Abstract
A dichapetalin-type triterpenoid and a dibenzylbutyrolactone-type lignan, together with five known lignans, a known aromatic diterpenoid, and a known acylated phytosterol, were isolated from the aerial parts of Phyllanthus songboiensis, collected in Vietnam. Their structures were determined by interpretation of the spectroscopic data, and the inhibitory activity toward the HT-29 human colon cancer cells of all isolates was evaluated by a cytotoxicity assay. The known arylnaphthalene lignan, (+)-acutissimalignan A, was highly cytotoxic toward HT-29 cells, with an IC50 value of 19 nM, but this compound was inactive as a DNA topoisomerase IIα (topo IIα) poison. The known phytosterol, (−)-β-sitosterol-3-O-β-D-(6-O-palmitoyl)glucopyranoside, was found to stimulate natural killer (NK) cells at a concentration of 10 μM in the presence of interleukin 12 (IL-12).
Keywords: Phyllanthus songboiensis, Phyllanthaceae, Lignan, Phytosterol, Cytotoxicity, DNA topoisomerase IIα, Natural killer cell
1. Introduction
Representative of the family Phyllanthaceae, Phyllanthus is a large genus containing over 700 species distributed throughout parts of South America, Asia, and Africa, with some members used in systems of Asian traditional medicine (Mabberley, 2008; Qi et al., 2014). For example, the fruits of Phyllanthus emblica and the whole plant of P. urinaria are utilized as traditional Chinese medicines to treat cancer and infectious diseases (Chang et al., 2003; Huang et al., 2006; Luo et al., 2011; Qi et al., 2014; Xu et al., 1996; Zhang et al., 2001). Both P. emblica and P. reticulatus have use in Ayurvedic traditional medicine in the Indian sub-continent for the treatment of cancer, diabetes, inflammation, and other diseases (Sharma and Kumar, 2013; Singh et al., 2011).
Phyllanthus species produce a diverse array of bioactive compounds, with lignans as one of the major groups of secondary metabolites elaborated (Qi et al., 2014). Several diphyllin-type arylnaphthalene lignans have been characterized from this genus and found to exhibit potent cytotoxicity toward a number of human cancer cell lines (Ren et al., 2014; Tuchinda et al., 2006, 2008; Wu and Wu, 2006). Among these, phyllanthusmin D, a potently cytotoxic diphyllin diacetylated arabinoside isolated from P. poilanei, exhibited selective cytotoxicity against the HT-29 human colon cancer cells in vitro and antitumor potency in vivo when evaluated in an in vivo hollow fiber assay (Ren et al., 2014). This compound activated caspase-3 and induced apoptosis, but did not inhibit DNA topoisomerase IIα activity (Ren et al., 2014). Also, several cytotoxic dichapetalin-type triterpenoids and aromatic diterpenoids have been reported from species in the genus Phyllanthus (Gunasekera et al., 1979; Qi et al., 2014; Sutthivaiyakit et al., 2003; Tuchinda et al., 2008).
Natural killer (NK) cells are an important component of innate immunity and serve as a crucial first line of defense against tumors and a diverse range of pathogens. Compounds that activate NK cells are of considerable interest (Gross et al., 2013; Maruyama et al., 2006; Masuda et al., 2008). For example, both β-sitosterol and β-sitosterol glucoside were found to increase NK cell activity (Bouic et al., 1996). When five-week-old C57BL/6 male mice injected with B16BL6 melanoma cells were treated orally with liposomal (composed of egg yolk phosphatidylcholine) β-sitosterol (4 μ mol/mouse, daily for seven days), NK cell activity was increased, and lung tumor metastasis was prevented, which was ascribed to the enhancement of the gut immune surveillance systems (Imanaka et al., 2008). In a previous study, phyllanthusmin C, a diphyllin arabinoside isolated from Phyllanthus poilanei, was found to selectively enhance interferon-γ (IFN-γ) production by human NK cells through upregulation of Toll-like receptor (TLR)-mediated NF-κB signaling (Deng et al., 2014).
As part of a search for novel anticancer agents from higher plants and other organisms (Kinghorn et al., 2009), the initial crude n-hexane and CHCl3-soluble extracts of the aerial parts of P. songboiensis N. N. Thin, collected in Vietnam, were found to be cytotoxic. There have been no chemical or biochemical investigations reported for this species, so it was selected as a target plant for further investigation. Using column chromatography guided by inhibitory activity against HT-29 cells, two new (1 and 2) and seven known compounds were isolated and evaluated for their cytotoxicity toward HT-29 cells. The highly cytotoxic compound, (+)-acutissimalignan A (3), was tested in a topo IIα inhibitory assay, and several of the compounds isolated were also evaluated for their human NK cell stimulatory effects.
2. Results and discussion
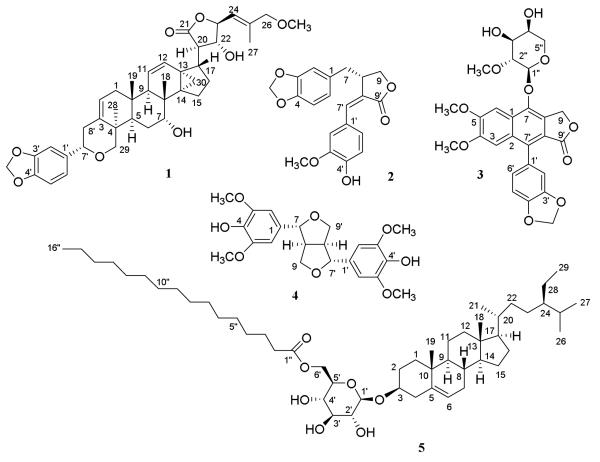
An n-hexane-soluble extract of the methanol extract of the dried aerial parts of P. songboiensis with low cytotoxicity toward HT-29 cells (IC50, 15.0 μg/mL) was subjected to passage over a silica gel column, and additional repeated silica gel column chromatography of combined fractions 4 and 5 afforded three known compounds, (−)-β-sitosterol-3-O-β-D-(6-O-palmitoyl)glucopyranoside (5) (Lin et al., 1976), (−)-pinoresinol (Fonseca et al., 1979), and (−)-spruceanol (Denton et al., 2001). The more potent CHCl3-soluble extract of this plant (IC50, 4.4 μg/mL toward HT-29 cells) was subjected to chromatographic separation by silica gel column chromatography, and repeated fractionation of fractions 8 and 9 produced (+)-songbodichapetalin (1), (+)-songbosin (2), and (−)-7'-hydroxydivanillyltetrahydrofuran (Estévez-Braun et al., 1995), respectively. Work up by silica gel column chromatography of combined fractions 11 and 12 gave (+)-acutissimalignan A (3) (Tuchinda et al., 2008), (−)-syringaresinol (4) (Abe and Yamauchi, 1988), and (+)-secoisolariciresinol (Xie et al., 2003). The known compounds isolated were identified by comparison of their spectroscopic data with literature values (Figure 1 and Figures S9 and S10, Supplementary Data). The assignments of their 1H and 13C NMR spectroscopic data are listed in Tables S1–S4 (Supplementary Data).
Fig. 1.
Chemical structures of compounds 1−5.
Compound 1 was isolated as an amorphous colorless powder, with a molecular formula of C40H50O8 determined by a combination of the 13C NMR spectroscopic data and the positive-ion sodiated HRESIMS (m/z 681.3412 [M + Na]+, calcd. for C40H50O8Na, 681.3403). The IR spectrum of 1 showed characteristic hydroxy (3419 cm−1), lactone ring (1771 cm−1), and benzene-methylenedioxy (1557, 1489, and 933 cm−1) group absorption bands (Tuchinda et al., 2008). The 1H NMR spectrum of 1 (Table 1) displayed signals for four tertiary methyl groups at δ 0.90 s, 1.05 s, 1.29 s, and 1.74 s, a pair of cyclopropyl methylene protons at δ 0.84 and 1.29, and four olefinic protons at δ 5.40 (2H), 5.64, and 6.82. The 13C NMR spectrum of 1 showed 40 signals (Table 1), which supported the presence of a triterpene unit connected with a methylenedioxyphenethyl group through a pyran ring system and a C-26 methoxy group. These spectroscopic characteristics were consistent with 1 being a dichapetalin-type triterpene, based on a 13,30-cyclo-dammarane skeleton, containing a five-membered lactone ring (C-20-C-23) and a phenethyl group linked through a pyran ring at the C-3, C-4, and C-29 positions (Achenbach et al., 1995; Addae-Mensah et al., 1996; Fang et al., 2006; Tuchinda et al., 2008; Weckert et al., 1996).
Table 1.
1H and 13C NMR spectroscopic data of 1a
| Position | δ C b | δ H c | Position | δ C b | δ H c |
|---|---|---|---|---|---|
| 1 | 40.0 CH2 | 2.08 m, 1.66 m | 21 | 176.4 C | |
| 2 | 118.0 CH | 5.40 m | 22 | 73.1 CH | 4.35 br s |
| 3 | 139.8 C | 23 | 79.3 CH | 5.06 br d (8.0) | |
| 4 | 38.2 C | 24 | 119.0 CH | 5.64 d (8.0) | |
| 5 | 43.8 CH | 2.03 m | 25 | 140.6 C | |
| 6 | 24.2 CH2 | 1.62 m, 1.84 m | 26 | 77.2 CH2 | 3.85 (overlapped) |
| 7 | 72.5 CH | 3.94 br s | 27 | 14.9 CH3 | 1.74 s |
| 8 | 36.6 C | 28 | 23.8 CH3 | 1.29 (overlapped) | |
| 9 | 45.4 CH | 1.96 m | 29 | 72.4 CH2 | 3.57 d (10.5), 3.75 d (10.5) |
| 10 | 38.2 C | 30 | 15.3 CH2 | 0.84 d (5.3), 1.29 (overlapped) | |
| 11 | 122.8 CH | 5.40 m | 1′ | 136.7 C | |
| 12 | 131.4 CH | 6.82 br d (9.2) | 2′ | 106.6 CH | 6.89 br s |
| 13 | 31.8 C | 3′ | 147.6 C | ||
| 14 | 36.2 C | 4′ | 146.8 C | ||
| 15 | 27.4 CH2 | 1.29 (overlapped), 1.84 m | 5′ | 108.1 CH | 6.76 d (7.9) |
| 16 | 24.8 CH2 | 2.03 m, 2.08 m | 6′ | 119.3CH | 6.82 br d (7.9) |
| 17 | 39.0 CH | 2.60 m | 7′ | 81.6 CH | 4.18 br d (9.0) |
| 18 | 17.6 CH3 | 0.90 s | 8′ | 40.8 CH2 | 2.12 m, 2.60 m |
| 19 | 18.1 CH3 | 1.05 s | OMe-26 | 58.4 CH3 | 3.36 s |
| 20 | 50.9 CH | 2.68 dd (4.0, 8.2) | OCH2O | 100.9 CH2 | 5.92 s |
All chemical shifts were assigned based on analysis of 1D and 2D-NMR spectra. CH3, CH2, CH, and C multiplicities were determined by DEPT 90, DEPT 135, and HSQC experiments. The overlapped signals were assigned from 1H–1H COSY, HSQC, and HMBC spectra without designating multiplicity. Proton coupling constant values (in parentheses) are presented in Hz and omitted if the signals overlapped as multiplets.
Data (δ) measured at 100.61 MHz in CDCl3.
Data (δ) recorded at 400.13 MHz in CDCl3.
Comparison of the NMR spectroscopic data of 1 (Table 1) with those of (+)-acutissimatriterpene A (Tuchinda et al., 2008) showed that both compounds contained a methylenedioxyphenyl residue. A signal for a tertiary spiro carbon at δ 113.3 (C-23) of (+)-acutissimatriterpene A was absent in the 13C NMR spectrum of 1, indicating the latter compound to be a non-spiro dichapetalin-type triterpene containing a methylenedioxyphenyl group linked at the C-7′ position, as implied by the HMBC correlations observed between H2-29/C-7′, H-2′/C-7′, and H-6′/C-7′ (Figure 2). Comparison of the NMR spectroscopic data of 1 with those of (+)-dichapetalin B (Addae-Mensah et al., 1996) showed the two substances to be structurally similar, except that compound 1 exhibited signals for a methylenedioxyphenyl residue while (+)-dichapetalin B contained a benzene group. In addition, resonances for a methoxy group (δC 58.4 and δH 3.36 s) observed in the NMR spectra of 1 were not apparent in analogous data of (+)-dichapetalin B (Addae-Mensah et al., 1996). This methoxy group was assigned at the C-26 position, as evidenced by the different C-26 chemical shifts for 1 (δC 77.2, δH 3.85 overlapped) and (+)-dichapetalin B (δC 67.1, δH 4.11 m) (Addae-Mensah et al., 1996), and the HMBC correlations observed between the protons of this methoxy group and C-26, H-24/C-26, and H2-26/C-27 of 1 (Figure 2). A five-membered lactone ring could be proposed for 1 from C-20 to C-23, as indicated by HMBC correlations between H-17/C-21, H-20/C-13 and C-16, H-22/C-21 and -24. These preliminary structural assignments were supported by 1H-1H COSY correlations between H2-1/H-2, H-5/H2-6, H2-6/H-7, H-9/H-11/, H-11/H-12, H-5'/H-6', H-7'/H-8', a long 1H-1H COSY sequence represented by H2-15/H2-16/H-17/H-20/H-22/H-23/H-24, and the HMBC correlations observed in the 2D HMBC NMR spectra of 1 (Figure 2).
Fig. 2.
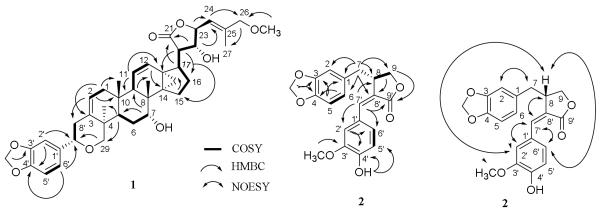
1H-1H COSY and key HMBC correlations for 1 and 2 and selected NOESY correlations for 2.
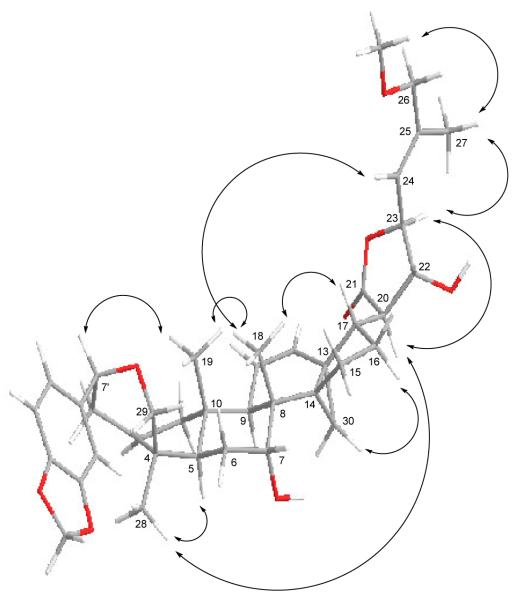
The relative configuration of 1 was determined by analysis of its 2D NOESY NMR spectrum, which showed cross correlations between H-5/H-28, H-17/H-18 and -22, H-18/H-19 and -24, H-19/H-7′, H-20/H-23 and -28, and H-23/H27 (Figure 3), indicating the same relative configuration of 1 as that of (+)-dichapetalin B (Addae-Mensah et al., 1996). An E geometry of the double bond at C-24 of 1 was indicated by the NOESY correlation between H-23/H-27. The absolute configuration of (+)-dichapetalin A has been determined by single-crystal X-ray diffraction using CuKα radiation (Weckert et al., 1996), and that of (+)-dichapetalin B was supported by analysis of its 2D NOESY NMR spectrum and ECD curve showing a strong positive Cotton effect around 217 nm and a weak negative Cotton effect around 235 nm, similar to that of dichapetalin A (Addae-Mensah et al., 1996). Compound 1 is a close analogue of (+)-dichapetalin B, containing a methoxy group as opposed to a hydroxy group at C-26 and a methylenedioxyphenyl moiety rather than a benzyl group at C-7′. The ECD curve of 1 showed a strong positive Cotton effect around 208 nm and a weak negative Cotton effect around 229 nm, suggestive of exciton coupling arising from the benzenoid chromophore at C-7′, similar to that of dichapetalin B (Addae-Mensah et al., 1996), indicating the same absolute configurations at C-7′ for both compounds. Therefore, the structure and absolute configuration of 1 [(+)-songbodichapetalin] were assigned as (2Z,4R,5R,7R,8R,9R,10S,11Z,13S,14S,17S,20R,22R,23S,24E,7'S)-4,2',5',6'-tetrahydro-7,22,23-trihydroxy-26-methoxy-4,8-dimethyl-6'-(1,3-benzodioxol-5-yl)-14,18-cyclocholesta-2,11,24-trieno[4,3-c]pyran-21-oic acid γ-lactone.
Fig. 3.
Molecular model and selected NOESY correlations for 1. Molecular model of 1 was obtained from Chem3D Pro 12.0 program, and the NOESY spectrum was measured on a Bruker DRX-800 MHz NMR spectrometer.
Compound 2 was isolated as an amorphous colorless powder, with a molecular formula of C20H18O6 established by its positive-ion HRESIMS at m/z 377.1014 (calcd. for C20H18O6Na, 377.1001) and its 13C NMR spectroscopic data (Table 2). The IR spectrum showed characteristic hydroxy group (3419 cm−1) and unsaturated lactone ring (1739, 1644 cm−1) absorption bands (Estévez-Reyes et al., 1992), and the intense UV maximum at 332 nm suggested the presence of an extended conjugated system in the molecule (Estévez-Reyes et al., 1992). The 13C NMR spectrum of 2 displayed 20 resolved signals for two benzyl groups, an olefinic group, a methoxy group, a methylenedioxy group, an oxygenated methylene carbon, a methylene carbon, a methine carbon, and a quaternary carbonyl carbon (Table 2). Consistent with these 13C NMR signals, the 1H NMR spectrum showed six signals for two 1,3,4-trisubstituted aromatic rings and other resonances for a conjugated olefinic proton, a methoxy group, a methylenedioxy group, and saturated methylene and methine groups (Table 2). Thus, compound 2 could be proposed as being a dibenzylbutyrolactone lignan (Estévez-Reyes et al., 1992), with a 1,3-benzodioxol-5-yl-methyl group connected to the C-8 position, as indicated by the HMBC correlations between the protons of a methylenedioxy group and C-3 and C-4, H-5/C-1 and -3, and H2-7/C-1, -2, -6, -8, -8′ and -9 (Figure 2). Also, a 4-hydroxy-3-methoxyphenyl group linked at the C-8′ position through a double bond between C-7′ and C-8′ was evident, as inferred by the HMBC correlations between the protons of a methoxy group and C-3′, an hydroxy group and C-4′ and C-5′, H-2′/C-4′ and -6′, and H-7′/C-8, -2′, -6′, and -9′ (Figure 2).
Table 2.
1H and 13C NMR spectroscopic data of 2a
| Position | δ C b | δ H c |
|---|---|---|
| 1 | 131.5 C | |
| 2 | 109.1 CH | 6.63 d (1.3) |
| 3 | 146.5 C | |
| 4 | 148.0 C | |
| 5 | 108.5 CH | 6.72 d (8.0) |
| 6 | 121.9 CH | 6.62 dd (1.3, 8.0) |
| 7 | 37.4 CH2 | 2.61 dd (10.0, 14.4) 3.03 dd (4.4, 14.4) |
| 8 | 39.7 CH | 3.79 dd (4.0, 8.4) |
| 9 | 69.5 CH2 | 4.24 m |
| 1′ | 125.3 C | |
| 2′ | 112.7 CH | 7.00 d (1.6) |
| 3′ | 146.7 C | |
| 4′ | 147.6 C | |
| 5′ | 115.0 CH | 6.99 d (8.0) |
| 6′ | 123.8 CH | 7.18 dd (1.6, 8.0) |
| 7′ | 137.6 CH | 7.49 d (1.6) |
| 8′ | 126.5 C | |
| 9′ | 172.7 C | |
| OMe-3′ | 56.0 CH3 | 3.91 s |
| OH-4′ | 5.91 (overlapped) | |
| OCH2O | 101.1 CH2 | 5.91 (overlapped) |
All chemical shifts were assigned based on analysis of 1D and 2D-NMR spectra. CH3, CH2, CH, and C multiplicities were determined by DEPT 90, DEPT 135, and HSQC experiments. The overlapped signals were assigned from 1H-1H COSY, HSQC, and HMBC spectra without designating multiplicity. Proton coupling constant values (in parentheses) are presented in Hz and omitted if the signals overlapped as multiplets.
Data (δ) measured at 100.61 MHz in CDCl3.
Data (δ) recorded at 400.13 MHz in CDCl3.
Comparison of the NMR spectroscopic data of 2 with those of 4′-O-demethylsuchilactone showed the signals for the lactone rings of both compounds to be closely comparable, but the resonances for the aromatic ring systems present to be different (Das et al., 1995), indicating that a 3′-methoxy-4′-hydroxyphenyl group was linked to C-7′ in 2 rather than to C-7 as in 4′-O-demethylsuchilactone. This was confirmed by MS2 and MS3 spectra of 2. As shown in Figure S3b and Scheme S2 (Supplementary Data), removal of a molecule of H2O from the [M+H]+ ion of 2 yielded [M+H-H2O]+ (C20H17O5+) at m/z 337. Breakage of the C-1′-C-7′ bond produced an ion fragment of C13H11O4+ at m/z 231, from which a neutral residue of CO was eliminated and a smaller ion fragment of C12H11O3+ at m/z 203 was produced. Elimination of a neutral residue by breaking the C-7-C-8 bond produced an ion fragment of C8H7O2+ at m/z 135.
Comparison of the NMR spectroscopic data of 2 with those of guamarol (Estévez-Reyes et al., 1992) showed the signals for C-1′-C-6′ of both compounds to be different, indicating that compound 2 contained a 3′-methoxy-4′-hydroxy rather than the 3′-hydroxy-4′-methoxy aromatic residue of guamarol. This was supported by NOESY correlations between 3′-methoxy group and H-2′, H-2′/H-7′ and H-8, H-7′/H-8 and H-6′, and H-8/H-6′ (Figure 2), as observed in the NOESY 2D NMR spectrum of 2 (Figure 4h, Supplementary Data).
Fig. 4.
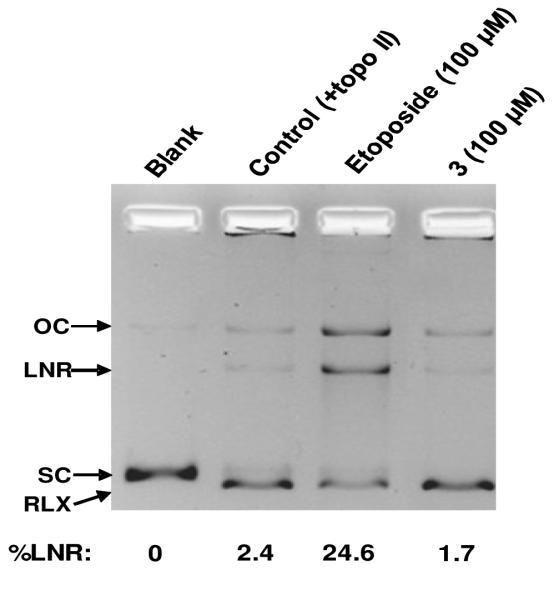
Evaluation of (+)-acutissimalignan A (3) as a topo IIα poison. Topo II-DNA covalent complexes induced by 3 and etoposide were trapped by rapidly denaturing the complexed enzyme with sodium dodecyl sulfate (SDS), digesting the enzyme, and releasing the cleaved DNA as linear DNA. The formation of linear DNA was detected by separating the SDS-treated reaction products using ethidium bromide gel electrophoresis and quantified by accounting for the relationship between fluorescence and relative band intensity for open circular (OC), linear (LNR), supercoiled (SC), and RLX (relaxed) configurations of DNA.
The absolute configuration of savinin, a close analogue of 2, was determined by a series of chemical reactions (Schrecker and Hartwell, 1954). Catalytic hydrogenation of (−)-savinin afforded dihydrosavinin, identical with (+)-isohinokinin, which was epimerized to (−)-hinokinin by a base-catalyzed epimerization (Schrecker and Hartwell, 1954). Since C-8 is not involved in the hydrogenation of (−)-savinin to (+)-isohinokinin, the absolute configuration at this carbon of (−)-savinin was determined as R, the same as that of (−)-hinokinin, which was synthesized enantioselectively (Van Oeveren et al., 1994), with the ECD spectra of some analogues also investigated (Tanoguchi et al., 1987). The 8S absolute configuration of 2 was inferred based on its proposed structure, which is closely comparable with that of (−)-savinin, containing a sole chiral center with the same substituent types, and gave a specific rotation value of +99.0 (c 0.1, CHCl3) [(−)-savinin, −88.0 (c 1.0, CHCl3)] (Schrecker and Hartwell, 1954). This determination was also supported by 4′-O-demethylsuchilactone and guamarol, both of which contain an 8R configuration and showed negative specific rotation values [−44 (c 0.04, CHCl3) for guamarol and −38.3 (c 0.2371, CHCl3) for 4′-O-demethylsuchilactone] (Das et al., 1995; Estévez-Reyes et al., 1992). Therefore, compound 2 was determined as (3E,4S)-4-(1,3-benzodioxol-5-ylmethyl)dihydro-3-[(4-hydroxy-3-methoxyphenyl)methylene]-2(3H)-furanone.
Compound 5 has been reported previously as “glycoside P1”, a mixture of β-sitosterol-3-O-β-D-(6-O-palmitoyl)glucopyranoside (5), β-campesterol-3-O-β-D-(6-O-palmitoyl)glucopyranoside, and β-stigmasterol-3-O-β-D-(6-O-palmitoyl)glucopyranoside, with the NMR spectroscopic data not being assigned (Lin et al., 1976). In the present study, HRESIMS of 5 showed this compound to have a molecular formula of C51H90O7 [positive-ion sodiated HRESIMS (m/z), 837.6586, calcd. for C51H90O7Na, 837.6584], and the IR, UV, and NMR spectra showed characteristics similar to those of β-sitosterol-3-O-β-D-glucopyranoside (Faizi et al., 2001). The connection of the β-sitosterol-3-O-β-D-glucopyranoside residue and the palmitoyl group of 5 was confirmed from the 1D and 2D NMR spectra (Figures S9 and S10, Supplementary Data). Thus, this compound was identified as β-sitosterol-3-O-β-D-(6-O-palmitoyl)glucopyranoside (Allerhand and Maple, 1988; Faizi et al., 2001).
All isolates of P. songboiensis were evaluated for their cytotoxicity against the HT-29 human colon cancer cell line, using a procedure reported previously (Ren et al. 2011), with paclitaxel being the positive control (Table 3). Compound 3 exhibited potent cytotoxicity toward HT-29 cells, with an IC50 value of 19 nM, and compound 4 was also active in this assay system. However, all other tested compounds were inactive.
Table 3.
Cytotoxicity against HT-29 cells and NK cell stimulation of 1–5
| Compound | HT-29a | NK stimulationb |
|---|---|---|
| 1 | >10 | NTc |
| 2 | >10 | inactive |
| 3 | 0.019 | NTc |
| 4 | 8.6 | inactive |
| 5 | >10 | active |
| Paclitaxeld | 0.001 | |
| IL-12e | active |
IC50 values (μM) toward the HT-29 cell line were calculated using nonlinear regression analysis with measurements performed in triplicate and representative of two independent experiments in which the values generally agreed within 10%.
Data from a NK stimulatory assay with a concentration of 10 μM.
NT: not tested.
Positive control used in cytotoxicity assay.
Positive control used in NK stimulation assay.
Several dichapetalin-type triterpenoids have been reported as potent cytotoxic compounds (Fang et al., 2006; Jing et al., 2014; Long et al., 2013; Tuchinda et al., 2008), and preliminary information about their structure-cytotoxicity requirements may be inferred from these previous studies. Acutissimatriterpenes A and B showed almost the same cytotoxic potencies toward a panel of human cancer cell lines (Tuchinda et al., 2008), suggesting that the methylenedioxy groups at C-3′ and C-4′ of these compounds are not determinants of cytotoxicity. Acutissimatriterpene E exhibited a much greater cytotoxicity (IC50, 0.005 μg/mL) than acutissimatriterpene A (IC50, 0.5 μg/mL) toward murine P-388 lymphocytic leukemia cells (Tuchinda et al., 2008), indicating that the presence of a hydroxy group at the C-22 position can enhance this activity. On comparing the cytotoxicity of dichapetalin A (Achenbach et al., 1995; Fang et al., 2006) and 1, it may be implied that a primary C-26 hydroxy group, when present, plays a key role in determining the cytotoxic potency of the dichapetalin-type triterpenes, and is probably more important than the C-22 hydroxy group in this regard.
The new lignan compound 2 was found to be non-cytotoxic toward HT-29 cells in the present study (Table 3), but one of its close analogues, enterolactone, a gut-derived mammalian metabolite of plant dibenzylbutyrolactone-type lignans, has been shown to inhibit the proliferation of LNCaP human prostate cancer cells, when cells were treated at a concentration of 60 μM for 72 h (McCann et al., 2008). (−)-Spruceanol was reported to be cytotoxic against murine P-388 lymphocytic leukemia cells (Gunasekera et al., 1979), but was non-cytotoxic toward HT-29 cells in the present study.
The enzyme DNA topoisomerase (topo II) is an established molecular target of etoposide (Meresse et al., 2004) causing DNA damage through interfacial inhibition or “poisoning” of topo II (Deweese and Osheroff, 2009; Pommier et al., 2010). In the current investigation, the highly cytotoxic arylnaphthalene lignan, (+)-acutissimalignan A (3), and etoposide were tested for their ability to induce topo IIα-mediated cleavage of plasmid DNA (Figure 4), using a method reported previously (Hasinoff et al., 2005). Consistent with previous reports (Hasinoff et al., 2012; Ren et al., 2014), the positive control etoposide (100 μM) exhibited topo IIα inhibitory activity, as assessed by cleavage of closed circular plasmid DNA to form linear double-stranded DNA, but the arylnaphthalene lignan lactone 3 did not show any topo II inhibition in this assay (Figure 4), indicating a different mechanism of action for 3 and etoposide.
Interleukin 12 (IL-12) is a potent cytokine produced by antigen-presenting cells (APCs) to stimulate interferon-γ (IFN-γ) production by NK cells (Masuda et al., 2008), and IFN-γ is essential for both innate and adaptive immune responses in the clearance of viral infections and for the host defense against malignant transformation (Dunn et al., 2004). In the present study, compounds 2, 4, 5, (−)-7'-hydroxydivanillyltetrahydrofuran, and (+)-secoisolariciresinol were tested for their NK stimulatory effect following a previous method (Yu et al., 2006). Compound 5 was found to be active, but all other compounds were inactive, when tested at 10 μM (Table 3). NK cells produced more IFN-γ when treated by IL-12 with or without 5, compared with the vehicle control (Figure 5). The production of IFN-γ by NK cells treated with IL-12 plus 5 was significantly higher than that treated by IL-12 alone (Figure 5), which indicates that 5 is able to induce NK cells to produce more IFN-γ in the presence of IL-12.
Fig. 5.
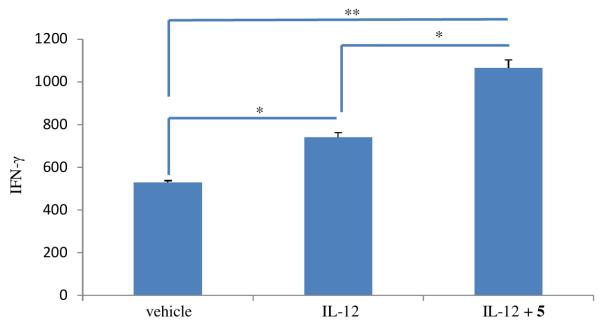
Production of IFN-γ by NK cells stimulated by IL-12 and 5. Human natural killer cells were treated by vehicle, IL-12 (10 ng/mL), or IL-12 (10 ng/mL) plus 5 (10 μM), for 24 h. Cells were harvested, and cell-free supernatants were collected. The level of IFN-γ protein in the collected cell-free supernatants was detected by an enzyme-linked immunosorbent assay (ELISA). The result showed that 5 stimulated NK cell to produce higher levels of IFN-γ in the presence of IL-12, compared to IL-12 alone (columns, mean values, n = 3; bars, SEM; * p ≤ 0.05 and ** p ≤ 0.01).
It is well documented that adaptive and innate immune cells, including NK cells, play critical roles in the host cancer immunosurveillance network (Dunn et al., 2004). IFN-α can inhibit normal or tumor cell proliferation in vitro and in vivo, and administration of IFN-α has a curative therapeutic action on cancer patients (Belardeli et al., 2002). Thus, this cytokine has been proposed as an immune adjuvant for the development of a cancer vaccine to be used in cancer immunotherapy (Belardeli et al., 2002). The present study here showed that the known phytosterol 5 exhibited a human NK cell stimulatory effect in vitro, hence, this agent may be worthy of future evaluation for efficacy in an in vivo mouse tumor model.
In summary, two new and seven known compounds were isolated and identified from Phyllanthus songboiensis (Phyllanthaceae), collected in Vietnam. The known arylnaphthalene lignan, (+)-acutissimalignan A (3), was highly cytotoxic toward HT-29 human colon cancer cells, but was not shown to be a topo IIα poison. The known phytosterol, (−)-β-sitosterol-3-O-β-D-(6-O-palmitoyl)glucopyranoside (5), was found to stimulate NK cells at a concentration of 10 μM in the presence of IL-12, and is one of only a few natural products reported to date with this kind of biological activity.
3. Experimental
3.1. General experimental procedures
Optical rotation values were obtained on a Perkin-Elmer model 343 polarimeter. UV spectra were recorded on a Hitachi U2910 UV spectrophotometer. Electronic circular dichroism (ECD) measurements were performed using a JASCO J-810 spectropolarimeter. IR spectra were recorded on a Nicolet 6700 FT-IR spectrometer. Molecular modeling was performed using the Chem3D Pro 12.0 program [Chemical Abstracts Service (CAS), Columbus, OH, USA and PerkinElmer, Inc., Waltham, MA, USA]. 1H and 13C, DEPT, COSY, HSQC, HMBC, and NOESY NMR spectra were recorded at room temperature on a Bruker Avance DRX-400, −600, or a −800 MHz NMR spectrometer. ESIMS and HRESIMS were measured on a LCT-TOF or a Q-TOF mass spectrometer in the positive-ion mode. MS2 and MS3 spectra were measured on a Bruker amaZon ETD instrument operating in the positive-ion mode using an ESI ion source. Column chromatography (CC) was conducted using silica gel (65 × 250 or 230 × 400 mesh, Sorbent Technologies, Atlanta, GA, USA). Analytical thin-layer chromatography (TLC) systems were performed on precoated silica gel 60 F254 plates (Sorbent Technologies). Sephadex LH-20 was purchased from Amersham Biosciences (Uppsala, Sweden). For visualization of TLC plates, sulfuric acid reagent was used. Fluorescence was tested using a Spectroline (model ENF-260C) UV light source. All procedures were carried out using anhydrous solvents purchased from commercial sources and employed without further purification.
3.2. Plant Material
A sample of the aerial parts of Phyllanthus songboiensis N. N. Thin was collected from an erect, free-standing plant 75 cm tall on the shore of Lake Kego (18o 09.033' N; 105o 59.843' E), Kego Nature Reserve, Cam Xuyen District, Hatinh Province, Vietnam, in December, 2008, by D.D.S., T.N.N., and V.T.T. A voucher herbarium specimen (DDS 14285) representing this collection has been identified by D.D.S. and is deposited at the John G. Searle Herbarium of the Field Museum of Natural History, Chicago, IL., under the accession number FM 2287532. Pham Hoang Ho, 2000, entry No. 4733d.
3.3. Extraction and Isolation
A sample of the milled, air-dried aerial parts of P. songboiensis (699 g) was extracted with MeOH (2 L × 6, 24 h each) at room temperature. The solvents were evaporated in vacuo, and the dried MeOH extract (71.0 g, 10.1%) was resuspended in 600 mL of MeOH mixed with H2O (H2O:MeOH, 10:90, v/v) and partitioned with n-hexane (500, 400, and 300 mL) to yield a n-hexane-soluble residue (D1, 7.0 g, 1.0%). The aqueous MeOH layer was then partitioned with CHCl3 (500, 300, and 300 mL) to afford a CHCl3-soluble extract (D2, 3.0 g, 0.43%), which was followed by washing with a 1% aqueous solution of NaCl to partially remove plant polyphenols. The n-hexane-soluble extract showed low cytotoxicity toward the HT-29 cell line (IC50, 15.0 μg/mL), with the CHCl3-soluble extract more active in this regard (IC50, 4.4 μg/mL). The water-soluble extract was inactive (IC50 > 20 μg/mL) in this bioassay system.
The n-hexane-soluble extract (6.8 g) was subjected to silica gel CC (4.5 × 45 cm), eluted with gradient mixtures of n-hexane–acetone (100:1→1:1; 500 mL each). The eluates were pooled by TLC analysis to give five combined fractions. Of these, active fractions 4 (IC50, 9.7 μg/mL) and 5 (IC50, 6.8 μg/mL) were combined and applied to another silica gel-containing column (2.5 × 20 cm), eluted with gradient mixtures of n-hexane–acetone (20:1→3:1, 200 mL each). Fractions were pooled by TLC analysis to give 16 combined fractions (D1F4F1–D1F4F16). Of these, fractions D1F4F5-D1F4F7 were combined and subjected to silica gel CC, eluted with a gradient of n-hexane-acetone and then purified by separation over a Sephadex LH-20 column, eluted with CH2Cl2-MeOH (1:1), affording (−)-spruceanol (1.5 mg). Fractions D1F4F10-D1F4F13 were combined and applied to a silica gel column, eluted with a gradient of n-hexane-acetone and then purified by separation over a Sephadex LH-20 column, eluted with CH2Cl2-MeOH (1:1), furnishing (−)-β-sitosterol-3-O-β-D-(6-O-palmitoyl)glucopyranoside (5, 6 mg) and (−)-pinoresinol (1 mg).
The CHCl3-soluble extract (2.8 g, IC50, 4.4 μg/mL) was subjected to silica gel CC (2.5 × 45 cm) by elution with a gradient of n-hexane-acetone. Fractions were pooled by TLC analysis to give 15 combined fractions (D2F1−D2F15). Of these, fractions D2F8, D2F9, D2F11, and D2F12 were found to be active, with IC50 values of 16.7, 15.5, 16.4, and 7.1 μg/mL, respectively. Fraction D2F8 was applied to a silica gel column, eluted with a gradient of n-hexane-acetone and then purified by separation over a Sephadex LH-20 column, eluted with CH2Cl2-MeOH (1:1), affording (+)-songbosin (2, 6 mg). Fraction D2F9 was subjected to silica gel CC, eluted with a gradient of n-hexane-acetone and then finally purified by separation over a Sephadex LH-20 column, eluted with CH2Cl2-MeOH (1:1), furnishing (+)-songbodichapetalin (1, 5 mg) and (−)-7'-hydroxydivanillyltetrahydrofuran (2 mg). Fractions D2F11 and D2F12 were applied to a silica gel column, eluted with a gradient of n-hexane-acetone and next purified by separation over a Sephadex LH-20 column, eluted with CH2Cl2-MeOH (1:1), affording (+)-acutissimalignan A (3, 2 mg), (−)-syringaresinol (4, 3 mg), and (+)-secoisolariciresinol (2 mg).
3.3.1. (+)-Songbodichapetalin 1: (2Z,4R,5R,7R,8R,9R,10S,11Z,13S,14S,17S,20R,22R,23S,24E,7'S)-4,2',5',6'-tetrahydro-7,22,23-trihydroxy-26-methoxy-4,8-dimethyl-6'-(1,3-benzodioxol-5-yl)-14,18-cyclocholesta-2,11,24-trieno[4,3-c]pyran-21-oic acid γ-lactone (1)
Amorphous colorless powder; [α]20D +35.0 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 285 (3.77), 231 (sh, 4.20), 204 (4.83) nm; ECD (MeOH, nm) λmax (Δε) 299 (−4.42), 229 (−0.44), 208 (+45.15); IR (dried film) νmax 3419, 2925, 1771, 1557, 1489, 933, 874 cm−1; for 1H and 13C NMR spectroscopic data, see Table 1; positive-ion sodiated HRESIMS m/z 681.3412, calcd. for C40H50O8Na, 681.3403.
3.3.2.1. (+)-Songbosin 2: (3E,4S)-4-(1,3-benzodioxol-5-ylmethyl)dihydro-3-[(4-hydroxy-3-methoxyphenyl)methylene]-2(3H)-furanone (2)
Amorphous colorless powder showing a blue color under UV light at 365 nm; [α]20D +99.0 (c 0.1, CHCl3); UV (MeOH) λmax (log ε) 332 (4.26), 295 (4.11), 236 (4.19), 203 (4.60) nm; ECD (MeOH, nm) λmax (Δε) 328 (−1.45), 283 (−1.52), 242 (+0.85), 209 (−5.75); IR (dried film) νmax 3419, 1739, 1644, 1592, 1516, 1489, 927, 856 cm−1; for 1H and 13C NMR spectroscopic data, see Table 2; positive-ion sodiated HRESIMS m/z 377.1014, calcd. for C20H18O6Na, 377.1001.
3.3.2.2. MS2, and MS3 spectra of (+)-Songbosin (2)
MS2 and MS3 spectra of 2 were measured on a Bruker amaZon ETD instrument operating in the positive-ion mode using an ESI ion source, with scans (m/z) from 100 to 1000. MS2 m/z (355.6), (rel. inten., %): 355.6 (3.8), 337.3 (100), 319.3 (11.5), 305.2 (9.2), 291.3 (8.4), 231.2 (39.7), 213.2 (2.3), 203.2 (77.1), 187.2 (7.6), 173.2 (3.1), 159.2 (3.1), 157.2 (3.1), 135.3 (22.1), 129.3 (2.3). The main peaks (with greater relative intensities) at m/z 337.3 (100), 231.2 (39.7), and 203.2 (77.1) from MS2 spectrum were selected by the mass spectrometer for MS3 measurements. MS3 m/z (337.3 of 355.6) (rel. inten., %): 337.3 (26.7), 319.3 (84.7), 305.2 (73.3), 291.3 (74.7), 277.2 (17.3), 261.2 (10.7), 247.2 (6), 233.2 (6), 213.2 (4.7), 187.2 (2), 177.2 (7.3), 160.2 (3.3), 135.3 (100). MS3 m/z (231.2 of 355.6), (rel. inten., %): 231.2 (0), 213.2 (34.9), 203.2 (9.4), 187.2 (100), 185.2 (22.1), 173.2 (20.1), 157.2 (33.6), 143.2 (1.3), 129.3 (5.4). MS3 m/z (203.2 of 355.6), (rel. inten., %): 203.2 (100), 185.2 (23.3), 175.2 (28.7), 173.2 (55.3), 161.2 (76), 145.3 (16.7), 135.2 (63.3), 131.2 (12.7), 122.3 (2), 117.3 (2.7).
3.3.3. Cytotoxicity Assay
The cytotoxicity of all isolates was tested against HT-29 cells, using a procedure reported previously (Ren et al., 2011), with paclitaxel used as positive control. Briefly, HT-29 cells were cultured under standard conditions and trypsinized. The harvested cells were treated by the test samples and incubated at 37 °C in 5% CO2 for 72 h. The cells were fixed, incubated at room temperature for 30 min, washed with tap water, and dyed using sulforhodamine B. Then, the dyed cells were lysed and read at 515 nm with an ELISA plate reader. Measurements were performed in triplicate and are representative of two independent experiments in which the values generally agreed within 10%.
3.3.4. Topoisomerase IIα Assay
Interfacial inhibition or poisoning of topo IIα can be evaluated by trapping topo II-plasmid DNA covalent complexes with sodium dodecyl sulfate, digesting away the enzyme, and releasing cleaved linear DNA. The topo IIα-inhibitory activity of etoposide and compound 3 was assessed using a procedure reported previously (Hasinoff et al., 2005; Ren et al., 2014). In brief, assay buffer containing pBR322 DNA and test compound/DMSO were mixed and allowed to sit at room temperature for 30 min after which topo IIα was added to initiate the reaction. The tubes were incubated at 37 °C for 15 min, and then quenched with 1% (v/v) SDS/10 mM disodium EDTA/200 mM NaCl. The mixture was treated subsequently with 0.77 mg/ml proteinase K (Sigma, St. Louis, MO, USA) at 55 °C for 60 min to digest the protein, and DNA bands were separated by electrophoresis (18 h at 2 V/cm) on an agarose gel (1.3% w/v) containing 0.7 μg/ml ethidium bromide. Then, DNA in the gel was imaged by its fluorescence on a Chemi-Doc XRS+ imager (Bio-Rad, Hercules, CA, USA). Percent linear DNA produced was quantified from total fluorescence of all bands accounting for differences in relative fluorescence of the different forms of DNA as previously reported (Projan et al., 1983).
3.3.5. Cell Culture and Isolation of Primary NK Cells
Human primary NK cells were isolated from peripheral blood leukopacks of healthy donors (American Red Cross, Columbus, OH, USA), as described previously (He et al., 2013). After incubation with RosetteSep–Human NK Cell Enrichment Cocktail (StemCell Technologies Inc., Vancouver, Canada) for 30 min at room temperature, enriched NK cells were isolated by Ficoll-Paque density gradient centrifugation. The enriched NK cells were approximately 80% CD56+CD3−, as determined by a flow cytometric analysis after staining with CD56-APC and CD3-FITC antibodies (BD Biosciences, San Jose, CA, USA). The enriched NK cells were further purified with CD56 magnetic beads and MACS LS Separation Columns (Miltenyi Biotec, Auburn, CA, USA). The purity of magnetic bead-purified NK cells was ≥ 99.0%.
3.3.6. NK Cell Stimulation and IFN-γ Immunoassay by ELISA
Following a previous procedure (Deng et al., 2014; Yu et al., 2006), purified human primary NK cells were treated with vehicle, IL-12 (R & D Systems, Minneapolis, MN, USA, 10 ng/ml), 5 (10 μM), or IL-12 plus 5 (10 μM) for 24 h. Cells were harvested, and cell-free supernatants were collected for detecting IFN-γ protein by enzyme-linked immunosorbent assay (ELISA) with commercially available mAb pairs (Endogen Inc., Woburn, MA, USA) according to the manufacturer’s protocol. Results are shown as a mean of triplicate paralleled wells with ± SD. Data were compared by Student’s two-tailed t test. A p value of less than 0.05 was considered statistically significant.
Supplementary Material
Highlights.
Nine compounds, seven known, were isolated from Phyllanthus songboiensis.
Structures were determined by analysis of their spectroscopic data.
The known arylnaphthalene lignan was highly cytotoxic toward HT-29 cells.
The known arylnaphthalene lignan did not act as a DNA topoisomerase IIα poison.
The known phytosterol stimulated NK cells in the presence of interleukin 12.
Acknowledgments
This investigation was supported by grant P01 CA125066 (to A.D.K.), grant R01 CA090787 (to J.C.Y.), and grant R01 CA155521 (to J.Y.), funded by the National Cancer Institute, NIH, Bethesda, MD, USA. The plant sample was collected under a collaborative agreement between the University of Illinois at Chicago and the Institute of Ecology and Biological Resources (IEBR), Vietnam Academy of Science and Technology, Hanoi. We thank Dr. Le Xuan Canh, Director of IEBR, for overseeing the collection, and the Director of Kego Nature Reserve, Cam Xuyen District, Hatinh Province, Vietnam, for permit of collection. We thank Dr. Craig McElroy, College of Pharmacy, The Ohio State University, for access to the NMR spectrometer. We also thank Mark Apsega and Andrew Van Schoiack, as well other staff members of the Mass Spectrometry and Proteomics Laboratory, Campus Chemical Instrument Center, The Ohio State University, for access to the MS instrumentation and MS2 and MS3 spectrum measurement.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary data
Supplementary data associated with this article, including the MS and NMR spectra of 1-3 and 5, MS2 and MS3 spectra of 2, structures and analytical data of the known compounds, and proposed biogenesis of the lignan compounds, can be found in the online version, at http://dx.doi.org/10.1016/j.phytochem.2014.
References
- Abe F, Yamauchi T. 9α-Hydroxypinoresinol, 9α-hydroxymedioresinol and related lignans from Allamanda neriifolia. Phytochemistry. 1988;27:575–577. [Google Scholar]
- Achenbach H, Asunka SA, Waibel R, Addae-Mensah I, Oppong IV. Dichapetalin A, a novel plant constituent from Dichapetalum madagascariense with potential antineoplastic activity. Nat. Prod. Lett. 1995;7:93–100. [Google Scholar]
- Addae-Mensah I, Waibel R, Asunka SA, Oppong IV, Achenbach H. The dichapetalins-a new class of triterpenoids. Phytochemistry. 1996;43:649–656. [Google Scholar]
- Allerhand A, Maple SR. Requirements for ultrahigh resolution NMR of large molecules on high-field instruments. J. Magn. Reson. 1988;76:375–379. [Google Scholar]
- Belardelli F, Ferrantini M, Proietti E, Kirkwood JM. Interferon-alpha in tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:119–134. doi: 10.1016/s1359-6101(01)00022-3. [DOI] [PubMed] [Google Scholar]
- Bouic PJD, Etsebeth S, Liebenberg RW, Albrecht CF, Pegel K, Van Jaarsveld PP. Beta-sitosterol and beta-sitosterol glucoside stimulate human peripheral blood lymphocyte proliferation: implications for their use as an immunomodulatory vitamin combination. Int. J. Immunopharmacol. 1996;18:693–700. doi: 10.1016/s0192-0561(97)85551-8. [DOI] [PubMed] [Google Scholar]
- Chang C-C, Lien Y-C, Liu KCSC, Lee S-S. Lignans from Phyllanthus urinaria. Phytochemistry. 2003;63:825–833. doi: 10.1016/s0031-9422(03)00371-6. [DOI] [PubMed] [Google Scholar]
- Das B, Rao SP, Srinivas KVNS, Yadav JS. Lignans, biflavones and taxoids from Himalayan Taxus baccata. Phytochemistry. 1995;38:715–717. [Google Scholar]
- Deng Y, Chu J, Ren Y, Fan Z, Ji X, Mundy-Bosse B, Yuan S, Hughes T, Zhang J, Cheema B, Camardo AT, Xia Y, Wu L-C, Wang L-S, He X, Kinghorn AD, Li X, Caligiuri MA, Yu J. The natural product phyllanthusmin C enhances IFN-γ production by human NK cells through upregulation of TLR-mediated NF-κB signaling. J. Immunol. 2014;193:2994–3002. doi: 10.4049/jimmunol.1302600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton RW, Harding WW, Anderson CI, Jacobs H, McLean S, Reynolds WF. New diterpenes from Jatropha divaricata. J. Nat. Prod. 2001;64:829–831. doi: 10.1021/np010098a. [DOI] [PubMed] [Google Scholar]
- Deweese JE, Osheroff N. The DNA cleavage reaction of topoisomerase II: wolf in sheep's clothing. Nucleic Acids Res. 2009;37:738–748. doi: 10.1093/nar/gkn937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Estévez-Braun A, Estévez-Reyes R, González-Pérez JA, González AG. Busaliol and busalicifol, two new tetrahydrofuran lignans from Bupleurum salicifolium. J. Nat. Prod. 1995;58:887–892. [Google Scholar]
- Estévez-Reyes R, Estévez-Braun A, González AG. Lignanolides from Bupleurum salicifolium. Phytochemistry. 1992;31:2841–2845. [Google Scholar]
- Faizi S, Ali M, Saleem R, Irfanullah Bibi. Complete 1H and 13C NMR assignments of stigma-5-en-3-O-β-glucoside and its acetyl derivative. Magn. Reson. Chem. 2001;39:399–405. S. [Google Scholar]
- Fang L, Ito A, Chai H-B, Mi Q, Jones WP, Madulid DR, Oliveros MB, Gao Q, Orjala J, Farnsworth NR, Soejarto DD, Cordell GA, Swanson SM, Pezzuto JM, Kinghorn AD. Cytotoxic constituents from the stem bark of Dichapetalum gelonioides collected in the Philippines. J. Nat. Prod. 2006;69:332–337. doi: 10.1021/np058083q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca SF, Nielsen LT, Rúveda EA. Lignans of Araucaria angustifolia and 13C NMR analysis of some phenyltetralin lignans. Phytochemistry. 1979;18:1703–1708. [Google Scholar]
- Gross E, Sunwoo JB, Bui JD. Cancer immunosurveillance and immunoediting by natural killer cells. Cancer J. 2013;19:483–489. doi: 10.1097/PPO.0000000000000005. [DOI] [PubMed] [Google Scholar]
- Gunasekera SP, Cordell GA, Farnsworth NR. Potential anticancer agents. XIV. Isolation of spruceanol and montanin from Cunuria spruceana (Euphorbiaceae) J. Nat. Prod. 1979;42:658–662. doi: 10.1021/np50006a012. [DOI] [PubMed] [Google Scholar]
- Hasinoff BB, Wu X, Krokhin OV, Ens W, Standing KG, Nitiss JL, Sivaram T, Giorgianni A, Yang S, Jiang Y, Yalowich JC. Biochemical and proteomics approaches to characterize topoisomerase IIα cysteines and DNA as targets responsible for cisplatin-induced inhibition of topoisomerase IIα. Mol. Pharmacol. 2005;67:937–947. doi: 10.1124/mol.104.004416. [DOI] [PubMed] [Google Scholar]
- Hasinoff BB, Wu X, Nitiss JL, Kanagasabai R, Yalowich JC. The anticancer multi-kinase inhibitor dovitinib also targets topoisomerase I and topoisomerase II. Biochem. Pharmacol. 2012;84:1617–1626. doi: 10.1016/j.bcp.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Chu J, Wu L-C, Mao H, Peng Y, Alvarez-Breckenridge CA, Hughes T, Wei M, Zhang J, Yuan S, Sandhu S, Vasu S, Benson DM, Hofmeister CC, He X, Ghoshal K, Devine SM, Caligiuri MA, Yu J. MicroRNAs activate natural killer cells through Toll-like receptor signaling. Blood. 2013;121:4663–4671. doi: 10.1182/blood-2012-07-441360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S-T, Yang R-C, Lee P-N, Yang S-H, Liao S-K, Chen T-Y, Pang J-HS. Anti-tumor and anti-angiogenic effects of Phyllanthus urinaria in mice bearing Lewis lung carcinoma. Int. Immunopharmacol. 2006;6:870–879. doi: 10.1016/j.intimp.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Imanaka H, Koide H, Shimizu K, Asai T, Shimizu NK, Ishikado A, Makino T, Oku N. Chemoprevention of tumor metastasis by liposomal β-sitosterol intake. Biol. Pharm. Bull. 2008;31:400–404. doi: 10.1248/bpb.31.400. [DOI] [PubMed] [Google Scholar]
- Jing S-X, Luo S-H, Li C-H, Hua J, Wang Y-L, Niu X-M, Li X-N, Liu Y, Huang C-S, Wang Y, Li S-H. Biologically active dichapetalins from Dichapetalum gelonioides. J. Nat. Prod. 2014;77:882–893. doi: 10.1021/np400971r. [DOI] [PubMed] [Google Scholar]
- Kinghorn AD, Carcache de Blanco EJ, Chai H-B, Orjala J, Farnsworth NR, Soejarto DD, Oberlies NH, Wani MC, Kroll DJ, Pearce CJ, Swanson SM, Kramer RA, Rose WC, Fairchild CR, Vite GD, Emanuel S, Jarjoura D, Cope FO. Discovery of anticancer agents of diverse natural origin. Pure Appl. Chem. 2009;81:1051–1063. doi: 10.1351/PAC-CON-08-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TD, Kondo N, Shoji J. Studies on the constituents of Panacis Japonici Rhizoma. V. The structures of chikusetsusaponin I, Ia, Ib, IVa and glycoside P1. Chem. Pharm. Bull. 1976;24:253–261. [Google Scholar]
- Long C, Aussagues Y, Molinier N, Marcourt L, Vendier L, Samson A, Poughon V, Chalo Mutiso PB, Ausseil F, Sautel F, Arimondo PB, Massiot G. Dichapetalins from Dichapetalum species and their cytotoxic properties. Phytochemistry. 2013;94:184–191. doi: 10.1016/j.phytochem.2013.03.023. [DOI] [PubMed] [Google Scholar]
- Luo W, Zhao M, Yang B, Ren J, Shen G, Rao G. Antioxidant and antiproliferative capacities of phenolics purified from Phyllanthus emblica L. fruit. Food Chem. 2011;126:277–282. [Google Scholar]
- Mabberley DJ. Mabberley's Plant-Book. 3rd Cambridge University Press; New York: 2008. pp. 659–660. [Google Scholar]
- Maruyama H, Tamauchi H, Iizuka M, Nakano T. The role of NK cells in antitumor activity of dietary fucoidan from Undaria pinnatifida sporophylls (Mekabu) Planta Med. 2006;72:1415–1417. doi: 10.1055/s-2006-951703. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Murata Y, Hayashi M, Nanba H. Inhibitory effect of MD-fraction on tumor metastasis: involvement of NK cell activation and suppression of intercellular adhesion molecule (ICAM)-1 expression in lung vascular endothelial cells. Biol. Pharm. Bull. 2008;31:1104–1108. doi: 10.1248/bpb.31.1104. [DOI] [PubMed] [Google Scholar]
- McCann MJ, Gill CIR, Linton T, Berrar D, McGlynn H, Rowland IR. Enterolactone restricts the proliferation of the LNCaP human prostate cancer cell line in vitro. Mol. Nutr. Food Res. 2008;52:567–580. doi: 10.1002/mnfr.200700052. [DOI] [PubMed] [Google Scholar]
- Meresse P, Dechaux E, Monneret C, Bertounesque E. Etoposide: discovery and medicinal chemistry. Curr. Med. Chem. 2004;11:2443–2466. doi: 10.2174/0929867043364531. [DOI] [PubMed] [Google Scholar]
- Pommier Y, Leo E, Zhang H-L, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010;17:421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Projan SJ, Carleton S, Novick RP. Determination of plasmid copy number by fluorescence densitometry. Plasmid. 1983;9:182–190. doi: 10.1016/0147-619x(83)90019-7. [DOI] [PubMed] [Google Scholar]
- Qi W, Hua L, Gao K. Chemical constituents of the plants from the genus Phyllanthus. Chem. Biodivers. 2014;11:364–395. doi: 10.1002/cbdv.201200244. [DOI] [PubMed] [Google Scholar]
- Ren Y, Matthew S, Lantvit DD, Ninh TN, Chai H-B, Fuchs JR, Soejarto DD, Carcache de Blanco EJ, Swanson SM, Kinghorn AD. Cytotoxic and NF-κB inhibitory constituents of the stems of Cratoxylum cochinchinense and their semisynthetic analogues. J. Nat. Prod. 2011;74:1117–1125. doi: 10.1021/np200051j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Lantvit DD, Deng Y, Kanagasabai R, Gallucci JC, Ninh TN, Chai H-B, Soejarto DD, Fuchs JR, Yalowich JC, Yu J, Swanson SM, Kinghorn AD. Potent cytotoxic arylnaphthalene lignan lactones from Phyllanthus poilanei. J. Nat. Prod. 2014;77:1494–1504. doi: 10.1021/np5002785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrecker AW, Hartwell JL. The structure of savinin. J. Am. Chem. Soc. 1954;76:4896–4899. [Google Scholar]
- Sharma S, Kumar S. Phyllanthus reticulatus Poir.-an important medicinal plant: a review of its phytochemistry, traditional uses and pharmacological properties. Int. J. Pharm. Sci. Res. 2013;4:2528–2534. [Google Scholar]
- Singh E, Sharma S, Pareek A, Dwivedi J, Yadav S, Sharma S. Phytochemistry, traditional uses and cancer chemopreventive activity of Amla (Phyllanthus emblica): the sustainer. J. Appl. Pharm. Sci. 2011;2:176–183. [Google Scholar]
- Sutthivaiyakit S, Nakorn NN, Kraus W, Sutthivaiyakit P. A novel 29-nor-3,4-seco-friedelane triterpene and a new guaiane sesquiterpene from the roots of Phyllanthus oxyphyllus. Tetrahedron. 2003;59:9991–9995. [Google Scholar]
- Tanoguchi M, Arimoto M, Saika H, Yamaguchi H. Studies on the constituents of the seeds of Hernandia ovigera L. VI. Isolation and structural determination of three lignans. Chem. Pharm. Bull. 1987;35:4162–4168. [Google Scholar]
- Tuchinda P, Kumkao A, Pohmakotr M, Sophasan S, Santisuk T, Reutrakul V. Cytotoxic arylnaphthalide lignan glycosides from the aerial parts of Phyllanthus taxodiifolius. Planta Med. 2006;72:60–62. doi: 10.1055/s-2005-873141. [DOI] [PubMed] [Google Scholar]
- Tuchinda P, Kornsakulkarn J, Pohmakotr M, Kongsaeree P, Prabpai S, Yoosook C, Kasisit J, Napaswad C, Sophasan S, Reutrakul V. Dichapetalin-type triterpenoids and lignans from the aerial parts of Phyllanthus acutissima. J. Nat. Prod. 2008;71:655–663. doi: 10.1021/np7007347. [DOI] [PubMed] [Google Scholar]
- Van Oeveren A, Jansen JFGA, Feringa BL. Enantioselective synthesis of natural dibenzylbutyrolactone lignans (−)-enterolactone, (−)-hinokinin, (−)-pluviatolide, (−)-enterodiol, and furofuran lignan (−)-eudesmin via tandem conjugate addition to γ-alkoxybutenolides. J. Org. Chem. 1994;59:5999–6007. [Google Scholar]
- Weckert E, Mattern G, Addae-Mensah I, Waibel R, Achenbach H. The absolute configuration of dichapetalin A. Phytochemistry. 1996;43:657–660. [Google Scholar]
- Wu S-J, Wu T-S. Cytotoxic arylnaphthalene lignans from Phyllanthus oligospermus. Chem. Pharm. Bull. 2006;54:1223–1225. doi: 10.1248/cpb.54.1223. [DOI] [PubMed] [Google Scholar]
- Xie L-H, Akao T, Hamasaki K, Deyama T, Hattori M. Biotransformation of pinoresinol diglucoside to mammalian lignans by human intestinal microflora, and isolation of Enterococcus faecalis strain PDG-1 responsible for the transformation of (+)-pinoresinol to (+)-lariciresinol. Chem. Pharm. Bull. 2003;51:508–515. doi: 10.1248/cpb.51.508. [DOI] [PubMed] [Google Scholar]
- Xu G-J, He H-X, Xu L-S, Jin R-Y. The Chinese Materia Medica. China Medical Science and Technology Press; Beijing: 1996. pp. 1105–1467. [Google Scholar]
- Yu J, Wei M, Becknell B, Trotta R, Liu S, Boyd Z, Jaung MS, Blaser BW, Sun J, Benson DM, Mao H, Yokohama A, Bhatt D, Shen L, Davuluri R, Weinstein M, Marcucci G, Caligiuri MA. Pro- and anti-inflammatory cytokine signaling: reciprocal antagonism regulates interferon-gamma production by human natural killer cells. Immunity. 2006;24:575–590. doi: 10.1016/j.immuni.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Zhang Y-J, Abe T, Tanaka T, Yang C-R, Kouno I. Phyllanemblinins A-F, new ellagitannins from Phyllanthus emblica. J. Nat. Prod. 2001;64:1527–1532. doi: 10.1021/np010370g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.