuates Autoimmune Inflammation. Journal of immunology. 2014;193(8):4203–13. doi: 10.4049/jimmunol.1400614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. The Journal of clinical investigation. 2004;114(2):280–90. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stern A, Wold AE, Ostman S. Neonatal mucosal immune stimulation by microbial superantigen improves the tolerogenic capacity of CD103(+) dendritic cells. PloS one. 2013;8(9):e75594. doi: 10.1371/journal.pone.0075594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Capietto AH, Kim S, Sanford DE, Linehan DC, Hikida M, Kumosaki T, et al. Down-regulation of PLCgamma2-beta-catenin pathway promotes activation and expansion of myeloid-derived suppressor cells in cancer. The Journal of experimental medicine. 2013;210(11):2257–71. doi: 10.1084/jem.20130281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghosh HS, Cisse B, Bunin A, Lewis KL, Reizis B. Continuous expression of the transcription factor e2-2 maintains the cell fate of mature plasmacytoid dendritic cells. Immunity. 2010;33(6):905–16. doi: 10.1016/j.immuni.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang A, Bloom O, Ono S, Cui W, Unternaehrer J, Jiang S, et al. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 2007;27(4):610–24. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oderup C, LaJevic M, Butcher EC. Canonical and noncanonical Wnt proteins program dendritic cell responses for tolerance. Journal of immunology. 2013;190(12):6126–34. doi: 10.4049/jimmunol.1203002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461(7264):614–20. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 35.Waaler J, Machon O, Tumova L, Dinh H, Korinek V, Wilson SR, et al. A novel tankyrase inhibitor decreases canonical Wnt signaling in colon carcinoma cells and reduces tumor growth in conditional APC mutant mice. Cancer research. 2012;72(11):2822–32. doi: 10.1158/0008-5472.CAN-11-3336. [DOI] [PubMed] [Google Scholar]
- 36.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nature reviews Cancer. 2013;13(1):11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 37.Villablanca EJ, Wang S, de Calisto J, Gomes DC, Kane MA, Napoli JL, et al. MyD88 and retinoic acid signaling pathways interact to modulate gastrointestinal activities of dendritic cells. Gastroenterology. 2011;141(1):176–85. doi: 10.1053/j.gastro.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang D, Du X. Crosstalk between tumor cells and microenvironment via Wnt pathway in colorectal cancer dissemination. World journal of gastroenterology : WJG. 2008;14(12):1823–7. doi: 10.3748/wjg.14.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larue L, Delmas V. The WNT/Beta-catenin pathway in melanoma. Frontiers in bioscience : a journal and virtual library. 2006;11:733–42. doi: 10.2741/1831. [DOI] [PubMed] [Google Scholar]
- 40.Manicassamy S, Pulendran B. Dendritic cell control of tolerogenic responses. Immunological reviews. 2011;241(1):206–27. doi: 10.1111/j.1600-065X.2011.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jian H, Shen X, Liu I, Semenov M, He X, Wang XF. Smad3-dependent nuclear translocation of beta-catenin is required for TGF-beta1-induced proliferation of bone marrow-derived adult human mesenchymal stem cells. Genes & development. 2006;20(6):666–74. doi: 10.1101/gad.1388806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato Y, Goto Y, Narita N, Hoon DS. Cancer Cells Expressing Toll-like Receptors and the Tumor Microenvironment. Cancer microenvironment : official journal of the International Cancer Microenvironment Society. 2009;2(Suppl 1):205–14. doi: 10.1007/s12307-009-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.