Abstract
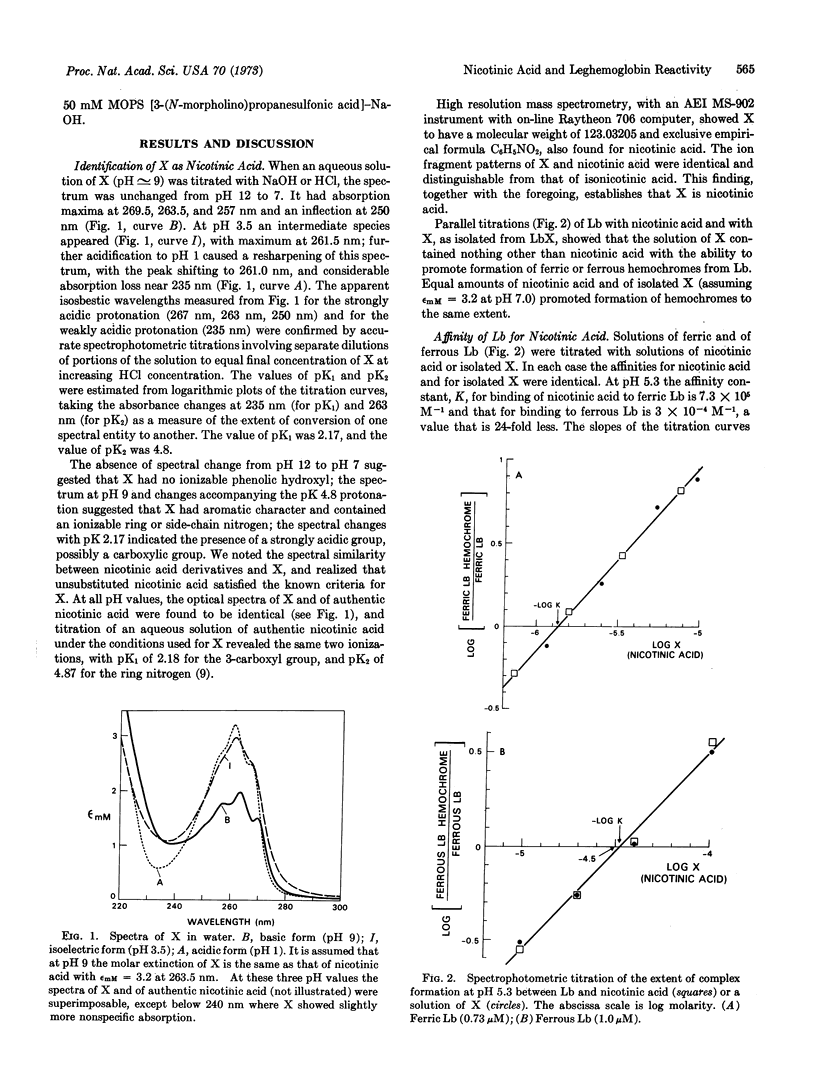
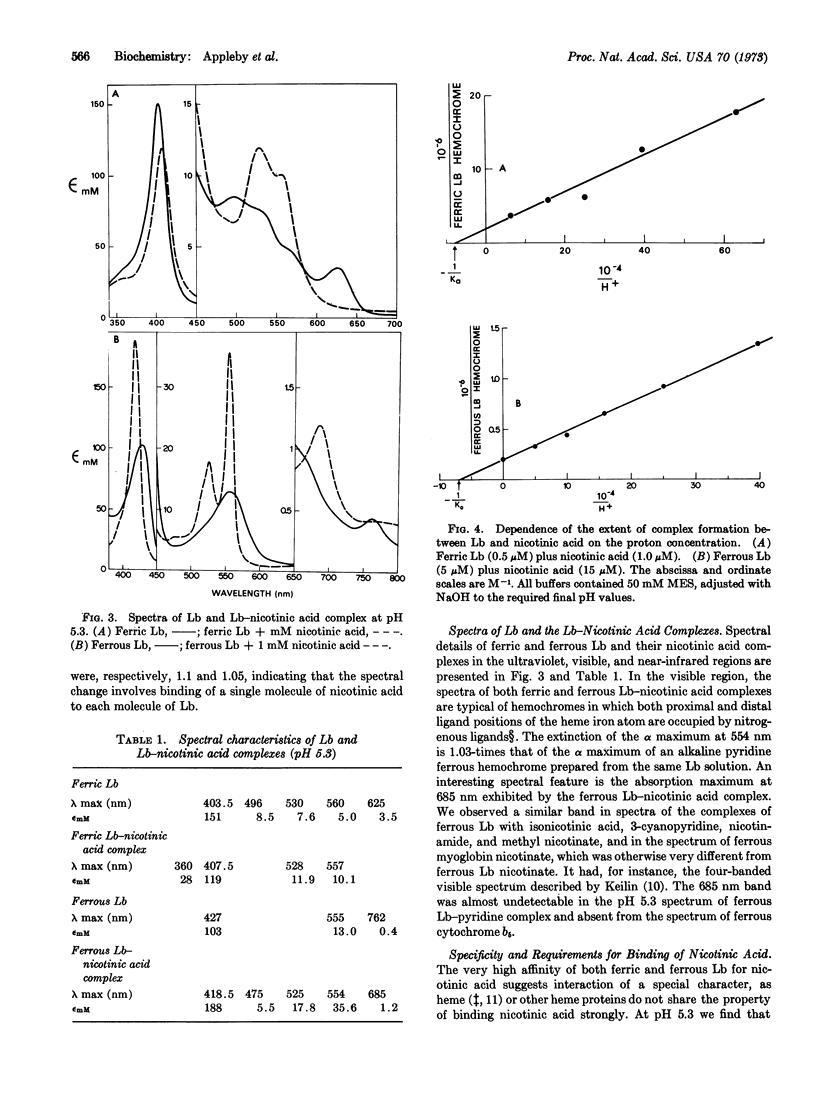
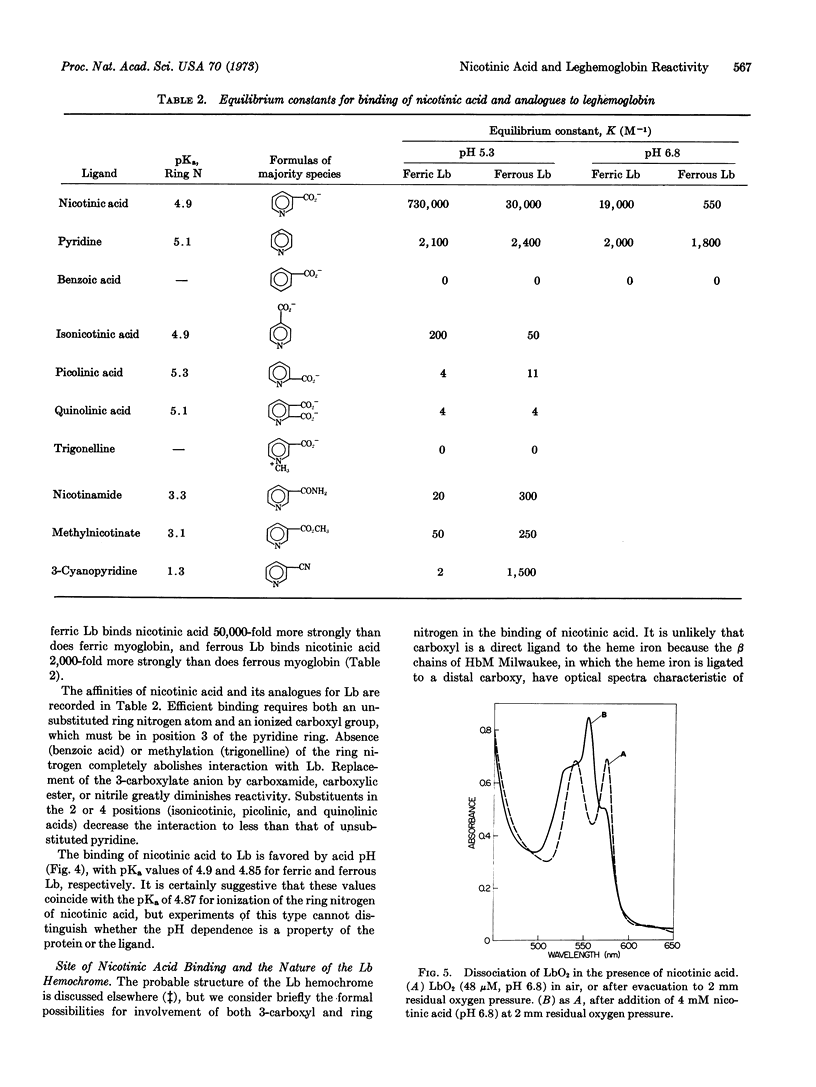
A small molecule, hitherto called X, which is present in legume root nodules and ligates reversibly to the monomeric protein, leghemoglobin, with formation of a hemochrome structure, is identified as nicotinic acid. The binding constants at pH 5.3 are K = 7.3 × 105 M-1 and K = 3.0 × 104 M-1 for combination of nicotinic acid with ferric and ferrous leghemoglobin, respectively. This high affinity binding of ligand requires an unsubstituted pyridine ring nitrogen atom and an ionized carboxyl group in the 3-position of the ring. Binding of nicotinic acid is favored at acid pH and is reflected by diminished apparent affinity of leghemoglobin for oxygen.
Keywords: nitrogen fixation, hemochrome, allosteric control
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AKOYUNOGLOU J. H., OLCOTT H. S., BROWN W. D. FERRIHEMOCHROME AND FERROHEMOCHROME FORMATION WITH AMINO ACIDS, AMINO ACID ESTERS, PYRIDINE DERIVATIVES, AND RELATED COMPOUNDS. Biochemistry. 1963 Sep-Oct;2:1033–1041. doi: 10.1021/bi00905a021. [DOI] [PubMed] [Google Scholar]
- APPLEBY C. A. The oxygen equilibrium of leghemoglobin. Biochim Biophys Acta. 1962 Jul 2;60:226–235. doi: 10.1016/0006-3002(62)90398-0. [DOI] [PubMed] [Google Scholar]
- Appleby C. A. Properties of leghaemoglobin in vivo, and its isolation as ferrous oxyleghaemoglobin. Biochim Biophys Acta. 1969;188(2):222–229. doi: 10.1016/0005-2795(69)90069-5. [DOI] [PubMed] [Google Scholar]
- Appleby C. A. The separation and properties of low-spin (haemochrome) and native, high-spin forms of leghaemoglobin from soybean nodule extracts. Biochim Biophys Acta. 1969 Oct 21;189(2):267–279. doi: 10.1016/0005-2728(69)90053-x. [DOI] [PubMed] [Google Scholar]
- Behlke J., Sievers G., Ellfolk N. Crystalline leghemoglobin. 13. Sedimentation studies. Acta Chem Scand. 1971;25(2):746–747. doi: 10.3891/acta.chem.scand.25-0746. [DOI] [PubMed] [Google Scholar]
- Bergersen F. J., Turner G. L., Appleby C. A. Studies of the physiological role of leghaemoglobin in soybean root nodules. Biochim Biophys Acta. 1973 Jan 18;292(1):271–282. doi: 10.1016/0005-2728(73)90271-5. [DOI] [PubMed] [Google Scholar]
- Ellfolk N., Sievers G. Crystalline leghemoglobin. X. The ferrihemochrome of leghemoglobin. Acta Chem Scand. 1967;21(6):1457–1461. doi: 10.3891/acta.chem.scand.21-1457. [DOI] [PubMed] [Google Scholar]
- Imamura T., Riggs A. Equilibria and kinetics of ligand binding by leghemoglobin from soybean root nodules. J Biol Chem. 1972 Jan 25;247(2):521–526. [PubMed] [Google Scholar]
- Perutz M. F., Pulsinelli P. D., Ranney H. M. Structure and subunit interaction of haemoglobin M Milwaukee. Nat New Biol. 1972 Jun 28;237(78):259–263. doi: 10.1038/newbio237259a0. [DOI] [PubMed] [Google Scholar]
- Wittenberg J. B., Appleby C. A., Wittenberg B. A. The kinetics of the reactions of leghemoglobin with oxygen and carbon monoxide. J Biol Chem. 1972 Jan 25;247(2):527–531. [PubMed] [Google Scholar]



