Abstract
Triplex-forming peptide nucleic acids (PNAs) facilitate gene editing by stimulating recombination of donor DNAs within genomic DNA via site-specific formation of altered helical structures that further stimulate DNA repair. However, PNAs designed for triplex formation are sequence restricted to homopurine sites. Herein we describe a novel strategy where next generation single-stranded gamma PNAs (γPNAs) containing miniPEG substitutions at the gamma position can target genomic DNA in mouse bone marrow at mixed-sequence sites to induce targeted gene editing. In addition to enhanced binding, γPNAs confer increased solubility and improved formulation into poly(lactic-co-glycolic acid) (PLGA) nanoparticles for efficient intracellular delivery. Single-stranded γPNAs induce targeted gene editing at frequencies of 0.8% in mouse bone marrow cells treated ex vivo and 0.1% in vivo via IV injection, without detectable toxicity. These results suggest that γPNAs may provide a new tool for induced gene editing based on Watson-Crick recognition without sequence restriction.
Keywords: β-globin, genome editing, GFP, nanoparticle, PLGA, PNA
INTRODUCTION
Site-specific genome modification using the host homologous recombination system has recently garnered attention as a possible strategy for the treatment of rare genetic diseases. Several strategies for gene editing have been developed based on the concept of activating the host DNA repair machinery by inducing DNA damage. One approach involves the use of engineered nucleases, including Meganucleases [1, 2], zinc-finger nucleases (ZFNs) [3, 4], TAL effector nucleases (TALENs) [5, 6], the CRISPR/Cas9 system [7, 8] and artificial DNA cutters [9]. However, off-target cleavage activity may hinder the clinical application of these molecules [10, 11].
An alternative approach uses triplex-forming oligonucleotides (TFOs) [12, 13]. TFOs bind duplex DNA to create an altered helical structure, thereby provoking DNA repair and further stimulating the recombination of short, single-stranded donor DNA molecules (containing the corrective nucleotide sequence) within nearby genomic sites [14]. Induction of recombination can occur over a distance of several hundred base pairs and has been shown to depend on the initiation of the nucleotide excision repair (NER) pathway by the triplex formation followed by engagement of the homology-dependent repair pathway [15, 16]. The ability of TFOs to induce recombination comes from tight binding at their cognate sites, as they do not possess any direct nuclease activity, and so the genomic safety profile of TFOs may be more favorable as compared to the engineered nucleases.
Recently we have shown that peptide nucleic acids (PNAs) (13,14), a specific class of TFOs, are capable of inducing recombination and gene modification [17, 18]. PNAs are synthetic analogues of DNA, comprised of a pseudopep-tide backbone and nucleobases connected via a flexible carboxymethylene bridge [19, 20]. The charge-neutral backbone allows PNAs to hybridize to DNA and RNA with high affinity as well as sequence specificity according to Watson-Crick base-pairing rules. Another important feature of PNAs is that they are enzymatically stable, in that they are not cleaved by proteases or nucleases. [21] Several promising PNA designs have been developed for gene targeting and induced gene editing including bisPNAs [22], tail clamp PNAs (tcPNAs) [14, 23] and pseudocomplementry PNAs (pcPNAs) [24]. However, each of these has some sequence limitations with respect to target selection. BisPNAs and tcPNAs are restricted to homopurine target sites [25, 26], and pcPNAs, while they can invade mixed-sequenced DNA, are restricted to sites with at least ~40% AT content [27].
We have shown that gamma PNAs (γPNAs), a chiral version of PNAs that adopt a right-handed helical motif [28], can invade mixed-sequence B-DNA without sequence restriction to form a heteroduplex, with the homologous strand locally displaced [29, 30]. In addition to conferring backbone pre-organization, making it possible for PNAs to invade mixed-sequence B-DNA in a sequence unrestricted manner at physiological temperature, introduction of a chiral mini-PEG substituent at the γ-position significantly improves the water solubility and specificity of the system [21, 31]. Though numerous in vitro studies have shown that γPNAs are capable of binding dsDNA with high affinity, none has demonstrated their potential for in vivo applications. Herein, we show that single-stranded γPNAs can recognize as well as bind genomic DNA in a sequence-unrestricted manner and induce gene correction in mammalian cells, both ex vivo and in vivo. Our rationale is that mini-PEG-substituted γPNAs (MP γPNAs) will target duplex DNA via strand invasion and thereby create altered helical structures consisting of PNA/DNA duplexes and displaced D-loops. We hypothesize that such structures will initiate recombination between the target genomic site and an introduced donor DNA.
To demonstrate proof of concept, we have used a transgenic mouse model containing a β-globin/EGFP fusion gene, consisting of intron 2 of human β-globin inserted within the GFP coding regions. The intron contains the IVS2-654 (C->T) mutation [32, 33], which is a most common cause of thalassemia in individuals of Southeast Asian heritage and creates a cryptic splice site that causes incorrect splicing of the intron and prevents expression. For delivery to mouse bone marrow cells both ex vivo and in vivo, we formulated the MP γPNAs and 60-nt donor DNAs into poly (lactide-co-glycolide) (PLGA) and Poly(beta-amino ester) polymer (PBAE) nanoparticles [14, 23], and we found substantial improvements in the encapsulation and subsequent release of MP γPNAs/donor DNA nucleic acids as compared to standard PNAs/donor DNA mixtures. In gene targeting assays, we report here that the MP γPNA/DNA-containing PBAE/PLGA nanoparticles mediated site-directed genome editing of the IVS2-654 (C->T) mutation site in vivo in mouse bone marrow at a frequency of 0.1%, with an off-target frequency at a partially homologous site more than 10,000 fold lower. Ex vivo treatment of mouse bone marrow revealed a gene editing frequency of 0.8% in a single nanoparticle dose treatment. Nanoparticle-mediated delivery of γPNAs is a promising strategy for treating genetic diseases through induced site-directed gene correction without sequence restriction.
MATERIAL AND METHODS
Regular and γPNA Oligomer Synthesis
MP γPNA monomers were prepared according to the procedures reported by Sahu et al. [21]. Regular PNA monomers were purchased from ASM Research Chemicals. All oligomers were synthesized via standard solid phase Boc chemistry [34]. Further oligomers were cleaved from the resin using cleavage cocktail; m-cresol:thioanisole: TFMSA:TFA (1:1:2:6). After precipitating the crude mixtures with ether (3x), purification was performed by using RP-HPLC and further characterization was done by MALDITOF. Purified PNA/γPNA oligos were dissolved in water to prepare stock solutions. The concentrations of PNAs and γPNAs were measured at 90 °C on a Bio spectrophotometer using the specific extinction coefficients of nucleobases at 260 nm: 8,600 M−1 cm−1 (T), 6,600 M−1 cm−1 (C), 13,700 M−1 cm−1 (A), 11,700 M−1 cm−1 (G) as done previously [35, 36].
ds DNA-Binding Gel Shift Assay
For gel electrophoresis, 120-bp dsDNA targets were incubated with indicated PNA/γPNA oligos at the indicated concentrations, and temperatures in low ionic strength buffer (10mM NaPi, pH 7.4). The 120-bp dsDNA target was prepared by using standard PCR procedure and the following DNA templates
Template 1
5’TAGATTCGATCTATCTCTAGTGATCTTAGAACT AGTGGATCGATATCTATTGTAACTGATGTAAGAGGTT -3’
Template 2
5’ATCTCTTCTATATATCTATTTCTGACGTTGACG TCAGCGTTCGAATTGCTAACCTCTTACATCAGTTACA3’
The incubated PNA/γPNA and DNA samples were run on 10% nondenaturing polyacrylamide gels (Catalog # 161-1110, Bio-Rad) at 120 V/cm for 2 hr at physiological temperature (37 °C) using 1X TBE buffer. Further the gels were stained with SYBR-Gold (Catalog #S11494, Invitrogen) for 10 min, washed twice with TBE buffer (1X) and imaged using a gel documentation system (BioDoc-It System) [37, 38]. The images were inverted using Adobe Photoshop 6.0 and the amounts of fraction-bound and-unbound DNA were quantified by using ImageJ software [37, 38].
PLGA Nanoparticle Synthesis
PLGA nanoparticles encapsulating PNA/donor DNA were formulated using the double-emulsion solvent evaporation technique as described previously [35]. PNAs and donor DNAs were dissolved in 60.8 μl DNAse free water. All nanoparticle batches had 2 nmole/mg of PNA or γPNA and 1nmole/mg of donor DNA. The encapsulant was added dropwise to a polymer solution containing 80 mg 50:50 ester-terminated PLGA dissolved in dichloromethane (800ul), then ultrasonicated (3 X 10 seconds) to formulate the first emulsion [23, 35]. To form second emulsion, first emulsion was added slowly dropwise to 1.6 ml of 5% aqueous polyvinyl alcohol and then ultrasonicated (3 × 10 seconds). This mixture was finally poured into 20 ml of 0.3% aqueous polyvinyl alcohol and stirred for 3 hr at room temperature [23, 35]. Nanoparticles were then thoroughly washed with 20 ml water (3X) and further collected each time by centrifugation (12,000 rpm for 10 min at 4°C). Nanoparticles were re-suspended in water, frozen at -80°C, and then lyophilized. Nanoparticles were stored at -20°C after lyophilization [23, 35].
PBAE/PLGA Nanoparticle Synthesis
PBAE polymer was synthesized using well known Michael addition reaction of 1,4-dutanediol diacrylate and 4,4’-trimethylenedipiperidine as previously reported [39]. Nanoparticle blends were further formulated using a double solvent emulsion method. Briefly, PBAE and PLGA polymers (40 mg) were co-dissolved in dichloromethane (800 ul) at an optimized ratio of 15:85 (wt:wt) [40, 41]. Nucleic acids in 1X TE buffer were added dropwise to the polymer blend solution and sonicated. The resulting solution was added dropwise to a 5% (w/v) solution of poly (vinyl alcohol) (PVA) (2 mL) while under vortex and sonicated again. The final emulsion was added to a stirring solution of 0.3% PVA (v/v) and stirred overnight, allowing the DCM to evaporate which further harden the nanoparticles. Nanoparticles were collected by centrifugation (3 ×, 15 min, 9500 rpm) and washed in diH2O. Particles were frozen in a cryoprotectant solution of 0.1% trehalose (w/v) at -80 °C and lyophilized for 72 hr. Resulting dry powders were stored at -20°C until used. For fluorescent particles, coumarin-6 (C6) was added to the polymer solution at a 2% C6: polymer ratio (wt:wt).
SEM Characterization
Nanoparticles were coated in platinum and imaged using scanning electron microscopy (XL-30, FEI, Hillsboro, Oregon). Image J was used to analyse particle size as well as morphology [35].
Loading Profile Studies of PLGA and PBAE/PLGA Nanoparticles
Nanoparticle loading was determined using a modified extraction method, as previously reported [40, 42]. Briefly, 1-3 mg of nanoparticles were dissolved in 0.5 mL of DCM and rotated at 300 rpm overnight. In order to extract the nucleic acid from the organic phase, 0.5 mL of 1X TE buffer was added to the organic phase, vortexed, and centrifuged (12,000 rpm for 5 min) [43]. After the first collection, the extraction was repeated a second time. The absorbance of the combined aqueous fractions was measured at 260nm and compared to a standard curve of a donor DNA correlating absorbance with nucleic acid concentration.
Controlled-Release Studies of PLGA Nanoparticles
Nucleic acid release was analyzed by incubating nanoparticles (4-6mg) in PBS (600 μl) in a 37°C shaker, spinning down, and removing supernatant. Further absorbance of the supernatant was measured at 260 nm at indicated time points [35]. At 25 hrs (for PLGA) and 48 hours (for PBAE:PLGA) nanoparticles, the residual nucleic acid in the nanoparticle pellet was extracted, total nucleic acid content was calculated as a sum of absorbance obtained from the pellet as well as supernatant [44]. Absorbances at 260 nm were measured with a Nanodrop 2000.
Ex vivo Experiments
Bone marrow cells were isolated by flushing of tibias and femurs with RPMI/10%FBS media from β-globin/EGFP transgenic mice. RBCs were separated from bone marrow, blood and spleen cells by using the Ficoll separation technique. 2 mg/ml of nanoparticles were used to treat 100,000 cells for 48 hr in RPMI/10% FBS media containing glutamine, in triplicate samples. After 48 hr, cells were fixed by using 4% PFA, and flow cytometry analyses were performed. Cells treated with blank nanoparticles were included as a control.
Mouse Models and In Vivo Treatments
The β-globin/EGFP transgenic mice were given generously by Drs. Ryszard Kole and Rudolph Juliano at the University of North Carolina and animals were raised and maintained at the Yale University Animal Facilities according to the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Yale University [23]. For nanoparticle treatment, nanoparticles (2mg) were resuspended in 150 μl of PBS buffer. Further resuspended nanoparticles were sonicated and injected via retro-orbital injection. Four doses were given at an interval of 48 hours. All the mice were sacrificed 5 days after the last treatment, and bone marrow, blood and spleen cells were harvested for further analysis. RBCs were separated from bone marrow, blood and spleen cells by Ficoll separation, and blood, spleen and bone marrow cells were fixed using 4% paraformaldehyde followed by flow cytometry analyses.
Colony-Forming Unit Assays
Bone marrow or spleen cells were plated in classic methylcellulose medium (MethoCult H3434), which is especially formulated to support the growth of mouse hematopoietic progenitor cells into macrophage or mixed colonies, erythroid, granulocyte as reported previously [23]. Further colonies were harvested as well as counted after 1-2 weeks.
Measurement of Inflammatory Cytokines Production after Nanoparticle Treatment
Level of inflammatory markers (Tumor necrosis factor-a and interleukin-6) was measured from their mRNA expression of bone marrow cells treated in vivo. Total RNA was extracted from bone marrow cells using the RNAeasy Plus kit. Further cDNA was synthesized with SuperScript II First-Strand Kit (Invitrogen) and quantitative PCR was performed using previously reported methods [23]. The primers sequences are as follows:
Mouse GAPDH: 5’-CGACTTCAACAGCAACTCCCA CTCTTCC-3’and 5’- TGGGTGGTCCAGGGTTTCTTACT CCTT-3’; mouse TNF alpha: 5’- CTGTAGCCCACGTCGTAGC-3’ and 5-TTGAGATCCATGCCGTTG-3’
Mouse IL-6 (Sense): AAAGAGTTGTGCAATGGCA ATTCT and mouse IL-6 (antisense): AAGTGCATCATCGTTGTTCATACA. The PCR conditions were 94 °C for 2 min, followed by 40 cycles of 94 °C for 30 sec, 50 °C for 30 sec and 72 °C for 1 min [23, 35]. The relative expression of glyceraldehyde 3-phosphate dehydrogenase mRNA was used as a reference gene [23, 35].
Genomic DNA Extraction
Genomic DNA from nanoparticles treated bone marrow cells was harvested using the Wizard Genomic Purification Kit (Promega) and further purified by agarose gel electrophoresis based protocols [14]. The desired molecular weight fragments, representing genomic DNA, isolate, extracted from agarose gel using the Wizard SV Gel and PCR Clean-Up System (Promega) [14].
Sample Preparation for Deep-Sequencing Analysis
PCR amplicons of a size 120-bp region of the β-globin gene and 150-bp regions in the RNF gene were generated. PCR amplicons were ligated to adapters and further sequenced on an Illumina HiSeq (Illumina Inc., San Diego, CA), with 75 base-pair (paired-end reads) at the Yale West Campus Keck Sequencing Facility [14]. Sequences were mapped against reference human genome (hg19) using BWA-MEM aligner (v0.7.5a). SAMTools (v0.1.19) to calculate the coverage of converted and reference bases, only high-quality calls (over 0.01% error rate) were used. Thus, only position 5247141 (IVS 654) showed alternative bases above the error rate of the Illumina calls. Percent genome editing was further calculated by counting precise numbers of amplicon copies of the IVS2-654 bp modification in -globin and donor DNA inserts in the RNF gene. Background frequency to the machine was no different between samples from untreated as well as treated mice [14].
RESULTS
Design of γPNA Oligomers
To quantify the potential of γPNAs to induce gene editing at the human β-globin locus ex vivo and in vivo, a β-globin/enhanced green fluorescent protein (EGFP) fusion gene carried within a transgenic reporter mouse was used. In this mouse, a mutation at nucleotide 654 of β-globin intron 2 activates an aberrant splice site and leads to retention of a β-globin intron fragment within the EGFP coding region mRNA, which in turn prevents proper translation of EGFP. Correction of the IVS2-654 mutation restores proper splicing and allows EGFP expression and cellular fluorescence. Hence, expression of EGFP provides a direct quantitative assessment of sequence-directed genome editing (Fig. 1).
Fig. (1).
Strategy for targeted correction of β-globin gene IVS2– 654 (C->T) mutation in β-globin/EGFP transgenic mice. Single-stranded MP γPNAs designed to bind to the coding strand of the β-globin/EGFP fusion gene invades genomic DNA and induces the recombination of donor DNA (complementary to sequences beginning 13 bp away from the γPNA binding site and containing corrected base modification which is indicated by black lines). Correction of the cryptic splice-site mutation enables EGFP expression.
We synthesized series of PNAs with or without gamma substitution and assessed their ability to induce gene correction (Fig. 2a and b). Two fully substituted MP PNAs, MP γPNA1 and MP γPNA2, were designed to bind the coding strand and template strand within the β-globin intron in the β-globin/EGFP fusion gene, respectively. For comparison, we also synthesized a mismatched MP γPNA4 and an unmodified PNA (PNA3) with the same sequence as MP γPNA1 (designed to bind to the coding strand) but without any gamma residues. Similarly, in order to test the extent to which γPNA can induce genome editing if designed to bind to sites at increased distances from the targeted mutation site to be corrected, we synthesized three more γPNAs: MP γPNA5, MP γPNA6 and MP γPNA7. These PNA and γPNA oligomers in the proposed studies were designed to target positions 694 to 714 (MP γPNA1, MP γPNA2, MP γPNA3, MP γPNA4), 782 to 802 (MP γPNA5), 904 to 924 (MP γPNA6) and 661 to 681 (MP γPNA7) in β-globin intron 2 within the β-globin/EGFP fusion gene. In all PNAs, we included 3 lysine residues at the C- and N-termini to enhance binding affinity and improve solubility [45]. In addition, we chose a 60-nucleotide sense donor DNA homologous to the β-globin/EGFP fusion gene except at the IVS2-654 site, where the donor DNA contained the wild-type nucleotide to correct the splicing mutation (Fig. 2c). For comparison, we also tested a 60-nucleotide antisense donor DNA strand for the proposed studies.
Fig. (2).
(a) Chemical structure of unmodified PNA and MP γPNA units. (b) Sequences of PNA and γPNA oligomers used in this study to target positions 694 to 714 (MP γPNA1, MP γPNA2, MP γPNA3, MP γPNA4), 782 to 802 (MP γPNA5), 904 to 924 (MP γPNA6) and 661 to 681 (MP γPNA7) in β-globin intron 2 within the β-globin/EGFP fusion gene. Bold letters indicate MP γPNA units. Bold underlines indicate mismatch region. (c) 60-nucleotide donor DNA (matching positions 622-681 in β-globin intron 2. Bold underline signifies corrective nucleotides that eliminate the aberrant splice site mutation.
DNA-Binding Properties of γPNA Oligomers
Rapireddy et al. have demonstrated that incorporation of an appropriate chiral substituent at the position in the PNA backbone induces right-handed helical pre-organization in the γPNA oligomers, enabling them to bind to double-stranded DNA targets via strand invasion with substantially increased affinity relative to standard PNAs [38]. In keeping with this, UV-melting analyses revealed that for both the MP γPNA1/DNA and MP γPNA2/DNA duplexes, the thermal stabilities were much higher than that of the PNA3/DNA (Supplemental Data, Table 1). MP γPNA4 showed no binding to the β-globin intron target, consistent with the sequence mismatch.
We also tested the binding of the γPNAs to a 120-bp DNA duplex containing the β-globin target sequence by gel-shift (Fig. 3). Our result showed that MP γPNA1 and MP γPNA2 were able to bind to their designated targets within the DNA duplex and to do so with much greater affinity than PNA3 (Fig. 3, lanes 2 and 3). However, we did not observe any retarded DNA band when MP γPNA1 and MP γPNA2 were incubated together in an equimolar amount (Fig. 3, lane 5), reflecting the much higher thermodynamic stability of γPNA/γPNA duplexes compared to γPNA/DNA duplexes.
Fig. (3).
Gel-shift analysis of PNA/γPNA oligomers binding to a 120-bp duplex DNA in 10 mm NaPi buffer at 37°C for 16 hr followed by separation on non-denaturing 10% PAGE gel followed by staining with SYBR-Gold. 0.1 uM of duplex DNA was used in each well.
PLGA Nanoparticle Formulations Containing γPNA/ Donor DNA and their Characterization
Previously we have demonstrated that systemic injection of PLGA nanoparticles can effectively deliver PNA/donor DNA combinations into primary human and mouse bone hematopoietic cells for site-directed genome editing of the β-globin and CCR5 genes [23]. Hence, to test their biological activity in mouse bone marrow cells, we encapsulated the γPNAs/DNAs into PLGA nanoparticles at a molar ratio of 2:1 in the starting mix using a double-emulsion solvent evaporation technique. Surprisingly, we noticed a substantial difference in the visual appearance of the encapsulants prepared with the γPNAs versus the samples containing regular PNAs. As shown, the MP γPNA1/donor DNA encapsulant formed a clear solution, in contrast to the PNA3/donor DNA encapsulant, which appeared as a suspension (data not shown). Even after incubating the encapsulants at room temperature over an extended period, we did not notice any precipitate within the MP γPNA1/donor DNA containing solution, in contrast to the persistent precipitate in the PNA3/donor DNA sample. This difference in encapsulant solubility likely reflects the presence of numerous ethylene glycol units in the γPNAs, which improve the overall γPNA/donor DNA complex solubility.
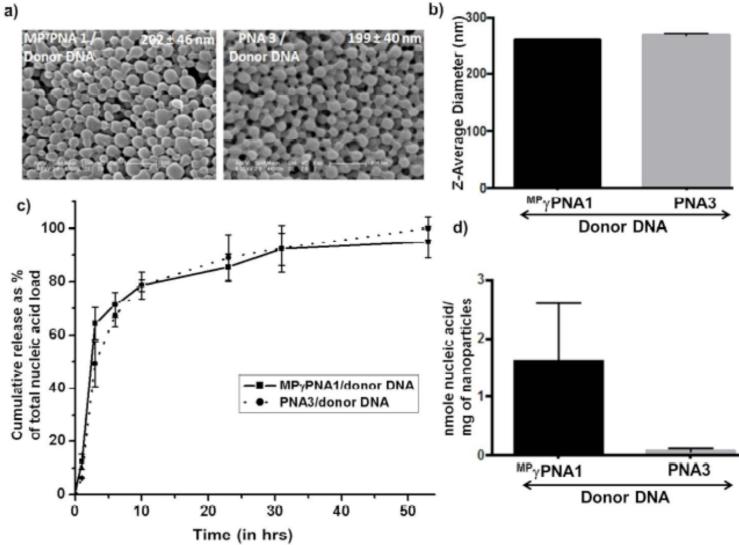
We next evaluated the morphology of formulated PLGA nanoparticles by scanning electron microscopy (SEM) (Fig. 4a) and measured the hydrodynamic diameter by dynamic light scattering (DLS) (Supplemental Data, Fig. S1). Based on our previous work, we desired nanoparticles of 200-300 nm diameter: all of the nanoparticles exhibited SEM and DLS sizes within this range. All nanoparticles were loaded with substantial quantities of nucleic acids (Supplemental Data, Fig. S2): the nucleic acids were slowly released from the nanoparticles during incubation in phosphate-buffered saline (PBS) (Fig. 4b). Importantly, we noticed higher loading of nucleic acids in the nanoparticles with MP γPNA1/donor DNA as compared to PNA3/donor DNA samples, again likely reflecting the improved solubility of γPNAs and greater stability of γPNAs in the emulsions used for nanoparticle synthesis.
Fig. (4).
Characterization of PLGA nanoparticles containing MP γPNA/donor DNA and treatment of mouse bone marrow cells ex vivo for gene editing. (a) Scanning electron microscope (SEM) images and size distribution of MP γPNA1/donor DNA containing PLGA nanoparticles. Average particle diameter and standard deviation are given. (b) Release of nucleic acids from PLGA nanoparticles during incubation at 37°C. At 24 hrs, the residual nucleic acid content in the nanoparticles pellet was extracted, total nucleic acid content was calculated as a sum of absorbance derived from the supernatant and pellet.
Ex vivo Studies Employing PLGA Nanoparticles in Mouse Bone Marrow Cells
Bone marrow cells harvested from the femurs of β-globin/EGFP transgenic mice and placed in culture were treated ex vivo with PLGA nanoparticles (2mg/ml). After 48 hr of nanoparticle treatment, the bone marrow cells were examined for EGFP expression. Flow cytometry confirmed that MP γPNA1/donor and MP γPNA2/donor DNA-containing nanoparticles mediated genome modification at frequencies of 0.1% and 0.05% respectively (Fig. 5a). Nanoparticless containing PNA3/donor DNA showed lower levels of gene modification (0.02%). The nanoparticles containing the mismatched MP γPNA4/donor DNA displayed no activity above nanoparticles with just donor alone (0.002%). In addition, fluorescence microscopy of MP γPNA1/donor DNA-containing nanoparticle-treated cells confirmed the presence of GFP-expressing cells. These results indicated the superiority of the MP γ-substituted PNAs; and based on the relative efficiencies, we focused our subsequent efforts on the MP γPNA1/donor DNA combination (Fig. 5b).
Fig. (5).
Ex vivo experiment results. (a) %EGFP-positive cells among mouse bone marrow cells (from β-globin/EGFP transgenic mice) after ex vivo treatment with PLGA nanoparticles. Data are shown as mean ± s.d., n = 3; statistical analysis was performed with Student's t-test, asterisk, P < 0.05; two asterisks, P < 0.01. (b) Fluorescence microscopy showing GFP+ cells in total population of uncorrected cells after ex vivo treatment of bone marrow cells with PLGA nanoparticles containing MP γPNA1 /donor DNA.
PBAE/PLGA Nanoparticle Formulation Containing γPNA/Donor DNA
To further improve delivery of the MP γPNA1/donor DNA molecules, we investigated an additional nanoparticle formulation that other work suggested might be favorable for encapsulation of nucleic acids [43]. This formulation consisted of 15% poly (beta-amino) ester (PBAE) blended with 85% PLGA. PBAE was synthesized by a reaction of 1,4-butanediol diacrylate and 4,4′- trimethylenedipiperidine [43]. Nanoparticles were loaded with similar PNA/DNA combinations as discussed above. We also synthesized PBAE/PLGA nanoparticles with γPNA/antisense donor DNA combinations. The PBAE/PLGA nanoparticles were characterized by SEM (Fig. 6a) and DLS (Fig. 6b). With this new formulation, we again observed improved loading of the MP γPNA1/donor DNA combination compared to the PNA3/donor DNA sample (Fig. 6d) and slow release of nucleic acids (Fig. 6c).
Fig. (6).
Characterization of PBAE/PLGA nanoparticles containing MP γPNA/donor DNA. (a) Scanning electron microscope (SEM) images of PBAE/PLGA nanoparticles. Average particle diameter and SD are given for each nanoparticle batch. (b) Hydrodynamic diameters of PBAE/PLGA nanoparticles. (c) Cumulative Release of nucleic acids from PBAE/PLGA nanoparticles during incubation at 37°C. At 53 hrs, the residual nucleic acid content in the nanoparticles pellet was extracted and calculated as a sum of absorbance derived from the supernatant and pellet. (d) Loading of nucleic acids in PBAE/PLGA nanoparticles. Data are shown as mean ± s.d., n = 3.
Ex Vivo Studies Employing PBAE/PLGA Nanoparticles in Mouse Bone Marrow Cells
The PBAE/PLGA nanoparticles containing PNA/donor DNA combinations were incubated with bone marrow cells harvested from EGFP transgenic mice, as above. After 48 hr of incubation, flow cytometry revealed that PBAE/PLGA nanoparticles containing the MP γPNA1/donor DNA combination induced gene editing at a frequency of 0.8%, significantly higher than the frequencies obtained with PLGA nanoparticles (Fig. 7a). Similarly, we also performed ex vivo experiments on bone marrow cells treated with MP γPNA/antisense donor DNA combinations to compare the effect of sense versus antisense donor DNAs (Supplemental Data, Fig. S3). Our results indicate that the MP γPNA1/antisense donor DNA combination also induces gene editing (0.18%), but overall the MP γPNA1/sense donor DNA combination gave higher gene-editing frequencies, a result which is consistent with our prior findings [23]. We also performed ex vivo treatment on bone marrow cells treated with other γPNAs designed to bind to the coding strand at more distant sites in the β-globin gene. We noticed γPNAs can induce gene editing at ~100 bp away (0.43%) and even ~250 bp away (0.23%) from the donor DNA binding site (Fig. 7b). Our results also reveal that MP γPNA1, alone, enclosed in nanoparticles in the absence of donor DNA does not exhibit any gene-editing effect nor does it produce any detectable mutagenic effect at its cognate binding site in the genomic β-globin site. We also performed an experiment in which different PBAE:PLGA formulations containing different molar ratios of PNA:DNA (2:0.5, 2:1 and 2:2) were formulated to study the effects of increased amounts of donor DNA on gene-editing frequencies. We observed that as we increased the proportion of donor DNA, the gene-editing frequency initially increases but then plateaus (Supplemental Data, Fig. S4).
Fig. (7).
Treatment of mouse bone marrow cells ex vivo for gene editing with PBAE/PLGA nanoparticles containing MP γPNA/donor DNA. (a & b) % EGFP-positive cells among mouse bone marrow cells (from β-globin/EGFP transgenic mice) after ex vivo treatment with PBAE/PLGA nanoparticles containing PNA and γPNA/donor DNA. (c) Bright field and fluorescence microscopy images of a GFP expressing colony derived from PBAE/PLGA nanoparticle containing MP γPNA1/donor DNA-treated bone marrow cells. (d) Colonies were counted as red (CFU/BFU-E) white (CFU-G/M/GM), or combined (CFU-GEMM). (e) Images of hematopoietic colonies. Treated mononuclear cells were plated in methylcellulose medium with selected cytokines.
Colony-Forming Unit Assays to Assess the Pluripotency of Bone Marrow Cells
To test the toxicity of the nanoparticle treatment and to evaluate possible effects on the pluripotency of the bone marrow cells, cells from MP γPNA1/donor DNA-treated bone marrow samples were examined in colony-forming assays. After nanoparticle treatment, the cells were plated in methyl-cellulose medium supplemented with selected cytokines for appropriate growth of granulocyte/macrophage [(CFU-G and CFU-GM), colony-forming unit erythroid (CFU-E)], erythroid [burst-forming unit (BFU)] or combined colonies (CFU-GEMM) [14]. Fluorescence microscopy of the hematopoietic colonies also revealed EGFP expression in some of colonies derived from cells treated with MP γPNA1/donor DNA-containing nanoparticles (Fig. 7c), consistent with the flow cytometry results. Mononuclear cells treated with MP γPNA1/donor DNA nanoparticles formed both erythroid and myeloid colonies with the same frequency as well as morphology as that of blank nanoparticle-treated cells (Figs.7d and e). These results suggest that nanoparticles containing MP γPNA1/donor DNA combinations are non-toxic to hematopoietic progenitors as well as do not affect their potential to differentiate.
In Vivo Studies to Measure Gene-Editing Potential through Systemic Delivery of PBAE/PLGA Nanoparticles
Next, we tested the activity of the MP γPNA1/donor DNA nanoparticles upon in vivo administration by intravenous injection in the β-globin/EGFP transgenic mice. In initial studies, we compared retro-orbital versus tail vein injection. PBAE/PLGA nanoparticles containing the dye Coumarin 6 (C6) were formulated using the double-solvent emulsion technique [43]. EGFP transgenic reporter mice were given a single 2mg dose of nanoparticles in 150 μl of PBS via either tail vein or retro-orbital injection. At 5 hr post-injection, bone marrow, blood and spleen cell samples were harvested and flow cytometry was performed to measure the biodistribution of C6 into each tissue. The histogram data of blood, bone marrow and spleen cells revealed that retro-orbital injection was a better route for delivering nanoparticles as compared to tail vein injection (Supplemental Data, Fig. S5).
To test for in vivo gene editing, we performed retro-orbital injections of blank PBAE/PLGA nanoparticles or PBAE/PLGA nanoparticles containing PNA3/donor DNA, MP γPNA1/donor DNA, or MP γPNA4/donor DNA into the globin/EGFP mice (Fig. 8a). Four nanoparticle injections of 2 mg each in 150 μl of PBS were given at intervals of 48 hr. We did not notice any signs of systemic toxicity or distress throughout these studies. Five days after the last treatment, bone marrow, blood and spleen cells were harvested for further analysis by flow cytometry. Mice treated with MP γPNA1/donor DNA nanoparticles showed significant EGFP expression in cells from bone marrow (0.11%), spleen (0.08%) and blood (0.09%) as compared to the blank nanoparticle-treated group (Fig. 8b, c and d, Supplemental Data, Fig. S6). Minimal genome modification was seen in mice treated with either PNA3/donor DNA or with the mismatched MP γPNA4/donor DNA combination.
Fig. (8).
(a) In vivo gene editing by treatment of β-globin/EGFP transgenic mice via intravenous injection of PBAE/PLGA nanoparticles containing MP γPNA/donor DNA. (b) β-globin/EGFP mice were treated by retro-orbital injections of blank PBAE/PLGA nanoparticles or PBAE/PLGA nanoparticles containing PNA3/donor DNA, MP γPNA1/donor DNA, or MP γPNA4/donor DNA. Four nanoparticle injections of 2 mg each in 150 μl of PBS were given at intervals of 48 hours. Five days after the last treatment, bone marrow, blood and spleen cells were harvested for EGFP expression analysis by flow cytometry (100,000 cells per sample). Each data point represents one mouse. (c) Deep-sequencing analysis to quantify on- and off-target in vivo editing frequencies in bone marrow cells from mice treated with PBAE/PLGA nanoparticles containing MP γPNA1/donor DNA.
Bone marrow cells harvested from in vivo-treated mice were also subjected to deep-sequencing analysis of the β-globin/EGFP alleles in the cell populations to quantify the frequency of gene editing. Deep-sequencing revealed a gene-editing frequency of 0.077% in bone marrow cells from the mice treated with PBAE/PLGA nanoparticles containing MP γPNA1/donor DNA .We further used deep-sequencing to assess off-target effects. By BLAST analysis, we found that the RING finger165 (RNF) gene possesses partial homology of 13 base pairs (ACTGATGTAAGAG) to the binding site of MP γPNA1 in the β-globin gene (Supplemental Data, Fig. S7). RNF domains function as ligases that link ubiquitination enzymes to their substrates [46]. Hence, considering the extent of sequence homology, we examined off-target effects on the RNF gene. Deep-sequencing revealed an extremely low frequency of γPNA/donor DNA-mediated sequence change in the RNF gene which is at or below the background frequency in the deep-sequencing assay (≤0.01%) (Fig. 8e).
In addition, we confirmed that bone marrow and spleen cells harvested from the mice retained their pluripotency after in vivo treatment with the PBAE/PLGA nanoparticles containing MP γPNA1/donor DNA, using colony-forming as-says (Figs. 9a and 9b). We also performed RT-PCR analyses on treated bone marrow cells to evaluate expression of inter-leukin 6 (IL-6) and tumor necrosis factor alpha (TNFα) levels as an inflammatory markers: we observed no difference between untreated cells and cells treated with PBAE/PLGA nanoparticles containing MP γPNA1/donor DNA, suggesting minimal inflammatory response (Fig. 9c & 9d).
Fig. (9).
(a) Colony-forming units derived after plating bone marrow cells from in vivo-treated mice. (b) Images of hematopoietic colonies arising from mononuclear cells from treated bone marrow as well as spleen cells plated in methylcellulose media. (c) qRT-PCR results to determine the inflammatory markers, TNF-alpha and interleukin-6 (IL-6), expression in bone marrow cells isolated from the treated mice. Data are shown as mean ± s.d., n = 3; statistical analysis performed with Student's t-test, asterisk, P < 0.05; two asterisks, P < 0.01.
DISCUSSION
Prior studies on γPNAs have highlighted their promising biophysical properties as well as in vitro -binding properties. The results described here illustrate the first demonstration of the gene-editing potential of single-stranded γPNAs to induce gene modification both ex vivo and in vivo in mice. A major advantage of γPNAs is that they can be used to induce gene editing at mixed-sequence target sites based on Watson-Crick recognition without sequence restriction. Importantly, we used a transgenic mouse carrying a β-globin/EGFP reporter gene so that editing of the splice site mutation in the β-globin intron within the EGFP gene can be detected by FACS-based analysis of EGFP protein expression for robust quantification. The work reported here also demonstrates the effectiveness of PBAE/PLGA nanoparticles for delivery of γPNAs/donor DNAs to target genomic DNA in primary hematopoietic cells ex vivo and in vivo.
Previous reports from our lab have shown that bisPNAs and tcPNAs can be used to induce gene editing [47, 48]. Although great strides have been made in improving the PNA design for site-directed gene editing, the application of tcPNAs and bisPNAs is still confined to homopurine regions of the target DNA. In prior work, we also demonstrated that the combination of pcPNAs and donor DNAs induces gene correction frequency of 0.012% in a single treatment in CHO-GFP/ IVS2-1(G->A) cell lines [24]. However, pcPNA-mediated DNA recognition employs two strands of complementary PNAs with modified bases that can target mixed sequence duplex DNA up to a certain extent (A+T content ≥40%). In contrast, the current study provides the first demonstration that single-stranded γPNAs, which can strand invade duplex DNA without sequence restriction, have the potential to induce recombination and gene editing in chromosomal DNA in primary blood cells.
Other strategies for targeting mixed-sequence duplex DNA involve the use of polyamides [49, 50] and engineered nucleases. Polyamides are minor groove-binding agents that have shown promise as transcription blockers and as in vivo antitumor agents but they have not been employed for targeted genome modification in cells. Engineered nucleases have shown enormous potential to induce double strand breaks in mammalian cells which further instigate recombination. However, engineered nucleases also possess numerous non-specific, off-target effects because of their nuclease activity. In contrast, γPNAs, which have no intrinsic nuclease activity, show very low off-target effects.
Intracellular delivery of PNA has been a challenge for its therapeutic applications. Nucleofection based protocols have been reported to induce gene editing in CCR5 as well as β-globin genes [22, 48]. Numerous studies have been also reported where PNAs have been delivered in vivo through systemic injections. In prior studies from our group, we found that Antennapedia-conjugated PNAs also yielded gene editing via systemic injection [51]. We have also shown that PLGA-based nanoparticles are able to deliver tcPNAs through systemic injection to target both the CCR5 and β-globin genes in blood cells [23]. In other prior work, we have demonstrated γPNA delivery via on bio-compatible nanoparticles for their antisense effect to suppress CCR5 gene expression [35].
During nanoparticle formulation, we noticed that inclusion of hydrophilic ethylene glycol chains at γ positions dramatically improves the solubility and stability of MP γPNA/donor DNA-containing encapsulants, which further increases loading into nanoparticles. Further, we found that PBAE/PLGA blended nanoparticles yielded better gene-editing frequencies than PLGA-only formulations (γPNA/donor DNA enclosed in PBAE/PLGA nanoparticles yielded gene editing frequencies of up to 0.8% following ex vivo treatment of mouse bone marrow cells versus 0.10% for the PLGA particles carrying the same γPNA/donor DNA molecules). We believe that the cationic character of PBAE, which facilitates the condensation of negatively charged donor DNAs more efficiently, contributes to this greater effectiveness of delivery. In addition, the tertiary amines present in PBAE nanoparticles may buffer the low pH milieu of endosomes leading to increased endosomal escape, which is considered one of the major limitations germane to cellular uptake and bioavailability of PNA [52].
We also tested the effect of varying the position of the PNA binding site relative to the site of donor homology. We found that γPNAs targeted 100 bp away from the donor site could induce site-directed genome modification as could γPNAs targeted ~250 bp away, but at a lower frequency. However at the same time, we noticed γPNAs that overlap with the donor DNA strand showed reduced gene-editing frequencies. We attribute this to the potential for partial γPNAs binding to the donor DNA through Watson Crick base pairing, thereby inhibiting their respective interactions with the genomic DNA target sites.
Our ex vivo studies also indicate that PNA3 induced some gene editing (~0.01%). However, the MP γPNAs clearly show increased intracellular activity for gene editing, likely reflecting the increased binding affinity and superior strand invasion properties compared to standard PNAs. The mechanism by which the single-stranded MP γPNAs induce recombination and gene editing is not fully worked out. Prior work has shown that triplex-forming PNAs create an altered helical structure that is recognized and processed by xeroderma pigmentosum (XPA) and other factors in the nucleotide excision repair (NER) pathway, followed by engagement of homology-dependent repair. The strand invasion complex created by MP γPNAs does not create a Hoogsteen triple helix, but is does disrupt the duplex and create a three-stranded structure consisting of a PNA/DNA duplex and a displaced DNA strand. We suspect that this structure is also recognized as a lesion by the cell and triggers the cell's repair and recombination pathways in a manner similar to the effect of triplexes, but further work will be needed to verify this.
We do know from prior work [16] that to stimulate DNA repair, PNAs and other triplex-forming oligomers must bind tightly with the target DNA. The gamma substitution creates chirality and provides helical pre-organization to the PNA oligomer, yielding substantially increased binding affinity to the target DNA [38]. The lack of editing by the mismatched γPNA, as well as the deep-sequencing results showing almost no effect at a partially homologous site, further demonstrates the specificity of binding by γPNAs. At the same time we underscore that caution needs to be exercised during design of gamma PNAs since their strong binding affinity can lead to intermolecular recognition events if there is too much self-complementarity. This can cause self-hybridization between γPNA nucleobases because of the very strong γPNA–γPNA interaction.
In vivo treatment by systemic delivery of nanoparticles containing γPNA/donor DNA combinations led to site-directed genome editing in cells from multiple tissues in the β-globin/EGFP reporter mice (blood, bone marrow and spleen). Hence, in addition to sequence-unrestricted targeting of genomic DNA, the combination of nanoparticle and γPNA technology possess a “minimally invasive” approach to achieve in vivo gene editing: intravenous injection is simple when compared to ex vivo therapy requiring cell mobilization, harvesting and further ex vivo manipulation, and transplantation. Retention of stem cell properties is important for therapies involved in blood- related disorders, and our results suggest that treatment with nanoparticles containing γPNAs does not yield toxic effects to the pluripotent nature of hematopoietic stem cells.
CONCLUSION
The work reported here demonstrates that PNA can bind to duplex DNA in a sequence-unrestricted manner in vivo and can target human β-globin gene sequences to promote editing of a thalassemia-associated mutation. This work may provide a basis for a new approach to treat not only haemoglobin disorders but also a variety of other genetic diseases.
Supplementary Material
ACKNOWLEDGEMENTS
We thank F. Rogers, M. Kaushik, Y. Dang, A. Gupta, and L. Cabral for discussions and suggestions, and K. Bliguvar for deep-sequencing.
FUNDING
R.B. is the recipient of a James Hudson Brown— Alexander Brown Coxe Postdoctoral Fellowship. This work was supported by the Doris Duke Charitable Foundation to P.M.G., the National Science Foundation (CHE-1012467) and DSF Charitable Foundation to D.H.L and National Institutes of Health grant (EB000487) to W.M.S.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
PATIENT CONSENT
Declared none.
REFERENCES
- 1.Arnould S, Delenda C, Grizot S, et al. The I-CreI meganuclease and its engineered derivatives: applications from cell modification to gene therapy. Protein Eng Des Sel. 2010;24(1-2):27–31. doi: 10.1093/protein/gzq083. [DOI] [PubMed] [Google Scholar]
- 2.Silva G, Poirot L, Galetto R, et al. Meganucleases and other tools for targeted genome engineering: perspectives and challenges for gene therapy. Curr Gene Ther. 2011;11(1):11–27. doi: 10.2174/156652311794520111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palpant NJ, Dudzinski DM. Zinc-finger nucleases: looking toward translation. Gene Ther. 2013;20(2):121–7. doi: 10.1038/gt.2012.2. [DOI] [PubMed] [Google Scholar]
- 4.Haendel E-M, Cathomen T. Zinc-finger nuclease based genome surgery: it's all about specificity. Curr Gene Ther. 2011;11(1):28–37. doi: 10.2174/156652311794520120. [DOI] [PubMed] [Google Scholar]
- 5.Joung JK, Sander JD. TALENs: a widely applicable technology for targeted genome editing. Nat Rev Mol Cell Biol. 2013;14(1):49–55. doi: 10.1038/nrm3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scharenberg AM, Duchateau P, Smith J. Genome engineering with TAL-effector nucleases and alternative modular nuclease technologies. Curr Gene Ther. 2013;13(4):291–303. doi: 10.2174/15665232113139990026. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Yang H, Shivalila CS, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153(4):910–8. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin H, Xue W, Chen S, et al. Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nat Biotechnol. 2014;32(6):551–3. doi: 10.1038/nbt.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katada H, Komiyama M. Artificial restriction DNA cutters to promote homologous recombination in human cells. Curr Gene Ther. 2011;11(1):38–45. doi: 10.2174/156652311794520094. [DOI] [PubMed] [Google Scholar]
- 10.Pattanayak V, Ramirez CL, Joung JK, Liu DR. Revealing off-target cleavage specificities of zinc-finger nucleases by in vitro selection. Nat Methods. 2011;8(9):765–70. doi: 10.1038/nmeth.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cradick TJ, Fine EJ, Antico CJ, Bao G. CRISPR/Cas9 systems targeting β-globin and CCR5 genes have substantial off-target activity. Nucleic Acids Res. 2013;41(20):9584–92. doi: 10.1093/nar/gkt714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knauert MP, Glazer PM. Triplex forming oligonucleotides: sequence-specific tools for gene targeting. Hum Mol Genet. 2001;10(20):2243–51. doi: 10.1093/hmg/10.20.2243. [DOI] [PubMed] [Google Scholar]
- 13.Vasquez KM, Narayanan L, Glazer PM. Specific mutations induced by triplex-forming oligonucleotides in mice. Science. 2000;290(5491):530–3. doi: 10.1126/science.290.5491.530. [DOI] [PubMed] [Google Scholar]
- 14.McNeer NA, Chin JY, Schleifman EB, Fields RJ, Glazer PM, Saltzman WM. Nanoparticles Deliver Triplex-forming PNAs for Site-specific Genomic Recombination in CD34+ Human Hematopoietic Progenitors. Mol Ther. 2011;19(1):172–80. doi: 10.1038/mt.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Datta HJ, Chan PP, Vasquez KM, Gupta RC, Glazer PM. Triplex-induced recombination in human cell-free extracts. Dependence on XPA and HsRad51. J Biol Chem. 2001;276(21):18018–23. doi: 10.1074/jbc.M011646200. [DOI] [PubMed] [Google Scholar]
- 16.Faruqi AF, Datta HJ, Carroll D, Seidman MM, Glazer PM. Triple-helix formation induces recombination in mammalian cells via a nucleotide excision repair-dependent pathway. Mol Cell Biol. 2000;20(3):990–1000. doi: 10.1128/mcb.20.3.990-1000.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim K-H, Nielsen PE, Glazer PM. Site-Specific Gene Modification by PNAs Conjugated to Psoralen. Biochemistry. 2006;45(1):314–23. doi: 10.1021/bi051379a. [DOI] [PubMed] [Google Scholar]
- 18.Faruqi AF, Egholm M, Glazer PM. Peptide nucleic acid-targeted mutagenesis of a chromosomal gene in mouse cells. Proc Natl Acad Sci U S A. 1998;95(4):1398–403. doi: 10.1073/pnas.95.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egholm M, Buchardt O, Christensen L, et al. PNA hybridizes to complementary oligonucleotides obeying the Watson-Crick hydrogen-bonding rules. Nature. 1993;365(6446):566–8. doi: 10.1038/365566a0. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen PE, Egholm M, Berg RH, Buchardt O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science. 1991;254(5037):1497–500. doi: 10.1126/science.1962210. [DOI] [PubMed] [Google Scholar]
- 21.Sahu B, Sacui I, Rapireddy S, et al. Synthesis and Characterization of Conformationally Preorganized, (R)-Diethylene Glycol-Containing γ-Peptide Nucleic Acids with Superior Hybridization Properties and Water Solubility. J Org Chem. 2011;76(14):5614–27. doi: 10.1021/jo200482d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chin JY, Kuan JY, Lonkar PS, et al. Correction of a splice-site mutation in the β-globin gene stimulated by triplex-forming peptide nucleic acids. Proc Natl Acad Sci U S A. 2008;105(36):13514–9. S/1–S/7. doi: 10.1073/pnas.0711793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNeer NA, Schleifman EB, Cuthbert A, et al. Systemic delivery of triplex-forming PNA and donor DNA by nanoparticles mediates site-specific genome editing of human hematopoietic cells in vivo. Gene Ther. 2013;20(6):658–69. doi: 10.1038/gt.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lonkar P, Kim K-H, Kuan JY, et al. Targeted correction of a thalassemia-associated -globin mutation induced by pseudo-complementary peptide nucleic acids. Nucleic Acids Res. 2009;37(11):3635–44. doi: 10.1093/nar/gkp217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panyutin IG, Panyutin IV, Demidov VV. Targeting linear duplex DNA with mixed-base peptide nucleic acid oligomers facilitated by bisPNA openers. Anal Biochem. 2007;362(1):145–7. doi: 10.1016/j.ab.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 26.Bentin T, Larsen HJ, Nielsen PE. Combined Triplex/Duplex Invasion of Double-Stranded DNA by “Tail-Clamp” Peptide Nucleic Acid. Biochemistry. 2003;42(47):13987–95. doi: 10.1021/bi0351918. [DOI] [PubMed] [Google Scholar]
- 27.Lohse J, Dahl O, Nielsen PE. Double duplex invasion by peptide nucleic acid: a general principle for sequence-specific targeting of double-stranded DNA. Proc Natl Acad Sci U S A. 1999;96(21):11804–8. doi: 10.1073/pnas.96.21.11804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dragulescu-Andrasi A, Rapireddy S, Frezza BM, Gayathri C, Gil RR, Ly DH. A Simple γ-Backbone Modification Preorganizes Peptide Nucleic Acid into a Helical Structure. J Am Chem Soc. 2006;128(31):10258–67. doi: 10.1021/ja0625576. [DOI] [PubMed] [Google Scholar]
- 29.Yeh JI, Shivachev B, Rapireddy S, et al. Crystal Structure of Chiral γPNA with Complementary DNA Strand: Insights into the Stability and Specificity of Recognition and Conformational Preorganization. J Am Chem Soc. 2010;132(31):10717–27. doi: 10.1021/ja907225d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He G, Rapireddy S, Bahal R, Sahu B, Ly DH. Strand Invasion of Extended, Mixed-Sequence B-DNA by γPNAs. J Am Chem Soc. 2009;131(34):12088–90. doi: 10.1021/ja900228j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bahal R, Sahu B, Rapireddy S, Lee C-M, Ly DH. Sequence-Unrestricted, Watson-Crick Recognition of Double Helical B-DNA by (R)-MiniPEG-γPNAs. ChemBioChem. 2012;13(1):56–60. doi: 10.1002/cbic.201100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts J, Palma E, Sazani P, Orum H, Cho M, Kole R. Efficient and Persistent Splice Switching by Systemically Delivered LNA Oligonucleotides in Mice. Mol Ther. 2006;14(4):471–5. doi: 10.1016/j.ymthe.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 33.Sazani P, Gemignani F, Kang S-H, et al. Systemically delivered antisense oligomers upregulate gene expression in mouse tissues. Nat Biotechnol. 2002;20(12):1228–33. doi: 10.1038/nbt759. [DOI] [PubMed] [Google Scholar]
- 34.Christensen L, Fitzpatrick R, Gildea B, Petersen KH, Hansen HF, Koch T, et al. Solid-phase synthesis of peptide nucleic acids. J Pept Sci. 1995;1(3):185–83. doi: 10.1002/psc.310010304. [DOI] [PubMed] [Google Scholar]
- 35.Bahal R, McNeer NA, Ly DH, Saltzman WM, Glazer PM. Nanoparticle for delivery of antisense γPNA oligomers targeting CCR5. Artif DNA PNA XNA. 2013;4(2):49–57. doi: 10.4161/adna.25628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crawford MJ, Rapireddy S, Bahal R, Sacui I, Ly DH. Effect of steric constraint at the γ-backbone position on the conformations and hybridization properties of PNAs. J Nucleic Acids. 2011:652702, 10. doi: 10.4061/2011/652702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chenna V, Rapireddy S, Sahu B, Ausin C, Pedroso E, Ly DH. A simple cytosine to G-clamp nucleobase substitution enables chiral γ-PNAs to invade mixed-sequence double-helical B-form DNA. ChemBioChem. 2008;9(15):2388–91. doi: 10.1002/cbic.200800441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rapireddy S, Bahal R, Ly DH. Strand Invasion of Mixed-Sequence, Double-Helical B-DNA by γ-Peptide Nucleic Acids Containing G-Clamp Nucleobases under Physiological Conditions. Biochemistry. 2011;50(19):3913–8. doi: 10.1021/bi2002554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lynn DM, Langer R. Degradable poly(beta-amino esters): Synthesis, characterization, and self-assembly with plasmid DNA. J Am Chem Soc. 2000;122(44):10761–8. [Google Scholar]
- 40.Little SR, Lynn DM, Puram SV, Langer R. Formulation and characterization of poly (beta amino ester) microparticles for genetic vaccine delivery. J Controlled Release. 2005;107(3):449–62. doi: 10.1016/j.jconrel.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 41.Fields RJ, Cheng CJ, Quijano E, et al. Surface modified poly(beta amino ester)-containing nanoparticles for plasmid DNA delivery. J Controlled Release. 2012;164(1):41–8. doi: 10.1016/j.jconrel.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Saltzman WM. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat Material. 2009;8(6):526–33. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fields RJ, Cheng CJ, Quijano E, et al. Surface modified poly(βamino ester)-containing nanoparticles for plasmid DNA delivery. J Controlled Release. 2012;164(1):41–8. doi: 10.1016/j.jconrel.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schleifman EB, McNeer NA, Jackson A,Y, et al. Site-specific Genome Editing in PBMCs With PLGA Nanoparticle-delivered PNAs Confers HIV-1 Resistance in Humanized Mice. Mol Ther Nucleic Acids. 2013;2:e135. doi: 10.1038/mtna.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abibi A, Protozanova E, Demidov VV, Frank-Kamenetskii MD. Specific versus nonspecific binding of cationic PNAs to duplex DNA. Biophys J. 2004;86(5):3070–8. doi: 10.1016/S0006-3495(04)74356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelly CE, Thymiakou E, Dixon JE, Tanaka S, Godwin J, Episkopou V. Rnf165/Ark2C enhances BMP-Smad signaling to mediate motor axon extension. PLoS Biol. 2013;11(4):e1001538. doi: 10.1371/journal.pbio.1001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang G, Xu X, Pace B, et al. Peptide nucleic acid (PNA) binding-mediated induction of human gamma-globin gene expression. Nucleic Acids Res. 1999;27(13):2806–13. doi: 10.1093/nar/27.13.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schleifman EB, Bindra R, Leif J, et al. Targeted Disruption of the CCR5 Gene in Human Hematopoietic Stem Cells Stimulated by Peptide Nucleic Acids. Chem Biol. 2011;18(9):1189–98. doi: 10.1016/j.chembiol.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mapp AK, Ansari AZ, Ptashne M, Dervan PB. Activation of gene expression by small molecule transcription factors. Proc Natl Acad Sci U S A. 2000;97(8):3930–5. doi: 10.1073/pnas.97.8.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.White S, Szewczyk JW, Turner JM, Baird EE, Dervan PB. Recognition of the four Watson-Crick base pairs in the DNA minor groove by synthetic ligands. Nature. 1998;391(6666):468–71. doi: 10.1038/35106. [DOI] [PubMed] [Google Scholar]
- 51.Rogers FA, Lin SS, Hegan DC, Krause DS, Glazer PM. Targeted gene modification of hematopoietic progenitor cells in mice following systemic administration of a PNA-peptide conjugate. Mol Ther. 2012;20(1):109–18. doi: 10.1038/mt.2011.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kamat CD, Shmueli RB, Connis N, Rudin CM, Green JJ, Hann CL. Poly(β-amino ester) Nanoparticle Delivery of TP53 Has Activity against Small Cell Lung Cancer In vitro and In vivo. Mol Cancer Ther. 2013;12(4):405–15. doi: 10.1158/1535-7163.MCT-12-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.