Abstract
Androgen receptor (AR) signaling is a critical pathway for prostate cancer cells, and androgen-deprivation therapy (ADT) remains the principal treatment for patients with locally advanced and metastatic disease. However, over time, most tumors become resistant to ADT. The view of castration-resistant prostate cancer (CRPC) has changed dramatically in the last several years. Progress in understanding the disease biology and mechanisms of castration resistance led to significant advancements and to paradigm shift in the treatment. Accumulating evidence showed that prostate cancers develop adaptive mechanisms for maintaining AR signaling to allow for survival and further evolution. The aim of this review is to summarize molecular mechanisms of castration resistance and provide an update in the development of novel agents and strategies to more effectively target the AR signaling pathway.
INTRODUCTION
The androgen dependency of prostate cancer (PCa) was first established in the 1940's when Huggins and Hodges demonstrated the antitumor activity of hormonal manipulation in the treatment of PCa.1 Since then androgen-deprivation therapy (ADT) has been a mainstay in the treatment of advanced PCa and remains without doubt the single most effective therapy. Although initial response rates exceed 80%, relapse invariably occurs with the transition to a more aggressive form of PCa that is termed castration-resistant prostate cancer (CRPC). Mechanisms of resistance to castration have historically been thought to be androgen independent. This misconception was dismissed and it is now apparent that signaling through the AR continues to be crucial for tumor growth under castrate condition in a significant proportion of patients.
Over the past decades preclinical studies and analyses of CRPC tumor samples have revealed several mechanisms by which the androgen receptor (AR) signaling pathway can be activated/ maintained in the presence of ADT. With a better understanding of the mechanisms resulting in castration resistance, novel therapeutic agents that target AR axis have been developed, and have contributed to significantly improve survival of CRPC patients.2–4 It appears that prostate cancers have selective pressures for maintaining AR signaling to allow for survival and further evolution.5 Therapies that are more efficient at blocking this crucial signaling pathway are therefore potentially promising approaches to further improve CRPC management.
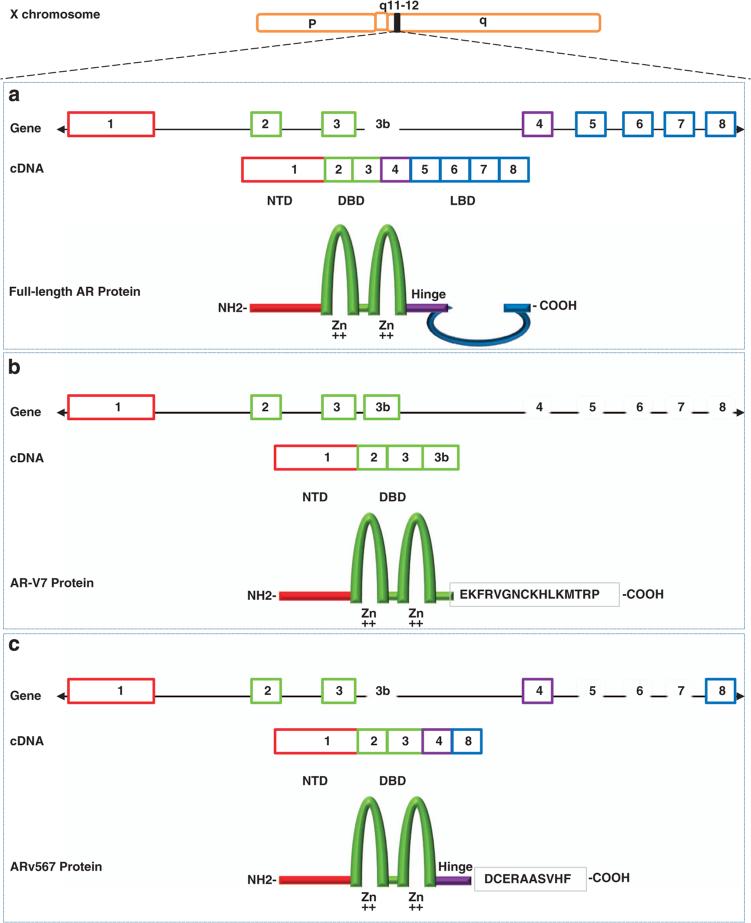
THE AR: STRUCTURE AND FUNCTION
Androgen exerts its biological effects through the AR, a 110-kDa protein that acts as a nuclear transcription factor. The AR gene is situated at Xq11–12 and consists of eight exons (Figure 1a). The N-terminal domain (NTD) contains the activation function 1 (AF-1) that includes two overlapping TAUs (transcription activation units): TAU-1 (amino acids 1–370), which supports AR transcriptional activity upon stimulation by full agonist, and TAU-5 (amino acids 360–528), which confers constitutive activity to the AR in the absence of the ligand-binding domain (LBD).6 The NTD contains several phosphorylation and sumoylation regulatory sites. The central region of the receptor contains the DNA-binding domain and the hinge region and harbors the nuclear localization signal. The carboxy-terminal end contains the LBD and the AF-2 function. The activity of both AF-1 and AF-2 is modulated by coregulatory proteins, which function to either upregulate (coactivators) or downregulate (corepressors) AR activity.7 AR activity largely depends on access to cognate binding sites on chromatin, facilitated in part by histone-modifying enzymes such as p300 and CREB-binding protein (which directly promote a chromatin landscape favorable for transcriptional activation) and pioneer factors such as FOXA1 and GATA2 (which promote open chromatin structure, subsequent nuclear receptor binding nearby and resultant initiation of specific transcriptional programs).
Figure 1.
Schematic structure of human AR and AR splice variant 7 (AR-V7) and 567(AR567es). (a) The human AR gene consists of eight exons with exon 1 encoding the N-terminal domain (NTD) and the entire 5′ untranslated region; exons 2 and 3 encoding the DNA-binding domain (DBD); and exons 4–8 encoding the ‘hinge’ region (HR) and ligand binding domain (LBD). The NTD contains the activation function 1 (AF-1) that includes two overlapping transcription activation units (TAU): TAU-1 (amino acids 1–370), which supports AR transcriptional activity upon stimulation by full agonist, and TAU-5 (amino acids 360–528), which confers constitutive activity to the AR in the absence of the LBD. The central region of the receptor contains the DBD and the HR and harbors the nuclear localization signal (NLS). The DBD is comprised of two cysteine-rich zinc finger motifs consisting of three alpha-helixes and a carboxy terminal extension (CTE) extending into the flexible hinge region. The first zinc finger defines DNA binding specificity, whereas the second zinc finger facilitates receptor dimerization and stabilization of the DNA-receptor complex. The carboxy-terminal end contains the LBD and the AF-2 function; (b) AR-V7 (also named AR3) encodes a protein with exons 1–3 and a terminal cryptic exon (CE3); (c) AR567es encodes a protein comprised of exons 1–4, and because of a frame-shift due to loss of exons 5–7, exon 8 has a stop codon generated after the first 10 amino acids resulting in a shortened exon 8.
In the absence of hormone ligand, the AR is located in the cytoplasm and associated in a complex with heat shock proteins HSP90, HSP70 and various cochaperones, which maintain the AR in a conformation capable of ligand binding and protect the AR from proteolysis.8,9 Upon ligand binding, AR dissociates from HSP90 and undergoes a conformational change whereby the highly flexible C-terminal helix 12 (H12) realigns over the ligand-binding pocket to yield a hydrophobic coactivator binding groove that serves as a platform for interaction with coactivators10 and facilitates NTD/LBD interactions.11–13 The interaction stabilizes the AR protein by slowing the dissociation rate of the ligand and is important for optimal transcriptional response.14 Several proteins mediate the import of AR into the nucleus including the nuclear importin cargo complex that translocates the AR through the nuclear pores.15 A role for HSP27 in enhancing AR stability, shuttling to the nucleus, and transcriptional activity was also observed upon treatment with androgens.16
In the canonical genomic pathway, AR translocates to the nucleus and binds to AR-response elements in the DNA, recruits coregulators to form a pre-initiation complex and together with the basal transcriptional machinery initiates transcription of specific AR target genes. In addition in PCa AR can drive the expression of oncogenes such as the ETS transcription factors (for example, ERG, ETV1) and uncommonly B-RAF or C-RAF as a consequence of gene rearrangements.17,18 Among these rearrangements, the most common is the fusion of the 3′ region of ERG with the 5′ region (containing the promoter/enhancer region) of the highly AR-regulated TMPRSS2 gene. ERG rearrangements have been identified in 40–60% of PCa.17,19,20 Evidence also suggests that the AR can activate transcription by binding to other transcription factors and essentially acts as a coactivator.21 Additional studies have also revealed that activated AR can stimulate downstream kinases (for example, MAPK and PI3K)22–24 and trigger rapid activation of a mitogenic response through nongenomic AR signaling.
MECHANISMS OF MAINTAINED AR ACTIVITY IN CRPC
Persistent androgen production: canonical and alternative pathways
Around 80% of circulating androgens arise in the testes and are effectively inhibited by castration (medical or surgical). However, castration cannot prevent the biosynthesis of androgen inside adrenals. Studies demonstrated that intra-tumoral testosterone in CRPC tumors are restored to equivalent or even higher levels than in non-castrate prostates.25–27 Intra-tumoral testosterone and/or dihydrotestosterone (DHT) in castrate men can be generated from conversion of circulating adrenal androgens27,28 or possibly by de novo synthesis of androgens by CRPC cells.29
Androgens are synthesized from cholesterol via multiple enzymatic steps, most of which are catalyzed by members of the cytochrome P450 (CYP) family (Figure 2). Gene expression studies have identified alterations in the expression of multiple steroidogenic enzymes in CRPC tissue including increased levels of SRD5A1, 3βHSD, 17β-HSD5 (also called AKR1C3) and reduced expression of SRD5A2.27,30,31 SRD5A1 has a preference for alternative substrates (for example, progesterone, 17OHP and AD) rather than testosterone and mediates the 5α-androstanedione pathway synthesis of DHT.32 A recently described, ubiquitously expressed SRD5A type 3 isoform is also present at higher levels in PCa.33,34 Increased levels of SRD5A1 and 3 may facilitate conversion of DHT via the alternative pathway. A gain-of-function mutation in 3βHSD1 has also been identified in CRPC.35 The mutation (N367T) renders the enzyme resistant to ubiquitination and degradation, leading to profound accumulation and increased flux to DHT. Taken together, these findings suggest that ADT can activate an adaptive response in PCa which augments its ability to convert adrenal precursors into testosterone and DHT through both canonical and alternative pathways that bypass testosterone.
Figure 2.
Canonical and alternative androgen biosynthesis pathways. Androgens are synthesized from cholesterol via multiple enzymatic steps, most of which are catalyzed by members of the cytochrome P450 (CYP) family. CYP11A1 is responsible for side chain cleavage of cholesterol, converting cholesterol to pregnenolone. Pregnenolone is then metabolized to dehydro-epiandrosterone (DHEA) and androstenedione (AD) via CYP17A1, which catalyzes both the 17 α-hydroxylation and the subsequent 17,20-lyase cleavage. The ‘canonical pathway’ for testosterone synthesis involves conversion of the major adrenal androgen DHEA and AD to testosterone in the testis, followed by irreversible 5α-reduction of testosterone to the higher affinity ligand DHT by 5α-reductases (SRD5A). 5α-reduction of upstream steroids, as opposed to 5α-reduction of testosterone, leads to DHT synthesis that bypasses testosterone through at least two pathways. In the ‘androstanedione pathway’, AD may be converted to 5-androstanedione by SRD5A that can then be converted into DHT by 17βHSD(s). Another alternative pathway to DHT occurs when 17-hydroxyprogesterone accumulates and SRD5A enzymes are present. In this alternative or ‘backdoor’ pathway, 17-hydroxyprogesterone is 5α- and 3α- reduced before the 17,20-lyase reaction of CYP17A1, yielding the 5α-reduced androgen androsterone. This pathway yields DHT without using DHEA, AD and testosterone as intermediates.
AR point mutations
More than 100 somatic mutations in the AR have been described so far, most of which occur either in the NTD or the LBD (http://androgendb.mcgill.ca/). Relevant mutations reported to date cluster in particular regions involved in critical ligand receptor and protein-receptor interactions.36,37 Mutations in the hinge region and LBD usually confer increased transactivational activity and reduced ligand specificity. A large number of AR mutations result in promiscuous activation of the AR by weak adrenal androgens and other steroid hormones; other mutations convert AR antagonists (flutamide and bicalutamide) into potent agonists.
AR mutations are rare in early stage untreated PCa but are common in CRPC, where they are present in approximately 10–30% of patients previously treated with the first-generation antiandrogens, bicalutamide and especially flutamide.38–42 It has been proposed that treatment with specific AR antagonists actually selects for tumors expressing AR mutants activated by the therapeutic agent.43 Therapy-specific selection of AR mutants represents a possible mechanism for the withdrawal syndrome in which tumors regress on antiandrogens discontinuation,44,45 and explains why tumors resistant to first-generation antagonist may still respond favorably to second-generation antiandrogens.46,47 Recently, a novel missense mutation in the LBD, F876L, which can convert second-generation antiandrogens enzalutamide and ARN-509 into AR agonist and drive resistance in preclinical models48,49 have been identified in patients treated with ARN-509.50
AR overexpression
Up to 80% of CRPC display a marked increase in AR mRNA and protein.51–54 In nearly one-third of patients progressing after castration and antiandrogens, the mechanism for increased AR expression is through amplification of the AR gene.51,52,55–57 In contrast, untreated PCa very rarely contain an AR gene amplification.51 Studies also reported high frequency of AR gene amplification in circulating tumor cells in CRPC.20,58 The mechanisms leading to AR overexpression without gene amplification are still elusive, but may include increased transcription rates, or stabilization of the mRNA or protein.59–61 Interestingly, tissue- and cell-specific AR mRNA regulation (both up and downregulation) by AR and other oncogenes, including cMYC, has been reported;62–64 and functional, exonic AR-response elements have been identified in exons 4 and 5 of the AR gene.65 AR overexpression can confer AR hypersensitivity to low levels of androgens and can result in continued tumor proliferation despite minimal androgen concentrations.56,66 In experimental PCa models, an increase in AR protein concentration can also cause antagonist–agonist conversion of bicalutamide.67
AR splice variants
We and others68–72 have established the existence of truncated AR splice variants (AR-Vs) that contain an intact AR NTD and DNA-binding domain, but lack the LBD, which is the target of current hormonal agents (Figures 1b and c). Increased expression of these AR-Vs in CRPC are often coupled with increased transcription from the AR gene locus (or loci when AR is amplified), but may arise due to aberrant splicing and/or structural rearrangements of the AR gene.71,73,74 Multiple AR-Vs have been fully decoded and proven to be expressed in CRPC, with differing properties.75,76 Key differences among the known variants include transcriptional activity and expression abundance. AR-V7 is constitutively active and the most abundant variant detected to date in CRPC.77 In some but not all cell line models, ligand stimulation negatively regulates AR-V7 expression, and elevated AR-V7 directs a distinct transcriptional program.77 In preclinical models, AR-V7 levels and function cannot be suppressed, and may be enhanced by AR-directed therapies including abiraterone acetate and enzalutamide.77–79 On the basis of mRNA levels, AR-Vs generally appear to be expressed at low levels relative to the full length AR in CRPC specimens. The full-length AR and AR-Vs appear to almost always coexist in PCa, introducing a challenge to dissect in translational studies of clinical specimens their relative roles in driving AR signaling.
Cross-talk with other signal transduction pathways
Although AR is a central player in PCa, other signaling pathways and their interactions with AR signaling are also critically implicated in PCa progression.80–84 The phosphatidylinositol 3-kinase (PI3K)/Akt pathway is one of the most commonly altered signaling pathway in PCa (Table 1). Loss-of-function mutations or deletions involving the tumor suppressor gene phosphatase and tensin homolog (PTEN) are commonly observed in advanced PCa (with loss of heterozygosity at PTEN locus present in up to 60% of patients).85–87 The frequency of PI3K pathway alteration rises substantially when PTEN alterations are considered together with alterations in the INPP4B and PHLPP phosphatases recently implicated in PI3K regulation, the PIK3CA gene itself, and the PIK3CA regulatory subunits.54 Whole genome sequencing studies have also recently identified complex rearrangements disrupting both PTEN and its interacting protein MAGI2.88 In PTEN-deficient preclinical models, it has been demonstrated that AR and PI3K/Akt pathways cross-regulate each other by reciprocal feedback, thereby maintaining tumor cell survival, and combined pharmacologic inhibition of PI3K and AR signaling axis is more effective than single agent AR or Akt inhibition.81,89 This strategy is currently under evaluation in clinical trials (Table 2).
Table 1.
Potentially actionable common genomic aberrations in prostate cancer
| Type | Frequency (ref. 86,92,172) | Potential targeted therapeutic approach | |
|---|---|---|---|
| Intracellular transduction pathways | |||
| Androgen receptor | Amplification, mutation | 40–50% | Novel AR-targeting agents |
| KRAS | Mutation | 1–2% | MEK inhibitor plus PI3K/AKT inhibitor |
| PI3K/AKT pathway | |||
| PTEN | Deletion, mutation | up to 60–65% | PI3K/Akt inhibitor ± AR-targeting agents |
| PIK3CA | Amplification, mutation | ||
| AKT | Mutation | ||
| PHLPP | Deletion | ||
| INPP4B | Amplification | ||
| RAF-MEK-Erk pathway | |||
| BRAF | Gene fusion, mutation | 1–2% | BRAF or MEK inhibitor |
| Transcription factors | |||
| N-MYC (concurrent with AURKA) | Amplification | 5% | Aurora-A inhibitor |
| 65% NEPC | |||
| ETS (ERG, ETV1, ETV4, FLI1) | Gene fusion | 50% | PARP inhibitors±AR-targeting agents |
| Wnt/β-catenin pathway | |||
| APC | Deletion, mutation | 4–16% | Wnt-targeting drug |
| B-catenin | Amplification, mutation | ||
| Cell cycle control | |||
| CDK4, CDK6, CyclinD1 | Mutation, amplification | 2–10% | CDK4/6 inhibitor |
| Chromatin remodeling | |||
| EZH2 | Amplification | 2–5% | EZH2 inhibitor |
| DNA damage repair | |||
| ATM, BRCA1 and BRCA2, RAD51 | Mutation, deletion | 20–25% | PARP inhibitors |
Table 2.
Selected phase I/II trials of signaling pathways inhibitor in combination with AR-targeting agents for the treatment of metastatic CRPC
| Phase | Study population | Regimen | Clinicaltrial.gov ID | |
|---|---|---|---|---|
| PI3K/Akt/mTOR inhibitors | ||||
| Pan-class I PI3K inhibitor | ||||
| BKM120 | I | Abiraterone-resistant CRPC | Plus abiraterone | NCT01634061 |
| I | CRPC, post-docetaxel | Plus abiraterone | NCT01741753 | |
| Pan AKT inhibitor | ||||
| GDC-0068 | II | CRPC, post-docetaxel | Plus abiraterone | NCT01485861 |
| AZD5363 | I-II | CRPC | Plus enzalutamide | NA |
| Dual PI3K/mTOR inhibitors | ||||
| BEZ235 | Ib | Abiraterone-resistant CRPC | Plus abiraterone | NCT01251861 |
| Tyrosine kinase inhibitors | ||||
| Cabozantinib (MET, RET, VEGFR2) | I | CRPC, post-docetaxel | Plus abiraterone | NCT01574937 |
| II | CRPC, chemo-naïve with bone mets | Plus abiraterone | NCT01995058 | |
| Dasatinib (ABL, SRC) | II R | CRPC, chemo-naive | Dasatinib plus abiraterone vs abiraterone | NCT01685125 |
| Sunitinib (VEGFR, PDGFR) | II R | CRPC | Sunitinib plus abiraterone vs abiraterone vs dasatinib plus abiraterone | NCT01254864 |
| Tivozanib (VEGFR) | II | CRPC, post-docetaxel | Plus enzalutamide | NCT01885949 |
| Dovitinib (VEGFR, FGFR, PDGFR) | CRPC, post-docetaxel | Plus abiraterone | NCT01994590 | |
| Aurora-A inhibitor | ||||
| Alisertib | I/II R | Abiraterone-resistant CRPC | Alisertib vs Alisertib plus abiraterone | NCT01848067 |
Abbreviations: CRPC, castration-resistant prostate cancer; NA, not available; R, randomized.
Alteration of coregulators and pioneer factors
Deregulation of expression and/or turnover of coactivators, corepressors and pioneer factors may provide a mechanism for increased AR transcriptional activity, promoting PCa cell survival.90, 91 The p160 SRCs have critical roles in AR transcriptional activity, cell proliferation and resistance to androgen deprivation. Interestingly, speckle-type POZ protein (SPOP) mutations found in 6–15% of PCa,92 can alter SRC-3 activity.93 Wild-type SPOP interacts directly with SRC-3 and regulates SRC-3 turnover acting as a tumor-suppressor in PCa cells. Somatic missense SPOP mutations lack the capacity to attenuate the coactivator function of SRC-3 on AR transcriptional activity. These studies provide a possible explanation for the role of SPOP mutations in PCa, and highlight the potential of SRC-3 as a therapeutic target in SPOP-mutated PCa. SRC-1 and SRC-3 coactivators are now the focus of new drug discovery programs.
Post-translational modification of AR
Multiple and diverse post-translational modifications of AR have been described including phosphorylation, acetylation, sumoylation, ubiquitination and methylation.94 AR is phosphorylated at multiple sites and studies suggest that this mechanism can enhance both ligand-dependent and ligand-independent activation capacity. Several preclinical studies have demonstrated that kinases activated by surface signals (cytokines and growth factors) or oncogenic mechanisms can directly enhance AR transcriptional function through modification of the AR itself, or by augmenting the activity of AR coactivators, contributing to the generation of a hypersensitive phenotype (reviewed in ref. 95). There are many examples of serine/threonine and tyrosine kinases implicated in AR phosphorylation and amongst the most interesting are Aurora kinase A (Aurora-A),96 cyclin-dependent kinase 1 (Cdk1),97,98 PIM1 kinase,99 Src and Ack1;100–102 all of these are targets of small molecule inhibitors in clinical development.
Cross talk with DNA damage pathways
The PARP (Poly ADP-ribose polymerase) family of enzymes modifies a subset of nuclear proteins by poly (ADP-ribose)-ylation, and is known to have a critical role in DNA damage repair and transcription. Inhibition of PARP1 is selectively lethal in tumors that harbor defective homologous recombination-mediated DNA repair.103,104 PARP inhibitors have shown impressive and durable responses in BRCA carrier patients suffering from advanced PCa.103,105 Recently, it has been shown that PARP-1 has a second major cellular function on chromatin as a transcriptional co-regulator, capable of modulating chromatin structure and transcription factor activity.106 PARP enzymatic activity is critical for AR occupancy on chromatin and for AR transcriptional activity in hormone-dependent and CRPC models107 and also mediates ETS transcription factor activity.108 These observations and next-generation sequencing studies identifying aberrations in DNA repair genes in up to 15–20% of sporadic CRPC86,109 have led to the clinical evaluation of PARP inhibitors as a strategy for simultaneously inhibiting AR activity and exploiting cancer-specific susceptibility to PARPi-genotoxic stresses.
Alternative steroid receptors
Glucocorticoids are widely used in the treatment of CRPC patients to slow disease progression, to palliate symptoms and to offset side effects of chemotherapy and hormonal therapy. The mechanisms underlying the antitumor activity seen in CRPC, also if still elusive, include suppression of adrenal steroidogenesis and a direct anti-proliferative effect mediated by modulation of TGF-β, IL-6 and IL-8.110 Few data are available on the expression and regulation of glucocorticoid receptor (GR) in PCa. Although AR is expressed in nearly all PCa, GR is only found in around 30%. This percentage, however, increases after ADT.111 Recent studies have demonstrated that AR and GR share the same chromatin binding sites and GR can regulate a large number of genes considered to be AR pathway-specific.112 It was also shown that resistance to novel antiandrogen enzalutamide can occur via increased expression of GR.113 This introduces the hypothesis that the GR, or potentially other nuclear steroid receptors, can bypass AR pathway blockade. Further work is needed to elucidate the context by which the various roles of GR are regulated. The six related steroid receptors, AR, estrogen receptor (ER)-α and -β, progesterone receptor (PR), GR, and mineralcorticoid receptor (MR), arose from a single ancestor and display high homology, in particular in the DNA-binding domain. Phylogenetic studies demonstrated that AR, GR, and PR are the most closely related.114,115 Given the evidence that GR can regulate a number of AR target genes and that it is itself increased during CRPC, it must be considered that redundancy may exist between AR and other steroid receptors.
NOVEL AGENTS AND STRATEGIES TO TARGET MAINTAINED AR SIGNALING
Until recently, the therapeutic options following development of CRPC were limited to second-line hormonal therapies with relatively limited clinical benefit. These included the addition of first-generation antiandrogens such as flutamide and bicalutamide, corticosteriods, estrogenic agents such as diethylstilbestrol, and the non-specific CYP inhibitor ketoconazole. After many years in which new developments were stalled, progress in translational research has finally brought us to an exciting era in the management of CRPC with several rationally developed new agents that target the AR signaling axis.
Androgen biosynthesis inhibitors
CYP17 inhibitors
Abiraterone (formerly known as CB7598) was identified in the 1990's in the Jarman lab using an iterative process to design and develop derivatives of the CYP17A1 substrate pregnenolone that were then evaluated in a human testicular microsome assay.116 Abiraterone significantly inhibited both activities of CYP17A1, that is, 17α-hydroxylase and C17–20 lyase, with similar potency (IC50 of 4 and 2.9 nm, respectively). Due to its poor bioavailability the 3-Beta-O-acetate form abiraterone acetate (formerly known as CB7630) that is rapidly deacetylated to the active drug in vivo was developed.117
Abiraterone acetate was the first-in-class selective CYP17A1 inhibitor118 to enter clinical development in the late 1990's, but development was discontinued due to lack of investment and safety concerns arising from an attenuated cortisol rise following an adrenocorticotropic hormone stimulation test seen in initial limited duration of exposure phase I studies.119 The clinical development was re-started in 2005 with a series of phase I/II studies that demonstrated significant prostate specific antigen (PSA) responses and anti-tumor activity in both chemotherapy-naive and post-docetaxel-treated CRPC patients, including in patients who have progressed on ketoconazole.120 Abiraterone acetate monotheraphy was associated with a syndrome of secondary mineralocorticoid excess due to a compensatory rise in adrenocorticotropic hormone, characterized by fluid retention, hypokalemia and lower-extremity edema, which was easily reversed with the use of eplerenone, a mineralocorticoid receptor antagonist, or with daily low-dose steroids that suppress adrenocorticotropic hormone.121 The improved tolerability when administered in combination with low-dose steroids led to its further development in combination with low-dose steroids. The encouraging phase II results rapidly led in 2008 to the initiation of a large randomized phase III study (COU-AA-301) in CRPC patients who had failed previous treatment with docetaxel. Median overall survival (OS) in the abiraterone and prednisone arm was significantly prolonged (15.8 months compared with 11.2 months in the placebo arm; P<0.0001).2,122 Abiraterone and prednisone also significantly delayed pain progression and skeletal-related events and improved quality of life and pain control.123 A second large randomized phase III trial started in 2009 (COU-AA-302) in chemotherapy-naive men with CRPC with rising PSA but minimal symptoms from their disease.3 At a median follow-up of 22.2 months, abiraterone produced a statistically significant improvement in radiological progression-free survival and increased OS. Abiraterone resulted in clinically and statistically significant effects on all secondary endpoints of the study.
Co-administration of low-dose prednisone with abiraterone acetate ameliorates hypertension, hypokalemia and fluid overload resulting from mineralocorticoid excess induced by CYP17A1 hydroxylase inhibition. Due to these aforementioned side-effects, there is increased interest in developing new CYP17A1 inhibitors that more specifically inhibit 17,20-lyase and are therefore less likely to require GC co-administration.
Orteronel (TAK-700; Millennium Pharmaceuticals, Cambridge, MA, USA) is a naphthylmethylimidazole derivative discovered by Takeda Pharmaceuticals (Osaka, Japan) that inhibits the 17,20-lyase activity 5.4 times more potently than 17α-hydroxylase activity.124 Two large randomized phase III trials in chemotherapy-naive (ELM-PC 4) and chemotherapy-treated CRPC (ELM-PC 5; NCT01193244 and NCT01193257, respectively) have completed recruitment. The ELM-PC 5 study comparing orteronel plus prednisone vs placebo plus prednisone in docetaxel pre-treated metastatic CRPC patient was recently unblinded after a scheduled interim analysis that indicated that orteronel would likely not meet the primary endpoint of improved OS when compared with the control arm (http://www.takeda.com/news/2013-/20130726_5894.html). Galeterone (VN/124-1, TOK-001; Tokai Pharmaceuticals, Cambridge, MA, USA) is a combined inhibitor of CYP17A1 and AR developed by the Brodie lab (Baltimore, MD, USA)125 currently in phase II testing (ARMOR2) (NCT01709734). VT-464 (Viamet Inc., Durham, NC, USA) is a potent, nonsteroidal CYP17 inhibitor discovered by Viamet with a 60-fold greater specificity for 17,20-lyase than 17α-hydroxylase and could therefore potentially not require concomitant exogenous glucocorticoids. Treatment of monkeys with VT-464 did not cause a rise in steroids upstream of CYP17A1 in contrast to abiraterone.126 This agent is also in early clinical trials (EudraCT Number: 2011-004103-2).
Targeting of other components of the androgen biosynthesis pathways
A number of drugs limiting androgen availability by blocking steroidal enzymes other than CYP17A1 have been developed. Critically, both the conventional and alternative pathways of androgen biosynthesis are dependent on CYP17A1 17,20-lyase for production of androgens and to date CYP17A1-independent mechanisms for androgen biosynthesis have not yet been identified. Thus the role of other inhibitors may be limited to combinations with CYP17A1 inhibitors when 17,20-lyase blockade is incomplete.
Increased levels of SRD5A1 and 3 is observed in CRPC30,31 and may facilitate conversion of DHT via the alternative pathway (Figure 2). Increased expression of SRD5A1 can have clinical implications as the commonly used 5α-reductase inhibitor finasteride is relatively specific for the type 2 enzyme, whereas dutasteride inhibits both enzymes. A phase II study is looking at the addition of dutasteride to abiraterone in metastatic CRPC (NCT01393730). AKR1C3 was reported to be highly expressed in CRPC.31 ASP9521 is a potent, selective and orally bioavailable AKR1C3 inhibitor discovered by Astellas Pharmaceutical by high throughput screening approaches for targeting enzyme activity.127 Results from a phase I/II study were recently reported but no activity was seen in a monotherapy study.128
Second-generation antiandrogens
Antiandrogens are agents that compete with endogenous androgens for the ligand-binding pocket of the AR and ‘actively’ antagonize the AR through a steric clash.129–131 Nonsteroidal first-generation AR antagonists (bicalutamide, nilutamide or flutamide) have been a standard treatment for advanced PCa for over three decades. However, first-generation antiandrogens are reversible inhibitors of the AR, have a several-fold lower affinity for the AR compared with androgens, do not result in significant degradation or cytoplasmic sequestration of the AR and can result in agonist behavior.132,133 Evidence supporting resistance to ‘first-generation’ antiandrogens following the emergence of mutations in the LBD or AR increased expression led to efforts to develop novel and more potent antiandrogens.67
Enzalutamide (formerly MDV3100) is a rationally designed potent second-generation antiandrogen developed in the Jung/ Sawyers lab utilizing as a starting point agonistic compounds previously crystalized bound to the AR LBD.134 Enzalutamide was selected for clinical development after showing activity in bicalutamide-resistant PCa models with overexpression of AR and mutant AR. A phase I/II study conducted in 140 men with metastatic CPRC showed evidence of antitumor activity, with 50% or greater decline of PSA in 62 and 51% of chemotherapy-naive patients and docetaxel-treated patients, and a median time to PSA progression of 41 and 21 weeks, respectively.135 The phase III AFFIRM study randomized men with metastatic CRPC who had previously received docetaxel chemotherapy. After a planned interim analysis, which showed a median of 4.8 months improvement in OS (hazard ratio = 0.631, P<0.0001) in favor of enzalutamide, the study was unblinded. The phase III PREVAIL study in men with chemotherapy-naive CRPC (NCT01212991) was recently unblinded and has met its co-primary endpoints of OS and radiological progression-free survival.136
From the same chemical series of enzalutamide, a second analogous diarylthiohydantoin ARN-509 (Aragon Pharmaceuticals, San Diego, CA, USA) showed similarly potent AR antagonism. In preclinical models, ARN-509 exhibited similar AR antagonism as enzalutamide and additional potentially advantageous features, including reduced crossing of the blood–brain barrier.137 Phase I/II analysis of ARN-509 (NCT01171898) has demonstrated that the drug is safe and well tolerated with evidence of promising preliminary activity in chemotherapy-naive CRPC both before and after treatment with abiraterone.138,139
Predictably, the significant activity reported in phase I/II studies of enzalutamide led to significant investment and interest in developing other novel antiandrogens. ODM-201 (Orion, Espoo, Finland), is a potent AR antagonist that does not cross the brain barrier in preclinical model.121 Two phase I/II trials (NCT01317641; NCT01784757) assessed the efficacy and safety of ODM-201 in metastatic CRPC patients. PSA decline and RECIST (response evaluation criteria in solid tumor) responses were observed across different patients groups with highest activity seen in the chemotherapy- and CYP17 inhibitor-naive group (response rate 30%; PSA decline >50% in >80% patients).140,141
Agents targeting AR NTD
An alternative to blocking the AR is targeting the NTD, which is essential for both ligand-dependent and ligand-independent AR activation. The AR NTD is flexible with a high degree of intrinsic disorder142,143 making it challenging to develop a therapeutic using rational structure-based drug design. One compound reported to target the AR NTD is EPI-001, isolated from a compound screen of a library of extracts from the marine sponge Geodia lindgreni.144 EPI-001 binds to the AR NTD without altering ligand binding or AR nuclear translocation. Instead EPI-001 inhibits AR transactivation by disrupting AF-1 function and inhibiting cofactor recruitment and N/C interaction regardless of the presence of androgen. As EPI-001 does not require the LBD, it may have antitumor activity in the setting of AR-Vs.
Alternative targeting of the AR
Traditional AR antagonists, such as flutamide or bicalutamide, act by binding to the ligand binding pocket of the receptor, resulting in a conformational change of the LBD and in an indirect blockade of transcriptional activity. Recently other chemical approaches have been pursued to develop improved AR inhibitors. EZN-4176 is a third-generation locked antisense oligonucleotides that binds the hinge region of the AR and results in downregulation of AR protein.145 The first-in-human clinical trial of EZN-4176 in CRPC patients, however, showed only modest activity with dosing limited by significant but reversible transaminitis.146 An alternative way to target AR is the use of small peptides that disrupt specific protein–protein interactions. Peptidomimetics are small organic molecules that do not possess a peptide backbone structure, but retain a capacity to interact with the same target protein by mimicking protein structures. Peptidomimetics combine the advantages of both peptides (that is, high efficacy, target selectivity) and small organic molecules (that is, cell permeability, stability from protease-mediated proteolytic degradation) and since peptidomimetics can mimic selective motifs in defined conformations, they may be designed to interfere with specific protein–protein interactions involving the different domains of the AR including the NTD.147
Therapies aimed to alter the stability and function of the AR
Another strategy currently being evaluated to disrupt continued AR signaling is the destabilization of the AR by inhibiting HSPs. We and others have tested HSP90 inhibitors in early clinical studies. Limited antitumor activity was reported to date in CRPC, although we have reported a durable response lasting more than a year in a patient treated with 17-DMAG (17-dimethylaminoethylamino-17-demethoxygeldanamycin).148 A phase II single agent study of Ganetespib (STA-9090, Synta Pharmaceuticals, Lexington, MA, USA), a synthetic next-generation HSP90 inhibitor, has been recently stopped at the first stage of enrollment due to lack of activity as per study design.149 A phase I/II study evaluating the novel resorcinol inhibitor AT13387 (Astex Pharmaceuticals, Dublin, CA, USA) alone or in combination with abiraterone and low-dose steroids in patients no longer responding to treatment with abiraterone is ongoing (NCT01685268).
OGX-427 (OncoGenex Pharmaceuticals, Bothell, WA, USA), a second-generation ASO drug targeting HSP27, has been evaluated in a randomized phase II study in combination with low-dose prednisone in patients with CRPC who have not previously received chemotherapy (NCT01120470). Preliminary results showed promising activity in terms of delaying disease progression (71% of patients were progression-free at 12 weeks compared with 31% in the prednisone-alone arm), ≥ 50% PSA falls (41% vs 20% in the prednisolone-alone arm), and decline in circulating tumor cell count (NCT01120470). The value of adding OGX-427 to continuing abiraterone and prednisone in patients with metastatic CRPC who have PSA progression will be evaluated in a randomized phase II study (NCT01681433).
Several histone deacetylase inhibitors, including depsipeptide, SAHA and LBH589 were tested in phase I/II clinical trials in CRPC with very modest activity observed. Recent reports show that sulforaphane, a derivative of glucoraphanin found in crucifers, may impair PCa growth through inhibition of histone deacetylase 6. Sulforaphane treatment increases HSP90 acetylation and leads to dissociation of AR from HSP90 and results in AR degradation and impaired expression of AR target genes.150 A novel sulforaphane–cyclodextrin complex orally bioavailable called Sulforadex (Evgen Limited, Liverpool, UK) has entered first-inhuman clinical trials.
PARP inhibitors
Emerging data suggest that homologous recombination DNA repair defects are common in PCa, potentially conferring a BRCAness phenotype.86 PARP is also implicated in ERG transcription and AR signaling, key drivers in PCa.107 Various PARP inhibitors are currently under development for the treatment of sporadic CRPC. Niraparib (formerly MK4827, Tesaro, Waltham, MA, USA), a potent PARP-1 and PARP-2 inhibitor, has been tested in a dose-escalation phase I/II trial in BRCA mutation carriers and patients with sporadic cancer. Nine of 21 (43%) patients with sporadic CRPC receiving active doses of niraparib (either 290/day or 300 mg/day) had stable disease for a median duration of 254 days. One patient had a greater than 50% decrease in concentration of PSA and remained on study for 306 days. No radiological responses were recorded, but decreases in circulating tumor cells of at least 30% were noted in eight patients.151 Olaparib (AZD2281, AstraZeneca, London, UK), a selective PARP-1 inhibitor, is currently being tested as a single agent in CRPC patients (TOPARP) (NCT01682772). A second randomized phase II study is evaluating veliparib (ABT-888, Abbott Laboratories, Abbott Park, IL, USA), a PARP-1 and PARP-2 inhibitor, in combination with abiraterone acetate and prednisone vs abiraterone acetate and prednisone alone (NCT01576172).
Therapies aimed to block cross-talk of the AR with other signaling pathways
A common and targetable set of alterations in PCa involves the PTEN/PI3K/Akt pathway. Pan-class I PI3K inhibitors and potent and isoform-selective PI3K inhibitors have entered clinical trials. Data indicated that PI3K isoform selectivity may be important to maximize therapeutic benefit and minimize toxicity. Preclinical data indicate that p110β, and possibly p110δ152 isoforms are important for the growth of PTEN-null tumors.153,154 p110β also appears to be essential for androgen-induced AR transactivation. Phase I studies are currently evaluating the p110β-specific-inhibitors AZD8186 (AstraZeneca) (NCT01884285) and GSK--2636771(GlaxoSmithKline, London, UK) (NCT01458067) in CRPC. There are also several classes of Akt inhibitors currently in development, including isoform-selective Akt catalytic-domain inhibitors. Although the importance of the individual Akt isoforms in PCa has yet to be fully elucidated a recent study demonstrated distinct roles for Akt-1 and Akt-2 in PCa cell growth and migration.155 Simultaneous targeting of Akt-1 and Akt-2 was shown to be superior to the inhibition of a single isozyme for induction of caspase-3 activity in tumor cells, suggesting that pan-Akt inhibitors are likely to be more promising, although toxicity may be a potential issue.156 Given the evidence of a reciprocal feedback loop between the AR and PI3K/Akt pathway,81 combination of novel AR-targeted drugs enzalutamide and abiraterone acetate with PI3K/Akt inhibitors appears to hold great promise (Table 1).
Various tyrosine kinase inhibitors are also under evaluation in combination with abiraterone acetate (Table 1). Cabozantinib (XL-184, Exelexis, San Francisco, CA, USA), a multitargeted tyrosine kinase inhibitor, that has shown encouraging antitumor activity as single agent in CRPC157 is currently being tested in two phase II studies in combination with abiraterone acetate (NCT01574937); (NCT01995058). The multitargeted tyrosine kinase inhibitor sunitinib (Sutent) and dasatinib (Sprycel) have been tested in combination with docetaxel in large phase III trial and failed to increase the efficacy of docetaxel158,159 despite encouraging results in phase II studies.160,161 Ongoing studies are exploring a different strategy and testing the hypothesis that suppression of androgens through CYP17 inhibition with abiraterone acetate in combination with dasatinib (NCT01254864) or sunitinib (NCT01254864) might result in improved efficacy.
PERSPECTIVES AND FUTURE DIRECTIONS
The realization that many prostate tumors are still dependent on AR activity after ADT has provided a paradigm shift in our understanding of the role of AR signaling in the progression of PCa. A number of cellular mechanisms have been described whereby the AR can be activated despite castration to significantly contribute to the progression of PCa, either alone or, more likely, in concert with each other. Myriad aberrations at various levels, from androgen synthesis to receptor binding on DNA, suggest mechanisms that contribute to disease progression. The challenge for researchers and clinicians now is to find the right therapeutic modalities to inhibit AR-driven PCa progression and identify biomarkers to guide treatment selection.
With many mechanisms at play, sometimes simultaneously, the next logical steps seem to be the use of combination regimens, which could potentially increase treatment efficacy and explore the utility of novel agents in earlier stages of the disease. Impressive responses have been reported with abiraterone and enzalutamide in the neoadjuvant setting162 and hormone-naïve PCa.163
Exciting opportunities have emerged in the field of combining AR axis–targeting agents with inhibitors of other oncogenic signaling pathways, however, this line of approach may be fraught with pitfalls. Redundancy between signaling pathways and negative-feedback loops within a single signaling pathway can make durable inhibition difficult to achieve. Studies to understand the potential of ‘horizontal’ and ‘vertical’ blockade as well as studies to understand the potential for stratification of patients, should be considered in targeted drug development and efforts made to use genomic abnormalities to optimally stratified patients for treatment (Table 1).
Despite their documented efficacy, neither abiraterone nor enzalutamide are curative and resistant disease eventually develops. In addition, not all patients respond to these treatments. Understanding the mechanisms that underlie acquired and primary resistance is a priority in PCa research to inform on the development of the next therapeutic strategies. In the case of abiraterone, preclinical models of resistance have suggested that treatment upregulates the expression of full-length AR, truncated AR-Vs and several steroidogenic enzymes, including CYP17A1 and AKR1C3.79,164–166 Translational studies to date have failed to identify a rise in circulating androgens on treatment.123,166 However, measurement of intracellular androgens has been limited by the availability of CRPC tissue and the technical and analytical challenges of controlling for losses during extraction and processing, and definitively separating, detecting and identifying particular steroids among highly related compounds.
Several preclinical studies have also shown that mutant AR can become promiscuously activated by very low levels of androgens (that could persist in patients treated with abiraterone), other steroid metabolites and drugs that bind the AR (such as prednisolone given concomitantly to abiraterone).38,166–168 These models support co-targeting combinations of CYP17 inhibitors with other enzymatic inhibitors or with potent second-generation AR antagonists. Combined therapy, particularly with abiraterone plus enzalutamide and/or related compounds, is a particularly interesting area of study (NCT01650194; NCT01792687; NCT01949337). However, it is possible that primary mechanisms of cross-resistance to both compounds exist.
Recently, a novel missense mutation in the AR LBD, F876LF, has been identified in plasma DNA from ARN-509–treated patients with progressive disease. This mutation confers agonistic activity to enzalutamide and ARN-509 in preclinical models.48–50 The identification of this novel amino acid change that bestows resistance to these second-generation antiandrogens may enable the design and/or discovery of next-generation agents targeting the LBD. Studies are required to determine the frequency of this mutation in patients with acquired resistance to enzalutanide, and to evaluate whether clonal selection of mutant AR-expressing clones also occurs on abiraterone.
The constitutively active AR-Vs have been proposed as mechanisms of resistance to both abiraterone and enzalutamide.78,79,165 However, in our view, investigations encom passing the full spectrum of molecular characterization and clinical translation of AR-V are still at a nascent stage. Nevertheless, the implications are clear and multifaceted. Because AR signaling mediated by these truncated AR molecules is not targeted by therapies targeting the AR LBD, such as enzalutamide and abiraterone, rational development of novel agents that target the NTD of AR could represent a key step to overcome endocrine resistance.
The majority of patients progressing on abiraterone and enzalutamide have a rise in PSA, suggesting reactivation of AR or other steroid signaling pathways that could increase PSA transcription. However, the hypothesis that continued activation of the AR receptor signaling pathways results in drug resistance in a significant proportion of CRPC patients progressing on abiraterone and enzalutamide is still unproven. Moreover, the widespread use of novel AR-targeted therapies may produce the emergence of PCa that no longer depends on AR signaling. Small retrospective reports on the use of abiraterone after enzalutamide failure and vice versa suggests only modest response rates and brief duration of effect.128,169,170 However, the benefit for a few is significant. Studies are required to elucidate mechanisms of resistance to these novel AR-targeted therapies.171 Procurement and study of tumors resistant to these therapies is necessary and should be prioritized.
Acknowledgments
JSdB received consulting fees from Ortho Biotech Oncology Research and Development (a unit of Cougar Biotechnology), consulting fees and travel support from Amgen, Astellas, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Dendreon, Enzon, Exelixis, Genentech, GlaxoSmithKline, Medivation, Merck, Novartis, Pfizer, Roche, Sanofi-Aventis, Supergen and Takeda, and grant support from AstraZeneca and Genentech. GA received consulting fees and travel support from Janssen-Cilag, Veridex, Roche/ Ventana and Millennium Pharmaceuticals, lecture fees from Janssen-Cilag, Ipsen, Takeda and Sanofi-Aventis and grant support from AstraZeneca and Genentech. GA is on the ICR rewards to inventors list of abiraterone acetate.
Footnotes
CONFLICT OF INTEREST
Abiraterone acetate was developed at The Institute of Cancer Research (ICR), which therefore has a commercial interest in the development of this agent.
REFERENCES
- 1.Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 2.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 5.Garraway LA, Sellers WR. Lineage dependency and lineage-survival oncogenes in human cancer. Nat Rev Cancer. 2006;6:593–602. doi: 10.1038/nrc1947. [DOI] [PubMed] [Google Scholar]
- 6.Jenster G, van der Korput HA, van Vroonhoven C, van der Kwast TH, Trapman J, Brinkmann AO. Domains of the human androgen receptor involved in steroid binding, transcriptional activation, and subcellular localization. Mol Endocrinol. 1991;5:1396–1404. doi: 10.1210/mend-5-10-1396. [DOI] [PubMed] [Google Scholar]
- 7.Heinlein CA, Chang C. Androgen receptor (AR) coregulators: an overview. Endocr Rev. 2002;23:175–200. doi: 10.1210/edrv.23.2.0460. [DOI] [PubMed] [Google Scholar]
- 8.Georget V, Terouanne B, Nicolas JC, Sultan C. Mechanism of antiandrogen action: key role of hsp90 in conformational change and transcriptional activity of the androgen receptor. Biochemistry. 2002;41:11824–11831. doi: 10.1021/bi0259150. [DOI] [PubMed] [Google Scholar]
- 9.Prescott J, Coetzee GA. Molecular chaperones throughout the life cycle of the androgen receptor. Cancer Lett. 2006;231:12–19. doi: 10.1016/j.canlet.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 10.van de Wijngaart DJ, Dubbink HJ, van Royen ME, Trapman J, Jenster G. Androgen receptor coregulators: recruitment via the coactivator binding groove. Mol Cell Endocrinol. 2012;352:57–69. doi: 10.1016/j.mce.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Askew EB, Minges JT, Hnat AT, Wilson EM. Structural features discriminate androgen receptor N/C terminal and coactivator interactions. Mol Cell Endocrinol. 2012;348:403–410. doi: 10.1016/j.mce.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaufele F, Carbonell X, Guerbadot M, Borngraeber S, Chapman MS, Ma AA, et al. The structural basis of androgen receptor activation: intramolecular and intermolecular amino-carboxy interactions. Proc Natl Acad Sci USA. 2005;102:9802–9807. doi: 10.1073/pnas.0408819102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Royen ME, Cunha SM, Brink MC, Mattern KA, Nigg AL, Dubbink HJ, et al. Compartmentalization of androgen receptor protein-protein interactions in living cells. J Cell Biol. 2007;177:63–72. doi: 10.1083/jcb.200609178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He B, Kemppainen JA, Wilson EM. FXXLF and WXXLF sequences mediate the NH2-terminal interaction with the ligand binding domain of the androgen receptor. J Biol Chem. 2000;275:22986–22994. doi: 10.1074/jbc.M002807200. [DOI] [PubMed] [Google Scholar]
- 15.Cutress ML, Whitaker HC, Mills IG, Stewart M, Neal DE. Structural basis for the nuclear import of the human androgen receptor. J Cell Sci. 2008;121:957–968. doi: 10.1242/jcs.022103. [DOI] [PubMed] [Google Scholar]
- 16.Zoubeidi A, Zardan A, Beraldi E, Fazli L, Sowery R, Rennie P, et al. Cooperative interactions between androgen receptor (AR) and heat-shock protein 27 facilitate AR transcriptional activity. Cancer Res. 2007;67:10455–10465. doi: 10.1158/0008-5472.CAN-07-2057. [DOI] [PubMed] [Google Scholar]
- 17.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 18.Palanisamy N, Ateeq B, Kalyana-Sundaram S, Pflueger D, Ramnarayanan K, Shankar S, et al. Rearrangements of the RAF kinase pathway in prostate cancer, gastric cancer and melanoma. Nat Med. 2010;16:793–798. doi: 10.1038/nm.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Attard G, de Bono JS, Clark J, Cooper CS. Studies of TMPRSS2-ERG gene fusions in diagnostic trans-rectal prostate biopsies. Clin Cancer Res. 2010;16:1340. doi: 10.1158/1078-0432.CCR-09-2253. author reply 1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Attard G, Swennenhuis JF, Olmos D, Reid AH, Vickers E, A'Hern R, et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 2009;69:2912–2918. doi: 10.1158/0008-5472.CAN-08-3667. [DOI] [PubMed] [Google Scholar]
- 21.Lu S, Jenster G, Epner DE. Androgen induction of cyclin-dependent kinase inhibitor p21 gene: role of androgen receptor and transcription factor Sp1 complex. Mol Endocrinol. 2000;14:753–760. doi: 10.1210/mend.14.5.0461. [DOI] [PubMed] [Google Scholar]
- 22.Cinar B, Mukhopadhyay NK, Meng G, Freeman MR. Phosphoinositide 3-kinase-independent non-genomic signals transit from the androgen receptor to Akt1 in membrane raft microdomains. J Biol Chem. 2007;282:29584–29593. doi: 10.1074/jbc.M703310200. [DOI] [PubMed] [Google Scholar]
- 23.Castoria G, Lombardi M, Barone MV, Bilancio A, Di Domenico M, De Falco A, et al. Rapid signalling pathway activation by androgens in epithelial and stromal cells. Steroids. 2004;69:517–522. doi: 10.1016/j.steroids.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Unni E, Sun S, Nan B, McPhaul MJ, Cheskis B, Mancini MA, et al. Changes in androgen receptor nongenotropic signaling correlate with transition of LNCaP cells to androgen independence. Cancer Res. 2004;64:7156–7168. doi: 10.1158/0008-5472.CAN-04-1121. [DOI] [PubMed] [Google Scholar]
- 25.Mostaghel EA, Page ST, Lin DW, Fazli L, Coleman IM, True LD, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67:5033–5041. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 26.Geller J, Albert J, Loza D, Geller S, Stoeltzing W, de la Vega D. DHT concentrations in human prostate cancer tissue. J Clin Endocrinol Metab. 1978;46:440–444. doi: 10.1210/jcem-46-3-440. [DOI] [PubMed] [Google Scholar]
- 27.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11:4653–4657. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 29.Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–6415. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 30.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 31.Mitsiades N, Sung CC, Schultz N, Danila DC, He B, Eedunuri VK, et al. Distinct patterns of dysregulated expression of enzymes involved in androgen synthesis and metabolism in metastatic prostate cancer tumors. Cancer Res. 2012;72:6142–6152. doi: 10.1158/0008-5472.CAN-12-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell DW, Wilson JD. Steroid 5 alpha-reductase: two genes/two enzymes. Annu Rev Biochem. 1994;63:25–61. doi: 10.1146/annurev.bi.63.070194.000325. [DOI] [PubMed] [Google Scholar]
- 33.Uemura M, Tamura K, Chung S, Honma S, Okuyama A, Nakamura Y, et al. Novel 5 alpha-steroid reductase (SRD5A3, type-3) is overexpressed in hormone-refractory prostate cancer. Cancer Sci. 2008;99:81–86. doi: 10.1111/j.1349-7006.2007.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Godoy A, Kawinski E, Li Y, Oka D, Alexiev B, Azzouni F, et al. 5alpha-reductase type 3 expression in human benign and malignant tissues: a comparative analysis during prostate cancer progression. Prostate. 2011;71:1033–1046. doi: 10.1002/pros.21318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang KH, Li R, Kuri B, Lotan Y, Roehrborn CG, Liu J, et al. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell. 2013;154:1074–1084. doi: 10.1016/j.cell.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brooke GN, Bevan CL. The role of androgen receptor mutations in prostate cancer progression. Curr Genomics. 2009;10:18–25. doi: 10.2174/138920209787581307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bergerat JP, Ceraline J. Pleiotropic functional properties of androgen receptor mutants in prostate cancer. Hum Mutat. 2009;30:145–157. doi: 10.1002/humu.20848. [DOI] [PubMed] [Google Scholar]
- 38.Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, et al. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332:1393–1398. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- 39.Taplin ME, Rajeshkumar B, Halabi S, Werner CP, Woda BA, Picus J, et al. Androgen receptor mutations in androgen-independent prostate cancer: Cancer and Leukemia Group B Study 9663. J Clin Oncol. 2003;21:2673–2678. doi: 10.1200/JCO.2003.11.102. [DOI] [PubMed] [Google Scholar]
- 40.Culig Z, Hobisch A, Cronauer MV, Cato AC, Hittmair A, Radmayr C, et al. Mutant androgen receptor detected in an advanced-stage prostatic carcinoma is activated by adrenal androgens and progesterone. Mol Endocrinol. 1993;7:1541–1550. doi: 10.1210/mend.7.12.8145761. [DOI] [PubMed] [Google Scholar]
- 41.Wallen MJ, Linja M, Kaartinen K, Schleutker J, Visakorpi T. Androgen receptor gene mutations in hormone-refractory prostate cancer. J Pathol. 1999;189:559–563. doi: 10.1002/(SICI)1096-9896(199912)189:4<559::AID-PATH471>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 42.Taplin ME, Bubley GJ, Ko YJ, Small EJ, Upton M, Rajeshkumar B, et al. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Res. 1999;59:2511–2515. [PubMed] [Google Scholar]
- 43.Steinkamp MP, O'Mahony OA, Brogley M, Rehman H, Lapensee EW, Dhanasekaran S, et al. Treatment-dependent androgen receptor mutations in prostate cancer exploit multiple mechanisms to evade therapy. Cancer Res. 2009;69:4434–4442. doi: 10.1158/0008-5472.CAN-08-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scher HI, Kelly WK. Flutamide withdrawal syndrome: its impact on clinical trials in hormone-refractory prostate cancer. J Clin Oncol. 1993;11:1566–1572. doi: 10.1200/JCO.1993.11.8.1566. [DOI] [PubMed] [Google Scholar]
- 45.Hara T, Miyazaki J, Araki H, Yamaoka M, Kanzaki N, Kusaka M, et al. Novel mutations of androgen receptor: a possible mechanism of bicalutamide withdrawal syndrome. Cancer Res. 2003;63:149–153. [PubMed] [Google Scholar]
- 46.Okegawa T, Nutahara K, Higashihara E. Alternative antiandrogen therapy in patients with castration-resistant prostate cancer: a single-center experience. Int J Urol. 2010;17:950–955. doi: 10.1111/j.1442-2042.2010.02620.x. [DOI] [PubMed] [Google Scholar]
- 47.Choi JI, Kim YB, Yang SO, Lee JK, Jung TY. Efficacy of alternative antiandrogen therapy for prostate cancer that relapsed after initial maximum androgen blockade. Korean J Urol. 2011;52:461–465. doi: 10.4111/kju.2011.52.7.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korpal M, Korn JM, Gao X, Rakiec DP, Ruddy DA, Doshi S, et al. An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide). Cancer Discov. 2013;3:1030–1043. doi: 10.1158/2159-8290.CD-13-0142. [DOI] [PubMed] [Google Scholar]
- 49.Balbas MD, Evans MJ, Hosfield DJ, Wongvipat J, Arora VK, Watson PA, et al. Overcoming mutation-based resistance to antiandrogens with rational drug design. Elife. 2013;2:e00499. doi: 10.7554/eLife.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joseph JD, Lu N, Qian J, Sensintaffar J, Shao G, Brigham D, et al. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. 2013;3:1020–1029. doi: 10.1158/2159-8290.CD-13-0226. [DOI] [PubMed] [Google Scholar]
- 51.Bubendorf L, Kononen J, Koivisto P, Schraml P, Moch H, Gasser TC, et al. Survey of gene amplifications during prostate cancer progression by high-throughout fluorescence in situ hybridization on tissue microarrays. Cancer Res. 1999;59:803–806. [PubMed] [Google Scholar]
- 52.Haapala K, Kuukasjarvi T, Hyytinen E, Rantala I, Helin HJ, Koivisto PA. Androgen receptor amplification is associated with increased cell proliferation in prostate cancer. Hum Pathol. 2007;38:474–478. doi: 10.1016/j.humpath.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 53.Linja MJ, Savinainen KJ, Saramaki OR, Tammela TL, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61:3550–3555. [PubMed] [Google Scholar]
- 54.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koivisto P, Kononen J, Palmberg C, Tammela T, Hyytinen E, Isola J, et al. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57:314–319. [PubMed] [Google Scholar]
- 56.Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinanen R, Palmberg C, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401–406. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 57.Miyoshi Y, Uemura H, Fujinami K, Mikata K, Harada M, Kitamura H, et al. Fluorescence in situ hybridization evaluation of c-myc and androgen receptor gene amplification and chromosomal anomalies in prostate cancer in Japanese patients. Prostate. 2000;43:225–232. doi: 10.1002/(sici)1097-0045(20000515)43:3<225::aid-pros9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 58.Leversha MA, Han J, Asgari Z, Danila DC, Lin O, Gonzalez-Espinoza R, et al. Fluorescence in situ hybridization analysis of circulating tumor cells in metastatic prostate cancer. Clin Cancer Res. 2009;15:2091–2097. doi: 10.1158/1078-0432.CCR-08-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang L, Altuwaijri S, Deng F, Chen L, Lal P, Bhanot UK, et al. NF-kappaB regulates androgen receptor expression and prostate cancer growth. Am J Pathol. 2009;175:489–499. doi: 10.2353/ajpath.2009.080727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma A, Yeow WS, Ertel A, Coleman I, Clegg N, Thangavel C, et al. The retinoblastoma tumor suppressor controls androgen signaling and human prostate cancer progression. J Clin Invest. 2010;120:4478–4492. doi: 10.1172/JCI44239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin PC, Chiu YL, Banerjee S, Park K, Mosquera JM, Giannopoulou E, et al. Epigenetic repression of miR-31 disrupts androgen receptor homeostasis and contributes to prostate cancer progression. Cancer Res. 2013;73:1232–1244. doi: 10.1158/0008-5472.CAN-12-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wiren KM, Zhang X, Chang C, Keenan E, Orwoll ES. Transcriptional up-regulation of the human androgen receptor by androgen in bone cells. Endocrinology. 1997;138:2291–2300. doi: 10.1210/endo.138.6.5163. [DOI] [PubMed] [Google Scholar]
- 63.Wolf DA, Herzinger T, Hermeking H, Blaschke D, Horz W. Transcriptional and posttranscriptional regulation of human androgen receptor expression by androgen. Mol Endocrinol. 1993;7:924–936. doi: 10.1210/mend.7.7.8413317. [DOI] [PubMed] [Google Scholar]
- 64.Grad JM, Dai JL, Wu S, Burnstein KL. Multiple androgen response elements and a Myc consensus site in the androgen receptor (AR) coding region are involved in androgen-mediated up-regulation of AR messenger RNA. Mol Endocrinol. 1999;13:1896–1911. doi: 10.1210/mend.13.11.0369. [DOI] [PubMed] [Google Scholar]
- 65.Grad JM, Lyons LS, Robins DM, Burnstein KL. The androgen receptor (AR) amino-terminus imposes androgen-specific regulation of AR gene expression via an exonic enhancer. Endocrinology. 2001;142:1107–1116. doi: 10.1210/endo.142.3.8049. [DOI] [PubMed] [Google Scholar]
- 66.Waltering KK, Helenius MA, Sahu B, Manni V, Linja MJ, Janne OA, et al. Increased expression of androgen receptor sensitizes prostate cancer cells to low levels of androgens. Cancer Res. 2009;69:8141–8149. doi: 10.1158/0008-5472.CAN-09-0919. [DOI] [PubMed] [Google Scholar]
- 67.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 68.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469–5477. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120:2715–2730. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Watson PA, Chen YF, Balbas MD, Wongvipat J, Socci ND, Viale A, et al. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci USA. 2010;107:16759–16765. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Y, Alsagabi M, Fan D, Bova GS, Tewfik AH, Dehm SM. Intragenic rearrangement and altered RNA splicing of the androgen receptor in a cell-based model of prostate cancer progression. Cancer Res. 2011;71:2108–2117. doi: 10.1158/0008-5472.CAN-10-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu LL, Xie N, Sun S, Plymate S, Mostaghel E, Dong X. Mechanisms of the androgen receptor splicing in prostate cancer cells. Oncogene. doi: 10.1038/onc.2013.284. (e-pub ahead of print 15 July 2013; doi: 10.1038/onc.2013.284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luo J, Pienta KJ. Words of wisdom: re: androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Eur Urol. 2013;64:339–340. doi: 10.1016/j.eururo.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 76.Plymate SR, Luo J. The expression signature of androgen receptor splice variants and their distinctive transcriptional activities in castration-resistant prostate cancer. In: Zhou W, editor. Androgen-Responsive Genes in Prostate Cancer. Springer; New York, NY, USA: 2013. [Google Scholar]
- 77.Hu R, Lu C, Mostaghel EA, Yegnasubramanian S, Gurel M, Tannahill C, et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012;72:3457–3462. doi: 10.1158/0008-5472.CAN-11-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KA, Dehm SM. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013;73:483–489. doi: 10.1158/0008-5472.CAN-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res. 2011;17:5913–5925. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Verras M, Lee J, Xue H, Li TH, Wang Y, Sun Z. The androgen receptor negatively regulates the expression of c-Met: implications for a novel mechanism of prostate cancer progression. Cancer Res. 2007;67:967–975. doi: 10.1158/0008-5472.CAN-06-3552. [DOI] [PubMed] [Google Scholar]
- 81.Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575–586. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu JD, Haugk K, Woodke L, Nelson P, Coleman I, Plymate SR. Interaction of IGF signaling and the androgen receptor in prostate cancer progression. J Cell Biochem. 2006;99:392–401. doi: 10.1002/jcb.20929. [DOI] [PubMed] [Google Scholar]
- 83.Signoretti S, Montironi R, Manola J, Altimari A, Tam C, Bubley G, et al. Her-2-neu expression and progression toward androgen independence in human prostate cancer. J Natl Cancer Inst. 2000;92:1918–1925. doi: 10.1093/jnci/92.23.1918. [DOI] [PubMed] [Google Scholar]
- 84.Di Lorenzo G, Tortora G, D'Armiento FP, De Rosa G, Staibano S, Autorino R, et al. Expression of epidermal growth factor receptor correlates with disease relapse and progression to androgen-independence in human prostate cancer. Clin Cancer Res. 2002;8:3438–3444. [PubMed] [Google Scholar]
- 85.Reid AH, Attard G, Ambroisine L, Fisher G, Kovacs G, Brewer D, et al. Molecular characterisation of ERG, ETV1 and PTEN gene loci identifies patients at low and high risk of death from prostate cancer. Br J Cancer. 2010;102:678–684. doi: 10.1038/sj.bjc.6605554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Robbins CM, Tembe WA, Baker A, Sinari S, Moses TY, Beckstrom-Sternberg S, et al. Copy number and targeted mutational analysis reveals novel somatic events in metastatic prostate tumors. Genome Res. 2011;21:47–55. doi: 10.1101/gr.107961.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berger MF, Lawrence MS, Demichelis F, Drier Y, Cibulskis K, Sivachenko AY, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470:214–220. doi: 10.1038/nature09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thomas C, Lamoureux F, Crafter C, Davies BR, Beraldi E, Fazli L, et al. Synergistic targeting of PI3K/AKT pathway and androgen receptor axis significantly delays castration-resistant prostate cancer progression in vivo. Mol Cancer Ther. 2013;12:2342–2355. doi: 10.1158/1535-7163.MCT-13-0032. [DOI] [PubMed] [Google Scholar]
- 90.Chmelar R, Buchanan G, Need EF, Tilley W, Greenberg NM. Androgen receptor coregulators and their involvement in the development and progression of prostate cancer. Int J Cancer. 2007;120:719–733. doi: 10.1002/ijc.22365. [DOI] [PubMed] [Google Scholar]
- 91.Jozwik KM, Carroll JS. Pioneer factors in hormone-dependent cancers. Nat Rev Cancer. 2012;12:381–385. doi: 10.1038/nrc3263. [DOI] [PubMed] [Google Scholar]
- 92.Barbieri CE, Baca SC, Lawrence MS, Demichelis F, Blattner M, Theurillat JP, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet. 2012;44:685–689. doi: 10.1038/ng.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Geng C, He B, Xu L, Barbieri CE, Eedunuri VK, Chew SA, et al. Prostate cancer-associated mutations in speckle-type POZ protein (SPOP) regulate steroid receptor coactivator 3 protein turnover. Proc Natl Acad Sci USA. 2013;110:6997–7002. doi: 10.1073/pnas.1304502110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coffey K, Robson CN. Regulation of the androgen receptor by post-translational modifications. J Endocrinol. 2012;215:221–237. doi: 10.1530/JOE-12-0238. [DOI] [PubMed] [Google Scholar]
- 95.Lamont KR, Tindall DJ. Minireview: Alternative activation pathways for the androgen receptor in prostate cancer. Mol Endocrinol. 2011;25:897–907. doi: 10.1210/me.2010-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shu SK, Liu Q, Coppola D, Cheng JQ. Phosphorylation and activation of androgen receptor by Aurora-A. J Biol Chem. 2010;285:33045–33053. doi: 10.1074/jbc.M110.121129. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 97.Chen S, Xu Y, Yuan X, Bubley GJ, Balk SP. Androgen receptor phosphorylation and stabilization in prostate cancer by cyclin-dependent kinase 1. Proc Natl Acad Sci USA. 2006;103:15969–15974. doi: 10.1073/pnas.0604193103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Willder JM, Heng SJ, McCall P, Adams CE, Tannahill C, Fyffe G, et al. Androgen receptor phosphorylation at serine 515 by Cdk1 predicts biochemical relapse in prostate cancer patients. Br J Cancer. 2013;108:139–148. doi: 10.1038/bjc.2012.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ha S, Iqbal NJ, Mita P, Ruoff R, Gerald WL, Lepor H, et al. Phosphorylation of the androgen receptor by PIM1 in hormone refractory prostate cancer. Oncogene. 2012;32:3992–4000. doi: 10.1038/onc.2012.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guo Z, Dai B, Jiang T, Xu K, Xie Y, Kim O, et al. Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell. 2006;10:309–319. doi: 10.1016/j.ccr.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 101.Mahajan NP, Liu Y, Majumder S, Warren MR, Parker CE, Mohler JL, et al. Activated Cdc42-associated kinase Ack1 promotes prostate cancer progression via androgen receptor tyrosine phosphorylation. Proc Natl Acad Sci USA. 2007;104:8438–8443. doi: 10.1073/pnas.0700420104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu Y, Karaca M, Zhang Z, Gioeli D, Earp HS, Whang YE. Dasatinib inhibits site-specific tyrosine phosphorylation of androgen receptor by Ack1 and Src kinases. Oncogene. 2010;29:3208–3216. doi: 10.1038/onc.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 104.Yap TA, Sandhu SK, Carden CP, de Bono JS. Poly(ADP-ribose) polymerase (PARP) inhibitors: Exploiting a synthetic lethal strategy in the clinic. CA Cancer J Clin. 2011;61:31–49. doi: 10.3322/caac.20095. [DOI] [PubMed] [Google Scholar]
- 105.Sandhu SK, Omlin A, Hylands L, Miranda S, Barber LJ, Riisnaes R, et al. Poly (ADP-ribose) polymerase (PARP) inhibitors for the treatment of advanced germline BRCA2 mutant prostate cancer. Ann Oncol. 2013;24:1416–1418. doi: 10.1093/annonc/mdt074. [DOI] [PubMed] [Google Scholar]
- 106.Kraus WL. Transcriptional control by PARP-1: chromatin modulation, enhancer-binding, coregulation, and insulation. Curr Opin Cell Biol. 2008;20:294–302. doi: 10.1016/j.ceb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schiewer MJ, Goodwin JF, Han S, Brenner JC, Augello MA, Dean JL, et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2012;2:1134–1149. doi: 10.1158/2159-8290.CD-12-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brenner JC, Ateeq B, Li Y, Yocum AK, Cao Q, Asangani IA, et al. Mechanistic rationale for inhibition of poly(ADP-ribose) polymerase in ETS gene fusion-positive prostate cancer. Cancer Cell. 2011;19:664–678. doi: 10.1016/j.ccr.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beltran H. DNA mismatch repair in prostate cancer. J Clin Oncol. 2013;31:1782–1784. doi: 10.1200/JCO.2012.48.4667. [DOI] [PubMed] [Google Scholar]
- 110.Montgomery B, Cheng HH, Drechsler J, Mostaghel EA. Glucocorticoids and prostate cancer treatment: friend or foe? Asian J Androl. doi: 10.4103/1008-682X.125392. (e-pub ahead of print 7 March 2014; doi: 10.4103/1008-682X.125392) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Szmulewitz RZ, Chung E, Al-Ahmadie H, Daniel S, Kocherginsky M, Razmaria A, et al. Serum/glucocorticoid-regulated kinase 1 expression in primary human prostate cancers. Prostate. 2012;72:157–164. doi: 10.1002/pros.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sahu B, Laakso M, Pihlajamaa P, Ovaska K, Sinielnikov I, Hautaniemi S, et al. FoxA1 specifies unique androgen and glucocorticoid receptor binding events in prostate cancer cells. Cancer Res. 2013;73:1570–1580. doi: 10.1158/0008-5472.CAN-12-2350. [DOI] [PubMed] [Google Scholar]
- 113.Arora VK, Schenkein E, Murali R, Subudhi SK, Wongvipat J, Balbas MD, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155:1309–1322. doi: 10.1016/j.cell.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brinkmann AO, Faber PW, van Rooij HC, Kuiper GG, Ris C, Klaassen P, et al. The human androgen receptor: domain structure, genomic organization and regulation of expression. J Steroid Biochem. 1989;34:307–310. doi: 10.1016/0022-4731(89)90098-8. [DOI] [PubMed] [Google Scholar]
- 115.Laudet V, Hanni C, Coll J, Catzeflis F, Stehelin D. Evolution of the nuclear receptor gene superfamily. EMBO J. 1992;11:1003–1013. doi: 10.1002/j.1460-2075.1992.tb05139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barrie SE, Potter GA, Goddard PM, Haynes BP, Dowsett M, Jarman M. Pharmacology of novel steroidal inhibitors of cytochrome P450(17) alpha (17 alpha-hydroxylase/C17-20 lyase). J Steroid Biochem Mol Biol. 1994;50:267–273. doi: 10.1016/0960-0760(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 117.Barrie SE, Haynes BP, Potter GA, Chan FC, Goddard PM, Dowsett M, et al. Biochemistry and pharmacokinetics of potent non-steroidal cytochrome P450 (17alpha) inhibitors. J Steroid Biochem Mol Biol. 1997;60:347–351. doi: 10.1016/s0960-0760(96)00225-7. [DOI] [PubMed] [Google Scholar]
- 118.Potter GA, Barrie SE, Jarman M, Rowlands MG. Novel steroidal inhibitors of human cytochrome P45017 alpha (17 alpha-hydroxylase-C17,20-lyase): potential agents for the treatment of prostatic cancer. J Med Chem. 1995;38:2463–2471. doi: 10.1021/jm00013a022. [DOI] [PubMed] [Google Scholar]
- 119.O'Donnell AI, Judson I, Dowsett M, Dowsett M, Raynaud F, Dearnaley D, et al. Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer. 2004;90:2317–2325. doi: 10.1038/sj.bjc.6601879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ferraldeschi R, de Bono J. Agents that target androgen synthesis in castration-resistant prostate cancer. Cancer J. 2013;19:34–42. doi: 10.1097/PPO.0b013e31827e0b6f. [DOI] [PubMed] [Google Scholar]
- 121.Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 122.Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–992. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 123.Logothetis CJ, Basch E, Molina A, Fizazi K, North SA, Chi KN, et al. Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: exploratory analysis of data from the COU-AA-301 randomised trial. Lancet Oncol. 2012;13:1210–1217. doi: 10.1016/S1470-2045(12)70473-4. [DOI] [PubMed] [Google Scholar]
- 124.Kaku T, Hitaka T, Ojida A, Matsunaga N, Adachi M, Tanaka T, et al. Discovery of orteronel (TAK-700), a naphthylmethylimidazole derivative, as a highly selective 17,20-lyase inhibitor with potential utility in the treatment of prostate cancer. Bioorg Med Chem. 2011;19:6383–6399. doi: 10.1016/j.bmc.2011.08.066. [DOI] [PubMed] [Google Scholar]
- 125.Handratta VD, Vasaitis TS, Njar VC, Gediya LK, Kataria R, Chopra P, et al. Novel C-17-heteroaryl steroidal CYP17 inhibitors/antiandrogens: synthesis, in vitro biological activity, pharmacokinetics, and antitumor activity in the LAPC4 human prostate cancer xenograft model. J Med Chem. 2005;48:2972–2984. doi: 10.1021/jm040202w. [DOI] [PubMed] [Google Scholar]
- 126.Eisner JR, Abbott DH, Bird IM, Rafferty SW, Moore MR, Schotzinger RJ. VT-464: A novel, selective inhibitor of P450c17(CYP17)-17,20 lyase for castration-refractory prostate cancer (CRPC). J Clin Oncol. 2012;30 abstr e15167. [Google Scholar]
- 127.Kikuchi A, Enjo K, Furutani T, Azami H, Nimi T, Kuromitsu S, et al. ASP9521, a novel, selective, orally bioavailable AKR1C3 (type 5, 17ß-hydroxysteroid dehydrogenase) inhibitor: In vitro and in vivo characterization. J Clin Oncol. 2013;31 doi: 10.1007/s10637-014-0130-5. suppl; abstr 5046. [DOI] [PubMed] [Google Scholar]
- 128.Loriot Y, Bianchini D, Ileana E, Sandhu S, Patrikidou A, Pezaro C, et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100). Ann Oncol. 2013;24:1807–1812. doi: 10.1093/annonc/mdt136. [DOI] [PubMed] [Google Scholar]
- 129.Bohl CE, Gao W, Miller DD, Bell CE, Dalton JT. Structural basis for antagonism and resistance of bicalutamide in prostate cancer. Proc Natl Acad Sci USA. 2005;102:6201–6206. doi: 10.1073/pnas.0500381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jarman M, Barrie SE, Llera JM. The 16,17-double bond is needed for irreversible inhibition of human cytochrome p45017alpha by abiraterone (17-(3-pyridyl) androsta-5, 16-dien-3beta-ol) and related steroidal inhibitors. J Med Chem. 1998;41:5375–5381. doi: 10.1021/jm981017j. [DOI] [PubMed] [Google Scholar]
- 131.Osguthorpe DJ, Hagler AT. Mechanism of androgen receptor antagonism by bicalutamide in the treatment of prostate cancer. Biochemistry. 2011;50:4105–4113. doi: 10.1021/bi102059z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kolvenbag GJ, Furr BJ, Blackledge GR. Receptor affinity and potency of nonsteroidal antiandrogens: translation of preclinical findings into clinical activity. Prostate Cancer Prostatic Dis. 1998;1:307–314. doi: 10.1038/sj.pcan.4500262. [DOI] [PubMed] [Google Scholar]
- 133.Simard J, Singh SM, Labrie F. Comparison of in vitro effects of the pure anti-androgens OH-flutamide, Casodex, and nilutamide on androgen-sensitive parameters. Urology. 1997;49:580–586. doi: 10.1016/s0090-4295(97)00029-0. discussion 586-589. [DOI] [PubMed] [Google Scholar]
- 134.Jung ME, Ouk S, Yoo D, Sawyers CL, Chen C, Tran C, et al. Structure-activity relationship for thiohydantoin androgen receptor antagonists for castration-resistant prostate cancer (CRPC). J Med Chem. 2010;53:2779–2796. doi: 10.1021/jm901488g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet. 2010;375:1437–1446. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Beer TM, Sternberg CN, Higano CS, Iversen P, Loriot Y, Rathkopf DE, et al. Enzalutamide in men with chemotherapy-naive metastatic prostate cancer (mCRPC): Results of phase III PREVAIL study. J Clin Oncol. 2014;32(suppl 4) abstr LBA1. [Google Scholar]
- 137.Clegg NJ, Wongvipat J, Joseph JD, Tran C, Ouk S, Dilhas A, et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 2012;72:1494–1503. doi: 10.1158/0008-5472.CAN-11-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rathkopf DE, Morris MJ, Fox JJ, Danila DC, Slovin SF, Hager JH, et al. Phase I study of ARN-509, a novel antiandrogen, in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2013;31:3525–3530. doi: 10.1200/JCO.2013.50.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Smith MR, Antonarakis ES, Ryan CJ, Berry W, Shore ND, Liu G, et al. Arn-509 in Men with High Risk Non-Metastatic Castration-Resistant Prostate Cancer. Ann Oncol. 2012;23:303–303. [Google Scholar]
- 140.Fizazi K, Massard C, James ND, Culine S, Jones RH, Oksala R, et al. ODM-201, a new generation androgen receptor inhibitor for castration-resistant prostate cancer: preclinical and phase I data. J Clin Oncol. 2013;31(Suppl 6) abstr 65. [Google Scholar]
- 141.Massard C, James N, Culine S, Jones R, Vuorela A, Mustonen M, et al. ARADES trial: A first-in-man, open-label, phase I/II safety, pharmacokinetic, and proof-of-concept study ofODM-201 in patients (pts) with progressive metastatic castration-resistant prostate cancer (mCRPC). Presented at the 2012 ESMO Congress; Vienna, Austria Abstract LBA25_PR. 2012 [Google Scholar]
- 142.Lavery DN, McEwan J. Functional characterization of the native NH2-terminal transactivation domain of the human androgen receptor: binding kinetics for interactions with TFIIF and SRC-1a. Biochemistry. 2008;47:3352–3359. doi: 10.1021/bi702220p. [DOI] [PubMed] [Google Scholar]
- 143.Lavery DN, McEwan IJ. Structural characterization of the native NH2-terminal transactivation domain of the human androgen receptor: a collapsed disordered conformation underlies structural plasticity and protein-induced folding. Biochemistry. 2008;47:3360–3369. doi: 10.1021/bi702221e. [DOI] [PubMed] [Google Scholar]
- 144.Andersen RJ, Mawji NR, Wang J, Wang G, Haile S, Myung JK, et al. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell. 2010;17:535–546. doi: 10.1016/j.ccr.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 145.Zhang Y, Castaneda S, Dumble M, Wang M, Mileski M, Qu Z, et al. Reduced expression of the androgen receptor by third generation of antisense shows antitumor activity in models of prostate cancer. Mol Cancer Ther. 2011;10:2309–2319. doi: 10.1158/1535-7163.MCT-11-0329. [DOI] [PubMed] [Google Scholar]
- 146.Bianchini D, Omlin A, Pezaro C, Lorente D, Ferraldeschi R, Mukherji D, et al. First-in-human Phase I study of EZN-4176, a locked nucleic acid antisense oligonucleotide to exon 4 of the androgen receptor mRNA in patients with castration-resistant prostate cancer. Br J Cancer. 2013;109:2579–2586. doi: 10.1038/bjc.2013.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ravindranathan P, Lee TK, Yang L, Centenera MM, Butler L, Tilley WD, et al. Peptidomimetic targeting of critical androgen receptor-coregulator interactions in prostate cancer. Nat Commun. 2013;4:1923. doi: 10.1038/ncomms2912. [DOI] [PubMed] [Google Scholar]
- 148.Pacey S, Wilson RH, Walton M, Eatock MM, Hardcastle A, Zetterlund A, et al. A phase I study of the heat shock protein 90 inhibitor alvespimycin (17-DMAG) given intravenously to patients with advanced solid tumors. Clin Cancer Res. 2011;17:1561–1570. doi: 10.1158/1078-0432.CCR-10-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Heath EI, Stein MN, Vaishampayan UN, Antonarakis ES, Liu G, Sheng S, et al. Phase II trial of single-agent ganetespib (STA-9090), a heat shock protein 90 (Hsp90) inhibitor in heavily pretreated patients with metastatic castration-resistant prostate cancer (mCRPC) post docetaxel-based chemotherapy: Results of a Prostate Cancer Clinical Trials Consortium (PCCTC) study. J Clin Oncol. 2013;31(suppl) abstr 5085. [Google Scholar]
- 150.Gibbs A, Schwartzman J, Deng V, Alumkal J. Sulforaphane destabilizes the androgen receptor in prostate cancer cells by inactivating histone deacetylase 6. Proc Natl Acad Sci USA. 2009;106:16663–16668. doi: 10.1073/pnas.0908908106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sandhu SK, Schelman WR, Wilding G, Moreno V, Baird RD, Miranda S, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;14:882–892. doi: 10.1016/S1470-2045(13)70240-7. [DOI] [PubMed] [Google Scholar]
- 152.Jiang X, Chen S, Asara JM, Balk SP. Phosphoinositide 3-kinase pathway activation in phosphate and tensin homolog (PTEN)-deficient prostate cancer cells is independent of receptor tyrosine kinases and mediated by the p110beta and p110delta catalytic subunits. J Biol Chem. 2010;285:14980–14989. doi: 10.1074/jbc.M109.085696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ni J, Liu Q, Xie S, Carlson C, Von T, Vogel K, et al. Functional characterization of an isoform-selective inhibitor of PI3K-p110beta as a potential anticancer agent. Cancer Discov. 2012;2:425–433. doi: 10.1158/2159-8290.CD-12-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhu Q, Youn H, Tang J, Tawfik O, Dennis K, Terranova PF, et al. Phosphoinositide 3-OH kinase p85alpha and p110beta are essential for androgen receptor transactivation and tumor progression in prostate cancers. Oncogene. 2008;27:4569–4579. doi: 10.1038/onc.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cariaga-Martinez AE, Lopez-Ruiz P, Nombela-Blanco MP, Motino O, Gonzalez-Corpas A, Rodriguez-Ubreva J, et al. Distinct and specific roles of AKT1 and AKT2 in androgen-sensitive and androgen-independent prostate cancer cells. Cell Signal. 2013;25:1586–1597. doi: 10.1016/j.cellsig.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 156.Rhodes N, Heerding DA, Duckett DR, Eberwein DJ, Knick VB, Lansing TJ, et al. Characterization of an Akt kinase inhibitor with potent pharmacodynamic and antitumor activity. Cancer Res. 2008;68:2366–2374. doi: 10.1158/0008-5472.CAN-07-5783. [DOI] [PubMed] [Google Scholar]
- 157.Smith DC, Smith MR, Sweeney C, Elfiky AA, Logothetis C, Corn PG, et al. Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J Clin Oncol. 2013;31:412–419. doi: 10.1200/JCO.2012.45.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Michaelson MD, Oudard S, Ou YC, Sengelov L, Saad F, Houede N, et al. Randomized, Placebo-Controlled, Phase III Trial of Sunitinib Plus Prednisone Versus Prednisone Alone in Progressive, Metastatic, Castration-Resistant Prostate Cancer. J Clin Oncol. 2014;32:76–82. doi: 10.1200/JCO.2012.48.5268. [DOI] [PubMed] [Google Scholar]
- 159.Araujo JC, Trudel GC, Saad F, Armstrong AJ, Yu EY, Bellmunt J, et al. Docetaxel and dasatinib or placebo in men with metastatic castration-resistant prostate cancer (READY): a randomised, double-blind phase 3 trial. Lancet Oncol. 2013;14:1307–1316. doi: 10.1016/S1470-2045(13)70479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yu EY, Wilding G, Posadas E, Gross M, Culine S, Massard C, et al. Phase II study of dasatinib in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. 2009;15:7421–7428. doi: 10.1158/1078-0432.CCR-09-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sonpavde G, Periman PO, Bernold D, Weckstein D, Fleming MT, Galsky MD, et al. Sunitinib malate for metastatic castration-resistant prostate cancer following docetaxel-based chemotherapy. Ann Oncol. 2010;21:319–324. doi: 10.1093/annonc/mdp323. [DOI] [PubMed] [Google Scholar]
- 162.Taplin ME, Montgomery RB, Logothetis C, Bubley GJ, Richie JP, Dalkin BL, et al. Effect of neoadjuvant abiraterone acetate (AA) plus leuprolide acetate (LHRHa) on PSA, pathological complete response (pCR), and near pCR in localized high-risk prostate cancer (LHRPC): Results of a randomized phase II study. J Clin Oncol. 2012;30(suppl) doi: 10.1200/JCO.2013.53.4578. abstr 4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tombal B, Borre M, Rathenborg P, Werbrouck P, Poppel HV, Heidenreich A, et al. Enzalutamide monotherapy: Extended follow-up of a phase II study in hormone-naive prostate cancer patients. J Clin Oncol. 2014;32(suppl 4) abstr 62. [Google Scholar]
- 164.Cai C, Chen S, Ng P, Bubley GJ, Nelson PS, Mostaghel EA, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011;71:6503–6513. doi: 10.1158/0008-5472.CAN-11-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yu Z, Chen S, Sowalsky AG, Voznesensky O, Mostaghel EA, Nelson PS, et al. Rapid Induction of Androgen Receptor Splice Variants by Androgen Deprivation in Prostate Cancer. Clin Cancer Res. 2014;20:1590–1600. doi: 10.1158/1078-0432.CCR-13-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Richards J, Lim AC, Hay CW, Taylor AE, Wingate A, Nowakowska K, et al. Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: a rationale for increasing abiraterone exposure or combining with MDV3100. Cancer Res. 2012;72:2176–2182. doi: 10.1158/0008-5472.CAN-11-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Veldscholte J, Ris-Stalpers C, Kuiper GG, Jenster G, Berrevoets C, Claassen E, et al. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem Biophys Res Commun. 1990;173:534–540. doi: 10.1016/s0006-291x(05)80067-1. [DOI] [PubMed] [Google Scholar]
- 168.Zhao XY, Malloy PJ, Krishnan AV, Swami S, Navone NM, Peehl DM, et al. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med. 2000;6:703–706. doi: 10.1038/76287. [DOI] [PubMed] [Google Scholar]
- 169.Bianchini D, Lorente D, Rodriguez-Vida A, Omlin A, Pezaro C, Ferraldeschi R, et al. Antitumour activity of enzalutamide (MDV3100) in patients with metastatic castration-resistant prostate cancer (CRPC) pre-treated with docetaxel and abiraterone. Eur J Cancer. 2014;50:78–84. doi: 10.1016/j.ejca.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 170.Schrader AJ, Boegemann M, Ohlmann CH, Schnoeller TJ, Krabbe LM, Hajili T, et al. Enzalutamide in Castration-resistant Prostate Cancer Patients Progressing After Docetaxel and Abiraterone. Eur Urol. 2013;65:30–36. doi: 10.1016/j.eururo.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 171.Noonan KL, North S, Bitting RL, Armstrong AJ, Ellard SL, Chi KN. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann Oncol. 2013;24:1802–1807. doi: 10.1093/annonc/mdt138. [DOI] [PubMed] [Google Scholar]
- 172.Mosquera JM1, Beltran H, Park K, MacDonald TY, Robinson BD, Tagawa ST, et al. Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatment-related neuroendocrine prostate cancer. Neoplasia. 2013;15:1–10. doi: 10.1593/neo.121550. [DOI] [PMC free article] [PubMed] [Google Scholar]