Abstract
In a living cell, oxidative stress resulting from an external or internal insult can result in mitochondrial DNA (mtDNA) damage and degradation. Here, we show that in HeLa cells, mtDNA can withstand relatively high levels of extracellular oxidant H2O2 before it is damaged to a point of degradation, and that mtDNA levels in these cells quickly recover after removal of the stressor. In contrast, mtDNA degradation in mouse fibroblast cells is induced at eight-fold lower concentrations of H2O2, and restoration of the lost mtDNA proceeds much slower. Importantly, mtDNA levels in HeLa cells continue to decline even after withdrawal of the stressor thus marking the “slow” mode of mtDNA degradation. Conversely, in mouse fibroblasts maximal loss of mtDNA is achieved during treatment, and is already detectable at 5 min after exposure, indicating the “fast” mode. These differences may modulate susceptibility to oxidative stress of those organs, which consist of multiple cell types.
Keywords: Circulating mtDNA, mtDNA repair, mtDNA degradation, oxidative mtDNA damage
Introduction
In mammalian cells, both the nucleus and mitochondria serve as repositories for genetic information (DNA). Mitochondrial DNA (mtDNA) is a circular DNA molecule that encodes 37 genes: 22 tRNA, 2 rRNA required for proper functioning of the mitochondria's own translational apparatus and 13 polypeptide components of respiratory complexes I, III, IV, and V, which are translated on mitochondrial ribosomes (Anderson et al., 1981).
In the cell, mitochondria perform numerous functions including, but not limited to producing the bulk of the ATP (Zu & Guppy, 2004), generating reactive oxygen species, which can be both damaging and play a role in normal cellular signaling (Alexeyev, 2009; Murphy, 2009; Al-Mehdi et al., 2012), and regulation of cell death (Carlsen, 1990; Wang & Youle, 2009). All these functions, directly or indirectly, are affected by the state of mitochondrial respiratory complexes, which in turn depends on mtDNA-encoded subunits, whose supply critically depends on mtDNA integrity. As a consequence, alterations in mtDNA integrity have been associated with various human pathologies, such as mitochondrial diseases (Schapira, 2012; Ylikallio & Suomalainen, 2012; Zheng et al., 2012), diabetes (Bannwarth et al., 2011; Maassen et al., 2005; Supale et al., 2012), cancer (Kurelac et al., 2013; Larman et al., 2012; Wallace, 2012; Yu, 2012) and neurodegenerative disorders (Milone, 2012).
While significant progress has been achieved in understanding the mechanisms involved in the maintenance of nuclear genome integrity, our understanding of corresponding mitochondrial mechanisms remains incomplete. The mitochondrial genome accumulates mutations at a faster rate than nuclear genome (Ballard & Whitlock, 2004; Brown et al., 1979; Tatarenkov & Avise, 2007). However, the exact mechanism behind this observation remains controversial. Three hypotheses have been put forward over the years: (1) the lack of “protective” histones in mitochondria; (2) a close proximity of the mtDNA to the electron transport chain, which is a major cellular source of reactive oxygen species (ROS); and (3) a smaller repertoire of DNA-repair pathways in mitochondria. However: (1) depending on the experimental conditions, histone proteins may either protect DNA from oxidative damage or promote it (Liang & Dedon, 2001; Liang et al., 1999). Also, mitochondrial nucleoid proteins have been shown to be just as “protective” as histones (Guliaeva et al., 2006); (2) a close proximity to the source of ROS may only contribute to mtDNA mutagenesis, if it is not efficiently counteracted by a combination of antioxidant defenses and DNA repair. Also, actual rates of mitochondrial ROS production in vivo remain controversial (Alexeyev, 2009); (3) while the full complement of mitochondrial DNA repair pathways remains to be elucidated, there is evidence for the presence of many nuclear pathways in mitochondria (Alexeyev et al., 2013; Gredilla et al., 2010; Kazak et al., 2012; Liu & Demple, 2010). Mitochondria are proficient in Base Excision Repair, the main pathway for the repair of oxidative base lesions and single-strand breaks, and at least one oxidative DNA lesion, 8-oxoguanine, is repaired more efficiently in mitochondria than it is in the nucleus (Thorslund et al., 2002). Moreover, mitochondria possess a unique mechanism for the degradation of damaged mtDNA molecules, which co-exists with DNA repair and may be activated by excessive mtDNA damage (Furda et al., 2012; Shokolenko et al., 2009, 2013b). This pathway, together with the high-redundancy of organellar genomes may enable effective management of even relatively high levels of mtDNA damage in both mitochondria and chloroplasts (Bendich, 2013).
Recently, we have demonstrated that in several cell lines of epithelial origin, mtDNA degradation coincides with repair and occurs predominantly after withdrawal of the stressor during the recovery phase (Shokolenko et al., 2009). mtDNA degradation is of particular interest because it may contribute to both the etiology of mtDNA depletion syndromes (Clay Montier et al., 2009; Rotig & Poulton, 2009) and to the activation of the innate immune system by circulating mtDNA (Oka et al., 2012; Zhang et al., 2010). Here, we investigated mtDNA degradation patterns in mouse fibroblasts and HeLa cells, and report that among the studied cell lines, fibroblasts are more sensitive to hydrogen peroxide (H2O2)-induced damage, that mtDNA degradation in these cells proceeds faster, and that mtDNA degradation process in these cells is largely completed during 30 min treatment with the stressor.
Methods
Cell lines, media and treatments
Unless specified otherwise, all cells were grown in Dulbecco's Modified Eagle Medium (DMEM) containing 10% Fetal Bovine Serum, 50 μg/ml gentamycin, 50 μg/ml uridine, and 1 mM sodium pyruvate in a humidified atmosphere containing 5% CO2 at 37 ° C. Cells were treated with H2O2 in Hank's Balanced Salt Solution (HBSS) under the same conditions. HeLa (cervical epithelial cell line) and L929 (areolar connective tissue cell line) were from laboratory collection. SV40 large T-antigen immortalized mouse embryonic fibroblast (MEF) cell lines Cre2 and 4B6 were derived in our lab (Shokolenko et al., 2013a), and 92TAg (Sobol et al., 2003) was kindly provided by Dr. R. Sobol.
Quantitative Southern Blotting
Quantitative Southern Blotting under non-denaturing conditions (QSBN) was performed as described earlier (Shokolenko et al., 2009), except mouse total DNA was digested with EcoRI. When blotting BamHI-digested total human DNA, the membrane was cut at the level of the 9 kb band of lambda/HindIII marker after transfer. The upper portion was then hybridized with the mtDNA probe (detects 16,569 bp fragment), and the lower portion was hybridized with the 18S rDNA probe (5102 bp fragment). Similarly, for mouse DNA the membrane was cut at the same level, and the upper portion was hybridized with a probe encompassing 6615–8053 bp of the mouse mtDNA (GenBank NC006914, detects 14,037 bp fragment), while the lower portion was hybridized with rDNA probe encompassing 12,949–13,738 bp of mouse rDNA (GenBank GU372691, detects 6627 bp fragment). After hybridization, membranes were exposed to an imaging screen to measure band intensities. The number of pixels per band was determined by encompassing bands with identical rectangular regions of interest and subtracting the background. It is important to note that both nuclear DNA (nDNA) and mtDNA are subjected to oxidative damage with H2O2, and therefore nDNA can not serve as true loading control in these experiments. However, it has been reported that nDNA is less susceptible to oxidative damage (Shokolenko et al., 2009; Yakes & Van Houten, 1997), and therefore it can serve as a useful reference in Southern hybridizations of oxidatively damaged total cellular DNA.
The percent mtDNA remaining was determined by means of QSBN as % = T/C*100%, where C is intensity of the band in the control lane and T is intensity of the band in the lane corresponding to a given time point.
Western blotting
Protein extracts from treated and control cells were prepared using lysis solution containing 10 mM Tris-HCl, 1% SDS, 1 × EDTA-free protease inhibitor cocktail (Roche, Indianapolis, IN). Protein concentrations were measured using the BCA assay (Pierce, Rockford, IL). Proteins were separated by PAAG electrophoresis and transferred to PVDF membranes, blocked and incubated with primary and secondary antibodies using standard techniques (Sambrook & Russel, 2001). Blots were developed with SuperSignal West Pico and exposed to CL-Xposure film (both Pierce). Primary antibodies were α-myc tag (Cell Signaling), α-HSP60 (mitochondrial, BD Biosciences).
Microscopy
For phase contrast imaging, cells were plated into 35 mm glass bottom MaTek dishes, allowed to attach overnight, and imaged with a Nikon TE200U microscope (10× objective).
Results
The extent and pattern of mtDNA degradation in HeLa cells depends on the level of oxidative stress
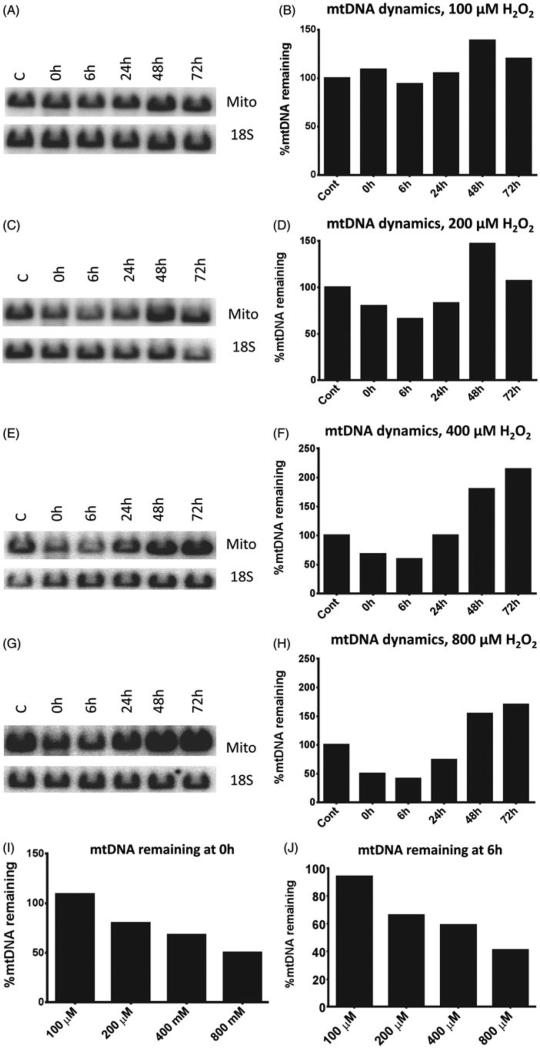
Our previous studies indicate that in the human adenocarcinoma cell line HCT116 (epithelial origin) the mtDNA was largely (>90%) preserved during a 30-min treatment with exogenous H2O2 despite the fact that during this period a substantial amount of lesions was accumulated. Instead, mtDNA degradation occurred after H2O2 withdrawal, during the recovery period (Shokolenko et al., 2009). During this period, both mtDNA repair and degradation were observed to occur simultaneously. In contrast, when oxidative stress was induced by the generation of H2O2 through enzymatic oxidation of hypoxanthine with xanthine oxidase, a substantial loss of mtDNA was observed at the end of the treatment (Shokolenko et al., 2009). One possible explanation for this discrepancy is in the dynamics of the H2O2 concentrations. When cells were exposed to pure H2O2, its concentration steadily decreased over the time, so that at the end of treatment most of the H2O2 has decomposed (Yakes & Van Houten, 1997). In contrast, when H2O2 was generated enzymatically, its concentration in the treatment medium rose steadily and was the highest at the end of treatment. This suggests that the pattern of mtDNA degradation may depend on the concentration of H2O2. To directly test this hypothesis, we exposed HeLa cells to different concentrations of H2O2 for 30 min, and followed mtDNA content in the exposed cells for up to 3 days after withdrawal. At the lowest concentration of H2O2 used, 100 μM, no mtDNA degradation was observed (Figure 1A and B), whereas higher concentrations led to a substantial mtDNA loss immediately after the treatment. This loss became more prominent after 6 h recovery period (Figure 1C–H). Importantly, there was a clear dose response in mtDNA degradation as measured both immediately after the treatment and after a 6 h recovery period (Figure 1I and J). Interestingly, at all concentrations of H2O2 used, a substantial increase in mtDNA content was observed in treated cells as compared to control at 48 h after the treatment. This increase became even more prominent at 72 h after the treatment when cells were treated with 40 μM or 800 μM of H2O2 (Figure 1F and H). This increase is reminiscent of the “overshoot repair” reported by us previously (Driggers et al., 1997).
Figure 1.
mtDNA degradation in HeLa cells. HeLa cells were exposed to indicated concentrations of H2O2 for 30 min in HBSS as described in “Methods” Section, and mtDNA degradation and recovery were assessed over 72 h period by QSBN. Mito, mtDNA band; 18S, 18S (nDNA) band. A, C, E, and G, representative gel images; B, D, F, and H, quantitation of band intensities. I, per cent of mtDNA remaining after 30 min treatment with various concentrations of H2O2. J, the same as I, except after 6 h recovery from H2O2 treatment. Note direct correlation between H2O2 concentration and the extent of mtDNA degradation.
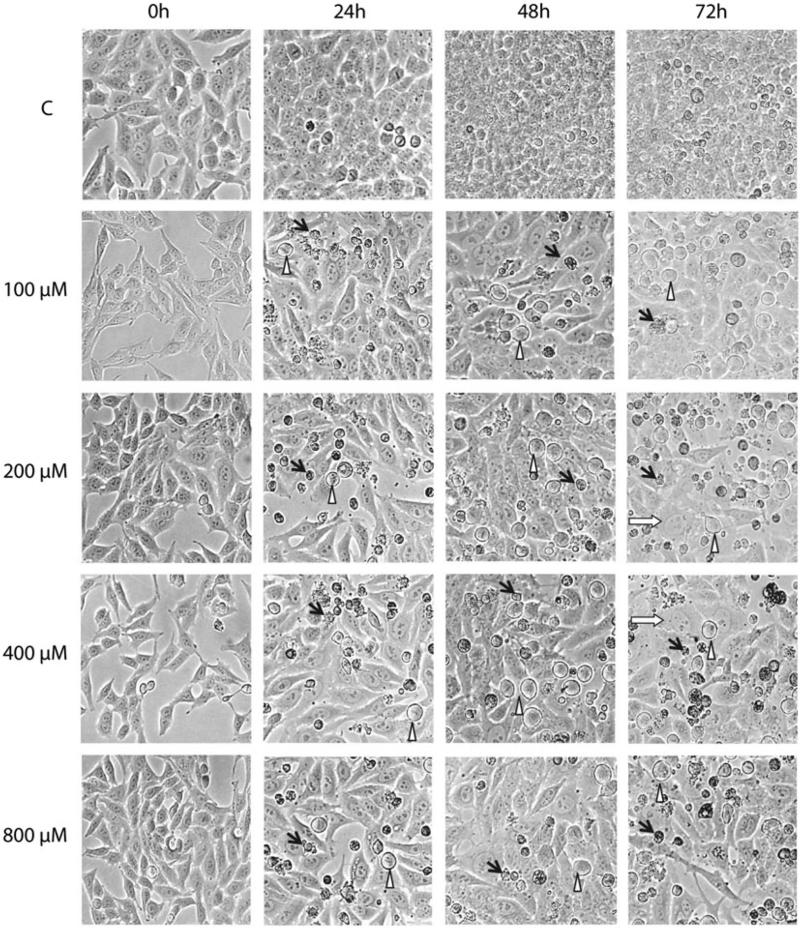
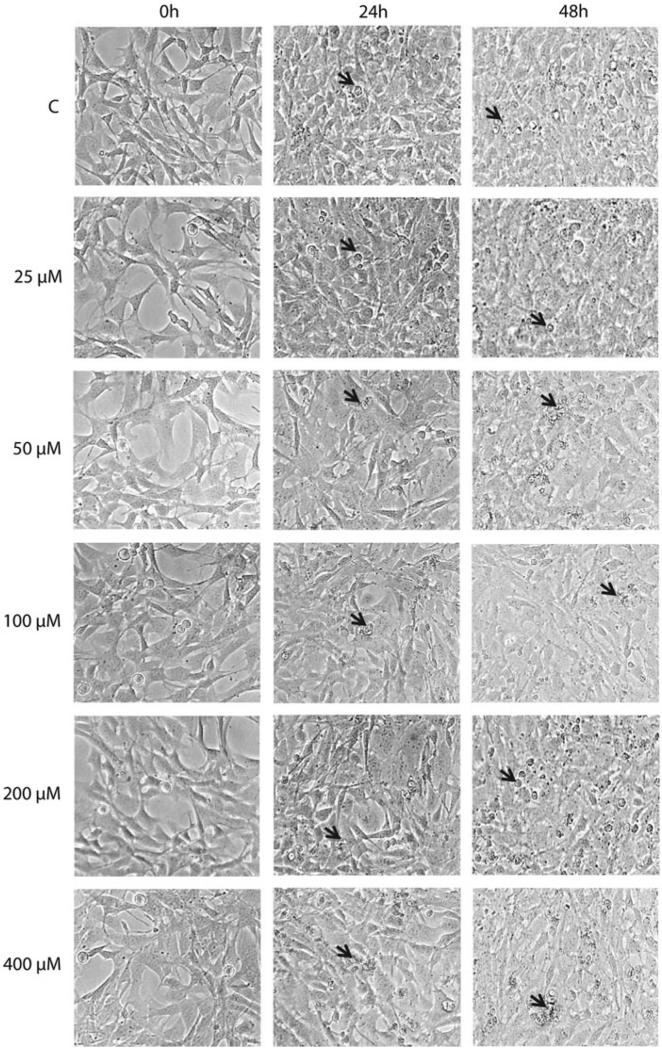
The exposure of HeLa cells to H2O2 was accompanied by growth arrest, cell swelling (especially at 400 and 800 μM H2O2), cell death and detachment (Figure 2). The detachment was most profound at 60–90 h after the treatment.
Figure 2.
Morphological changes in HeLa cells following oxidative stress. HeLa cells were treated with the indicated concentrations of H2O2 for 30 min in HBSS as described in “Methods” Section, and morphological changes in population were followed with phase contrast microscopy. Black arrows, detached apoptotic cells; open triangles, growth arrested cells; white arrows, swollen cells.
mtDNA in mouse fibroblast cell lines is highly susceptible to oxidative stress-induced degradation
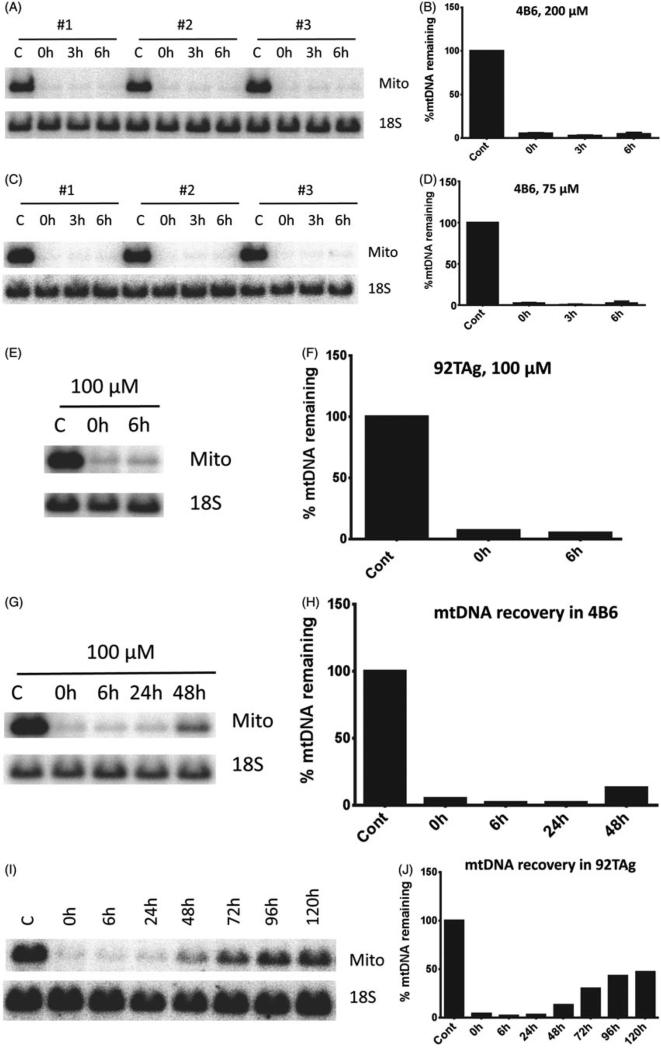
Our initial attempts to characterize mtDNA degradation in cell types other than human epithelial cell lines led to the observation that, in the immortalized MEF cell line 4B6, mtDNA loss was dramatic not only at 200 μM H2O2, which is a threshold concentration for mtDNA degradation in Hela (Figure 3A and B), but also at 75 μM H2O2, a concentration which does not induce mtDNA degradation in HeLa cells (Figure 3C and D). Similar rapid and extensive mtDNA loss was also observed in another mouse fibroblast cell line, 92 TAg (Figure 3E and F). In sharp contrast to HeLa cells, mtDNA recovery after degradation in both 4B6 and 92Tag was much slower. Whereas in Hela cells mtDNA “overshot” its baseline 48 h after treatment, mtDNA recovery in mouse fibroblasts just became noticeable at this time point (Figure 3G–J), and was only around 50% at 120 h after the treatment in 92TAg (Figure 3I and J).
Figure 3.
mtDNA degradation in connective tissue cells. 4B6 or 92TAg cells were exposed to the indicated concentrations of H2O2 for 30 min in HBSS as described in “Methods”, and mtDNA degradation and recovery were assessed by QSBN. Mito, mtDNA band; 18S, 18S (nDNA) band. A, C, and E, representative gel images; B, D, and F, quantitation of band intensities. G, H, I and J, time course of mtDNA recovery in 92TAg cells after exposure to 100 μM H2O2 for 30 min.
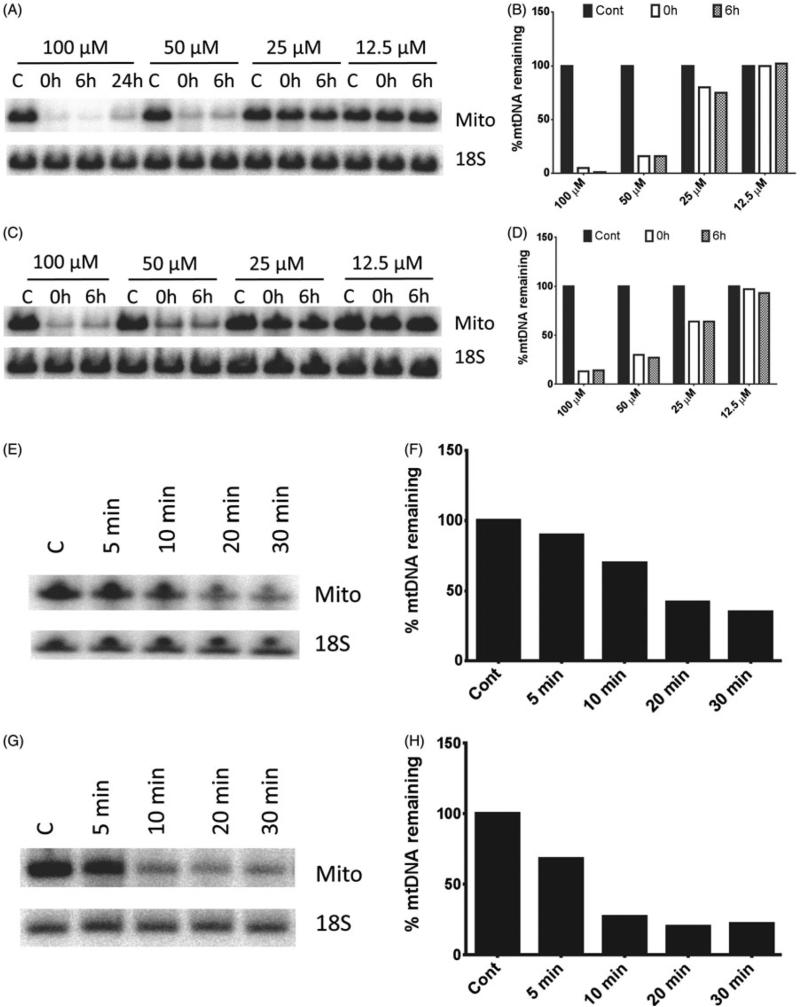
To obtain a better quantitative insight into the differences in susceptibility to mtDNA degradation between HeLa and MEFs, we conducted a dose response study of mtDNA degradation to H2O2 concentration in 4B6. In these cells, mtDNA degradation was detectable at 25 μM H2O2 compared to 200 μM H2O2 in HeLa cells (Figure 4A and B). Therefore, it appears that mtDNA in MEFs is about 8 times more sensitive to H2O2 than in HeLa cells. We then determined whether the extent of mtDNA degradation was dependent of the duration of exposure to H2O2. As expected, a 15 min incubation generally resulted in lesser mtDNA degradation as compared to a 30-min incubation (Figure 4C and D). Finally, a time course of mtDNA degradation in response to the treatment with 100 μM H2O2 was determined in 92TAg and L929 cells. In both cell lines, mtDNA loss was detectable within 5–10 min upon exposure to the stressor (Figure 4E–H).
Figure 4.
Time course and dose response of “fast” mtDNA degradation in connective tissue cells. 4B6 or 92TAg cells were exposed to the indicated concentrations of H2O2 for indicated periods of time in HBSS as described in “Methods” Section, and mtDNA degradation was assessed by QSBN. A–D, dose response of mtDNA degradation in 4B6 cells exposed to various concentrations of H2O2 for either 30 min (A and B) or 15 min (C and D). Mito, mtDNA band; 18S, 18S (nDNA) band. A, C, E, and G, representative gel images; B, D, F, and H, quantitation of band intensities. E–H, time course of the “fast” mtDNA degradation in 92TAg (E and F) and L929 (G and H). Note that mtDNA degradation is noticeable at the earliest time point taken, 5 min.
Similar to HeLa cells, exposure of 92TAg cells to H2O2 was accompanied by cell death and growth arrest (Figure 5), although to a much lesser extent. No cell swelling or significant detachment was observed. The experiment was terminated at 48 h after the treatment when cells reached confluency.
Figure 5.
Morphological changes in 92TAg cells following oxidative stress. 92TAg cells were treated with the indicated concentrations of H2O2 for 30 min in HBSS as described in “Methods” Section, and morphological changes in population were followed with phase contrast microscopy. Black arrows, apoptotic cells.
Discussion
MtDNA depletion has been observed in a variety of pathological conditions such as biliary atresia (Tiao et al., 2007), viral infections (Corcoran et al., 2009; Wiedmer et al., 2008), statin-induced myopathy (Stringer et al., 2013), anti-retroviral drug toxicity (Feeney et al., 2012; Koczor & Lewis, 2010; Nasi et al., 2011; Stankov et al., 2009), mtDNA depletion syndromes (Barthelemy et al., 2001; El-Hattab & Scaglia, 2013; Rotig & Poulton, 2009), metabolic syndrome (Huang et al., 2011), diabetes (Monickaraj et al., 2012), metastatic cancer (Cloos et al., 2009; Guo et al., 2011; Koochekpour et al., 2013; Moro et al., 2008; Potenza et al., 2011; Yu et al., 2010), and aging (Barazzoni et al., 2000; Cree et al., 2008; Kaaman et al., 2007). Also, mtDNA content strongly correlates with lipogenesis in adipocytes (Kaaman et al., 2007). However, the detailed mechanism behind this phenomenon remains poorly understood.
In mice, intragastric administration of ethanol induced oxidative stress, which was accompanied by a reversible loss of mtDNA (Mansouri et al., 1999). The loss of mtDNA was approximately 50% in all organs studied. It could be partially prevented by the antioxidants melatonin, vitamin E and coenzyme Q and was followed by adaptive mtDNA resynthesis (Mansouri et al., 2001). Lipopolysaccharide, a known inducer of in vivo oxidative stress, also induced mtDNA depletion (Suliman et al., 2003). Angiotensin II induced mitochondrial ROS production and decreased skeletal muscle mtDNA content in mice (Mitsuishi et al., 2008). Degradation of mtDNA was observed in the rat model of cerebral ischemia/reperfusion (Chen et al., 2001). Similar to mtDNA depletion induced by intragastric ethanol administration, mtDNA levels returned to normal within 24 h of cerebral ischemia/reperfusion (Chen et al., 2001). Finally, H2O2-induced oxidative stress in hamster fibroblasts was accompanied by a Ca2+-dependent degradation of mtDNA (Crawford et al., 1998). Taken together, these findings strongly suggested a link between oxidative stress (which may result in oxidative mtDNA damage) and mtDNA degradation.
In this study, we observed that mtDNA degradation in response to oxidative stress proceeds with different kinetics in different cell types. In terms of mtDNA degradation, the human epithelial cell line HeLa, similar to other human epithelial cell lines studied previously (Shokolenko et al., 2009), was relatively resistant to oxidative stress, and underwent mtDNA degradation only at H2O2 concentrations in excess of 100 μM. In contrast, under the same experimental conditions the threshold H2O2 concentration was eight times lower in fibroblasts, which are connective tissue cells. Moreover, in HeLa cells mtDNA degradation continued after the removal of H2O2, whereas in connective tissue mouse fibroblasts, degradation reached its maximum during 30-min treatment. We designate patterns of mtDNA loss observed in HeLa and connective tissue cells as “slow” and “fast” modes of mtDNA degradation, respectively. Recovery of mtDNA levels occurred faster in HeLa cells, and original levels of mtDNA per cell were achieved and even exceeded within 48 h of recovery in complete medium even at H2O2 concentrations as high as 800 μM. In contrast, in connective tissue cells, recovery of mtDNA levels only became noticeable at 48 h after the treatment, and was still incomplete at 120 h. Previous studies have demonstrated that other epithelial cell lines like HCT116 and A549 behave in a way similar to HeLa and lose their mtDNA by a “slow” mechanism (Shokolenko et al., 2009). Similar to HeLa cells, exposure of connective tissue cells to H2O2 was accompanied by cell death and growth arrest. However, no cell swelling or massive detachment was observed in connective tissue cells even though mtDNA loss, under the experimental conditions employed, was much more extensive in these cells. This suggests that mtDNA retention is not likely to be the main determinant of cell swelling, growth arrest or survival in our experiments. Since H2O2 has access to both nDNA and mtDNA, it was likely that the nDNA damage was responsible for growth arrest in our experiments, even though nDNA was less susceptible to oxidative damage (Yakes & Van Houten, 1997).
This study demonstrates that mtDNA degradation can proceed with either “fast” or “slow” kinetics as extreme examples, and that mode of degradation may be determined by the cell type. It is likely that future studies will uncover other cell type-specific intermediate modes. The “fast” mode which is described here for the first time may play a regulatory or signaling role, e.g. it may contribute to the pool of extracellular fragmented mtDNA (damage-associated molecular patterns or DAMPs), which was shown to play a leading role in sterile inflammation (Zhang et al., 2010). It is also tempting to speculate that this “fast” mode contributes to the raise in the pool of circulating mtDNA observed after myocardial ischemiareperfusion injury, a pathological condition associated with oxidative stress (Bliksoen et al., 2012).
Conclusions
Overall, this study provides mechanistic insight into how oxidative stress in a particular organ composed of different tissues and cell types can lead to predominant mtDNA damage in some cell type(s), while relatively sparing other cell type(s).
Acknowledgements
The authors acknowledge Dr Rob Sobol for providing 92TAg cell line.
Footnotes
Declaration of interest
The authors declare no conflict of interest in the writing of this paper. These studies were supported by the National Institutes of Health grants ES03456, PO1 HL66299 and OD010944.
References
- Al-Mehdi AB, Pastukh VM, Swiger BM, Reed DJ, Patel MR, Bardwell GC, Pastukh VV, et al. Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci Signal. 2012;5:ra47. doi: 10.1126/scisignal.2002712. doi: 10.1126/scisignal.2002712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexeyev M, Shokolenko I, Wilson G, Ledoux S. The maintenance of mitochondrial DNA integrity – critical analysis and update. Cold Spring Harb Perspect Biol. 2013;5:a012641. doi: 10.1101/cshperspect.a012641. doi: 10.1101/cshperspect.a012641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexeyev MF. Is there more to aging than mitochondrial DNA and reactive oxygen species? FEBS J. 2009;276:5768–87. doi: 10.1111/j.1742-4658.2009.07269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Bankier AT, Barrell BG, De Bruijn MH, Coulson AR, Drouin J, Eperon IC, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–65. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Ballard JW, Whitlock MC. The incomplete natural history of mitochondria. Mol Ecol. 2004;13:729–44. doi: 10.1046/j.1365-294x.2003.02063.x. [DOI] [PubMed] [Google Scholar]
- Bannwarth S, Abbassi M, Valero R, Fragaki K, Dubois N, Vialettes B, Paquis-Flucklinger V. A novel unstable mutation in mitochondrial DNA responsible for maternally inherited diabetes and deafness. Diabetes Care. 2011;34:2591–3. doi: 10.2337/dc11-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barazzoni R, Short KR, Nair KS. Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart. J Biol Chem. 2000;275:3343–7. doi: 10.1074/jbc.275.5.3343. [DOI] [PubMed] [Google Scholar]
- Barthelemy C, Ogier De Baulny H, Diaz J, Cheval MA, Frachon P, Romero N, Goutieres F, et al. Late-onset mitochondrial DNA depletion: DNA copy number, multiple deletions, and compensation. Ann Neurol. 2001;49:607–17. [PubMed] [Google Scholar]
- Bendich AJ. DNA abandonment and the mechanisms of uniparental inheritance of mitochondria and chloroplasts. Chromosome Res. 2013;21:287–96. doi: 10.1007/s10577-013-9349-9. [DOI] [PubMed] [Google Scholar]
- Bliksoen M, Mariero LH, Ohm IK, Haugen F, Yndestad A, Solheim S, Seljeflot I, et al. Increased circulating mitochondrial DNA after myocardial infarction. Int J Cardiol. 2012;158:132–4. doi: 10.1016/j.ijcard.2012.04.047. [DOI] [PubMed] [Google Scholar]
- Brown WM, George M, Jr, Wilson AC. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci USA. 1979;76:1967–71. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen O. Root complex and root canal system: A correlation analysis using one-rooted mandibular second molars. Scand J Dent Res. 1990;98:273–85. doi: 10.1111/j.1600-0722.1990.tb00973.x. [DOI] [PubMed] [Google Scholar]
- Chen H, Hu CJ, He YY, Yang DI, Xu J, Hsu CY. Reduction and restoration of mitochondrial dna content after focal cerebral ischemia/reperfusion. Stroke. 2001;32:2382–7. doi: 10.1161/hs1001.097099. [DOI] [PubMed] [Google Scholar]
- Clay Montier LL, Deng JJ, Bai Y. Number matters: Control of mammalian mitochondrial DNA copy number. J Genet Genomics. 2009;36:125–31. doi: 10.1016/S1673-8527(08)60099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloos CR, Daniels DH, Kalen A, Matthews K, Du J, Goswami PC, Cullen JJ. Mitochondrial DNA depletion induces radioresistance by suppressing G2 checkpoint activation in human pancreatic cancer cells. Radiat Res. 2009;171:581–7. doi: 10.1667/RR1395.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran JA, Saffran HA, Duguay BA, Smiley JR. Herpes simplex virus UL12.5 targets mitochondria through a mitochondrial localization sequence proximal to the N terminus. J Virol. 2009;83:2601–10. doi: 10.1128/JVI.02087-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DR, Abramova NE, Davies KJ. Oxidative stress causes a general, calcium-dependent degradation of mitochondrial poly-nucleotides. Free Radic Biol Med. 1998;25:1106–11. doi: 10.1016/s0891-5849(98)00143-9. [DOI] [PubMed] [Google Scholar]
- Cree LM, Patel SK, Pyle A, Lynn S, Turnbull DM, Chinnery PF, Walker M. Age-related decline in mitochondrial DNA copy number in isolated human pancreatic islets. Diabetologia. 2008;51:1440–3. doi: 10.1007/s00125-008-1054-4. [DOI] [PubMed] [Google Scholar]
- Driggers WJ, Holmquist GP, Ledoux SP, Wilson GL. Mapping frequencies of endogenous oxidative damage and the kinetic response to oxidative stress in a region of rat mtDNA. Nucleic Acids Res. 1997;25:4362–9. doi: 10.1093/nar/25.21.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hattab AW, Scaglia F. Mitochondrial DNA depletion syndromes: Review and updates of genetic basis, manifestations, and therapeutic options. Neurotherapeutics. 2013;10:186–98. doi: 10.1007/s13311-013-0177-6. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney ER, Van Vonderen MG, Wit F, Danner SA, Van Agtmael MA, Villaroya F, Domingo P, et al. Zidovudine/lamivudine but not nevirapine in combination with lopinavir/ritonavir decreases subcutaneous adipose tissue mtDNA. AIDS. 2012;26:2165–74. doi: 10.1097/QAD.0b013e328358b279. 2012. [DOI] [PubMed] [Google Scholar]
- Furda AM, Marrangoni AM, Lokshin A, Van Houten B. Oxidants and not alkylating agents induce rapid mtDNA loss and mitochondrial dysfunction. DNA Repair (Amst) 2012;11:684–92. doi: 10.1016/j.dnarep.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gredilla R, Bohr VA, Stevnsner T. Mitochondrial DNA repair and association with aging – an update. Exp Gerontol. 2010;45:478–88. doi: 10.1016/j.exger.2010.01.017. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guliaeva NA, Kuznetsova EA, Gaziev AI. Proteins associated with mitochondrial DNA protect it against the action of X-rays and hydrogen peroxide. Biofizika. 2006;51:692–7. [PubMed] [Google Scholar]
- Guo J, Zheng L, Liu W, Wang X, Wang Z, French AJ, Kang D, et al. Frequent truncating mutation of TFAM induces mitochondrial DNA depletion and apoptotic resistance in microsatellite-unstable colorectal cancer. Cancer Res. 2011;71:2978–87. doi: 10.1158/0008-5472.CAN-10-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CH, Su SL, Hsieh MC, Cheng WL, Chang CC, Wu HL, Kuo CL, et al. Depleted leukocyte mitochondrial DNA copy number in metabolic syndrome. J Atheroscler Thromb. 2011;18:867–73. doi: 10.5551/jat.8698. [DOI] [PubMed] [Google Scholar]
- Kaaman M, Sparks LM, Van Harmelen V, Smith SR, Sjolin E, Dahlman I, Arner P. Strong association between mitochondrial DNA copy number and lipogenesis in human white adipose tissue. Diabetologia. 2007;50:2526–33. doi: 10.1007/s00125-007-0818-6. [DOI] [PubMed] [Google Scholar]
- Kazak L, Reyes A, Holt IJ. Minimizing the damage: Repair pathways keep mitochondrial DNA intact. Nat Rev Mol Cell Biol. 2012;13:659–71. doi: 10.1038/nrm3439. [DOI] [PubMed] [Google Scholar]
- Koczor CA, Lewis W. Nucleoside reverse transcriptase inhibitor toxicity and mitochondrial DNA. Expert Opin Drug Metab Toxicol. 2010;6:1493–504. doi: 10.1517/17425255.2010.526602. [DOI] [PubMed] [Google Scholar]
- Koochekpour S, Marlowe T, Singh KK, Attwood K, Chandra D. Reduced mitochondrial DNA content associates with poor prognosis of prostate cancer in african american men. PLoS One. 2013;8:e74688. doi: 10.1371/journal.pone.0074688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurelac I, Mackay A, Lambros MB, Di Cesare E, Cenacchi G, Ceccarelli C, Morra I, et al. Somatic complex I disruptive mitochondrial DNA mutations are modifiers of tumorigenesis that correlate with low genomic instability in pituitary adenomas. Hum Mol Genet. 2013;22:226–38. doi: 10.1093/hmg/dds422. [DOI] [PubMed] [Google Scholar]
- Larman TC, Depalma SR, Hadjipanayis AG, Protopopov A, Zhang J, Gabriel SB, Chin L, et al. Spectrum of somatic mitochondrial mutations in five cancers. Proc Natl Acad Sci USA. 2012;109:14087–91. doi: 10.1073/pnas.1211502109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Q, Dedon PC. Cu(II)/H2O2-induced DNA damage is enhanced by packaging of DNA as a nucleosome. Chem Res Toxicol. 2001;14:416–22. doi: 10.1021/tx0002278. [DOI] [PubMed] [Google Scholar]
- Liang R, Senturker S, Shi X, Bal W, Dizdaroglu M, Kasprzak KS. Effects of Ni(II) and Cu(II) on DNA interaction with the N-terminal sequence of human protamine P2: Enhancement of binding and mediation of oxidative DNA strand scission and base damage. Carcinogenesis. 1999;20:893–8. doi: 10.1093/carcin/20.5.893. [DOI] [PubMed] [Google Scholar]
- Liu P, Demple B. DNA repair in mammalian mitochondria: Much more than we thought? Environ Mol Mutagen. 2010;51:417–26. doi: 10.1002/em.20576. [DOI] [PubMed] [Google Scholar]
- Maassen JA, Janssen GM, Hart LM. Molecular mechanisms of mitochondrial diabetes (MIDD). Ann Med. 2005;37:213–21. doi: 10.1080/07853890510007188. [DOI] [PubMed] [Google Scholar]
- Mansouri A, Demeilliers C, Amsellem S, Pessayre D, Fromenty B. Acute ethanol administration oxidatively damages and depletes mitochondrial DNA in mouse liver, brain, heart, and skeletal muscles: Protective effects of antioxidants. J Pharmacol Exp Ther. 2001;298:737–43. [PubMed] [Google Scholar]
- Mansouri A, Gaou I, De Kerguenec C, Amsellem S, Haouzi D, Berson A, Moreau A, et al. An alcoholic binge causes massive degradation of hepatic mitochondrial DNA in mice. Gastroenterology. 1999;117:181–90. doi: 10.1016/s0016-5085(99)70566-4. [DOI] [PubMed] [Google Scholar]
- Milone M. Mitochondria, diabetes, and Alzheimer's disease. Diabetes. 2012;61:991–2. doi: 10.2337/db12-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuishi M, Miyashita K, Muraki A, Itoh H. Angiotensin II reduces mitochondrial content in skeletal muscle and affects glycemic control. Diabetes. 2008;58:710–17. doi: 10.2337/db08-0949. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monickaraj F, Aravind S, Gokulakrishnan K, Sathishkumar C, Prabu P, Prabu D, Mohan V, Balasubramanyam M. Accelerated aging as evidenced by increased telomere shortening and mitochondrial DNA depletion in patients with type 2 diabetes. Mol Cell Biochem. 2012;365:343–50. doi: 10.1007/s11010-012-1276-0. 2012. [DOI] [PubMed] [Google Scholar]
- Moro L, Arbini AA, Marra E, Greco M. Mitochondrial DNA depletion reduces PARP-1 levels and promotes progression of the neoplastic phenotype in prostate carcinoma. Cell Oncol. 2008;30:307–22. doi: 10.3233/CLO-2008-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasi M, Pinti M, Chiesa E, Fiore S, Manzini S, Del Giovane C, D'amico R, et al. Decreased mitochondrial DNA content in subcutaneous fat from HIV-infected women taking antiretroviral therapy as measured at delivery. Antivir Ther. 2011;16:365–72. doi: 10.3851/IMP1764. [DOI] [PubMed] [Google Scholar]
- Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–5. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza L, Calcabrini C, De Bellis R, Guescini M, Mancini U, Cucchiarini L, Nappo G, et al. Effects of oxidative stress on mitochondrial content and integrity of human anastomotic colorectal dehiscence: A preliminary DNA study. Can J Gastroenterol. 2011;25:433–9. doi: 10.1155/2011/741073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotig A, Poulton J. Genetic causes of mitochondrial DNA depletion in humans. Biochim Biophys Acta. 2009;1792:1103–8. doi: 10.1016/j.bbadis.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russel DW. A laboratory manual. Cold Spring Harbor Laboratory Press; New York: 2001. Molecular cloning. [Google Scholar]
- Schapira AH. Mitochondrial diseases. Lancet. 2012;379:1825–34. doi: 10.1016/S0140-6736(11)61305-6. [DOI] [PubMed] [Google Scholar]
- Shokolenko I, Venediktova N, Bochkareva A, Wilson GL, Alexeyev MF. Oxidative stress induces degradation of mitochondrial DNA. Nucleic Acids Res. 2009;37:2539–48. doi: 10.1093/nar/gkp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokolenko IN, Fayzulin RZ, Katyal S, Mckinnon PJ, Wilson GL, Alexeyev MF. Mitochondrial DNA ligase is dispensable for the viability of cultured cells but essential for mtDNA maintenance. J Biol Chem. 2013a;288:26594–605. doi: 10.1074/jbc.M113.472977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokolenko IN, Wilson GL, Alexeyev MF. Persistent damage induces mitochondrial DNA degradation. DNA Repair (Amst) 2013b;12:488–99. doi: 10.1016/j.dnarep.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobol RW, Kartalou M, Almeida KH, Joyce DF, Engelward BP, Horton JK, Prasad R, et al. Base excision repair intermediates induce p53-independent cytotoxic and genotoxic responses. J Biol Chem. 2003;278:39951–9. doi: 10.1074/jbc.M306592200. [DOI] [PubMed] [Google Scholar]
- Stankov MV, Lucke T, Das AM, Schmidt RE, Behrens GM. Mitochondrial DNA depletion and respiratory chain activity in primary human subcutaneous adipocytes treated with nucleoside-analogue reverse transcriptase inhibitors. Antimicrob Agents Chemother. 2009;54:280–7. doi: 10.1128/AAC.00914-09. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer HA, Sohi GK, Maguire JA, Cote HC. Decreased skeletal muscle mitochondrial DNA in patients with statin-induced myopathy. J Neurol Sci. 2013;325:142–7. doi: 10.1016/j.jns.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Suliman HB, Carraway MS, Piantadosi CA. Postlipopolysaccharide oxidative damage of mitochondrial DNA. Am J Respir Crit Care Med. 2003;167:570–9. doi: 10.1164/rccm.200206-518OC. [DOI] [PubMed] [Google Scholar]
- Supale S, Li N, Brun T, Maechler P. Mitochondrial dysfunction in pancreatic beta cells. Trends Endocrinol Metab. 2012;23:477–87. doi: 10.1016/j.tem.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Tatarenkov A, Avise JC. Rapid concerted evolution in animal mitochondrial DNA. Proc Biol Sci. 2007;274:1795–8. doi: 10.1098/rspb.2007.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorslund T, Sunesen M, Bohr VA, Stevnsner T. Repair of 8-oxoG is slower in endogenous nuclear genes than in mitochondrial DNA and is without strand bias. DNA Repair (Amst) 2002;1:261–73. doi: 10.1016/s1568-7864(02)00003-4. [DOI] [PubMed] [Google Scholar]
- Tiao MM, Lin TK, Kuo FY, Huang CC, Du YY, Chen CL, Chuang JH. Early stage of biliary atresia is associated with significant changes in 8-hydroxydeoxyguanosine and mitochondrial copy number. J Pediatr Gastroenterol Nutr. 2007;45:329–34. doi: 10.1097/MPG.0b013e3180cc2c0f. [DOI] [PubMed] [Google Scholar]
- Wallace DC. Mitochondria and cancer. Nat Rev Cancer. 2012;12:685–98. doi: 10.1038/nrc3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Youle RJ. The role of mitochondria in apoptosis. Annu Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedmer A, Wang P, Zhou J, Rennekamp AJ, Tiranti V, Zeviani M, Lieberman PM. Epstein-Barr virus immediate-early protein Zta co-opts mitochondrial single-stranded DNA binding protein to promote viral and inhibit mitochondrial DNA replication. J Virol. 2008;82:4647–55. doi: 10.1128/JVI.02198-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci USA. 1997;94:514–19. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylikallio E, Suomalainen A. Mechanisms of mitochondrial diseases. Ann Med. 2012;44:41–59. doi: 10.3109/07853890.2011.598547. [DOI] [PubMed] [Google Scholar]
- Yu M. Somatic mitochondrial DNA mutations in human cancers. Adv Clin Chem. 2012;57:99–138. doi: 10.1016/b978-0-12-394384-2.00004-8. [DOI] [PubMed] [Google Scholar]
- Yu M, Wan Y, Zou Q. Decreased copy number of mitochondrial DNA in Ewing's sarcoma. Clin Chim Acta. 2010;411:679–83. doi: 10.1016/j.cca.2010.01.035. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–7. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Ji Y, Guan MX. Mitochondrial tRNA mutations associated with deafness. Mitochondrion. 2012;12:406–13. doi: 10.1016/j.mito.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Zu XL, Guppy M. Cancer metabolism: Facts, fantasy, and fiction. Biochem Biophys Res Commun. 2004;313:459–65. doi: 10.1016/j.bbrc.2003.11.136. [DOI] [PubMed] [Google Scholar]