Abstract
The proto-oncogene c-MYC, is rearranged in about 15% of patients with multiple myeloma (MM).
We identified 23 patients with MM and c-MYC. Primary objectives were to describe the clinical characteristics, response to therapy, progression free survival and overall survival (OS). 12/23 patients presented with or progressed to either plasma cell leukemia (PCL) and/or extramedullary disease (EMD). Induction therapy consisted of an immunomodulatory, proteasome inhibitor-based or conventional chemotherapy regimens. 15 patients achieved a PR and 3 achieved a VGPR. 16 patients received an autologous and, 1 patient an allogeneic HSCT. Median OS from diagnosis was 20.2 months. Patients with PCL or EMD had significantly shorter OS (15.5 vs. 40.4 months, p=0.0005). This is the first report describing the clinical characteristics of patients with MM and c-MYC. These abnormalities are associated with an aggressive form of MM, high incidence of PCL/EMD and short OS.
Index Words: c-MYC/8q24, multiple myeloma, plasma cell leukemia, high risk
Introduction
Multiple myeloma (MM) is the second most common hematologic malignancy after non- Hodgkin’s Lymphoma with a median age at diagnosis of about 65 years1, 2. It is characterized by chromosomal abnormalities that can be detected by either conventional cytogenetic (CC) or fluorescence in situ hybridization (FISH) studies3–5. A number of these chromosomal abnormalities are associated with shorter progression-free survival (PFS) and overall survival (OS). The high-risk (HR) abnormalities include hypodiploidy with del(13q) identified by CC, t(4;14)(p16;q32), t(14;16)(q32;q23), t(14;20)(q32;q11.2), and del(17)(p13) by either CC or FISH analyses5–8.
c-MYC, a proto-oncogene discovered initially in Burkitt’s lymphoma and one of the first oncogenes to be described, is dysregulated or overexpressed in many different cancers9. Mapped to the long arm of chromosome 8 at 8q24.1 region, it encodes a transcription factor that regulates cell proliferation, growth, protein translation, metabolism, and apoptosis9, 10. Recently, c-MYC rearrangement has been reported in approximately 15% of patients with MM, either at diagnosis or at relapse11, 12. In addition, there is evidence that c-MYC plays an important role in the pathogenesis of MM. Increased c-MYC expression appears to be involved in the progression from monoclonal gammopathy of undetermined significance or smoldering multiple myeloma to MM13, 14. Overexpression of c-MYC is also associated with more aggressive forms of MM, such as plasma cell leukemia (PCL) and extramedullary disease (EMD)15. PCL is defined as the presence of >2×109/L peripheral blood plasma cells or plasmacytosis accounting for >20% of the differential white cell count, and does not arise from pre-existing multiple myeloma (MM)16. Secondary PCL, a leukemic transformation of end-stage MM, has been reported to be associated with the upregulation of c-MYC15. Furthermore, in patients with EMD, which has been associated with plasmablastic morphology17, 18%–20% have overexpression of c-MYC17, 18. High-dose therapy with autologous stem-cell transplantation (auto-HCT) has been reported to neutralize the negative prognostic impact of extramedullary disease in selected patients19.
Here we report the clinical characteristics and outcomes of myeloma patients with c-MYC rearrangements who were treated at our institution.
Methods
Patients
Between 8/1998 and 12/2012, we identified 23 patients with MM and c-MYC rearrangements that were treated at The University of Texas MD Anderson Cancer Center. This may be a significant under-representation of patients with 8q24.1/c-MYC rearrangement because these tests were not routinely performed for each patient with MM. Disease, treatment, outcome, and patient demographic data were obtained from a retrospective chart review after IRB approval of the protocol (informed consent waved by IRB). . In addition, we identified 46 patients with MM and 32 patients with PCL in our database, who did not have c-MYC rearrangement and were matched to the 23 patients with c-MYC abnormality in age and disease characteristics.
Routine cytogenetic and FISH analyses for the assessment of c-MYC rearrangement
Chromosomal abnormalities involving 8q24.1 were detected by CC analyses, which were performed either at initial diagnosis or when patients first presented to our institution. A minimum of 20 metaphases were analyzed, and a clonal abnormality was defined as the presence of at least 2 abnormal metaphases with the same structural abnormality17. FISH analyses were performed for the presence of c-MYC rearrangement, as well as for IgH rearrangement, TP53 gene deletion, and RB1 gene deletion using probes from Abbott Molecular, Inc.(Abbott Park, Illinois, USA). A total of 200 interphases were analyzed for each FISH abnormality. c-MYC testing was performed using an LSI c-MYC dual-color, break apart rearrangement probe, which hybridizes to 8q24.1 region (spectrum orange on the centromeric side and spectrum green on the telomeric side of the c-MYC gene breakpoint)17. The 95% (p<0.05) confidence limit of the LSI c-MYC probe established in the MD Anderson Cancer Center Cytogenetics Laboratory is 0.6%–3.4%.
Response and outcome measures
Clinical response, relapse, and progression were defined by International Myeloma Working Group (IMWG) criteria20. OS was measured from the date of diagnosis to the date of last follow-up or death, and PFS was measured from the date of the last treatment (including auto-HCT) to the date of disease progression, last follow-up, or death. For the purpose of this analysis, EMD was defined as soft tissue plasmacytoma not involving the bones. PCL was defined by IMWG criteria16, 19.
Statistical analysis
The primary outcomes were response to therapy, PFS, and OS. PFS and OS were estimated by the Kaplan–Meier method21. Differences between categorical variables were determined using Fisher’s exact test or the Pearson χ2 test. Analyses were performed using STATA software (2005; StataCorp, College Station, TX).
Results
The patient characteristics are summarized in Table 1. Twelve patients (52%) had either PCL (8) and/or EMD (10) either at diagnosis or at relapse. In 8 patients with PCL, 4 had primary PCL and 4 had secondary PCL. Patients in both control groups were fairly well matched with the study cohort.
Table I.
Patient Characteristics
| Characteristic | Patients with c- MYC abnormality (n=23) |
Patients with multiple myeloma, no c-MYC abnormality and no PCL (n=46) |
Patients with PCL and no c- MYC abnormality (n=32) |
|---|---|---|---|
| Median age, (range) | 57 (21–72) | 59 (36–70) | 59.5 (21–74) |
| Male: Female | 13:10 | 34:12 | 19:13 |
| Hemoglobin*, g/dL (range) | 10.9 (5.6 – 14) | 11.8 (6.5–16) | 9.6 (6–15.5) |
| Calcium*, mg/dL (range) | 9.7 (6.3 – 13.7) | 9.5 (7.9 – 16.9) | 10.0 ( 7.9–14.3) |
| Creatinine*, mg/dL (range) | 1.0 (0.61 – 2.27) | 1.0 (0.5–9.3) | 1.15 (0.7–5.0) |
| International Staging System for myeloma I:II:III | 10:5:8 | 22:13:11 | 11:6:15 |
| % Bone marrow plasma cell* | 70 (30–95) | 30 (4–90) | 70 (5–95) |
| Plasma cell leukemia* | 8 (35%) | 0 (0%) | 32 (100%) |
| Extramedullary disease* | 10 (43%) | 4 (7%) | 8 (25%) |
| Other high risk cytogenetic abnormalities | 8 (35%) | 5(11%) | 14 (43%) |
| Treatment with immunomodulatory agent or proteasome inhibitor | 16 (69%) | 30 (65%) | 23 (71%) |
| Autologous or allogeneic stem cell transplantation | 17 (74%) | 46 (100%) | 22 (68%) |
at diagnosis
Normal laboratory values: Hemoglobin, 12.0– 16.0 g/dL; Calcium, 8.4–10.2 mg/dL; Creatinine, 0.60–1.00 mg/dL
Cytogenetic and FISH abnormalities
All 23 patients had an 8q24.1 abnormality detected by CC. Seventeen patients (74%) had a balanced translocation involving 8q24.1, whereas 6 patients had unbalanced abnormalities involving 8q24.1 including add(8)(q24.1) (4 cases) leading to 3’c-MYC deletion, t(1;8)(p21;q24.1) (one case) and t(8;20(q24.1;q13.1) (one case) resulting in typical c-MYC rearrangement confirmed by FISH. The most common balanced translocation involving 8q24.1 was t(8;14)(q24.1;q32) (8 patients), followed by t(8;22)(q24.1;q11.2) (6 patients) and t(2;8)(p12;q24) (3 patients). FISH analyses were available in 22 of the 23 cases confirming c-MYC rearrangement. The single case without FISH performance had a complex karyotype including a typical t(8;22)(q24;q11.2), a variant of t(8;14)(q24.1;q32). Seven patients (30%) had the following additional high- risk (HR) abnormalities detected by conventional cytogenetics: del(13q) in 5; and del(17)(p13) in two patients, which were also confirmed by FISH.
Treatment and outcome
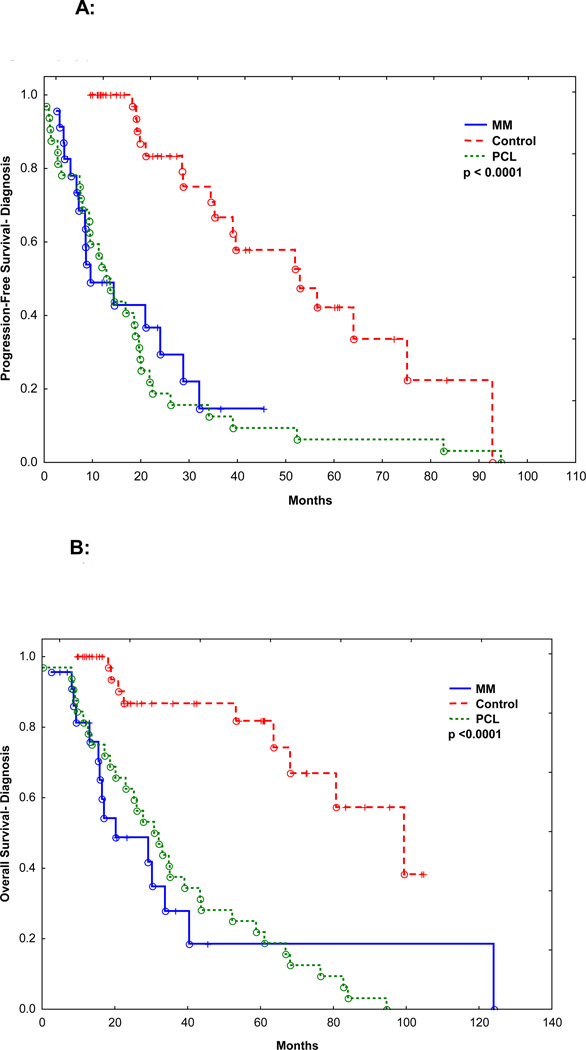
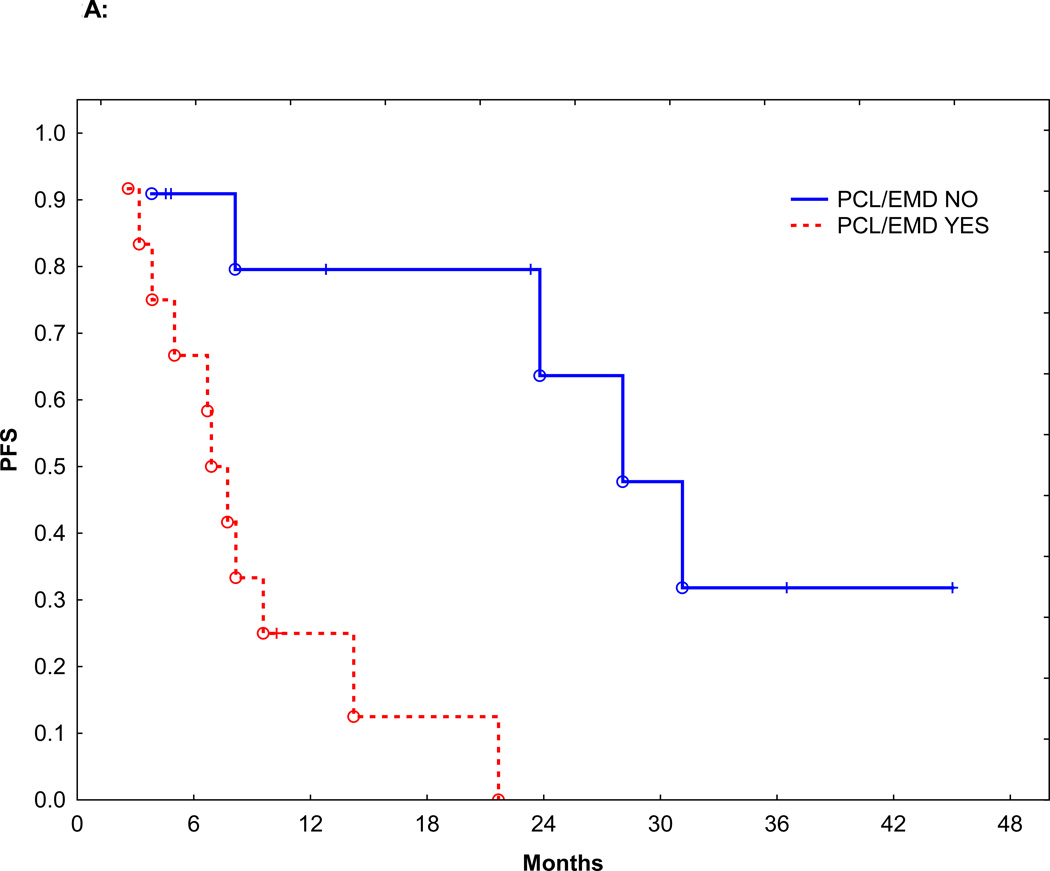
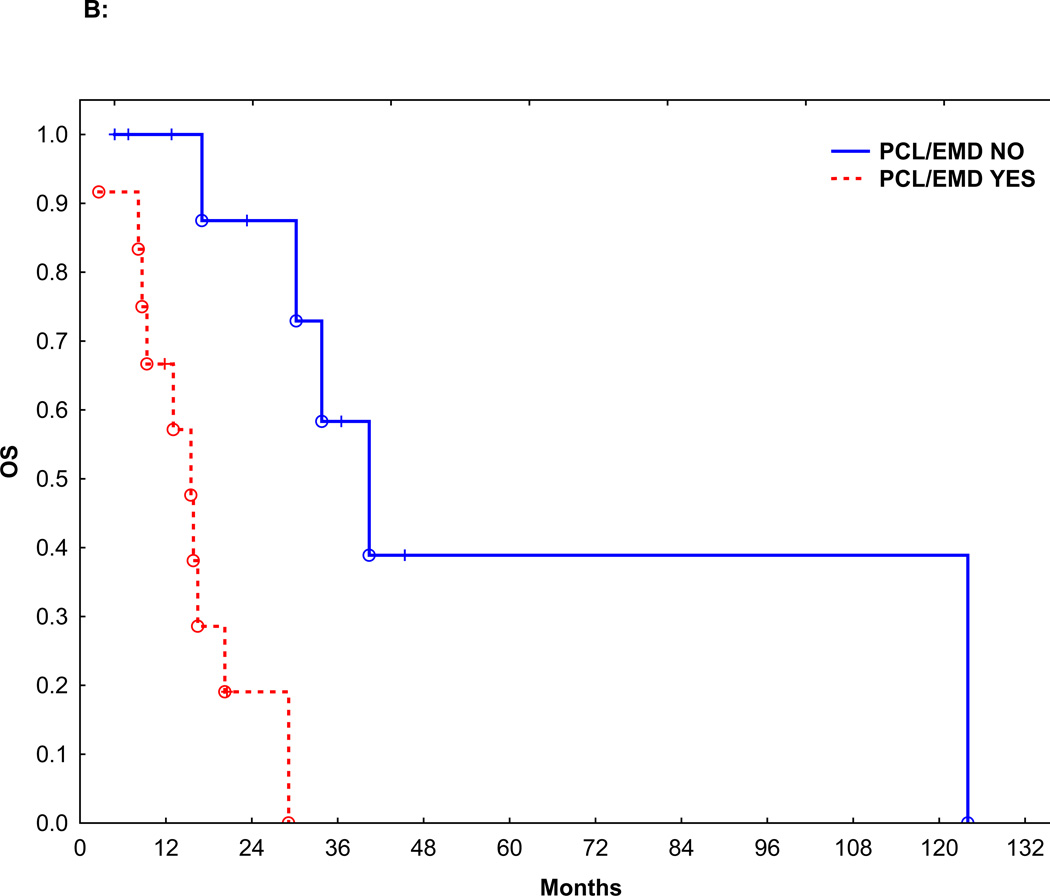
Sixteen patients (69%) received induction therapy with IMiDs or PI. Seven patients (30%) received induction therapy with a conventional chemotherapy regimen CVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone): 3, pulsed steroids: 2, EPOCH (etoposide, doxorubicin, vincristine, prednisone, and cyclophosphamide): 1, and melphalan plus prednisone: 1. Fifteen patients (65%) achieved a partial response (PR), and 3 patients (13%) achieved a very good partial remission (VGPR), for an overall response to induction rate of 78%. There was no significant difference in overall response between patients who received IMiDs or PI (14/16, 87%) vs. conventional chemotherapy (4/7, 57%, p=0.14). The median follow-up was 16.5 months (range: 1.8–125.4 months). Median PFS from the start of induction therapy was 9.5 months and the median OS from diagnosis was 20.2 months. The PFS and OS were significantly worse than patients with MM without c-MYC and similar to patients with PCL without c-MYC (Figure 1A and B, p<0.0001). Median PFS of patients with or without PCL or EMD was 7.7 and 28.0 months, respectively (p=0.004) (Figure 2A). Median OS of patients with or without PCL or EMD was 15.5 vs. 40.4 months (p=0.002), respectively (Figure 2B). The PFS and OS were not affected by additional HR abnormalities by CC (p=0.35 and 0.70), International Staging System (ISS).stage at diagnosis (p=0.22 and 0.21), or the use of IMiDs or PI for induction (p=0.83 and 0.29).
Figure 1.
A: PFS for patients with c-MYC abnormality (“MM”, n=23), matched patients with MM but no c-MYC abnormality (“control”, n=46) and patients with PCL without c-MYC rearrangement (“PCL”, n=32).
B: OS for patients with c-MYC abnormality (“MM”, n=23), matched patients with MM but no c-MYC abnormality (“control”, n=46) and patients with PCL without c-MYC rearrangement (“PCL”, n=32).
Figure 2.
A: PFS in patients with or without PCL/EMD
B: OS in patients with or without PCL/EMD
Seventeen patients (74%) went on to receive high-dose chemotherapy and autologous (auto-HCT; n=16) or allogeneic (allo-HCT; n=1) hematopoietic stem cell transplantation. Five patients (22%) died of rapid disease progression before HCT (median age 66, range 21–72 years). Patients who received an auto- or allo-HCT had a longer median PFS (21.6 vs. 3.8 months, p=0.021) and OS (30.2 vs. 9.3 months, p=0.028). Eight (67%) with EMD or PCL received auto or allo-HCT. Their median PFS or OS was not significantly different from the 4 patients who did not receive an HCT (p=0.06 and 0.11, respectively).
Discussion
This is the first study to describe the clinical characteristics and outcomes of MM patients with 8q24.1/c-MYC rearrangements. Though it has been reported that t(8;14)(q24.1;q32) and its variants t(8;22)(q24.1;q11.2) and t(2;8)(p12;q24.1) resulting in c-MYC rearrangement play important roles in tumorigenesis in Burkitt‘s lymphoma22 and a subset of acute lymphoblastic leukemia23, the significance of 8q24.1/c-MYC rearrangements in myeloma has not well studied. In this retrospective analysis, we demonstrated that 8q24.1/c-MYC rearrangement is diverse and includes typical changes as seen in Burkitt’s lymphoma and B-ALL, as well as partial 8q24.1/c-MYC deletion and a typical 8q24.1/c-MYC rearrangement involving different chromosome partner. We also showed that I8q24.1/c-MYC rearrangement t is a high-risk marker in MM and is characterized by an increased incidence of PCL, EMD, and plasmablastic morphology. Patients with c-MYC rearrangement also had short duration of remission and OS24.
Of the 23 patients reported here, almost 50% presented with aggressive features, including PCL, EMD, and/or plasmablastic morphology. Despite treatment with novel agents and aggressive chemotherapy regimens patients with aggressive disease features had shorter durations of OS and PFS than the patients with less-aggressive MM. Overall, the outcome of patients with c-MYC abnormality was similar to patients with PCL, a known high-risk group, but significantly worse than patients with MM without c-MYC abnormality. These results underscore the aggressive nature of MM with c-MYC abnormality.
The OS of patients with MM has improved considerably over the past decade, with the 5-year OS rate now surpassing 70% in transplant-eligible patients25, 26. However, patients with certain HR cytogenetic abnormalities, such as t(14;16), t(14;20), and del(17p), continue to have poor outcomes, with a median OS duration between 2 and 3 years, despite the use of novel agents and auto-HCT27–30. The OS of patients with 8q24.1 abnormalities was comparable to that of patients with other HR cytogenetic features. Patients who went on to receive an auto-HCT had somewhat better outcomes than those who did not, perhaps due to less aggressive disease that responded to induction therapy, thereby allowing them to proceed to auto-HCT. Despite aggressive treatment, most of the patients with c-MYC abnormalities had short remissions and died of progressive disease within 2–3 years, highlighting the need for early recognition and more effective therapeutic approaches in these patients.
In view of the increased likelihood of aggressive disease, frequent occurrence of PCL and plasmablastic morphology, patients with these abnormalities should be enrolled in prospective clinical trials for high-risk myeloma. Patients ineligible for clinical trials should be offered aggressive induction regimens incorporating novel agents used for lymphoma, followed by high-dose therapy and auto-HCT.
This report has the inherent limitations of a retrospective, chart-review analysis. These include missing vital pieces of information such as FISH studies in some patients, patient and treatment heterogeneity, and potential bias in treatment selection due to physician or patient preference. These limitations can be overcome in a prospective study that should include all patients with HR myeloma treated with a uniform approach.
In conclusion, this is the first clinical report describing the outcome of myeloma patients with 8q24.1/c-MYC abnormality, which is associated with an overall aggressive course and poor outcome.
REFERENCES
- 1.Rajkumar SV, Gahrton G, Bergsagel PL. Approach to the treatment of multiple myeloma: a clash of philosophies. Blood. 2011;118:3205–3211. doi: 10.1182/blood-2011-06-297853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Dispenzieri A, Rajkumar SV, Gertz MA, et al. Treatment of newly diagnosed multiple myeloma based on Mayo Stratification of Myeloma and Risk-adapted Therapy (mSMART): consensus statement. Mayo Clin Proc. 2007;82:323–341. doi: 10.4065/82.3.323. [DOI] [PubMed] [Google Scholar]
- 4.Fonseca R, Barlogie B, Bataille R, et al. Genetics and cytogenetics of multiple myeloma: a workshop report. Cancer Res. 2004;64:1546–1558. doi: 10.1158/0008-5472.can-03-2876. [DOI] [PubMed] [Google Scholar]
- 5.Munshi NC, Anderson KC, Bergsagel PL, et al. Consensus recommendations for risk stratification in multiple myeloma: report of the International Myeloma Workshop Consensus Panel 2. Blood. 2011;117:4696–4700. doi: 10.1182/blood-2010-10-300970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar SK, Mikhael JR, Buadi FK, et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines. Mayo Clin Proc. 2009;84:1095–1110. doi: 10.4065/mcp.2009.0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart AK, Bergsagel PL, Greipp PR, et al. A practical guide to defining high-risk myeloma for clinical trials, patient counseling and choice of therapy. Leukemia. 2007;21:529–534. doi: 10.1038/sj.leu.2404516. [DOI] [PubMed] [Google Scholar]
- 8.Rajkumar SV. Multiple myeloma: 2013 update on diagnosis, risk-stratification, and management. Am J Hematol. 2013;88:226–235. doi: 10.1002/ajh.23390. [DOI] [PubMed] [Google Scholar]
- 9.Prochownik EV. c-Myc: linking transformation and genomic instability. Curr Mol Med. 2008;8:446–458. doi: 10.2174/156652408785747988. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman B, Liebermann DA. Apoptotic signaling by c-MYC. Oncogene. 2008;27:6462–6472. doi: 10.1038/onc.2008.312. [DOI] [PubMed] [Google Scholar]
- 11.Avet-Loiseau H, Gerson F, Magrangeas F, Minvielle S, Harousseau JL, Bataille R. Rearrangements of the c-myc oncogene are present in 15% of primary human multiple myeloma tumors. Blood. 2001;98:3082–3086. doi: 10.1182/blood.v98.10.3082. [DOI] [PubMed] [Google Scholar]
- 12.Holien T, Sundan A. Oncogene addiction to c-MYC in myeloma cells. Oncotarget. 2012;3:739–740. doi: 10.18632/oncotarget.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tonon G. Molecular pathogenesis of multiple myeloma. Hematol Oncol Clin North Am. 2007;21:985–1006. vii. doi: 10.1016/j.hoc.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Chiecchio L, Dagrada GP, Protheroe RK, et al. Loss of 1p and rearrangement of MYC are associated with progression of smouldering myeloma to myeloma: sequential analysis of a single case. Haematologica. 2009;94:1024–1028. doi: 10.3324/haematol.2008.004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiecchio L, Dagrada GP, White HE, et al. Frequent upregulation of MYC in plasma cell leukemia. Genes Chromosomes Cancer. 2009;48:624–636. doi: 10.1002/gcc.20670. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez de Larrea C, Kyle RA, Durie BG, et al. Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia. 2013;27:780–791. doi: 10.1038/leu.2012.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taddesse-Heath L, Meloni-Ehrig A, Scheerle J, Kelly JC, Jaffe ES. Plasmablastic lymphoma with MYC translocation: evidence for a common pathway in the generation of plasmablastic features. Mod Pathol. 2010;23:991–999. doi: 10.1038/modpathol.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Billecke L, Murga Penas EM, May AM, et al. Cytogenetics of extramedullary manifestations in multiple myeloma. Br J Haematol. 2013;161:87–94. doi: 10.1111/bjh.12223. [DOI] [PubMed] [Google Scholar]
- 19.Blade J, Fernandez de Larrea C, Rosinol L, Cibeira MT, Jimenez R, Powles R. Soft-tissue plasmacytomas in multiple myeloma: incidence, mechanisms of extramedullary spread, and treatment approach. J Clin Oncol. 2011;29:3805–3812. doi: 10.1200/JCO.2011.34.9290. [DOI] [PubMed] [Google Scholar]
- 20.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan ELM P. Nonparametric estimation from incomplete observations. J. Amer. Statist. Assn. 1958;53:457–481. [Google Scholar]
- 22.Carbone A, Gloghini A. AIDS-related lymphomas: from pathogenesis to pathology. Br J Haematol. 2005;130:662–670. doi: 10.1111/j.1365-2141.2005.05613.x. [DOI] [PubMed] [Google Scholar]
- 23.Delgado MD, Leon J. Myc roles in hematopoiesis and leukemia. Genes Cancer. 2010;1:605–616. doi: 10.1177/1947601910377495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palumbo A, Attal M, Roussel M. Shifts in the therapeutic paradigm for patients newly diagnosed with multiple myeloma: maintenance therapy and overall survival. Clin Cancer Res. 2011;17:1253–1263. doi: 10.1158/1078-0432.CCR-10-1925. [DOI] [PubMed] [Google Scholar]
- 25.Cavo M, Tacchetti P, Patriarca F, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010;376:2075–2085. doi: 10.1016/S0140-6736(10)61424-9. [DOI] [PubMed] [Google Scholar]
- 26.Nair B, van Rhee F, Shaughnessy JD, Jr, et al. Superior results of Total Therapy 3 (2003–33) in gene expression profiling-defined low-risk multiple myeloma confirmed in subsequent trial 2006–66 with VRD maintenance. Blood. 2010;115:4168–4173. doi: 10.1182/blood-2009-11-255620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergsagel PL, Mateos MV, Gutierrez NC, Rajkumar SV, San Miguel JF. Improving overall survival and overcoming adverse prognosis in the treatment of cytogenetically high-risk multiple myeloma. Blood. 2013;121:884–892. doi: 10.1182/blood-2012-05-432203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avet-Loiseau H, Attal M, Moreau P, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood. 2007;109:3489–3495. doi: 10.1182/blood-2006-08-040410. [DOI] [PubMed] [Google Scholar]
- 29.Neben K, Lokhorst HM, Jauch A, et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood. 2012;119:940–948. doi: 10.1182/blood-2011-09-379164. [DOI] [PubMed] [Google Scholar]
- 30.Sonneveld P, Schmidt-Wolf IG, van der Holt B, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/ GMMG-HD4 trial. J Clin Oncol. 2012;30:2946–2955. doi: 10.1200/JCO.2011.39.6820. [DOI] [PubMed] [Google Scholar]