Abstract
The objective of this study was to identify the ERK 1/2 involvement in the changes in compressive and tensile mechanical properties associated with hydrostatic pressure treatment of self-assembled cartilage constructs. In study 1, ERK 1/2 phosphorylation was detected by immunoblot following application of hydrostatic pressure (1 hour of static 10MPa) applied at day 10-14 of self-assembly culture. In study 2, ERK 1/2 activation was blocked during hydrostatic pressure application on days 10-14. With pharmacological inhibition of the ERK pathway by the MEK1/ERK inhibitor U0126 during hydrostatic pressure application on days 10-14, the increase in Young’s modulus induced by hydrostatic pressure was blocked. Furthermore, this reduction in Young’s modulus with U0126 treatment during hydrostatic pressure application corresponded with a decrease in total collagen expression. However, U0126 did not inhibit the increase in aggregate modulus or GAG induced by hydrostatic pressure. These findings demonstrate a link between hydrostatic pressure application, ERK signaling, and changes in biomechanical properties of a tissue engineered construct.
Keywords: Self-assembly, extracellular signal-regulated kinase 1/2 (ERK 1/2), hydrostatic pressure, chondrocyte, tissue engineering, cartilage
1. Introduction
Within the body articular cartilage is found at the ends of long bones and functions as a load dissipating, low friction bearing surface. These functions are dependent on the composition of a dense extracellular matrix composed of 70-80% water and a collagen type II tensile network interlaced with compression-resisting proteoglycans. Cartilage is both avascular and aneural and has poor self-repair capabilities, with destruction of the tissue resulting in reduced mobility and pain. Tissue engineered replacement cartilage is designed to fill this need, and our group has developed a scaffoldless self-assembly technology to produce cartilage constructs with clinically relevant properties approaching those of native articular cartilage (Hu and Athanasiou 2006).
During normal motion articular cartilage encounters various forces including shear, compression and hydrostatic pressure (Guilak et al. 2000). These forces are necessary to maintain tissue function, as immobilization results in matrix degradation (Buckwalter 1995). Of these, hydrostatic pressure represents a major factor in modulating cartilage mechanobiology (Elder and Athanasiou 2009), with the physiological range in articular cartilage lying between 3-15MPa (Afoke et al. 1987). Application of these mechanical stimuli have therefore been applied to improve matrix functional properties during cartilage tissue engineering (Elder and Athanasiou 2009). Previously, our group has identified a regimen of 10 MPa of hydrostatic pressure, statically applied for 1 hour from days 10 to 14, that induced increases in both tensile and compressive mechanical properties in self-assembled chondrocyte constructs (Elder and Athanasiou 2009). The addition of TGF-β1 prior to and during hydrostatic pressure application further improved construct properties in a synergistic manner (Elder and Athanasiou 2008).
The mechanism by which hydrostatic pressure enhances self-assembled chondrocyte construct properties is not well understood. Multiple studies have produced conflicting reports on the signaling mechanisms involved during hydrostatic pressure application and the outcome on production of extracellular matrix components (Kraft et al. 2011). This is possibly a result of the wide range of hydrostatic pressure loading regimens, time scales, and model systems used (Elder and Athanasiou 2009). In chondrocytes results ranging from ion channel activation and intracellular Ca2+ release to modulation of extracellular signal-regulated kinase (ERK) activity have been detected depending on hydrostatic pressure regimen (Kopakkala-Tani et al. 2004; Mio et al. 2007). Of the mitogen-activated protein kinases (MAPKs) the ERK pathway has been observed to respond to mechanical signals in multiple cell types (Reusch et al. 1997; Jessop et al. 2002; Hatton et al. 2003; Laboureau et al. 2004; Bastow et al. 2005; Liu et al. 2006; Kook et al. 2009). MAPKs have been identified as signaling pathway components that transduce external signals into a plethora of cellular responses (Krishna and Narang 2008). Downstream effects of ERK activation are context specific (Schaeffer and Weber 1999), and both stimulatory and inhibitory roles for ERK in cartilage have been reported (Nakamura et al. 1999; Hung et al. 2000; Murakami et al. 2000; Yoon et al. 2002; Bobick and Kulyk 2004; Zakany et al. 2005; Ryan et al. 2009; Prasadam et al. 2010). For example, activation of the ERK pathway by FGF has been demonstrated to induce SOX9 expression in mouse primary chondrocytes (Murakami et al. 2000) and is required for TGF-β signaling during chondrogenesis (Li et al. 2010). Conversely activation of ERK in primary chondrocytes or cartilage (following impact (Ryan et al. 2009; Ding et al. 2010) or TGF-α application (Appleton et al. 2010)) has been linked to down-regulation of matrix components.
Therefore, in this study we sought to determine the activation state of ERK following hydrostatic pressure application and the role this plays in modulating the mechanical properties of self-assembled constructs. We hypothesized that 1) ERK is activated by application of hydrostatic pressure, and 2) ERK is necessary for the enhanced self-assembled construct properties observed by the hydrostatic pressure treatment regimen.
2. Materials and Methods
2.1 Chondrocyte isolation and construct seeding
Bovine stifle (knee) joints of 1 week old calves were obtained (Research 87, Boston, MA) and opened under aseptic conditions to expose the femoral condyles. Articular cartilage was removed, minced and digested with 0.2% collagenase P (Worthington, Lakewood, NJ) in culture medium containing 3% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA) for 24 hours as described previously (Hu and Athanasiou 2006). 5.5 million chondrocytes were seeded into 5 mm agarose wells and allowed to self-assemble as previously described (Hu and Athanasiou 2006; Elder and Athanasiou 2009). Constructs were cultured in a humidified incubator at 37°C and 10% CO2 for a total of four weeks, and then assayed. All cell culture media components were purchased from Invitrogen (Carlsbad, CA) or Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
2.2 Hydrostatic pressure
Hydrostatic pressure was applied to constructs as described previously (Elder and Athanasiou 2009). Briefly, control and pressurized constructs were placed into sterile, heat sealed bags containing media. Specimens to be pressurized were transferred into the hydrostatic pressure chamber, which was maintained at 37°C during testing, while non-pressurized specimens (controls) were transferred into an adjacent container which was not pressurized. 10MPa of static pressure was applied for 1 hour during days 10-14. Daily, after hydrostatic pressure application, constructs were transferred back to individual agarose lined wells (Elder and Athanasiou 2009) and maintained in a humidified incubator at 37°C and 10% CO2.
2.3 Study 1: Identification of ERK activation
On day 10 post-seeding, self-assembled constructs for all groups were removed from the initial seeding wells and transferred to sterile, heat sealed bags as described above. Non-pressurized specimens were bagged and placed into an adjacent non-pressurized container. As previous studies in bovine cartilage (Ryan et al. 2009) have determined ERK activation is maximal between 30 min and 2 hours following mechanical loading, constructs were collected 1 hour after hydrostatic pressure treatment and flash frozen in liquid nitrogen. To determine the initial change in signaling following hydrostatic pressure application, and if this was still activated after multiple hydrostatic pressure applications, samples were collected on day 10 (first day) and day 14 (last day) of the hydrostatic pressure regimen. Samples were processed for immunoblot as described below.
2.4 Protein isolation and immunoblot
Protein was extracted using RIPA lysis buffer [50 mM sodium fluoride, 0.5% Igepal CA-630 (NP-40), 10 mM sodium phosphate, 150 mM sodium chloride, 25 mM Tris (pH 8.0), 1 mM phenylmethylsulfonyl fluoride, 2 mM ethylenediaminetetraacetic acid (EDTA), and 1.2 mM sodium vanadate supplemented with protease inhibitor cocktail (Sigma–Aldrich, St. Louis, MO)]. Protein concentrations were measured using the Protein Assay kit (Bio-Rad). Protein was electrophoresed on a 12% SDS–polyacrylamide gel and transferred to polyvinylidene difluoride membrane by electroblotting (Bio-Rad). The membranes were blocked with 5% dried, non-fat milk in TBST (25 mM Tris–HCl, 125 mM NaCl, and 0.1% Tween 20) for 2 hours, probed with primary antibody overnight, and then incubated with horseradish peroxidase-conjugated secondary antibody for 1 hour. Antibodies were procured from Cell Signaling (Beverly, MA). Immunoblot signal was visualized using goat anti-rabbit HRP (Pierce) and SuperSignal® West Pico Substrate (SuperSignal West Pico Chemiluminescent Substrate, Pierce) according to the manufacturer’s instructions.
2.5 Study 2: Inhibition of ERK activation
U0126 functions to block ERK activity by inhibiting MEK1, the immediate upstream activator of ERK. Previous studies (Bobick and Kulyk 2004; Elluru et al. 2009) have indicated 20 μM of U0126 is sufficient to block ERK activation, therefore 20 μM of U0126 was added to constructs 1 hour prior to hydrostatic pressure application. All other constructs were treated with the vehicle control (3 μl/ml ethanol). Inhibitor or vehicle control was added from Days 10-14 at the same time as hydrostatic pressure treatment.
2.6 Quantitative biochemistry and histology
Briefly, construct pieces were weighed and lyophilized for 96 hours. After lyophilization, specimens were reweighed. Specimens were then digested to completion using a sequential pepsin-elastase digestion. This digest was used for both collagen and glycosaminoglycan (GAG) content assays. Collage content was assayed using the chloramine-T hydroxyproline assay (Woessner 1961). GAG content was assayed using the Biocolor Biglycan GAG assay kit according to manufacturer’s instructions (Biocolor, UK) (Hu and Athanasiou 2006). For histology, specimens were frozen in OCT cutting media and cut into 14 μm thick sections on a cryotome. All sections were adhered to Superfrost Plus slides and fixed in formalin prior to staining. Sections were stained for Safranin-O/Fast green and Picosirius Red as previously described (Hu and Athanasiou 2006).
2.7 Creep indentation and tensile mechanical testing
Compressive and tensile mechanical properties were determined as previously described (Elder and Athanasiou 2008). Briefly, compressive aggregate modulus was determined using a creep indentation apparatus (Athanasiou et al. 1994) using a 0.8mm flat porous indenter tip, applying a tare weight of 0.2 g, and a test load of 0.7 g (Elder and Athanasiou 2009). Discs were bathed in PBS during the duration of testing. Data were modeled using the linear biphasic theory (Mow et al. 1989).
Tensile tests consisted of an uniaxial pull-apart test until failure as previously described (Aufderheide and Athanasiou 2007). Specimens were cut into dogbone-shaped pieces and both ends glued to paper test strips. Gauge length represented the distance between the paper test strips. Sample thickness and gauge length were measured using digital calipers (Hu and Athanasiou 2006). The rate of displacement was 1% of the gauge length per second and achieved using an Instron 5565 materials testing system (Instron, Norwood, MA). Using the cross-sectional area, stress-strain curves were calculated from the load-displacement curves. Young’s modulus was determined from the linear region of the stress-strain curve.
2.8 Statistical analysis
For experimental groups in study 1, 3 samples were used per group for immunoblotting. For study 2, 5-6 samples were used per group for both biomechanical and biochemical analysis. Groups were analyzed by one-way ANOVA and Tukey-Kramer post-hoc test was conducted when appropriate using the statistical analysis software package JMP (SAS, Cary, NC) with p<0.05 being defined as being statistically significant. All data are reported as mean ± standard deviation.
3. Results
3.1 Study 1
3.1.1 Hydrostatic pressure activates ERK
Activation of ERK was detected by immunoblot following initial application of hydrostatic pressure at day 10 of culture (first application of hydrostatic pressure) through day 14 of culture (last application of hydrostatic pressure) (Figure 1). Activation at day 14 of culture by hydrostatic pressure was less than that at day 10. Minimal ERK activation was detected in control constructs at day 10 or 14, indicating the basal activity of this pathway in control constructs remains low or non-existent during this time. As ERK was activated by hydrostatic pressure, the relevance of inhibiting ERK activation during hydrostatic pressure treatment was chosen to carry forward into study 2.
Figure 1.
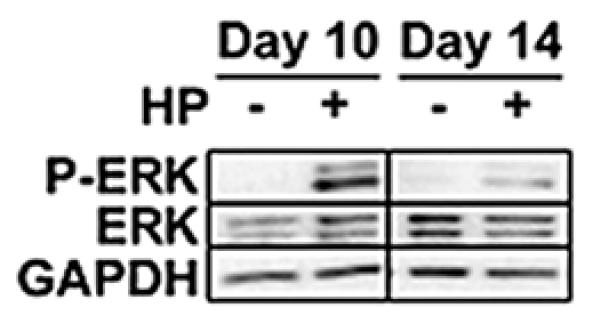
Immunoblot of phosphorylated ERK (P-ERK) with hydrostatic pressure (HP) treatment. Assembled from a single larger gel, equal amounts of protein were loaded per lane. Hydrostatic pressure induced ERK activation in self-assembled constructs at day 10 and weakly at day 14.
3.2 Study 2
3.2.1 Gross appearance and histology
ERK inhibition did not alter gross morphology
Constructs from all groups reached a diameter of approximately 5mm, and a thickness of approximately 0.66 (0.66±0.08, 0.69±0.08, 0.65±0.05, 0.67±0.06, for control, U0126, hydrostatic pressure and U0126 + hydrostatic pressure groups, respectively; p>0.05). Addition of U0126 did not grossly alter morphology or matrix accumulation. Representative constructs for diameter, thickness, and histological staining are displayed in Figure 2.
Figure 2.
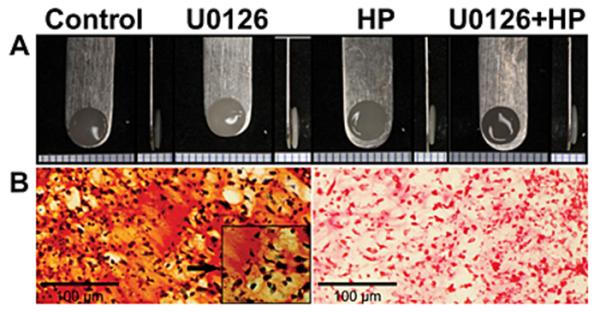
Gross morphology and representative histological staining of constructs. (A) Gross morphology and thickness appeared similar between all groups. (B) Histological images are representative of all groups, (Left Safranin-O, Fast Green; Right Picrosirius Red) 20X magnification. Left Safranin-O inset is 40X magnification.
3.2.2 Quantitative biochemistry
Inhibition of ERK during hydrostatic pressure reduced total collagen accumulation, but did not reduce GAG accumulation
Hydrostatic pressure resulted in a significant increase in total collagen content (Figure 3) from control levels (4.91±0.24 %/ww to 5.79±0.62%ww). Constructs treated with U0126 blocked the increase in collagen induced by hydrostatic pressure treatment (4.96±0.63%ww) and were not significantly different from control or U0126 alone (4.53±0.33%ww). GAG accumulation in U0126 treated constructs was significantly more than control (Figure 3). Addition of U0126 did not significantly affect accumulation of GAGs in hydrostatic pressure treated constructs (Figure 3), compared to hydrostatic pressure treatment alone, though.
Figure 3.
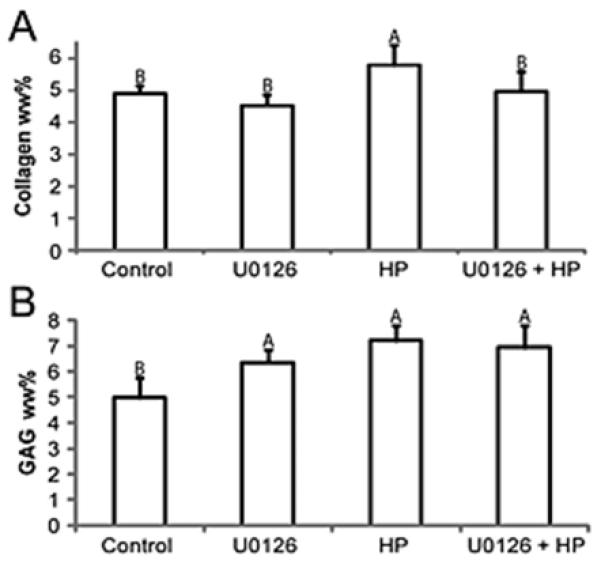
Biochemistry for total collagen wet weight % (A) and total GAG wet weight % (B). Application of hydrostatic pressure increased total collagen; this was inhibited by application of U0126 during hydrostatic pressure treatment. (B) GAG was increased by application of hydrostatic pressure or U0126 alone. Application of U0126 during hydrostatic pressure treatment did not inhibit GAG accumulation. Groups with different letters are significantly different p<0.05.
3.2.3 Mechanical evaluation
Inhibition of ERK during hydrostatic pressure application inhibited the increase in tensile properties induced by hydrostatic pressure, but did not inhibit changes in compressive properties
Hydrostatic pressure treated constructs had a significantly increased tensile modulus (791±204 kPa) over control (no treatment) constructs (461±108 kPa). The tensile modulus of constructs treated with U0126 during hydrostatic pressure application (438±149 kPa) was not significantly different from control constructs. Addition of U0126 by itself did not significantly alter tensile modulus compared to control constructs. Compressive properties did not significantly change with addition of U0126 in any group, although a trend for increased compressive properties (aggregate modulus) was observed (Figure 4).
Figure 4.
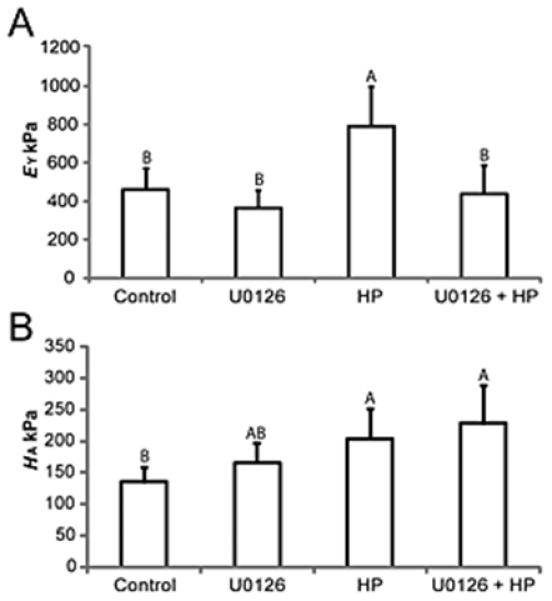
Hydrostatic pressure induces increases in Young’s modulus (EY) of self-assembled constructs, this was blocked by ERK inhibitor U0126 (A), but does not inhibit the aggregate modulus (HA) (B) induced by hydrostatic pressure. Groups with different letters are significantly different p<0.05.
4. Discussion
This study identified ERK-mediated changes in mechanical properties of tissue engineered articular cartilage constructs following hydrostatic pressure treatment. This study was designed to 1) identify the signaling state of ERK activation during initial hydrostatic pressure application and 2) quantify the effect of ERK inhibition during hydrostatic pressure application on the mechanical and biochemical properties of self-assembled constructs. Application of 1 hour of static 10MPa of hydrostatic pressure on day 10 was sufficient to activate ERK, although activation of ERK at day 14 was much less than at day 10. Given the low level of basal ERK activation seen in the control, this is likely due to an ERK negative feedback loop following multiple applications of stimuli (Shin et al. 2009).
The hypothesis that ERK activation is required for tensile property enhancements due to hydrostatic pressure application was confirmed. Changes in tensile properties with hydrostatic pressure application were inhibited with the addition of the ERK activation inhibitor U0126. Treatment with U0126 during hydrostatic pressure application resulted in decreased collagen wet weight (collagen/ww%) compared to hydrostatic pressure application alone; this decrease in collagen may have resulted in the decreased tensile properties of this group.
However, ERK signaling does not explain the change in compressive properties induced by hydrostatic pressure in self-assembled cartilage, as inhibition of ERK did not inhibit changes in compressive properties. The increase in compressive properties corresponds to the increase in GAG content seen in both the U0126 and hydrostatic pressure groups. As the changes in compressive properties by hydrostatic pressure did not appear to be negatively altered by inhibiting ERK, it can be concluded that these may be mediated by a different pathway. As GAG content increased with U0126 addition this may indicate that ERK activation is counterproductive to increased GAG content.
Changes in GAG content with U0126 application may be due to multiple scenarios. It is possible that U0126 may inhibit low basal levels of ERK activation in the nonhydrostatic pressure treated constructs. Another possibility is that U0126 may increase GAG content through unknown secondary effects. Although U0126 has extremely high specificity, other protein kinases can be weakly inhibited (Davies et al. 2000). Interestingly, this U0126-induced increase in GAG content appears to be overshadowed when hydrostatic pressure is applied, as the combination of hydrostatic pressure and U0126 did not significantly increase GAG content over hydrostatic pressure alone.
Mechanosensitive ion channels have been documented in multiple cell systems (Martinac 2004), with multiple ion channels responding to hydrostatic pressure stimulation (Browning et al. 1999; Hall 1999; Olsen et al. 2011). In chondrocytes ion flow has been proposed as a signal transducer of mechanical loading (Mow et al. 1999). Application to chondrocytes of hydrostatic pressure in the 10MPa range induces inhibition of the Na/K ion channel and activation of the Na/H pump (Browning et al. 1999; Hall 1999). Natoli et. al. (Natoli et al. 2010) have shown Ca+2 or Na/K ion channel modulation by ionomycin and ouabain in self-assembled cartilage constructs resulted in mechanical changes tantamount to those resulting from application of hydrostatic pressure. In multiple systems, activation of ERK by ion channel activity has been described (Rane 1999), and both ionomycin and ouabain have been reported in other systems to modulate ERK activity (Hanson and Ziegler 2002; Soltoff and Hedden 2008; Sweadner 2008), and increase intracellular Ca+2 levels. Synthesizing these data, a model can be proposed that hydrostatic pressure may act to modulate ion channel activity and therefore intracellular ion flux (mainly Ca+2), with a portion of this signal transducing through the ERK pathway, resulting in upregulation of extracellular matrix molecules (Elder and Athanasiou 2009).
The changes in mechanical and biochemical properties of tissue engineered cartilage seen with ERK activation are likely not limited to hydrostatic pressure application. The ERK pathway is known to be regulated by multiple external stimuli, especially growth factors such as TGF-β1 and FGF2, and may thus play a role in the beneficial effects of these stimuli on tissue engineered constructs (Elder and Athanasiou 2008; Elder and Athanasiou 2009). Further studies should be undertaken to identify the mechanism of upstream ERK activation, and downstream gene activation (for example SOX9 in chondrocytes) during hydrostatic pressure application, within the context of ion channel activity. This mechanism also likely functions in native tissues that are exposed to hydrostatic pressure, and may have relevance in other tissue engineered materials.
Modulation of mechanical properties in tissue engineering remains an important objective, as the need for functional tissue replacements requires that engineered tissues exhibit biomechanical properties on par with native tissue. Identification of signaling pathways that regulate mechanical properties will further this goal.
Acknowledgements
The authors would like to acknowledge the support for this work of NIH R01AR053286, and the Arthritis Foundation Postdoctoral Fellowship of which G. DuRaine is a recipient.
References
- Afoke NY, Byers PD, Hutton WC. Contact pressures in the human hip joint. J Bone Joint Surg Br. 1987;69(4):536–541. doi: 10.1302/0301-620X.69B4.3611154. [DOI] [PubMed] [Google Scholar]
- Appleton CT, Usmani SE, Mort JS, Beier F. Rho/ROCK and MEK/ERK activation by transforming growth factor-alpha induces articular cartilage degradation. Lab Invest. 2010;90(1):20–30. doi: 10.1038/labinvest.2009.111. [DOI] [PubMed] [Google Scholar]
- Athanasiou KA, Agarwal A, Dzida FJ. Comparative study of the intrinsic mechanical properties of the human acetabular and femoral head cartilage. J Orthop Res. 1994;12(3):340–349. doi: 10.1002/jor.1100120306. [DOI] [PubMed] [Google Scholar]
- Aufderheide AC, Athanasiou KA. Assessment of a bovine co-culture, scaffold-free method for growing meniscus-shaped constructs. Tissue Eng. 2007;13(9):2195–2205. doi: 10.1089/ten.2006.0291. [DOI] [PubMed] [Google Scholar]
- Bastow ER, Lamb KJ, Lewthwaite JC, Osborne AC, Kavanagh E, Wheeler-Jones CP, Pitsillides AA. Selective activation of the MEKERK pathway is regulated by mechanical stimuli in forming joints and promotes pericellular matrix formation. J Biol Chem. 2005;280(12):11749–11758. doi: 10.1074/jbc.M414495200. [DOI] [PubMed] [Google Scholar]
- Bobick BE, Kulyk WM. The MEK-ERK signaling pathway is a negative regulator of cartilage-specific gene expression in embryonic limb mesenchyme. J Biol Chem. 2004;279(6):4588–4595. doi: 10.1074/jbc.M309805200. [DOI] [PubMed] [Google Scholar]
- Browning JA, Walker RE, Hall AC, Wilkins RJ. Modulation of Na+ × H+ exchange by hydrostatic pressure in isolated bovine articular chondrocytes. Acta Physiol Scand. 1999;166(1):39–45. doi: 10.1046/j.1365-201x.1999.00534.x. [DOI] [PubMed] [Google Scholar]
- Buckwalter JA. Osteoarthritis and articular cartilage use, disuse, and abuse: experimental studies. J Rheumatol Suppl. 1995;43:13–15. [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351(Pt 1):95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Heying E, Nicholson N, Stroud NJ, Homandberg GA, Buckwalter JA, Guo D, Martin JA. Mechanical impact induces cartilage degradation via mitogen activated protein kinases. Osteoarthritis Cartilage. 2010;18(11):1509–1517. doi: 10.1016/j.joca.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder BD, Athanasiou KA. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PLoS One. 2008;3(6):e2341. doi: 10.1371/journal.pone.0002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder BD, Athanasiou KA. Effects of temporal hydrostatic pressure on tissue-engineered bovine articular cartilage constructs. Tissue Eng Part A. 2009;15(5):1151–1158. doi: 10.1089/ten.tea.2008.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder BD, Athanasiou KA. Hydrostatic pressure in articular cartilage tissue engineering: from chondrocytes to tissue regeneration. Tissue Eng Part B Rev. 2009;15(1):43–53. doi: 10.1089/ten.teb.2008.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder BD, Athanasiou KA. Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs. Osteoarthritis Cartilage. 2009;17(1):114–123. doi: 10.1016/j.joca.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elluru RG, Thompson F, Reece A. Fibroblast growth factor 18 gives growth and directional cues to airway cartilage. Laryngoscope. 2009;119(6):1153–1165. doi: 10.1002/lary.20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilak F, Setton LA, Kraus VB, Garrett WE, Speer KP, Kirkendall DT, Kitkowski MD. Principles And Practice Of Orthopaedic Sports Medicine. Lippincott Williams & Wilkins; Philadelphia, PA: 2000. Structure and function of articular cartilage. [Google Scholar]
- Hall AC. Differential effects of hydrostatic pressure on cation transport pathways of isolated articular chondrocytes. J Cell Physiol. 1999;178(2):197–204. doi: 10.1002/(SICI)1097-4652(199902)178:2<197::AID-JCP9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Hanson DA, Ziegler SF. Regulation of ionomycin-mediated granule release from rat basophil leukemia cells. Mol Immunol. 2002;38(16-18):1329–1335. doi: 10.1016/s0161-5890(02)00083-4. [DOI] [PubMed] [Google Scholar]
- Hatton JP, Pooran M, Li CF, Luzzio C, Hughes-Fulford M. A short pulse of mechanical force induces gene expression and growth in MC3T3-E1 osteoblasts via an ERK 1/2 pathway. J Bone Miner Res. 2003;18(1):58–66. doi: 10.1359/jbmr.2003.18.1.58. [DOI] [PubMed] [Google Scholar]
- Hu JC, Athanasiou KA. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 2006;12(4):969–979. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- Hung CT, Henshaw DR, Wang CC, Mauck RL, Raia F, Palmer G, Chao PH, Mow VC, Ratcliffe A, Valhmu WB. Mitogen-activated protein kinase signaling in bovine articular chondrocytes in response to fluid flow does not require calcium mobilization. J Biomech. 2000;33(1):73–80. doi: 10.1016/s0021-9290(99)00176-1. [DOI] [PubMed] [Google Scholar]
- Jessop HL, Rawlinson SC, Pitsillides AA, Lanyon LE. Mechanical strain and fluid movement both activate extracellular regulated kinase (ERK) in osteoblast-like cells but via different signaling pathways. Bone. 2002;31(1):186–194. doi: 10.1016/s8756-3282(02)00797-4. [DOI] [PubMed] [Google Scholar]
- Kook SH, Hwang JM, Park JS, Kim EM, Heo JS, Jeon YM, Lee JC. Mechanical force induces type I collagen expression in human periodontal ligament fibroblasts through activation of ERK/JNK and AP-1. J Cell Biochem. 2009;106(6):1060–1067. doi: 10.1002/jcb.22085. [DOI] [PubMed] [Google Scholar]
- Kopakkala-Tani M, Elo MA, Sironen RK, Helminen HJ, Lammi MJ. High hydrostatic pressure induces ERK and PI3 kinase phosphorylation in human HCS-2/8 chondrosarcoma cells. Cell Mol Biol (Noisy-le-grand) 2004;50(4):485–490. [PubMed] [Google Scholar]
- Kraft JJ, Jeong C, Novotny JE, Seacrist T, Chan G, Domzalski M, Turka CM, Richardson DW, Dodge GR. Effects of Hydrostatic Loading on a Self-Aggregating, Suspension Culture–Derived Cartilage Tissue Analog. Cartilage. 2011;2(3):254–264. doi: 10.1177/1947603510383686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna M, Narang H. The complexity of mitogen-activated protein kinases (MAPKs) made simple. Cell Mol Life Sci. 2008;65(22):3525–3544. doi: 10.1007/s00018-008-8170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laboureau J, Dubertret L, Lebreton-De Coster C, Coulomb B. ERK activation by mechanical strain is regulated by the small G proteins rac-1 and rhoA. Exp Dermatol. 2004;13(2):70–77. doi: 10.1111/j.0906-6705.2004.00117.x. [DOI] [PubMed] [Google Scholar]
- Li J, Zhao Z, Liu J, Huang N, Long D, Wang J, Li X, Liu Y. MEK/ERK and p38 MAPK regulate chondrogenesis of rat bone marrow mesenchymal stem cells through delicate interaction with TGF-beta1/Smads pathway. Cell Prolif. 2010;43(4):333–343. doi: 10.1111/j.1365-2184.2010.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zou L, Zheng Y, Yang P, Luo S, Zhao Z. ERK, as an early responder of mechanical signals, mediates in several mechanotransduction pathways in osteoblast-like cells. Biochem Biophys Res Commun. 2006 [Google Scholar]
- Martinac B. Mechanosensitive ion channels: molecules of mechanotransduction. J Cell Sci. 2004;117(Pt 12):2449–2460. doi: 10.1242/jcs.01232. [DOI] [PubMed] [Google Scholar]
- Mio K, Kirkham J, Bonass WA. Possible role of extracellular signal-regulated kinase pathway in regulation of Sox9 mRNA expression in chondrocytes under hydrostatic pressure. J Biosci Bioeng. 2007;104(6):506–509. doi: 10.1263/jbb.104.506. [DOI] [PubMed] [Google Scholar]
- Mow VC, Gibbs MC, Lai WM, Zhu WB, Athanasiou KA, A numerical algorithm and an experimental study Biphasic indentation of articular cartilage--II. J Biomech. 1989;22(8-9):853–861. doi: 10.1016/0021-9290(89)90069-9. [DOI] [PubMed] [Google Scholar]
- Mow VC, Wang CC, Hung CT. The extracellular matrix, interstitial fluid and ions as a mechanical signal transducer in articular cartilage. Osteoarthritis Cartilage. 1999;7(1):41–58. doi: 10.1053/joca.1998.0161. [DOI] [PubMed] [Google Scholar]
- Murakami S, Kan M, McKeehan WL, de Crombrugghe B. Up-regulation of the chondrogenic Sox9 gene by fibroblast growth factors is mediated by the mitogen-activated protein kinase pathway. Proc Natl Acad Sci U S A. 2000;97(3):1113–1118. doi: 10.1073/pnas.97.3.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Shirai T, Morishita S, Uchida S, Saeki-Miura K, Makishima F. p38 mitogen-activated protein kinase functionally contributes to chondrogenesis induced by growth/differentiation factor-5 in ATDC5 cells. Exp Cell Res. 1999;250(2):351–363. doi: 10.1006/excr.1999.4535. [DOI] [PubMed] [Google Scholar]
- Natoli RM, Skaalure S, Bijlani S, Chen KX, Hu J, Athanasiou KA. Intracellular Na(+) and Ca(2+) modulation increases the tensile properties of developing engineered articular cartilage. Arthritis Rheum. 2010;62(4):1097–1107. doi: 10.1002/art.27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SM, Stover JD, Nagatomi J. Examining the role of mechanosensitive ion channels in pressure mechanotransduction in rat bladder urothelial cells. Ann Biomed Eng. 2011;39(2):688–697. doi: 10.1007/s10439-010-0203-3. [DOI] [PubMed] [Google Scholar]
- Prasadam I, van Gennip S, Friis T, Shi W, Crawford R, Xiao Y. ERK-1/2 and p38 in the regulation of hypertrophic changes of normal articular cartilage chondrocytes induced by osteoarthritic subchondral osteoblasts. Arthritis Rheum. 2010;62(5):1349–1360. doi: 10.1002/art.27397. [DOI] [PubMed] [Google Scholar]
- Rane SG. Ion channels as physiological effectors for growth factor receptor and Ras/ERK signaling pathways. Adv Second Messenger Phosphoprotein Res. 1999;33:107–127. doi: 10.1016/s1040-7952(99)80007-x. [DOI] [PubMed] [Google Scholar]
- Reusch HP, Chan G, Ives HE, Nemenoff RA. Activation of JNK/SAPK and ERK by mechanical strain in vascular smooth muscle cells depends on extracellular matrix composition. Biochem Biophys Res Commun. 1997;237(2):239–244. doi: 10.1006/bbrc.1997.7121. [DOI] [PubMed] [Google Scholar]
- Ryan JA, Eisner EA, Duraine G, You Z, Hari Reddi A. Mechanical compression of articular cartilage induces chondrocyte proliferation and inhibits proteoglycan synthesis by activation of the ERK pathway: implications for tissue engineering and regenerative medicine. J Tissue Eng Regen Med. 2009;3(2):107–116. doi: 10.1002/term.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19(4):2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SY, Rath O, Choo SM, Fee F, McFerran B, Kolch W, Cho KH. Positive- and negative-feedback regulations coordinate the dynamic behavior of the Ras-Raf-MEK-ERK signal transduction pathway. J Cell Sci. 2009;122(Pt 3):425–435. doi: 10.1242/jcs.036319. [DOI] [PubMed] [Google Scholar]
- Soltoff SP, Hedden L. Regulation of ERK1/2 by ouabain and Na-KATPase-dependent energy utilization and AMPK activation in parotid acinar cells. Am J Physiol Cell Physiol. 2008;295(3):C590–599. doi: 10.1152/ajpcell.00140.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweadner KJ. A third mode of ouabain signaling. Focus on “Regulation of ERK1/2 by ouabain and Na-K-ATPase-dependent energy utilization and AMPK activation in parotid acinar cells. Am J Physiol Cell Physiol. 2008;295(3):C588–589. doi: 10.1152/ajpcell.00388.2008. [DOI] [PubMed] [Google Scholar]
- Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Yoon YM, Kim SJ, Oh CD, Ju JW, Song WK, Yoo YJ, Huh TL, Chun JS. Maintenance of differentiated phenotype of articular chondrocytes by protein kinase C and extracellular signal-regulated protein kinase. J Biol Chem. 2002;277(10):8412–8420. doi: 10.1074/jbc.M110608200. [DOI] [PubMed] [Google Scholar]
- Zakany R, Szijgyarto Z, Matta C, Juhasz T, Csortos C, Szucs K, Czifra G, Biro T, Modis L, Gergely P. Hydrogen peroxide inhibits formation of cartilage in chicken micromass cultures and decreases the activity of calcineurin: implication of ERK1/2 and Sox9 pathways. Exp Cell Res. 2005;305(1):190–199. doi: 10.1016/j.yexcr.2004.12.016. [DOI] [PubMed] [Google Scholar]


