Summary
Emerging evidence suggests that when cancer cells hijack normal stem cell properties, they acquire the ability to invade, metastasize to distant sites, and evade therapy. Thus, eliminating cancer cells with stem cell properties, or cancer stem cells, is of prime importance for the successful treatment of cancer, regardless of the tissue of origin. Previous efforts to target cancer stem cells, however, have been largely unsuccessful. Recent studies led to the discovery of a novel role for the high mobility group A1 protein as a master regulator in both cancer stem cells and normal embryonic stem cells. Here, we present exciting new work unveiling the HMGA1 as a promising target for therapies directed at eradicating cancer stem cells.
Keywords: High mobility group A1 protein, HMGA1, cancer stem cells, epithelial-mesenchymal transition, embryonic stem cells, chromatin remodeling proteins
Cancers become deadly when tumor cells develop the ability to evade therapy and spread to distant sites. Thus, understanding why some cells acquire these properties and how to effectively target resistant, metastatic cancer cells are the most important hurdles confronted by cancer biologists and clinicians treating cancer patients today. While several proposed mechanisms for drug resistance exist, the cancer stem cell theory has gained momentum with emerging knowledge of both normal embryonic development and tumor progression [1-4]. The cancer stem cell theory proposes that cancers exist as a cellular hierarchy, which is maintained by cells known as cancer stem cells (CSCs) or tumor-initiating cells (TICs; Figure 1A). Unlike the clonal evolution theory, which contends that all cells within the tumor have an equal likelihood of acquiring mutations that confer a selective advantage, the cancer stem cell theory holds that only CSCs are capable of reconstituting the tumor. Like normal stem cells, CSCs/TICs have unique “stem cell” properties, including: 1.) long-term self-renewal, which enables them to divide for long periods of time, and, 2.) plasticity, or the ability to alter their appearance, growth patterns, and function. In embryonic stem cells (ESCs), these properties are essential for normal development, while in CSCs, these properties are required for invasion, metastatic progression, and survival following exposure to therapy.
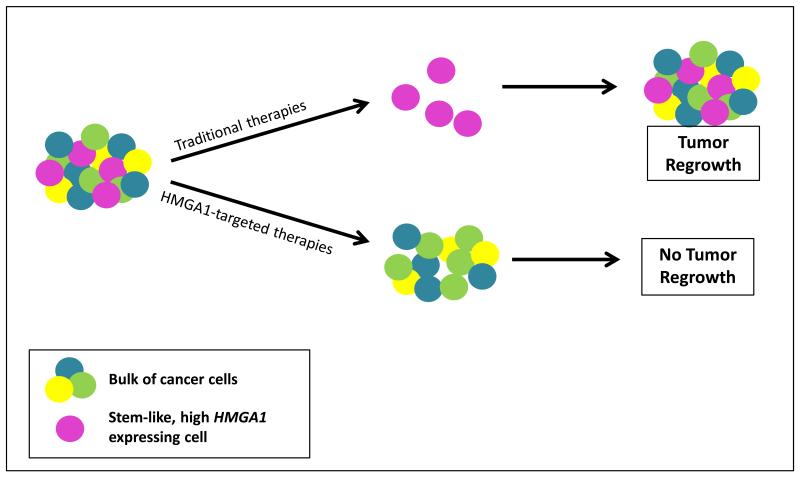
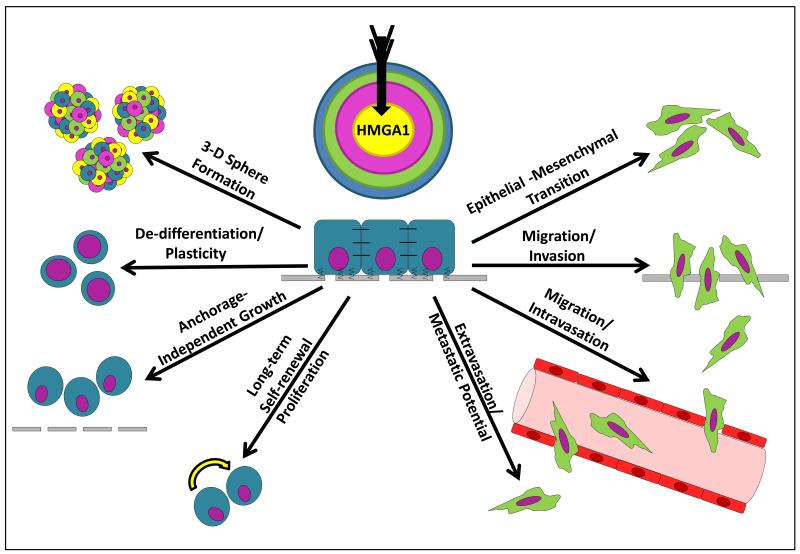
Figure 1. HMGA1 in Cancer Stem Cells.
A.) Traditional therapies target the more common “bulk” tumor cells, leaving therapy-resistant, stem-like cells that express high levels of HMGA1 and other stem cell genes. With time, the CSC/TICs repopulate the tumor and lead to relapse. However, therapies that target the stem-like HMGA1 expressing cells would eliminate those cells which are capable of repopulating the tumor and could result in cures when the bulk tumor cells are eliminated with traditional therapies.
B.) HMGA1 drives cancer stem cell phenotypes, and targeting HMGA1 disrupts these properties, including: (1) growth as 3-dimensional spheres (2) a de-differentiated (high-grade), plastic state, (3) anchorage independent growth, (4) long-term self-renewal and proliferation, (5) an epithelial-mesenchymal transition (EMT), (5) invasion/migration, (6) intravasation/migration, (7) extravasation and metastatic potential, after which the process could repeat itself from the metastatic site.
Increasing evidence suggests that some tumors have a small population of CSCs that are responsible for maintaining the tumor, with a larger population of “bulk” tumor cells that respond to therapy [1-2]. For example, many hematologic tumors can be eliminated by induction chemotherapy alone, but will invariably relapse if no further treatment is given. This behavior is consistent with the CSC model whereby the bulk tumor responds to therapy, while a small number of CSCs are refractory to therapy and maintain the tumor unless additional, effective therapy is administered. Alternatively, some tumors appear to be comprised predominantly of refractory, poorly differentiated stem-like tumor cells at presentation. To illustrate, most patients with pancreatic ductal adenocarcinomas will succumb to this malignancy, even when the tumors are small and surgically resectable at the time of diagnosis [5-6]. This suggests that pancreatic ductal adenocarcinoma cells are refractory, metastatic, and “stem-like” prior to clinical presentation in virtually all cases. In addition, recent studies indicate that remarkable heterogeneity exists within a single tumor, as evident by the varying tumorigenic potential of cells [1-4] and varied gene expression profiles from the same tumor [7]. It is plausible that CSCs, clonal evolution, and tumor heterogeneity all contribute to relapse and resistant disease, even within a single tumor. Regardless of the mechanism for resistance, each scenario requires anticancer treatment to be effective, not only at removing the bulk tumor burden, but also at preventing relapse by eliminating cells that can initiate and maintain the tumor (CSCs/TICs). Complicating this issue further is the fact that CSCs, similar to their normal counterparts (ESCs and adult stem cells), have inherent properties that confer resistance to many drugs commonly used to treat cancer, including specialized channels and enzymes to remove toxins, and the ability to remain quiescent or dormant [1,8]. As such, there is an urgent need to discover treatment approaches to target CSCs and/or stem-like cancer cells.
Attempting to better characterize and ultimately target CSCs has therefore been an area of active investigation. Several groups have identified cell surface markers or enzyme activities found in CSCs/TICs, and not surprisingly, many of these markers are shared by normal ESCs and adult stem cells [1-2]. Unfortunately, the CSC/TIC markers can be transient and non-specific, and therefore have remained elusive therapeutic targets [8]. An alternative approach to target CSCs is to modulate key regulators in signaling cascades that maintain the CSC/TIC phenotype. Recent work has identified the high mobility group A1 (HMGA1) gene as such a potential target [9-12; Figure 1B and Table 1]. This gene encodes the HMGA1a and HMGA1b protein isoforms that result from alternatively spliced mRNA [13-18]. HMGA1 was first discovered in highly proliferative, widely metastatic cervical cancer cells (HeLa) over 30 years ago [19] and has since been identified as a master regulator of transcriptional networks in normal ESCs and poorly differentiated, stem-like cancer cells [9-15, 20-22].
Table 1. HMGA1 in Normal Stem Cells and Cancer Stem Cells.
| Model System | Finding | Citation | |
|---|---|---|---|
|
HMGA1 in normal embryogenesis, ESCs, and adult stem cells |
Murine embryonic stem cells and postnatal tissues |
HMGA1 is highly expressed in embryonic development and silenced after birth |
Chiappetta et al, 1996 [40] |
| Heterozygous and homozygous HMGA1-null mice |
HMGA1-null mice develop aberrant hematopoiesis, a myeloproliferative disorder, and a cardiomyopathy |
Fedele et al, 2006 [46] | |
| Human embryonic stem cells and primary tumors |
HMGA1 is among a 9 gene transcription factor stem cell signature |
Ben-Porath et al, 2008 [9] | |
| Human hematopoietic stem cells |
CD34+ hematopoietic stem cells express HMGA1 |
Zhou et al, 2001 [41] Karp et al, 2011 [43], Nelson et al, 2011 [44], Chou et al, 2011 [42] |
|
| Lgr5-knock-in (Lgr5-ki) mice |
HMGA1 mRNA and protein are enriched in Lgr5+ intestinal stem cells |
Munoz et al, 2012 [45] | |
| Human embryonic stem cells and induced pluripotent stem cells |
HMGA1 is highly expressed in ESCs and iPSCs, and expression falls with differentiation |
Shah et al, 2012 [10] | |
| HMGA1 in CSCs | Murine model of AML |
HMGA1 is among the leukemia stem cell signature |
Somervaille et al, 2009 [53] |
| Colon cancer cell lines |
HMGA1 is required for tumor progression and colon cancer stem cells/tumor-initiator cells |
Belton et al, 2012 [11] | |
| Breast cancer cell lines |
HMGA1 is required for tumor progression and breast cancer stem cells/tumor-initiator cells |
Shah et al, 2013 [12] |
HMGA1 proteins belong to the class of chromatin remodeling proteins which modulate gene expression and cellular function by altering chromatin structure [13-34]. In fact, they are among the most abundant, nonhistone chromatin binding proteins found in cancer cells [13-16, 19]. HMGA1 proteins are named in part based on their small size (~10 kd) and thus rapid mobility (or highly mobility group) when separated on polyacrylamide electrophoretic gels. They also bind to chromatin at AT-rich regions (thus HMGA) in regulatory regions upstream of many genes important in development and cancer. After binding to DNA, HMGA1 bends chromatin and recruits additional transcription factors, forming a higher order transcriptional complex or “enhanceosome” that modulates gene expression. In vitro studies found that HMGA1 remodels chromatin by changing the rotational setting of DNA on the surface of isolated nucleosomes [35]. Elegant work subsequently demonstrated that HMGA1 plays a fundamental role in repositioning nucleosomes to facilitate gene expression with T cell activation [36-38]. HMGA1 also recruits additional chromatin remodeling complexes to DNA. For example, HMGA1 proteins are required for the recruitment of SWI/SNF chromatin remodeling complex to the HIV promoter, which results in histone acetylation and transcription from the LTR promoter [39]. Although HMGA1 proteins do not appear to have transcriptional activity alone, they alter gene expression by orchestrating the assembly of transcription factor complexes to DNA. Because histone H1 proteins maintain chromatin in a tightly bound, inactive state, HMGA1 proteins can globally activate gene expression by dislodging repressive histone H1 proteins [30-34]. In fact, sequence homology analysis of HMGA1 in plants suggests that histone H1 and HMGA1 evolved from the same ancestral protein.
HMGA1 is highly expressed during embryogenesis, with low or undetectable levels in most tissues postnatally [40]. HMGA1 is also highly expressed in adult stem cells, such as hematopoietic stem cells [41-44] and intestinal stem cells [45]. Mice deficient in HMGA1 develop aberrant hematopoiesis and a cardiomyopathy [46-48], while mice overexpressing HMGA1 develop diverse tumors including hematopoietic malignancies as well as pituitary, gastrointestinal, and uterine tumors [49-51]. HMGA1 was identified among a stem cell signature comprised of 9 genes highly expressed in embryonic stem cells that encode transcription factors [9]. This signature was derived by comparing multiple gene expression profiles from independent studies of ESCs. Interestingly, the HMGA1 stem cell signature predicts refractory disease in diverse solid tumors, including breast, bladder, and brain cancers [9]. In normal ESCs, HMGA1 maintains stem cells in an undifferentiated, pluripotent state by regulating key stem cell genes, including Lin28, Nanog, cMyc, and Sox2 [10]. In fact, HMGA1 is required for cellular reprogramming of somatic cells to induced pluripotent stem cells (iPSCs). These studies suggest that HMGA1 could drive stem cell properties in CSCs through molecular pathways active in normal ESCs. In keeping with the shared transcription factor signature in normal ESCs and resistant cancer cells or CSCs, previous studies have also uncovered stem cell transcriptional networks and signaling pathways activated during tumor progression [10-12,20-22]. For example, stem cell pathways are activated by HMGA1 in a transgenic model of lymphoid tumorigenesis [22]. Deletion of tumor suppressor genes (INK4A/ARF) that are normally silenced in stem cells leads to accelerated lymphoid tumorigenesis in an HMGA1 transgenic model [52]. An HMGA1 gene signature of 63 genes was also identified in poorly differentiated, triple-negative breast cancer cells. Strikingly, over half of the genes in this signature are highly enriched in embryonic stem cells [12]. Although there are limited published reports of CSC gene expression profiles, HMGA1 was identified in a leukemic stem cell signature identified in a murine model of acute myeloid leukemia [53]. In unpublished studies, we found that HMGA1 is enriched in the leukemic stem cell fraction of myeloid leukemias. Together, these studies indicate that HMGA1 regulates fundamental pathways in normal embryonic developmental, which become reactivated and dysregulated in cancer. Indeed, HMGA1 is not only up-regulated in diverse cancers, but it also portends a poor prognosis in many tumors [6, 9,11-15, 49, 51-65].
CSCs behave like “corrupted” normal stem cells during tumor initiation and progression. Although long-term self-renewal and pluripotency, or the ability to differentiate into other lineages, are hallmarks of normal ESCs, these properties are distorted in CSCs. For example, untethered self-renewal could account for high proliferative rates observed in some CSCs and aggressive tumors. While pluripotency, per se, is uncommon in most cancers and CSCs outside of some germ cell tumors, most resistant cancer cells have some degree of plasticity, or the ability to alter their appearance and behavior, including growth patterns, metabolism, and other fundamental properties. Increasing evidence suggests that epithelial tumor progression occurs, at least in part, from changes resembling an epithelial-mesenchymal transition (EMT) whereby cuboidal, immobile epithelial cells become elongated, invasive and mobile (mesenchymal) [11-12,66-68]. EMT occurs normally during embryonic development with gastrulation when embryos develop from a single-layered spherical collection of cells (blastula) to a three-layered structure (gastrula) [66-68]. Many epithelial tumors (carcinomas) undergo changes consistent with EMT during tumor invasion and migration prior to metastatic progression to distant sites. Although less well-studied, metastatic cancer cells must subsequently undergo additional alterations to establish residence at the metastatic site, some of which resembles a mesenchymal-epithelial transition (or MET).
Emerging evidence indicates that HMGA1 is a master regulator of EMT and tumor progression in diverse tumor models [11-12,20]. For example, experimental models of tumor progression demonstrate that inhibiting HMGA1 expression blocks proliferation, anchorage-independent cell growth, migration, invasion, and metastatic progression [11-12,20,51,54-55,59-64,69-70]. More recently, we found that silencing HMGA1 rapidly and dramatically reprograms aggressive, poorly differentiated breast cancer cells into cells with a more normal appearance and behavior [12]. Uncontrolled cell growth was halted in cancer cells after viral-mediated delivery of a short hairpin RNA to “switch off” HMGA1. Within a few days of silencing HMGA1, there were striking changes in appearance from spindle-shaped, mesenchymal tumor cells to more differentiated-appearing, cuboidal-shaped, epithelial cells. The tumor cells with knock-down of HMGA1 no longer metastasized from mammary fat pads to the lungs. Moreover, the cells with HMGA1 “switched-off” could no longer form foci in the lungs following tail vein injections [12]. Applying this same technology to colorectal and pancreatic cancer cells also disrupted cell growth and morphology, inducing changes consistent with MET (unpublished data). HMGA1 is also important in CSCs. Inhibiting HMGA1 expression in poorly differentiated cancer cells blocks 3-dimensional sphere formation, a defining property of CSCs and normal epithelial stem cells. In poorly differentiated breast cancer cells, not only is primary sphere formation impaired, but silencing HMGA1 also prevents the formation of secondary and tertiary sphere formation. In addition, tumors do not form when lower numbers of cancer cells were injected, indicating that the CSC/TIC population was depleted in cells with HMGA1 knock-down [11-12]. In both colon and breast cancer, downstream transcriptional targets of HMGA1 that function in stem cells and EMT were identified [11-12]. Interestingly, a study from over a decade earlier identified CD44 as an HMGA1 gene target, long before it was known that CD44 is an important marker of some CSCs [71]. The ever increasing list of genes regulated by HMGA1 indicates that it orchestrates transcriptional networks important in normal development and in resistant cancer cells. Together, these findings demonstrate that targeting HMGA1 in cancer reprograms poorly differentiated, metastatic cancer cells into cells with a more differentiated appearance and slower growth rates. These results also suggest that disrupting HMGA1 expression will also target cancer stem cell properties, and even the CSC/TICs population in tumors with this hierarchical organization.
Expert Commentary & Five-Year View
Because recent work has identified a unique role for HMGA1 as a master regulator of tumor progression and a stem cell phenotype, future research is needed to develop approaches to target HMGA1 in cancer therapy. A few compounds have been described that block HMGA1 function by crosslinking it to DNA, although none have proven to be specific and some are associated with significant toxicity that could result from disrupting the function of other AT DNA binding proteins, such as AKNA [72-75]. Of note, one crosslinking agent resulted in toxicity by enhancing expression of HMGA1 target genes [73]. Some studies have attempted to block transcriptional targets or pathways downstream of HMGA1 with promising results [51,63-64,76-77]. For example, both the cyclo-oxygenase-2 (COX-2) and signal transducer and activator of transcription 3 (STAT3) genes are induced by HMGA1 and they have been targeted pharmacologically in preclinical studies of tumors overexpressing HMGA1 [51,63,76-77]. While the results are promising, this approach will affect only a subset of HMGA1 downstream pathways. Recent technology to deliver short hairpin RNA plasmids is an exciting approach because it should be highly specific for HMGA1, as observed in our studies with in vitro cell culture models or murine preclinical models [11-12,78-79]. Results from early clinical trials with nanoparticles to alter gene expression have been encouraging and suggest that this approach could be used to deliver shRNA to modulate HMGA1 expression in tumors [77-79]. Another approach to target HMGA1 will be to deliver microRNAs that repress its expression and/or translation [80-82]. Because HMGA1 is highly expressed and likely plays an important role in adult stem and progenitor cells, future efforts will need to elucidate the molecular underpinnings that distinguish normal adult progenitor/stem cells from CSCs with the goal of targeting only the abnormal, cancer cells. Our recent work identifying HMGA1 as a key factor in orchestrating gene expression required for normal development and tumor progression is an important first step in this direction. Developing technology to target HMGA1 in resistant cancer cells promises to be an important strategy in the war on cancer.
Key Issues.
HMGA1 encodes the HMGA1a and HMGA1b chromatin remodeling proteins, which alter chromatin structure and modulate gene expression.
HMGA1 is highly expressed in all aggressive, poorly differentiated tumors studied to date and high levels portend a poor prognosis in diverse tumors
HMGA1 is enriched in normal embryonic stem cells, adult stem cells, and cancer stem cells.
HMGA1 induces oncogenic transformation in cultured cells and aggressive tumors in transgenic mice
Silencing HMGA1 results in a dramatic reprogramming of proliferative, invasive, spindle-shaped, mesenchymal cancer cells into non-invasive cells with slow proliferation rates and a more differentiated, cuboidal, epithelial appearance.
Switching off HMGA1 also blocks tumor progression and depletes cancer stem cells/tumor-initiator cells in murine models
HMGA1 is required for cellular reprogramming of somatic cells into induced pluripotent stem cells by the Yamanaka factors.
HMGA1 induces stem cell transcriptional networks during development in normal, pluripotent stem cells and with tumor progression
Recent work suggests that targeting HMGA1 will eliminate cancer stem cells in diverse tumors
References
- 1.Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–4807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- 2.McDermott SP, Wicha MS. Targeting breast cancer stem cells. Mol Oncol. 2010;4:404–419. doi: 10.1016/j.molonc.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marjanovic ND, Weinberg RA, Chaffer CL. Cell Plasticity and Heterogeneity in Cancer. Clinical Chemistry. 2013;59(1):168–179. doi: 10.1373/clinchem.2012.184655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: Impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21:283–296. doi: 10.1016/j.ccr.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koorstra JB, Hustinx SR, Offerhaus GJ, Maitra A. Pancreatic carcinogenesis. Pancreatology. 2008;8(2):110–125. doi: 10.1159/000123838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hristov A, Cope L, Di Cello F, et al. HMGA1 correlates with advanced tumor grade and decreased survival in pancreatic ductal adenocarcinoma. Mod Pathol. 2010;23:98–104. doi: 10.1038/modpathol.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerber JM, Smith DB, Ngwang B, et al. Clinically relevant population of leukemic CD34+CD38-cells in acute myeloid leukemia. Blood. 2012;119(12):3571–3577. doi: 10.1182/blood-2011-06-364182. *Of interest: This group discovered intermediate levels of expression of ALDH as a marker for leukemic stem cells.
- 9.Ben-Porath MW, Thomson VJ, Carey R, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. **Of considerable interest: This group defined an embryonic stem cell signature of genes expression transcription factors (including HMGA1) that is enriched in diverse solid tumors with adverse clinical outcomes.
- 10.Shah SN, Kerr C, Cope L, et al. HMGA1 reprograms somatic cells into pluripotent stem cells by inducing stem cell transcriptional networks. PLoS ONE. 2012;7(11):e48533. doi: 10.1371/journal.pone.0048533. **Of considerable interest: This landmark paper showed that HMGA1 is required for cellular reprogramming of somatic cells to induced pluripotent stem cells and identified a novel role for HMGA1 as a key transcriptional regulator in embryonic stem cells.
- 11.Belton A, Gabrovsky A, Kyung Bae Y, et al. HMGA1 induces intestinal polyposis in transgenic mice and drives tumor progression and stem cell properties in colon cancer cells. PLoS ONE. 2012;7(1):e30034. doi: 10.1371/journal.pone.0030034. **Of considerable interest: This is the first report demonstrating a unique role for HMGA1 in cancer stem cells.
- 12.Shah SN, Cope L, Poh W, et al. HMGA1: A master regulator of tumor progression in triple negative breast cancer. PLoS ONE. 2013;8(5):e63419. doi: 10.1371/journal.pone.0063419. *Of interest: This work showed that silencing HMGA1 results in a dramatic reprogramming of highly proliferative, invasive cancer cells into cells with markedly slower growth rates and a more differentiated appearance.
- 13.Reeves R, Beckerbauer L. HMGI/Y proteins: flexible regulators of transcription and chromatin structure. Biochim Biophys Acta. 2001;1519:13–29. doi: 10.1016/s0167-4781(01)00215-9. [DOI] [PubMed] [Google Scholar]
- 14.Fusco A, Fedele M. Roles of HMGA proteins in cancer. Nat Rev Cancer. 2007;7:899–910. doi: 10.1038/nrc2271. [DOI] [PubMed] [Google Scholar]
- 15.Resar LMS. High mobility group A1 gene: Transforming inflammation into cancer? Cancer Res. 2010;70(2):436–439. doi: 10.1158/0008-5472.CAN-09-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson KR, Lehn DA, Elton TS, Barr PJ, Reeves R. Complete murine cDNA sequence, genomic structure, and tissue expression of the high mobility group protein HMG-I(Y) The J Biol Chem. 1988;263:18338–18342. [PubMed] [Google Scholar]
- 17.Friedmann M, Holth LT, Zoghbi HY, Reeves R. Organization, inducible-expression and chromosome localization of the human HMG-I(Y) nonhistone protein gene. Nuc Acids Res. 1993;21:4259–4267. doi: 10.1093/nar/21.18.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedulla ML, Treff NR, Resar LMS, Reeves R. Sequence and analysis of the murine Hmgiy (Hmga1) gene. Gene. 2001;271:51–58. doi: 10.1016/s0378-1119(01)00500-5. [DOI] [PubMed] [Google Scholar]
- 19.Lund T, Holtlund J, Fredriksen M, Laland SG. On the presence of two new high mobility group-like proteins in HeLa S3 cells. FEBS Lett. 1983;152:163–167. doi: 10.1016/0014-5793(83)80370-6. *Of interest: This paper describes the discovery of HMGA1 (HMG-I/Y) proteins in aggressive cervical cancer cells.
- 20.Reeves R, Edberg DD, Li Y. Architectural transcription factor HMGI(Y) promotes tumor progression and mesenchymal transition of human epithelial cells. Mol Cell Biol. 2001;21:575–594. doi: 10.1128/MCB.21.2.575-594.2001. **Of considerable interest: This is the first report demonstrating that HMGA1 proteins induce genes and molecular changes consistent with an epithelial-mesenchymal transition.
- 21.Martinez Hoyos J, Fedele M, Battista S, et al. Identification of the genes up- and down-regulated by the high mobility group A1 (HMGA1) proteins: tissue specificity of the HMGA1-dependent gene regulation. Cancer Res. 2004;64:5728–5735. doi: 10.1158/0008-5472.CAN-04-1410. [DOI] [PubMed] [Google Scholar]
- 22.Schuldenfrei A, Belton A, Kowalski J, et al. HMGA1 drives inflammatory pathways, cell cycle progression, and embryonic stem cell genes during lymphoid tumorigenesis. BMC Genomics. 2011;12:549. doi: 10.1186/1471-2164-12-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thanos D, Maniatis T. The high mobility group protein HMG I(Y) is required for NF-kappa B-dependent virus induction of the human IFN-beta gene. Cell. 1992;71:777–789. doi: 10.1016/0092-8674(92)90554-p. 1992. **Of considerable interest: This is the first report demonstrating the role of HMGA1 as a key factor in orchestrating an enhanceosome required to induce gene expression.
- 24.Du W, Maniatis T. The high mobility group protein HMG I(Y) can stimulate or inhibit DNA binding of distinct transcription factor ATF-2 isoforms. Proc Natl Acad Sci USA. 1994;91:11318–11322. doi: 10.1073/pnas.91.24.11318. 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falvo JV, Thanos D, Maniatis T. Reversal of intrinsic DNA bends in the IFN beta gene enhancer by transcription factors and the architectural protein HMG I(Y) Cell. 1995;83:1101–1111. doi: 10.1016/0092-8674(95)90137-x. [DOI] [PubMed] [Google Scholar]
- 26.Thanos D, Maniatis T. Virus induction of human IFN beta gene expression requires the assembly of an enhanceosome. Cell. 1995;83:1091–1100. doi: 10.1016/0092-8674(95)90136-1. [DOI] [PubMed] [Google Scholar]
- 27.Munshi N, Agalioti T, Lomvardas S, Merika M, Chen G, Thanos D. Coordination of a transcriptional switch by HMGI(Y) acetylation. Science. 2001;293:1133–1136. doi: 10.1126/science.293.5532.1133. **Of considerable interest: This report demonstrates the role of acetylation as a post-translational modification important in modulating the transcriptional activity of HMGA1.
- 28.Panne D, Maniatis T, Harrison SC. An atomic model of the interferon-beta enhanceosome. Cell. 2007;129:1111–1123. doi: 10.1016/j.cell.2007.05.019. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ford E, Thanos D. The transcriptional code of human IFN-β gene expression. Biochemica Biophysi Acta. 2010;1799:328–336. doi: 10.1016/j.bbagrm.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Saitoh Y, Laemmli UK. Metaphase chromosome structure: bands arise from a differential folding path of the highly AT-rich scaffold. Cell. 1994;76:609–622. doi: 10.1016/0092-8674(94)90502-9. [DOI] [PubMed] [Google Scholar]
- 31.Girard F, Bello B, Laemmli UK, Gehring WJ. In vivo analysis of scaffold-associated regions in Drosophila: a synthetic high-affinity SAR binding protein suppresses position effect variegation. EMBO J. 1998;7:2079–2085. doi: 10.1093/emboj/17.7.2079. 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao K, Kas E, Gonzalez E, Laemmli UK. SAR-dependent mobilization of histone H1 by HMG-I/Y in vitro: HMG-I/Y is enriched in H1-depleted chromatin. EMBO J. 1993;12:3237–3247. doi: 10.1002/j.1460-2075.1993.tb05993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strick R, Laemmli UK. SARs are cis DNA elements of chromosome dynamics: synthesis of a SAR repressor protein. Cell. 1995;83:1137–1148. doi: 10.1016/0092-8674(95)90140-x. [DOI] [PubMed] [Google Scholar]
- 34.Mirkovitch J, Mirault ME, Laemmli UK. Organization of the higher-order chromatin loop: specific DNA attachment sites on nuclear scaffold. Cell. 1984;39:223–232. doi: 10.1016/0092-8674(84)90208-3. [DOI] [PubMed] [Google Scholar]
- 35.Reeves R, Wolffe AP. Substrate structure influences binding of the non-histone protein HMG-I(Y) to free nucleosomal DNA. Biochemistry. 1996;35:5063–74. doi: 10.1021/bi952424p. ** This is the first published evidence that HMGA1 has chromatin remodeling activity. In vitro studies showed that HMGA1 binds to DNA and changes the rotational setting of DNA on the surface of isolated nucleosome core particles.
- 36.Reeves R, Leonard WJ, Nissen MS. Binding of HMG-I(Y) imparts architectural specificity to a positioned nucleosome on the promoter of the human interleukin-2 receptor alpha gene. Mol Cell Biol. 2000;20:4666–79. doi: 10.1128/mcb.20.13.4666-4679.2000. 2000. * This work elegantly demonstrated that HMGA1 remodels chromatin in vivo by repositioning nucleosomes to facilitate gene expression in activated T-lymphoid cells.
- 37.Himes SR, Reeves R, Attema J, Nissen M, Li Y, Shannon MF. The role of high-mobility group I(Y) proteins in expression of IL-2 and T cell proliferation. J Immunol. 2000;164:3157–68. doi: 10.4049/jimmunol.164.6.3157. [DOI] [PubMed] [Google Scholar]
- 38.Attema JL, Reeves R, Murray V, Levichkin I, Temple MD, Tremethick DJ, Shannon MF. The human IL-2 gene promoter can assemble a positioned nucleosome that becomes remodeled upon T cell activation. J Immunol. 2002;169:2466–76. doi: 10.4049/jimmunol.169.5.2466. [DOI] [PubMed] [Google Scholar]
- 39.Henderson A, Holloway A, Reevers R, Tremethick DJ. Recruitment of SWI/SNF to the human immunodeficiency virus type 1 promoter. Mol Cell Biol. 2004;24:389–397. doi: 10.1128/MCB.24.1.389-397.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiappetta G, Avantaggiato V, Visconti R, et al. High level expression of the HMGI (Y) gene during embryonic development. Oncogene. 1996;13:2439–2446. *Of interest: This paper describes the high expression of HMGA1 (HMG-I/Y) during embryogenesis.
- 41.Zhou G, Chen J, Lee S, Clark T, Rowley JD, Wang SM. The pattern of gene expression in human CD34+ stem/progenitor cells. Proc Natl Acad Sci. USA. 2001;98:13966–13971. doi: 10.1073/pnas.241526198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chou B-K, Mali P, Huang X, et al. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with a unique epigenetic and gene expression signatures. Cell Research. 2011;11:518–529. doi: 10.1038/cr.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karp JE, Smith BD, Resar LMS, et al. Phase I and pharmacokinetic study of “hybrid” (bolus-infusion) flavopiridol administered followed in time sequence by cytosine arabinoside and mitoxantrone for adults with relapsed and refractory acute leukemias. Blood. 2011;117:3302–3310. doi: 10.1182/blood-2010-09-310862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nelson DM, Joseph B, Hillion J, Segal J, Karp J, Resar LMS. Flavopiridol induces BCL-2 expression and represses oncogenic transcription factors in leukemic blasts from adults with refractory acute myeloid leukemia. Leuk Lymphoma. 2011;52:1999–2006. doi: 10.3109/10428194.2011.591012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munoz J, Stange DE, Schepers AG, et al. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent +4 markers. EMBO J. 2012;31:3079–3091. doi: 10.1038/emboj.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Battista S, Pentimalli F, Baldassarre G, et al. Loss of hmga1 gene function affects embryonic stem cell lympho-hematopoietic differentiation. Faseb J. 2003;17:1496–1498. doi: 10.1096/fj.02-0977fje. [DOI] [PubMed] [Google Scholar]
- 47.Foti D, Chiefari E, Fedele M, et al. Lack of the architectural factor HMGA1 causes insulin resistance and diabetes in humans and mice. Nature Medicine. 2005;11:765–773. doi: 10.1038/nm1254. **Of considerable interest: This is the first report of Hmga1 deficient mice, which develop insulin resistance.
- 48.Fedele M, Fidanza V, Battista S, et al. Haploinsufficiency of the Hmga1 gene causes cardiac hypertrophy and myelo-lymphoproliferative disorders in mice. Cancer Res. 2006;66:2536–2543. doi: 10.1158/0008-5472.CAN-05-1889. [DOI] [PubMed] [Google Scholar]
- 49.Xu Y, Sumter Felder T, Bhattacharya R, et al. The HMG-I oncogene causes highly penetrant, metastatic lymphoid malignancy in transgenic mice and is overexpressed in human lymphoid malignancy. Cancer Res. 2004;64:3371–3375. doi: 10.1158/0008-5472.CAN-04-0044. **Of considerable interest: This is the first report demonstrating that HMGA1 induces aggressive tumors in vivo in a transgenic mouse model.
- 50.Fedele M, Pentimalli F, Baldassarre G, et al. Transgenic mice overexpressing the wild-type form of the HMGA1 gene develop mixed growth hormone/prolactin cell pituitary adenomas and natural killer cell lymphomas. Oncogene. 2005;24:3427–3435. doi: 10.1038/sj.onc.1208501. *Of interest: In this paper, the Fusco group describes a transgenic model overexpressing Hmga1b.; this mouse also develops lymphoid tumors as well as pituitary adenomas.
- 51.Tesfaye A, Di Cello F, Hillion J, et al. The High-Mobility Group A1 gene up-regulates Cyclooxygenase-2 expression in uterine tumorigenesis. Cancer Res. 2007;67:3998–4004. doi: 10.1158/0008-5472.CAN-05-1684. [DOI] [PubMed] [Google Scholar]
- 52.Di Cello F, Dhara S, Hristov A, et al. Inactivation of the Cdkn2a locus cooperates with HMGA1 to drive T-cell leukemogenesis. Leuk Lymphoma. 2013;54(8):1762–1768. doi: 10.3109/10428194.2013.764422. [DOI] [PubMed] [Google Scholar]
- 53.Somervaille TC, Matheny CJ, Spencer GJ, et al. Hierarchical maintenance of MLL myeloid leukemia stem cells employs a transcriptional program shared with embryonic rather than adult stem cells. Cell Stem Cell. 2009;4:129–140. doi: 10.1016/j.stem.2008.11.015. *Of interest: This work identified HMGA1 in a leukemic stem cell signature.
- 54.Wood LJ, Mukherjee M, Dolde CE, et al. HMG-I/Y: A new c-Myc target gene and potential human oncogene. Mol Cell Biol. 2000;20:5490–5502. doi: 10.1128/mcb.20.15.5490-5502.2000. **Of considerable interest: This is the first report demonstrating oncogenic properties of HMGA1 (formerly HMG-I/Y) in cultured cells.
- 55.Wood LJ, Maher J, Bunton TE, Resar LMS. The oncogenic properties of the HMG-I gene family. Cancer Res. 2000;60:4256–4261. [PubMed] [Google Scholar]
- 56.Pomeroy SL, Tamayo P, Gaasenbeek M, et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 2002;415:436–442. doi: 10.1038/415436a. [DOI] [PubMed] [Google Scholar]
- 57.Takaha N, Resar LMS, Vindivich D, Coffey D. High mobility protein HMGI(Y) enhances tumor cell growth, invasion, and matrix metalloproteinase-2 expression in prostate cancer cells. The Prostate. 2004;60:160–167. doi: 10.1002/pros.20049. [DOI] [PubMed] [Google Scholar]
- 58.Hommura F, Katabami M, Leaner VD, et al. HMG-I/Y is a cJun/AP-1 responsive gene and is necessary for cJun induced anchorage-independent growth. Mol Cancer Res. 2004;2:303–314. [PubMed] [Google Scholar]
- 59.Shah S, Resar LMS. HMGA1 in Cancer: Potential biomarker & therapeutic target. Histol Histopath. 2012;27:567–579. doi: 10.14670/HH-27.567. [DOI] [PubMed] [Google Scholar]
- 60.Roy S, Di Cello F, Kowalski J, et al. HMGA1 overexpression correlates with relapse in childhood B-lineage acute lymphoblastic leukemia. Leuk Lymphoma. 2013 Apr 30; doi: 10.3109/10428194.2013.782610. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dolde CE, Mukherjee M, Cho C, Resar LMS. The role of HMG-I/Y in human breast cancer cells. Breast Cancer Res Treat. 2002;71:181–191. doi: 10.1023/a:1014444114804. [DOI] [PubMed] [Google Scholar]
- 62.Hillion J, Wood LJ, Mukerjee M, et al. Up-regulation of matrix metalloproteinase-2 by HMGA1 promotes transformation in undifferentiated, large cell human lung cancer. Mol Cancer Res. 2009;7:1803–1812. doi: 10.1158/1541-7786.MCR-08-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hillion J, Dhara S, Sumter TF, et al. The HMGA1a-STAT3 axis: an “Achilles heel” for hematopoietic malignancies? Cancer Res. 2008;68:10121–10127. doi: 10.1158/0008-5472.CAN-08-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liau SS, Jazag A, Whang EE. HMGA1 is a determinant of cellular invasiveness and in vivo metastatic potential in pancreatic adenocarcinoma. Cancer Res. 2006;66:11613–11622. doi: 10.1158/0008-5472.CAN-06-1460. *Of interest: This is the first report showing that inhibiting HMGA1 expression in a murine cancer cell model blocks metastatic progression in vivo.
- 65.Sarhadi VK, Wikman H, Salmenkivi K, et al. Increased expression of high mobility group A proteins in lung cancer. J Pathol. 2006;209:206–212. doi: 10.1002/path.1960. [DOI] [PubMed] [Google Scholar]
- 66.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lim J, Thiery JP. Epithelial-mesenchymal transitions: insights from development. Development. 2012;139(19):3471–3486. doi: 10.1242/dev.071209. [DOI] [PubMed] [Google Scholar]
- 68.Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: Concepts and molecular links. Seminars in Cancer Biology. 2012;22:396–401. doi: 10.1016/j.semcancer.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dhar A, Hu J, Reeves R, Resar L, Colburn N. Dominant negative c-Jun (TAM67) target genes: HMGA1 is required for tumor promoter-induced transformation. Oncogene. 2004;23:4466–4476. doi: 10.1038/sj.onc.1207581. [DOI] [PubMed] [Google Scholar]
- 70.Scala S, Portella G, Fedele M, Chiappetta G, Fusco A. Adenovirus-mediated suppression of HMGI(Y) protein synthesis as potential therapy of human malignant neoplasias. Proc Natl Acad Sci U SA. 2000;97:4256–4261. doi: 10.1073/pnas.070029997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Foster LC, Wiesel P, Huggins GS, et al. Role of activating protein-1 and high mobility group-I(Y) protein in the induction of CD44 gene expression by interleukin-1beta in vascular smooth muscle cells. FASEB J. 2000;14:368–378. doi: 10.1096/fasebj.14.2.368. [DOI] [PubMed] [Google Scholar]
- 72.Beckerbauer L, Tepe JJ, Cullison J, Reeves R, Williams RM. FR900482 class of anti-tumor drugs cross-links oncoprotein HMG I/Y to DNA in vivo. Chem Biol. 2000;7:805–812. doi: 10.1016/s1074-5521(00)00028-4. [DOI] [PubMed] [Google Scholar]
- 73.Beckerbauer L, Tepe JJ, Eastman RA, Mixter PF, Williams RM, Reeves R. Differential effects of FR900482 and FK317 on apoptosis, IL-2 gene expression, and induction of vascular leak syndrome. Chem Biol. 2002;9:427–441. doi: 10.1016/s1074-5521(02)00122-9. **Of considerable interest: This is the first report of a clinical trial with an HMGA1 inhibitor.
- 74.Ma W, Oriz-Quintero B, Rangel R, et al. Innate activation of inflammatory gene networks, alveolar destruction and neonatal death in AKNA deficient mice. Cell Res. 2011;21:1564–1577. doi: 10.1038/cr.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moliterno A, Resar L. AKNA: Another AT-hook transcription factor hooking up with inflammation. Cell Res. 2011;21:1528–1530. doi: 10.1038/cr.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Di Cello F, Hillion J, Aderinto A, et al. COX-2 inhibitors block uterine tumorigenesis in HMGA1a transgenic mice and human uterine cancer xenografts. Mol Cancer Ther. 2008;7(7):2090–2095. doi: 10.1158/1535-7163.MCT-07-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hillion J, Di Cello F, Belton A, et al. Nanoparticle delivery of inhibitory STAT3 GQ-oligonucleotides blocks tumor growth in an HMGA1 transgenic model of T-cell leukemia. Leuk Lymphoma. 2013 Jul 5; doi: 10.3109/10428194.2013.821202. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boylan NJ, Suk JS, Lai SK, et al. Highly compacted DNA nanoparticles with low MW PEG coatings: In vitro, ex vivo and in vivo evaluation. J Control Release. 2012;157:72–79. doi: 10.1016/j.jconrel.2011.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 80.Pramanik D, Campbell NR, Karikari C, et al. Resititution of tumor suppressor microRNAs using a systemic nanovector inhibits pancreatic cancer growth in mice. Mol Cancer Ther. 2011;10:1470–1480. doi: 10.1158/1535-7163.MCT-11-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mussnich P, D’Angelo D, Leone V, Croce CM, Fusco A. The high mobility group A proteins contribute to thyroid cell transformation by regulating miR-603 and miR-10b expression. Mol Oncol. 2013;7:531–542. doi: 10.1016/j.molonc.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Resar LMS, Brodsky RA. “Let”-ing go with clonal expansion? Blood. 2011;117:5788–5790. doi: 10.1182/blood-2011-04-346668. [DOI] [PubMed] [Google Scholar]