Abstract
Objective
Awareness of motor functioning is most likely a complex process that requires integration of sensory-motor feedback in order to constantly update the system on the functioning of the limb during motor behavior. Using lesion mapping procedures and behavioral measures, the current study aimed to evaluate neural correlates of anosognosia for hemiplegia (AHP) in the acute stage (first 48 hours) of right hemisphere stroke.
Method
Thirty-five individuals with right hemisphere stroke who presented to an urban medical center within 24 hours of symptom onset were included in the study. All thirty-five individuals had hemiplegia, and eight of these individuals exhibited AHP.
Results
Fisher exact test statistical map of lesion-deficit association (range is between-log(p) 4 to 11) found maximal value of 10.9 located in pars orbitalis (Brodmann’s Area 47; BA). In this selected location, six out of eight patients with AHP had tissue abnormality, whereas none of the unaffected subjects had tissue abnormality in BA 47. Right BA 44/45 was also found to be lesioned more frequently in individuals with AHP (75%) than without AHP (11%).
Conclusions
The current study findings provide preliminary support for unique involvement of the right inferior frontal gyrus (IFG), pars orbitalis (BA 47) in AHP. The current data suggest that frontal operculum may play a key role in awareness of limb functioning.
Keywords: Anosognosia, Awareness, Stroke, Imaging
Introduction
Anosognosia means “without disease knowledge.” It has been defined as a neurologic impairment in which the individual lacks or has reduced awareness of impaired functioning or symptoms, illustrated through behavior or statement (Prigatano & Wong, 1999). One type of anosognostic syndrome following stroke is anosognosia for hemiplegia (AHP). Because these individuals believe that there is nothing wrong with their motor functioning or over-estimate their abilities, they do not understand the need for therapeutic interventions leading to refusals to participate in rehabilitation activities (Hartman-Maeir, Soroker & Katz, 2001). It has been shown that anosognosia worsens the rehabilitation prognosis significantly in patients with hemiplegia who also have neglect (Gialanella, Monguzzi, Santoro, & Rocchi, 2005). Additionally, individuals with AHP do not follow precautions resulting in safety risk increase (Hartman-Maeir, et al., 2001). Overall, AHP has been found to be associated with longer rehabilitation stays and poorer functional outcomes following stroke (Gialnella, et al, 2005; Maeshima, et al., 1997; Pedersen, Jørgensen, Nakayam, & Raaschou, 1996).
Incidence rates for AHP following stroke range from 17% to 58% (Appelros, Karlsson, Seiger, & Nydevik; 2002; Bisiach, Vallar, Perani, & Papagno, 1986; Cutting, 1978; Maeshima, et al., 1997; Nathanson, Bergman, & Gordon, 1952; Starkstein, Fedoroff, Price, Leiguarda, & Robinson, 1992; Stone, Halligan, & Greenwood, 1993). However, when more stringent criteria are used that do not include patients who do not spontaneously report the hemiplegia, but acknowledge it when asked about it directly, the incidence ranges from 10% to 18% (Baier & Karnath, 2005; Vocat, Staub, Stroppini, & Vuilleumier, 2010). In approximately 10% to 15% of these individuals, the anosognosia resolves within the two to three days of stroke onset (Pia, Neppi-Modona, Ricci, & Berti, 2004), but persists from weeks to months for the remaining 85–90% (Berti, Ladavas, & Della Corte, 1996; Berti, Ladavas, Stracciari, Giannarelli, & Ossola, 1998; Cocchini, Beschin, & Sala, 2002; Levine, Calvanio, & Rinn, 1991; Maeshima, et al., 1997; Marcel, Tegnér, & Nimmo-Smith, 2004; Venneri & Shanks, 2004). Given the potential for persistent symptoms across the acute and subacute recovery process, approximately one-sixth of stroke patients will present with AHP in some form.
A variety of neuropathological theories have been proposed to explain AHP. An early hypothesis proposed by Geschwind (1965a, 1965b) was that AHP represented a disconnection syndrome between the sensory processing hemisphere (right) and the verbal expression hemisphere (left). However, it has been demonstrated in a number of studies that even when the left hemisphere is provided information regarding the hemiplegia, the level of awareness does not necessarily change (Heilman, Barrett & Adair, 1998). Other possible contributory factors have also been proposed. Based upon clinical observations, AHP appears to co-occur frequently with other impairments that present following stroke, including hemispatial neglect (personal and extra-personal neglect), impaired somatosensory processing (proprioception), impaired cognitive functioning, and poor psychological adjustment. A review of the current literature reveals that all of these impairments can co-occur with AHP, but are not necessary for and do not explain AHP (Adair, et al., 1995; Bisiach, et al., 1986; Heilman, et al., 1998; Maeshima, et al., 1997; Small & Ellis, 1996; for a review, see Heilman & Harciarek, 2010). The evidence suggests that the frequent co-occurrence with neglect, impaired somatosensory functioning, and cognition deficits may be a product of shared anatomical regions rather than functional contiguity (Bisiach, et al., 1986; Dauriac-Le Masson, et al., 2002). It is acknowledged, however, that these concomitant impairments may contribute to the clinical presentation of persistent AHP (Heilman et al., 1998).
AHP has been shown to occur following both right hemisphere and left hemisphere damage (Cocchini, Beschin, Cameron, Fotopoulou, & Della Sala, 2009; Pia, et al., 2004). However, it more commonly occurs following right hemisphere stroke suggesting that there may be a pivotal role of the right hemisphere in self-awareness and self-monitoring of motor behavior (Pia, et al., 2004). Damage to multiple brain structures has been implicated; however, this line of research has provided few firm conclusions, in part because many of the earlier studies were single case studies. Additionally, although most studies relied on a consistent definition of AHP characterized by a specific behavioral presentation, the severity of the anosognosia is not always clearly defined. The timing of the assessment of AHP has also varied across studies and some used retrospective data for the diagnosis of AHP. In addition, the timing of the assessment of AHP was not always during the same period as when neuropathological evidence was collected (via neuroradiologic study or post-mortum analysis). The majority of the studies have relied on neuroradiologic or pathologic evidence collected during the acute through the subacute phases of stroke recovery. Existing evidence suggests that by weeks to months post-stroke some reorganization of structure-function relationships may have already occurred (Marshall, 1984; Merzenich, et al., 1983; Wall, 1988). One recent study is an exception. Vocat and colleagues (Vocat, et al., 2010) investigated the possible neuropathological underpinnings of AHP in the hyperacute phase of recovery (within first week) as well as in the more subacute phase. Using a voxel-based statistical mapping method for infarcted tissue found on CT or MRI, they found that those with infarcts in the insula and/or anterior internal capsule had more severe AHP in the hyperacute phase. In contrast, patients with infarcts in the premotor, anterior cingulate, internal capsule, insular, temporo-parietal junction, and hypocampus/amygdale had more severe AHP into the subacute phase of recovery. However, this study only investigated infarcted tissue. In the hyperacute to acute phases of stroke, some deficits are due to hypoperfused tissue around the infarct, as well as the infarct itself. Therefore, the relationship between the early behavioral presentation and the lesion is most likely a function of: the size and site of the infarcted tissue; the size and site of the hypoperfused tissue at the time of assessment, co-morbid factors, and stage in recovery process when it is assessed.
Given that the brain operates on a circuitry-function relationship, as opposed to a direct one-to-one relationship between structures and functions (Mesulam, 2012), AHP is most likely not a product of damage to just one structure or region. Thus, connections among the regions of the brain may form the basis for circuitry underlying limb movement awareness with disruption of the circuit leading to anosognosia for hemiplegia. Some have proposed a circuit that includes the inferior parietal lobe and its connections with the inferior lateral premotor cortex via the anterior cingulate, arguing that this circuit underlies motor preparation and motor imagery (Krams, Rushworth, Deiber, Frackowiak, & Passingham, 1998; Stephan, et al., 1995). It has been hypothesized that normal functioning of this circuit plays a role in self-monitoring of motor movement, such that when the expected movement does not match the implemented movement, then a mismatch is detected and the error is brought to conscious awareness (Frith, Blakemore & Wolpert, 2002; Jenkinson, Edelstyn, & Ellis, 2009). Others have speculated that the insular cortex also plays a role in this circuit. It has been argued that lesions in the circuit that includes the insular, dorsal premotor, somatosensory, and primary motor cortex leads to a disconnection between the mental representation of the motor movement and the detection of whether the intended movement occurred (motor monitoring; Berti, et al., 2005; Coslett, 2005; Karnath, Baier, & Nägele, 2005). The right frontal operculum (right IFG) has been found to be strongly implicated in motor response inhibition and action monitoring (Rubia, Smith, Brammer, & Taylor, 2003). The right IFG has been hypothesized to underlie body ownership similarly to the right posterior insular cortex and is involved in detection and resolution of various conflicting signals between internal and external representations of body-related events (Tsarkiris, et al., 2007).
There is some evidence that lesions in the frontal operculum are associated with AHP. Berti and colleagues (Berti, et al., 2005) found that lesions in BA 44 (inferior frontal gyrus, pars opercularis) occur more frequently in individuals with AHP. More recently, Fotopoulou and colleagues (Fotopoulou, Pernigo, Maeda, Rudd, Kopelman, 2010) found that the frequency of lesions between those with AHP and those with hemiplegia without AHP was significant in areas extending from the rolandic operculum and anterior insula to the basal ganglia and the superior temporal pole. This study goes beyond lesion analysis by investigating the potential differences between these two groups based upon their implicit versus explicit processing of deficit-related information. These researchers find that individuals with AHP were significantly slower at processing deficit-related sentences than controls, which suggests that the behavioral manifestation of AHP may go beyond motor monitoring of the errors and failures in movement. There may be implicit knowledge of the impairment, but that knowledge does not necessarily help one’s self-representation.
Using lesion mapping procedures, the current study aims to identify cortical and subcortical locations associated with AHP in the acute stage (first 48 hours) of right hemisphere stroke. By conducting the behavioral assessment in conjunction with the neuroradiologic studies within the acute phase of stroke (before substantial recovery or reorganization of structure-function relationships), the ability to identify the network normally critical for awareness and monitoring of motor output is enhanced. Additionally, by studying hypoperfused tissue as well as the infarct, we capture the entire area of tissue abnormality that may be causing the deficit. We hypothesize that dysfunctional tissue in the right rolandic operculum, insula, basal ganglia, and/or superior temporal pole would be associated with AHP.
Methods
Participants
Participants were selected from a pool of 148 individuals who presented to an urban medical center within 24 hours of right hemisphere stroke symptoms. To be included in the study, individuals had to exhibit clinical evidence of acute, unilateral, right hemisphere stroke (i.e. exhibit left hemiplegia), be age 18 or older, and be able to give informed consent or to indicate a family member who could do so. Individuals were excluded from the study if their MRI showed no stroke or they had a stroke limited to the brain-stem or cerebellum; were hemiparetic, but not hemiplegic; had evidence of prior stroke or other neurologic injury or illness; or had contraindications for MRI (e.g. implanted metal) or allergy to gadolinium contrast agents. Of the 148 individuals who presented consecutively to the medical center with right, unilateral hemisphere stroke symptoms, 54 met inclusion criteria (see Figure 1). Among these 54 individuals, 9 could not be included because MRI was not obtained; and 1 was not included because the MRI could not be used to obtain infarct volume due to motion artifact. Among the remaining 44 individuals, 27 had no AHP, 8 had moderate to severe AHP, and 9 had mild AHP. Medical record review indicated that all individuals were oriented to self and situation, and symptom presentation could not be explained by other factors (e.g. dementia, delirium).
Figure 1.
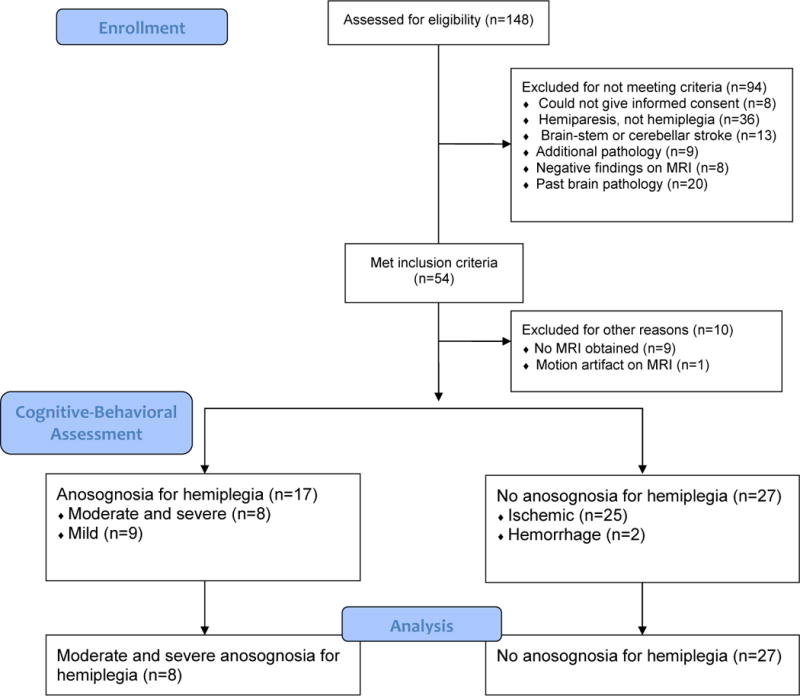
Study Enrollment and Process
Three groups (AHP Group, Non-AHP Group, and mild AHP) were created from the sample of 44 individuals. The mild AHP Group, based upon cut-off criterion established by Baier and Karnath (2005), were excluded from further study given the ambiguity of their presentation. Thus, the final AHP Group consisted of eight individuals with moderate to severe AHP. The non-AHP group consisted of 27 individuals with unilateral, ischemic or hemorrhagic stroke and hemiplegia.
All patients with AHP had unilateral, ischemic stroke. Among the Non-AHP Group, 25 individuals had ischemic strokes (including two with hemorrhagic transformation of the infarct), and two had primary hemorrhages. Analyses were conducted with and without participants with hemorrhagic components to their stroke and no significant difference were obtained. Therefore, all 27 Non-AHP Group participants were included in the final analyses presented in this study.
Procedure
Prior to commencing the study, IRB approval was obtained from the institutional review board. Following informed consent, individuals who met study criteria were interviewed to obtain demographic information. Within the first 48 hours following onset of stroke symptoms, participants completed the study measures and received medical and neurological examinations by physicians, including MRI as part of routine clinical care to evaluate stroke characteristics. Each subject completed an assessment of their awareness, sensory and proprioceptive functioning, and for signs of hemispatial and personal neglect.
Measures
Anosognosia for Hemiplegia
Anosognosia for motor impairment was examined by asking subjects a series of questions about their upper limb functioning and through having them demonstrate use of their upper limbs in a simple task (i.e., raising their arms) both in the left hemispace and in the right hemispace (by moving the limb across the subject’s body). Evaluation of their awareness was ascertained using the anosognosia scale proposed by Bisiach and colleagues (Bisiach, et al, 1986), which has been frequently used in clinical studies (Appelros, et al., 2002; Jehkonen, Ahonen, Dastidar, Laippala, & Vilkki, 2000; Starkstein, et al., 1992). The scale rates the degree of anosognosia from grade 0 to grade 3.
-
○
grade 0 (no anosognosia): the disorder is spontaneously reported or mentioned by the subject following a general question about the current medical complaints
-
○
grade 1 (mild): the disorder is reported only following a specific question about the strength of the subject’s limb
-
○
grade 2 (moderate): the disorder is acknowledged only after demonstrations through routine techniques of neurological examination
-
○
grade 3 (severe): no acknowledgement of the disorder can be obtained after questioning and demonstration of motor impairment.
Consistent with previous investigations of the validity of cut-off criterion (Baier & Karnath, 2005) ratings of grade 2 (moderate) or higher were used to identify AHP in the present study. Individuals with grade 1 (mild AHP) were not included given the ambiguity of their presentation. All individuals in the non-AHP group had a rating of 0.
Neglect battery
A multi-task approach was taken to assess personal neglect, which included (1) asking the subject to identify each of his/her limbs (e.g., point to right leg), (2) asking if each limb is his/hers (e.g., is this your left arm), (3) directly observing if the subject ignored any limbs during the evaluation, and (4) asking the subject to reach over to his/her left arm with his/her right arm to indicate where his/her left arm was located (a similar procedure outlined by Bisiach et al., 1986). If the subject was able to cross midline toward his/her left upper limb and there were no other signs of personal neglect when asked to identify his/her limbs, then the subject was concluded not to have personal neglect.
The hemispatial neglect battery included multiple measures (both perceptual and perceptual-motor tasks) in order to distinguish between viewer-centered, stimulus-centered, and object-centered neglect (types of neglect based on spatial coordinate frame; see references Hillis & Caramazza, 1995; Hillis, et al, 2002; Medina, et al., 2009; Ota, Fujii, Suzuki, Fukatsu, & Yamadori, 2001). The neglect protocol utilized in the present study is part of a larger neglect battery that has previously been described in the literature (Hillis, et al., 2002). Among the tasks included in the battery were a gap detection test (which requires detecting gaps on the left or right of circles scattered across the page; Ota, et al., 2001), reading and writing of words on both sides of a paper (see Hillis & Caramazza, 1995 for detailed description of these tests) and three perceptual-motor tasks (line cancellation; line bisection; scene copying). Not all parts of the battery were administered to every participant, given assessment constraints such as fatigue. All information that was obtained from the neglect battery was reviewed for each individual, and type of neglect was coded based on the results (See reference Medina, et al., 2009 for criteria for each type of neglect).
Sensation
Light-touch sensation in both hands was assessed using the Semmes-Weinstein monofilament test (Bell-Krotoski & Tomancik, 1987). The test site is located on the center of the anterior pad of each finger distal to the distal interphalangeal joint of the hand. While the subject’s hand is lying flat, palm face up a stable surface, and vision occluded, the 10 g-force (5.07) monofilament is pressed perpendicular to the test site at a 90 degree angle with enough pressure to bend the monofilament for 1 sec. Participants are asked to identify when they feel the press of the monofilament by answering “yes.” There were a total of five monofilaments of differing diameters to be trialed at each randomly sequenced testing site. The smallest size of the monofilament that participants were able to feel was recorded for each finger and based on the size; a determination was made by an Occupational Therapist whether the performance was consistent with sensory impairment. If yes, then the Occupational Therapist categorized the impairment into the following categories: diminished light touch, diminished protective sensation, loss of protective sensation, and deep pressure sensation only.
Proprioception
The Judgment of Movement task, a test of proprioceptive functioning requires participants to judge whether the limb is moved and what direction (Carey, Matyas, & Oke, 2002; Carey, Oke, & Matyas, 1996). Each joint (elbow, wrist, index finger) is put in flexion and extension of 10 degrees (small amplitude movement) and then flexion and extension of 45 degrees (large amplitude movement). Each position at each joint is tested six times (3 flexion and 3 extension). The participant is instructed to close his/her eyes in order to eliminate visual cues. The examiner passively moves one joint to the determined degree (as measured by a goniometer). The participant is asked whether his/her arm/wrist/finger moved and if so, what direction (up or down). The six experimental trials are interspersed with no-movement trials resulting in a total of 10 trials. Ten trials for each targeted joint in the upper limb (elbow, wrist, and index finger) result in 30 trials. This task is performed in the left arm and the right arm. Two scores are calculated for each upper limb: a total accuracy of movement judgment score (out of 30) and a total accuracy of direction judgment score (out of 30). It has been well demonstrated that neurologically normal controls can perform this task without error. Therefore, individuals with errors in movement detection or direction detection were deemed to have proprioception impairments.
Magnetic resonance imaging
Diffusion Weighted Imaging (DWI) and Perfusion Weighted Imaging (PWI), and additional sequences to rule out old lesions and non-stroke lesions (T1, T2, FLAIR, ADC) were obtained on echo plannar imaging (EPI) capable scanners. DWI trace images were obtained using a multi-slice, isotropic, single shot EPI sequence, with bmax=1000 s/mm2. Imaging parameters were: TR of 10,000msec and TE of 120. Dynamic susceptibility weighted perfusion imaging was performed to derive time-to-peak map (TTP). To achieve the best possible homogeneity across subjects, each image was registered to a brain template, a digital form of the Talairach atlas. Three dimensional image registration and warping was used to identify spatial correspondence between individual subject brain and the template space (Woods, Grafton, Watson, Sicotte, & Mazziotta, 1998). Registered images were stored and analyzed in the Brain Image Database (BRAID) for statistical analysis (Herskovits, 2000). We carried out both region of interest (ROI) analysis and a voxel-wise analysis of the association between AHP and areas of tissue abnormality (abnormality on diffusion weight imaging and/or hypoperfusion on dynamic contrast perfusion weight imaging), as both have limitations and strengths (see Crinion et al., 2013, for discussion). Standard atlases, software systems, and template drawings (Damasio & Damasio, 1989) were used to identify the presence or absence of abnormality on DWI and/or TTP maps in each of 10 ROIs for all participants. The 10 ROIs (precuneus, pars orbitalis, pars triangularis/opercularis, premotor, pre-central gyrus, post-central gyrus, basal ganglia, thalamus, internal capsule) were selected on the basis of previous literature review. Hypoperfusion was defined as >4 sec in TTP arrival in contrast to the same ROI in the normal left hemisphere. MRI scans were viewed with ImageJ software (http://rsb.info.nih.gov/ij/index.html) and 10 Brodmann’s areas (ROIs) were examined for the presence or absence of acute ischemia and/or hypoperfusion. We selected a threshold of TTP < 4 second delay relative to the other side as a measure of hypoperfused tissue as this threshold has been shown to correspond to tissue dysfunction in previous studies by ourselves and others using both behavioral data and PET (Hillis et al., 2001, Sobesky et al., 2004). For each MRI image, at least two researchers independently analyzed and coded the scan, to ensure reliability. On instances when differences between coders emerged, a third party was consulted, and the scan was reviewed as a group.
Additionally, to obtain volume of tissue abnormality (infarct and/or hypoperfusion), technicians masked to the behavioral data undertook volumetric analysis of DWI and PWI images using Image J software. Infarct (on DWI) or areas of hypoperfusion (on PWI) were traced on each MRI slice, and areas were multiplied by slice thickness and then added across all slices affected by the stroke.
Finally, for the whole brain voxel-wise analysis of the regions of tissue abnormality associated with AHP, we registered each individual’s lesion onto a Montreal Neurological Institute template (Rorden & Brett, 2000). Spatial correspondence between patient brain and stereotactic atlas was established using mutual information using 12 degree of freedom registration (FSL – FLIRT) (Jenkinson, Bannister, Brady, & Smith, 2002). Lesions were subsequently translated to common atlas space for statistical analyses. Spatial probability maps of lesions in each group (AHP+ and AHP−) were computed in common atlas space and visualized on Figure 1.
Statistical Analyses
Statistical analyses were conducted using SPSS Software version 19. Independent t-tests, ANOVAs, and chi square tests were conducted to demonstrate statistically significant group differences based upon demographic and stroke specific variables (i.e. lesion volume, vascular involvement). To determine if there were group differences among the two groups based upon tissue dysfunction in specific brain regions, Fisher’s exact tests were conducted for each ROI. An alpha level of p<0.05 after Bonferroni correction for multiple comparisons was selected as a threshold for significance. Voxel-wise Fisher Exact tests (Chen, Hillis, Pawlak, & Herskovits, 2008) were applied to evaluate the association between presence of AHP and presence of tissue abnormality in a given voxel to create a negative p-value log lesion-deficit association map and using Liebermeister measure (non-parametric mapping) with correction for false discovery rate (Cavada, Company, Tejedor, Cruz-Rizzolo, & Reinoso-Suarez, 2000; Rorden, Karnath, & Bonilha, 2007).
Results
Sample Descriptives
The sample characteristics are summarized in Table 1. Education level was not available for four participants. There was no significant difference between groups based on sex [χ2(1, N=37) = .01, p=.92], race [χ2(1, N=37) = 1.40, p=.24], or years of education [t(31)=.11, p=.91]. There was a modest, but statistically significant difference between groups based on age [t(35) = −2.40, p=.02]. The majority of individuals in both groups (AHP Group and Non-AHP Group) had middle cerebral arterial (MCA) territory involvement, including 24 out of 27 individuals in the Non-AHP Group and all 8 individuals in the AHP Group. Individuals without MCA territory involvement (3 in the Non-AHP Group) presented with posterior cerebral arterial (PCA) territory involvement, including motor thalamus. Although not statistically significant ([t(35) = −1.11, p=.27]), individuals within the AHP Group had a larger average lesion volume. The two groups included individuals with comparable large and small lesions.
Table 1.
Sample Description
| Group | Non-AHP Group (N=27) |
AHP Group (N=8) |
Total (N=35) |
|---|---|---|---|
| Age* | |||
| Mean (SD) | 62.30 (14.37) | 77.50 (7.76) | 65.77 (14.57) |
|
| |||
| Sex | |||
| Male | 13 | 4 | 17 |
| Female | 14 | 4 | 18 |
|
| |||
| Education | |||
| Mean (SD) | 11.57 (3.05) | 11.50 (2.83) | 11.62 (3.04) |
|
| |||
| Racial Group | |||
| Caucasian | 13 | 6 | 19 |
| African American | 14 | 2 | 16 |
|
| |||
| Vascular Involvement | |||
| MCA | 24 | 8 | 32 |
| PCA | 3 | 0 | 3 |
|
| |||
| Lesion Volume | |||
| Total (mean/sd) | 35.15 (70.15) | 68.09 (107.49) | 42.68 (79.62) |
| Minimum | 19.28 | 21.95 | 19.28 |
| Maximum | 314.12 | 324.21 | 324.21 |
|
| |||
| Sensory Impairment | |||
| Moderate-Severe | 5 | 3 | 8 |
| Mild | 7 | 2 | 9 |
| None | 15 | 3 | 18 |
|
| |||
| Proprioception Impairment | |||
| Yes | 4 | 2 | 6 |
| No | 23 | 6 | 29 |
|
| |||
| Viewer-Centered Neglect* | |||
| Yes | 5 | 4 | 9 |
| No | 22 | 4 | 26 |
|
| |||
| Stimulus-Centered Neglect | |||
| Yes | 2 | 2 | 4 |
| No | 25 | 6 | 31 |
|
| |||
| Object-Centered Neglect | |||
| Yes | 0 | 0 | 0 |
| No | 27 | 8 | 35 |
p<.05 Significant difference between group means
To rule out the potential of co-morbid behavioral impairments explaining any group differences, we evaluated all subjects for signs of neglect, sensation impairment, and proprioception impairment. The only difference found was that there was a higher percentage of individuals with viewer-centered neglect in the AHP group (50%) than in the Non-AHP group (18.5%; χ2 (3, N=37) = 6.39, p=.041). There were no differences found for stimulus-centered neglect or object-centered neglect. Similarly, there were no significant group differences for percentage with sensory impairments or proprioceptive impairments. No subjects showed any distinct signs of personal neglect.
Primary Analyses
A series of Fisher’s exact tests for each ROI revealed statistically significant group differences in two areas after Bonferroni correction for multiple comparisons: BA 44/45 and BA 47 – all in the posterior inferior frontal cortex (Table 2). The only area found to be uniquely involved for the AHP Group was BA 47. Six out of the eight individuals in the AHP Group had BA 47 involvement, as opposed to none of the individuals in the Non-AHP Group (p<.0001).
Table 2.
Regions of Interest
| Non-AHP Group Dysfunctional tissue | AHP Group Dysfunctional tissue | ||||
|---|---|---|---|---|---|
| Brain area | Not Present | Present | Not Present | Present | Fisher’s Exact (corrected) |
| Precuneus (BA7) | 22 | 5 | 5 | 3 | p=.35 |
| Inferior Frontal Gyrus (IFG), Pars Orbitalis (BA 47)** | 27 | 0 | 2 | 6 | p<.0005 |
| IFG, Pars Triangularis, Pars Opercularis* (BA44 & BA45) | 24 | 3 | 2 | 6 | p=.012 |
| Post-Central Gyrus (Somatosensory Cortex) (BA 1/2/3) | 19 | 8 | 1 | 7 | p=.11 |
| Precentral Gyrus (Primary Motor Cortex) (BA4) | 24 | 3 | 4 | 4 | p=.33 |
| Premotor (BA6) | 19 | 8 | 2 | 6 | p=.39 |
| Internal Capsule | 12 | 15 | 0 | 8 | p=.30 |
| Basal Ganglia | 13 | 14 | 1 | 7 | p=1.0 |
| Thalamus | 24 | 3 | 7 | 1 | p=1.0 |
| Insular Cortex | 15 | 12 | 1 | 7 | p=.47 |
p<.05
p<.001
Significant difference between groups in number of patients with tissue abnormality in the area, after Bonferroni correction for multiple comparisons
Figure 1 shows an spatial probability maps of tissue abnormality lesions for the AHP Group (Panel A) and the Non-AHP Group (Panel B). Fisher exact test statistical map of lesion-deficit association (range is between-log(p) 4 to 11) found maximal value of 10.9 located in pars orbitalis (BA 47). In this selected location, six out of eight patients with anosognosia had lesion, whereas none of the unaffected subjects had a lesion in BA 47. See Figure 2 for the images associated with this voxel-wise analysis. Fisher Exact test results are provided for voxels with at least three lesions (281 360 voxels – Panel F), Liebermeister test results are provided with false discovery rate corrected thresholds (Panel Panel G- Liebermeister Z=2.45 − p<0.01). Other voxels significantly associated with AHP after correction were in anterior insula.
Figure 2.
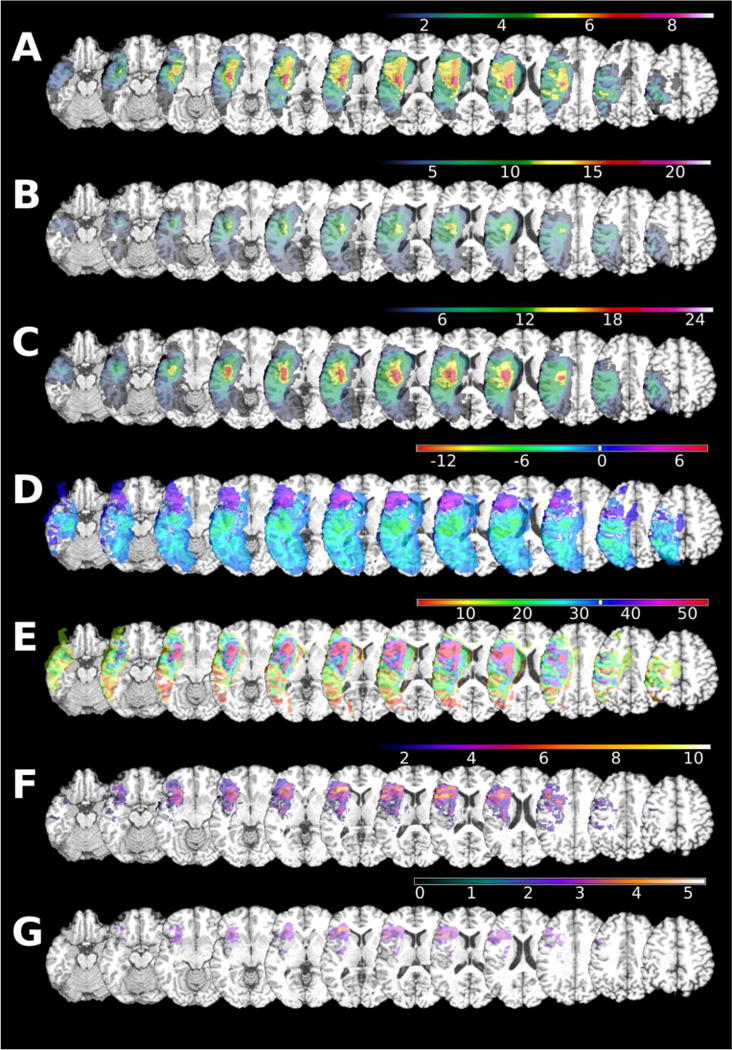
Mapping of voxel-bases analysis for presence of tissue abnormality for a given voxel.
Image orientation is in radiological convention. Maxima location is reported in Montreal Neurological Institute coordinate space. A – frequency of the lesions in AHP group (n=8); B – spatial distribution of lesion in non-AHP (n=27); C – spatial distribution of lesions in all patients (n=35); D – Difference in lesion count between AHP group and non-AHP group in absolute lesion count; E – difference in percentage of lesion count (single case is ~13% in AHP group, single case is 4% in non-AHP group); F – Fischer exact map -log(p) map; G – FDR corrected Liebermeister test results
Discussion
The current study findings provide preliminary support for unique involvement of the pars orbitalis (BA 47) of the right inferior frontal gyrus (IFG) in AHP. Seventy-five percent (six out of eight) of individuals with AHP had dysfunctional tissue in that area, whereas no subjects without AHP had any involvement in this region. Our whole brain, voxel-wise analysis confirmed the results of our ROI analysis, revealing the strong association between AHP and tissue abnormality in the IFG, particularly the pars orbitalis (BA 47). There were no other areas outside of our ROIs, or parts of our ROIs, that were strongly associated with AHP. Pars orbitalis (BA 47) has been shown to be involved in motor attention (evaluation of task relevant motor behavior; Enriquez-Geppert, et al., 2013) and to be integrated into a network for motor inhibition (Rubia, et al. 2001). It has been found to coactivate with the superior temporal gyrus and inferior parietal lobule in motor inhibition tasks (Enriquez-Geppert, et al., 2013; Rubia, et al., 2001) as well as with the dorsal anterior cingulate cortex for detection of motor errors and feedback for evaluation of task relevant behavior (inhibit or not to inhibit; Enriquez-Geppert, et al., 2013).
Along with pars orbitalis (BA 47), pars opercularis (BA44) and pars triangularis (BA45) make up the right IFG in the orbital frontal region that is also known as the frontal operculum. In the current study, right BA 44 and 45 (pars opercularis and pars triangularis) were also found to be lesioned more frequently in individuals with AHP (75%) than without AHP (11%). BA44 and 45 have tight connections within a fronto-subcortical circuit (rIFG, striatum, pallidum, thalamus, and motor regions) that underlie a motor inhibition network (Nambu, Tokuno, & Takada, 2002). Lesions in BA 44 (IFG, pars opercularis), known to be involved in the action monitoring, has previously been shown to be associated with AHP (Berti, et al., 2005).
Consistently in the literature, the right frontal operculum (right IFG) has been found to be strongly implicated in motor response inhibition and action monitoring (Rubia, Smith, Brammer, & Taylor, 2003). Tsakiris and colleagues (Tsakiris, Hesse, Boy, Haggard, & Fink, 2007) suggest that this region underlies body ownership similarly to the right posterior insular cortex and is involved in detection and resolution of various conflicting signals between internal and external representations of body-related events (Tsarkiris, et al., 2007). The frontal operculum receives inputs from multiple sensory modalities, and these cells respond to characteristics of stimuli in regards to emotional latency leading to reward/aversion behaviors (Cavada, et al., 2000; O’Doherty, Critchley, Deichmann, & Dolan, 2003). Enriquez-Geppert and colleagues (Enriquez-Geppert, et al. 2013) suggest that the frontal operculum is involved in a larger functional network of motor attention, monitoring, and inhibition that allows human beings “to differentiate between relevant and irrelevant information to guide performance, [which] often require[s] selective stops and changes of motor responses” (p. 1512). Overall, the current data suggest that frontal operculum may play a key role in awareness of limb functioning. By studying hypoperfused tissue as well as the infarct, we were able to capture the entire area of tissue abnormality that may be causing the deficit in the acute phase of recovery from stroke. We believe that this approach allowed us to provide further evidence that the frontal operculum in particular is a critical part of the circuitry underlying awareness and monitoring of motor output.
Despite our predictions, we did not find a statistically significant difference between groups in the percentage of individuals with dysfunctional tissue in the insular cortex, after correction for multiple comparisons. However, there was a trend for an association with AHP: dysfunctional tissue in the insular cortex was found in 86% of subjects with AHP, but only in 44% of subjects with hemiplegia without AHP. Our failure to detect a significant association between insular tissue abnormality and AHP may be due to the high rate of tissue abnormality in the insula even in participants without AHP. The insula is one of the most commonly infarcted areas in large MCA stroke. In this analysis, we did not segment the insular cortex; leaving open the possibility that only anterior insular cortex or only posterior insular cortex has a critical role in AHP. In fact, our voxel-based analysis of infarcted and/or hypoperfused tissue did reveal an association between a small part of the anterior insula and AHP. This finding is consistent with at least some previous literature, although earlier studies have been mixed regarding the role of the insula in AHP. Some have reported a clear association between right anterior insular lesions and AHP (Berti, et al., 2005; Fotopoulou, et al., 2010; Vocat et al., 2010), while others have reported that right posterior insular lesions are disproportionately associated with AHP (Baier & Karnath, 2008; Karnath, Baier, & Nägele, 2005). It appears that at least part of right anterior insular cortex is involved in this circuitry, consistent with the widely accepted role of the insula in re-representation of sensory, motor, and interoceptive information (see Tsakiris, 2010, for a review) and its proposed role in a first-person “self-representation” (Craig, 2009). There are also strong reciprocal connections between the insular cortex and the frontal operculum through efferent and afferent projections (for a review, see Augustine, 1996).
Consistent with recent findings of Fotopoulou and colleagues’ (Fotopoulou, et al., 2010), we did not find a significant association between AHP and the dorsal premotor area (BA 6). This finding contrasts with the 2005 study findings of Berti and colleagues (Berti, et al, 2005) in which BA 6 was the area most frequently associated with AHP. However, like Berti and colleagues (Berti, et al., 2005), we did find that areas 44/45 were more frequently involved in the AHP Group than the Non-AHP Group. Areas 4, 6, and 44 are all part of the agranular frontal cortex that is known to be involved in motor programming. BA 4 and 6 are often infarcted along with 44, 45 and 47, and may cause the motor deficit, while the lesion in BA 44, 45, and 47 may be responsible for the anosognosia. Obviously, one cannot have AHP without both the hemiplegia and the anosognosia. It should be noted that one of the main differences between the current study and the 2005 study by Berti and colleagues (Berti, et al., 2005) is consideration of chronicity of the patients studied. Berti and colleagues did not specify how far post onset of stroke were the subjects in their study. More importantly, it is clear in their study that they only assessed infarcted tissue whereas in the current study, we evaluated the infarct and the hypoperfused tissue around it as both are known to lead to deficits. This difference may explain, at least in part, the differences in findings between the two studies.
Hemispatial neglect (any type) was found to co-occur in 50% of individuals with AHP and 18.5% of individuals without AHP in the current study. It should be noted that none of the subjects in this study showed behavioral signs of personal neglect upon evaluation. Of potentially more importance, two subjects with AHP and lesion/hypoperfused tissue in BA47 did not have any type of neglect. The current data support past findings that hemispatial neglect and proprioceptive deficits can co-occur with AHP, but are not necessary, nor sufficient for AHP (Adair, et al, 1995; Bisiach, et al., 1986; Heilman, Barrett, & Adair, 1998; Maeshima, et al., 1997; Small & Ellis, 1996; Starkstein, et al., 1992; Vocat, et al., 2010). It is acknowledged, however, that these concomitant impairments may contribute to the clinical presentation of persistent AHP (Heilman, et al., 1998).
The current data do not support the importance of some regions of the brain that have been previously hypothesized to play a key role in awareness of limb movement. In the literature, there is some evidence that damage to the basal ganglia are associated with AHP (Small & Ellis, 1996; Fotopoulou, et al., 2010; Vocat, et al., 2010). In the current study, 86% of the subjects with AHP had lesions in the basal ganglia, but so did 52% of the subjects in the non-AHP group, resulting in a non-significant association. However, we did not distinguish which specific structure in the basal ganglia was lesioned in each case (which would have yielded even lower power to detect an association). It may be that specific structures are part of the motor awareness circuitry, and if we had distinguished between the different areas, and had had enough patients with lesions in each structure, an association with AHP might have been detected. It is also possible that given the shared vascular supply to the regions hypothesized to be included in the AHP circuit and the basal ganglia (middle cerebral artery), it may be argued that the cooccurrence is more a product of anatomical contiguity or shared vascular supply. However, the basal ganglia subserve many complex cognitive and motor abilities, and so future studies with larger numbers of patients with basal ganglia strokes should investigate whether involvement of particular structures of the basal ganglia play key roles in the impaired awareness of the motor impairment.
In considering the differences in this study in light of the previous literature, there are two main methodological differences that need to be considered. First, in the current study, we identified both areas of infarct and areas of significant hypoperfusion to determine the entire area of dysfunctional tissue, and then evaluated group differences (AHP versus non-AHP); whereas earlier studies identified infarcted tissue alone. Second, most past studies have been conducted in the subacute or chronic phase of recovery (with the exception of the Vocat, et al, 2010 study), and there is evidence that the underlying areas of “damage” associated with AHP may change over time (Vocat, et al., 2010). So it may be that the findings from the current study reflect the critical areas of dysfunctional tissue that result in AHP in the hyperacute phase of recovery (before reorganization or recovery), whereas previous research more collectively reflects the relationship between infarcted tissue and AHP in the acute (Vocat et al., 2010) or subacute/chronic phase of recovery.
One limitation of the current study was that the current sample was characterized by limited spatial distribution of tissue abnormality and therefore did not afford us the opportunity to evaluate all the areas that have been found or at least proposed in the past to be involved in the circuitry underlying motor awareness. Within the current sample, all eight individuals with AHP had involvement of the MCA. This is likely the result of several factors, with the most notable being that most anterior cerebral artery (ACA) and posterior cerebral artery (PCA) strokes do not result in hemiplegia (although PCA strokes can result in hemiplegia with involvement of the ventolateral or “motor” thalamus; and ACA strokes often result in leg weakness). Another factor may be that ACA and PCA strokes do not occur as frequently as MCA stroke (Suzuki, et al., 1987). Consequently, our sample did not afford us access to a large enough sample of individuals with ACA or PCA strokes to investigate the role of all areas of interest for AHP. It should be noted that the one individual in the non-AHP group with cingulate involvement secondary to PCA hypoperfusion did not demonstrate any signs of anosognosia. There were also three individuals in the non-AHP group with thalamic involvement who also did not demonstrate AHP. It may be that circumscribed lesions in these sections of the circuit may not be sufficient to lead to AHP without involvement of other areas of the circuitry. It also may be that the specific lesions in these individuals did not include the essential nuclei or portion of the brain that completes the AHP circuitry. Future research needs to include a larger sample of individuals with right hemisphere stroke who have involvement of PCA and ACA, as well as MCA territories. Additionally, further research should investigate whether specific nuclei and regions of structures play a specific role in AHP.
Another consideration when interpreting the current findings is the size of the lesion and the possibility that in this hyperacute phase of recovery, there may be down-regulation of both hemispheres. This is important to consider metabolic down-regulation of brain functioning as it can lead to behavioral impairment (Perani, Vallar, Paulesu, Alberoni, & Fazio, 1993). It may be that the behavioral manifestation of AHP is not just rooted in the areas found, but also secondary to the metabolic down-regulation in both hemispheres in this hyperacute phase of recovery.
Finally, future research should more closely investigate the impact of age at time of stroke to the presentation of AHP. In the current study, the AHP group was, on average, a solid decade older than the non-AHP group. It is known that aging is associated with atrophic changes in the brain across the healthy aging process and that these changes are associated with cognitive decline (Elderkin-Thompson, Ballmaier, Hellemann, Pham, & Kumar, 2008). Additionally, there is evidence to suggest that age is related to the severity of impairment. Most relevant to the current study, age has been found to be related to severity of neglect (Gottesman, et al., 2008) with increasing age being associated with more severe neglect independent of the size of the stroke. As noted previously, concomitant impairments, including neglect may contribute to the clinical presentation of persistent AHP (Heilman et al., 1998). It may be that there is more of a vulnerability of the brain in later decades of life to certain impairments because normal aging is associated with atrophic changes in the regions that underlie that cognitive function. Thus, one contribution to the individuals in the AHP group developing AHP after their stroke was the age-related changes in the frontal system that had already occurred. If this is the case, then age would then be a risk factor for developing AHP in those that suffer stroke.
In sum, converging data from our ROI analysis and our voxel-based analysis support the hypothesis that a circuit that includes the right inferior frontal gyrus, particularly pars orbitalis, contributes to AHP by possibly leading to disconnection between the monitoring of motor functioning and the evaluation of task relevant behavior (motor feedback to inform whether to inhibit or not inhibit a motor behavior). The IFG’s function is to monitor motor action in goal-oriented behavior and make in-the-moment changes by inhibiting based upon feedback (Enriquez-Geppert, et al., 2013). Without the ability to attend to the details of motor relevant behavior secondary to the dysfunctional pars orbitalis, decisions on whether to inhibit and not inhibit cannot be accurately made, particularly if the rest of the IFG is also dysfunctional. This finding is consistent with the feedback hypothesis of AHP proposed by Frith, Blakemore, and Wolport (2000) in which patients with AHP fail to register the incongruence between the predicted and actual motor-sensory experience. Without proper evaluation of the motor relevant behavior, the mis-match between the mental representation of the motor movement and intended movement is not detected (Fotopoulou, et al., 2008; Jehkonen, et al., 2000; Krams, et al., 1998; Stephan, et al., 1995; Tsakiris, et al., 2007). Consequently, the expectations of how the limb should “feel” during the movement are not updated and the motor plan is not changed accordingly (Fotopoulou, et al., 2008; Frith, Blakemore, & Wolpert, 2002). As such, the patient believes that the limb moved. Therefore, awareness of motor functioning is most likely a complex process that requires integration of sensory-motor feedback in order to constantly update the system on the functioning of the limb during motor behavior.
Acknowledgments
We would like to thank Dr. Amy Bastian for her contributions made in study design and implementation, particularly for the evaluation of motor and sensory impairments. The research reported in this study was supported by HD052774-01A1 to KBK and by R01 NS047691 to AEH, and by the Polish National Science Centre grant 2011/01/D/NZ4/05801 to MAP.
This work was supported in part by grants from the National Institutes of Health, K23 HD052774-04 to KK and R01 NS04691 to AH and by the National Center for Medical Rehabilitation Research
References
- Adair JC, Na DL, Schwartz RL, Fennell EM, Gilmore RL, Heilman KM. Anosognosia for hemiplegia: Test of the personal neglect hypothesis. Neurology. 1995;45:2195–2199. doi: 10.1212/wnl.45.12.2195. [DOI] [PubMed] [Google Scholar]
- Appelros P, Karlsson GM, Seiger Å, Nydevik I. Neglect and anosognosia after first-ever stroke: Incidence and relationship to disability. Journal of Rehabilitation Medicine. 2002;34:215–220. doi: 10.1080/165019702760279206. [DOI] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research: Brain Research Reviews. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Baier B, Karnath HO. Incidence and diagnosis of anosognosia for hemiparesis revisited. Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76:358–361. doi: 10.1136/jnnp.2004.036731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier B, Karnath HO. Tight link between our sense of limb ownership and self-awareness of actions. Stroke; a Journal of Cerebral Circulation. 2008;39:486–488. doi: 10.1161/STROKEAHA.107.495606. [DOI] [PubMed] [Google Scholar]
- Bell-Krotoski J, Tomancik E. The repeatability of testing with semmes-weinstein monofilaments. The Journal of Hand Surgery. 1987;12:155–161. doi: 10.1016/s0363-5023(87)80189-2. [DOI] [PubMed] [Google Scholar]
- Berti A, Bottini G, Gandola M, Pia L, Smania N, Stracciari A, Castiglioni I, Vallar G, Paulesu E. Shared cortical anatomy for motor awareness and motor control. Science (New York, NY) 2005;309:488–491. doi: 10.1126/science.1110625. [DOI] [PubMed] [Google Scholar]
- Berti A, Ladavas E, Della Corte M. Anosognosia for hemiplegia, neglect dyslexia, and drawing neglect: Clinical findings and theoretical considerations. Journal of the International Neuropsychological Society. 1996;2:426–440. doi: 10.1017/s135561770000151x. [DOI] [PubMed] [Google Scholar]
- Berti A, Ladavas E, Stracciari A, Giannarelli C, Ossola A. Anosognosia for motor impairment and dissociations with patients’ evalutaion of the disorder: Theoretical considerations. Cognitive Neuropsychology. 1998;3:21–44. [Google Scholar]
- Bisiach E, Vallar G, Perani D, Papagno C. Unawareness of disease following lesions of the right hemisphere: Anosognosia for hemiplegia and anosognosia for hemianopia. Neuropsychologia. 1986;24:471–482. doi: 10.1016/0028-3932(86)90092-8. [DOI] [PubMed] [Google Scholar]
- Carey LM, Matyas TA, Oke LE. Evaluation of impaired fingertip texture discrimination and wrist position sense in patients affected by stroke: Comparison of clinical and new quantitative measures. Journal of Hand Therapy: Official Journal of the American Society of Hand Therapists. 2002;15:71–82. doi: 10.1053/hanthe.2002.v15.01571. [DOI] [PubMed] [Google Scholar]
- Carey LM, Oke LE, Matyas TA. Impaired limb position sense after stroke: A quantitative test for clinical use. Archives of Physical Medicine and Rehabilitation. 1996;77:1271–1278. doi: 10.1016/s0003-9993(96)90192-6. [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cerebral Cortex (New York, NY: 1991) 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Chen R, Hillis AE, Pawlak M, Herskovits EH. Voxelwise Bayesian lesion-deficit analysis. Neuroimage. 2008;40:1633–42. doi: 10.1016/j.neuroimage.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchini G, Beschin N, Cameron A, Fotopoulou A, Della Sala S. Anosognosia for motor impairment following left brain damage. Neuropsychology. 2009;23:223–230. doi: 10.1037/a0014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchini G, Beschin N, Sala SD. Chronic anosognosia: A case report and theoretical account. Neuropsychologia. 2002;40:2030–2038. doi: 10.1016/s0028-3932(02)00054-4. [DOI] [PubMed] [Google Scholar]
- Coslett HB. Anosognosia and body representations forty years later. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2005;41:263–270. doi: 10.1016/s0010-9452(08)70912-2. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel–now? The anterior insula and human awareness. Nature Review: Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Crinion J, Holland AL, Copland DA, Thompson CK, Hillis AE. Neuroimaging in aphasia treatment research: quantifying brain lesions after stroke. Neuroimage. 2013;73:208–14. doi: 10.1016/j.neuroimage.2012.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting J. Study of anosognosia. Journal of Neurology, Neurosurgery, and Psychiatry. 1978;41:548–555. doi: 10.1136/jnnp.41.6.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H, Damasio A. Lesion analysis in neuropsychology. New York: Oxford University Press; 1989. [Google Scholar]
- Dauriac-Le Masson V, Mailhan L, Louis-Dreyfus A, De Montety G, Denys P, Bussel B, Azouvi P. Double dissociation between unilateral neglect and anosognosia. [Double dissociation entre negligence unilaterale gauche et anosognosie] Revue Neurologique. 2002;158:427–430. [PubMed] [Google Scholar]
- Elderkin-Thompson V, Ballmaier M, Hellemann G, Pham D, Kumar A. Executive function and MRI prefrontal volumes among healthy older adults. Neuropsychology. 2008;22:626–37. doi: 10.1037/0894-4105.22.5.626. [DOI] [PubMed] [Google Scholar]
- Enriquez-Geppert S, Eichele T, Specht K, Kugel H, Pantev C, Huster RJ. Functional parcellation of the inferior frontal and midcingulate cortices in a flanker-stop-change paradigm. Human Brain Mapping. 2013;34:1501–1514. doi: 10.1002/hbm.22002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotopoulou A, Pernigo S, Maeda R, Rudd A, Kopelman MA. Implicit awareness in anosognosia for hemiplegia: unconscious interference without conscious re-representation. Brain: A Journal of Neurology. 2010;133:3564–3577. doi: 10.1093/brain/awq233. [DOI] [PubMed] [Google Scholar]
- Fotopoulou A, Tsakiris M, Haggard P, Vagopoulou A, Rudd A, Kopelman M. The role of motor intention in motor awareness: An experimental study on anosognosia for hemiplegia. Brain: A Journal of Neurology. 2008;131:3432–3442. doi: 10.1093/brain/awn225. [DOI] [PubMed] [Google Scholar]
- Frith C, Blakemore S, Wolpert D. Abnormalities in the awareness and control of actions. Philosophical Translational Royal Society of London Biological Sciences. 2002;355:1771–88. doi: 10.1098/rstb.2000.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. I. Brain: A Journal of Neurology. 1965a;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. II. Brain: A Journal of Neurology. 1965b;88:585–644. doi: 10.1093/brain/88.3.585. [DOI] [PubMed] [Google Scholar]
- Gialanella B, Monguzzi V, Santoro R, Rocchi S. Functional recovery after hemiplegia in patients with neglect: The rehabilitative role of anosognosia. Stroke; a Journal of Cerebral Circulation. 2005;36:2687–2690. doi: 10.1161/01.STR.0000189627.27562.c0. [DOI] [PubMed] [Google Scholar]
- Gottesman R, Kleinman J, Davis C, Heidler-Gary J, Newhart M, Kannan V, Hillis A. Unilateral neglect is more severe and common in older patients with right hemispheric stroke. Neurology. 2008;71:1439–1444. doi: 10.1212/01.wnl.0000327888.48230.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman-Maeir A, Soroker N, Katz N. Anosognosia for hemiplegia in stroke rehabilitation. Neurorehabilitation & Neural Repair. 2001;15:213–222. doi: 10.1177/154596830101500309. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Barrett AM, Adair JC. Possible mechanisms of anosognosia: A defect in self-awareness. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 1998;353:1903–1909. doi: 10.1098/rstb.1998.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KM, Harciarek M. Anosogonosia and anosdiaphoria of weakness. In: Prigatano GP, editor. The Study of Awareness. New York New York: Oxford University Press; 2010. pp. 89–112. [Google Scholar]
- Herskovits EH. An architecture for a brain-image database. Methods of Information in Medicine. 2000;39:291–297. [PubMed] [Google Scholar]
- Hillis AE, Caramzza A. The compositionality of lexical semantic representations: clues from semantic errors in object naming. Memory. 1995;3:333–358. doi: 10.1080/09658219508253156. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Wityk RJ, Tuffiash E, Beauchamp NJ, Jacobs MA, Barker PB, Selnes OA. Hypoperfusion of Wernicke’s area predicts severity of semantic deficit in acute stroke. Annals of Neurology. 2001;50:561–566. doi: 10.1002/ana.1265. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Wityk RJ, Barker PB, Beauchamp NJ, Gailloud P, Murphy K, Cooper O, Metter EJ. Subcortical aphasia and neglect in acute stroke: The role of cortical hypoperfusion. Brain: A Journal of Neurology. 2002;125:1094–1104. doi: 10.1093/brain/awf113. [DOI] [PubMed] [Google Scholar]
- Jehkonen M, Ahonen J, Dastidar P, Laippala P, Vilkki J. Unawareness of deficits after right hemisphere stroke: Double-dissociations of anosognosias. Acta Neurologica Scandinavica. 2000;102:378–384. doi: 10.1034/j.1600-0404.2000.102006378.x. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady JM, Smith SM. Improved Optimisation for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson PM, Edelstyn NM, Ellis SJ. Imagining the impossible: Motor representations in anosognosia for hemiplegia. Neuropsychologia. 2009;47:481–488. doi: 10.1016/j.neuropsychologia.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Karnath H, Baier B, Nägele T. Awareness of the functioning of one’s own limbs mediated by the insular cortex? The Journal of Neuroscience. 2005;25:7134–7138. doi: 10.1523/JNEUROSCI.1590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krams M, Rushworth MF, Deiber MP, Frackowiak RS, Passingham RE. The preparation, execution and suppression of copied movements in the human brain. Experimental Brain Research. Experimentelle Hirnforschung. Experimentation Cerebrale. 1998;120:386–398. doi: 10.1007/s002210050412. [DOI] [PubMed] [Google Scholar]
- Levine DN, Calvanio R, Rinn WE. The pathogenesis of anosognosia for hemiplegia. Neurology. 1991;41:1770–1781. doi: 10.1212/wnl.41.11.1770. [DOI] [PubMed] [Google Scholar]
- Maeshima S, Dohi N, Funahashi K, Nakai K, Itakura T, Komai N. Rehabilitation of patients with anosognosia for hemiplegia due to intracerebral haemorrhage. Brain Injury. 1997;11:691–697. doi: 10.1080/026990597123232. [DOI] [PubMed] [Google Scholar]
- Marcel AJ, Tegnér R, Nimmo-Smith I. Anosognosia for plegia: Specificity, extension, partiality and disunity of bodily unawareness. Cortex. 2004;40:19–40. doi: 10.1016/S0010-9452(08)70919-5. [DOI] [PubMed] [Google Scholar]
- Marshall J. Brain function: Neural adaptations and recovery from injury. Annual Review of Psychology. 1984;35:377-377–408. doi: 10.1146/annurev.ps.35.020184.001425. [DOI] [PubMed] [Google Scholar]
- Medina J, Kannan V, Pawlak MA, Kleinman JT, Newhart M, Davis C, Heidler-Gary JE, Herskovits EH, Hillis AE. Neural substrates of visuospatial processing in distinct reference frames: evidence from unilateral spatial neglect. Journal of Cognitive Neuroscience. 2009;21:2073–2084. doi: 10.1162/jocn.2008.21160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M. The evolving landscape of human cortical connectivity: Facts and inferences. Neuroimage. 2012;62:1282–1289. doi: 10.1016/j.neuroimage.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Wall J, Nelson RJ, Sur M, Felleman D. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983;8:33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuna H, Takada M. Functional significance of the cortico-subthalamo-pallidal “hyperdirect” pathway. Neuroscience Research. 2002;43:11–117. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- Nathanson M, Bergman PS, Gordon GG. Denial of illness; its occurrence in one hundred consecutive cases of hemiplegia. AMA Archives of Neurology and Psychiatry. 1952;68:380–387. [PubMed] [Google Scholar]
- O’Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2003;23:7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota H, Fujii T, Suzuki K, Fukatsu R, Yamadori A. Dissociation of body-centered and stimulus-centered representations in unilateral neglect. Neurology. 2001;57:2064–2069. doi: 10.1212/wnl.57.11.2064. [DOI] [PubMed] [Google Scholar]
- Pedersen PM, Jørgensen HS, Nakayama H, Raaschou HO. Frequency, determinants, and consequences of anosognosia in acute stroke. Journal of Neurologic Rehabilitation. 1996;10:243–250. doi: 10.1177/154596839601000404. [DOI] [Google Scholar]
- Perani D, Vallar G, Paulesu E, Alberoni M, Fazio F. Left and right hemisphere contribution to recovery from neglect after right hemisphere damage–an [18F]FDG pet study of two cases. Neuropsychologia. 1993;31:115–25. doi: 10.1016/0028-3932(93)90040-7. [DOI] [PubMed] [Google Scholar]
- Pia L, Neppi-Modona M, Ricci R, Berti A. The anatomy of anosognosia for hemiplegia: A meta-analysis. Cortex. 2004;40:367–377. doi: 10.1016/S0010-9452(08)70131-X. [DOI] [PubMed] [Google Scholar]
- Prigatano GP, Wong JL. Cognitive and affective improvement in brain dysfunctional patients who achieve inpatient rehabilitation goals. Archives of Physical Medicine and Rehabilitation. 1999;80:77–84. doi: 10.1016/s0003-9993(99)90311-8. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behavioural Neurology. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha LJ. Improving lesion-symptom mapping. Cognitive Neuroscience. 2007;19:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Rorden C, Bonilha L, Nichols TE. Rank-order versus mean based statistics for neuroimaging. Neuroimage. 2007;35:1531–1537. doi: 10.1016/j.neuroimage.2006.12.043. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Simmons A, Williams SC, Giampietro V, Andrew CM, Taylor E. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Small M, Ellis S. Denial of hemiplegia: An investigation into the theories of causation. European Neurology. 1996;36:353–363. doi: 10.1159/000117293. [DOI] [PubMed] [Google Scholar]
- Sobesky J, Weber OZ, Lehnhardt FG, Hesselmann V, Thiel A, Dohmen C, Heiss WD. Which time-to-peak threshold best identifies penumbral flow? A comparison of perfusion-weighted magnetic resonance imaging and positron emission tomography in acute ischemic stroke. Stroke. 2004;35(12):2843–2847. doi: 10.1161/01.STR.0000147043.29399.f6. [DOI] [PubMed] [Google Scholar]
- Starkstein S, Fedoroff J, Price T, Leiguarda R, Robinson R. Anosognosia in patients with cerebrovascular lesions. A study of causative factors. Stroke. 1992;23:1446–1453. doi: 10.1161/01.str.23.10.1446. [DOI] [PubMed] [Google Scholar]
- Stephan KM, Fink GR, Passingham RE, Silbersweig D, Ceballos-Baumann AO, Frith CD, Frackowiak RS. Functional anatomy of the mental representation of upper extremity movements in healthy subjects. Journal of Neurophysiology. 1995;73:373–386. doi: 10.1152/jn.1995.73.1.373. [DOI] [PubMed] [Google Scholar]
- Stone SP, Halligan PW, Greenwood RJ. The incidence of neglect phenomena and related disorders in patients with an acute right or left hemisphere stroke. Age and Ageing. 1993;22:46–52. doi: 10.1093/ageing/22.1.46. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Kutsuzawa T, Takita K, Ito M, Sakamoto T, Hirayama A, Ito T, Ishida T, Ooishi H, Kawakami K. Clinico-epidemiologic study of stroke in Akita, Japan. Stroke. 1987;18:402–406. doi: 10.1161/01.str.18.2.402. [DOI] [PubMed] [Google Scholar]
- Tsakiris M. My body in the brain: A neurocognitive model of body-ownership. Neuropsychologia. 2010;48:703–712. doi: 10.1016/j.neuropsychologia.2009.09.034. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Hesse MD, Boy C, Haggard P, Fink GR. Neural signatures of body ownership: A sensory network for bodily self-consciousness. Cerebral Cortex. 2007;17(10):2235–2244. doi: 10.1093/cercor/bhl131. [DOI] [PubMed] [Google Scholar]
- Venneri A, Shanks MF. Belief and awareness: Reflections on a case of persistent anosognosia. Neuropsychologia. 2004;42:230–238. doi: 10.1016/S0028-3932(03)00171-4. [DOI] [PubMed] [Google Scholar]
- Vocat R, Staub F, Stroppini T, Vuilleumier P. Anosognosia for hemiplegia: a clinical-anatomical prospective study. Brain. 2010;133:3578–97. doi: 10.1093/brain/awq297. [DOI] [PubMed] [Google Scholar]
- Wall JT. Variable organization in cortical maps of the skin as an indication of the lifelong adaptive capacities of circuits in the mammalian brain. Trends in Neurosciences. 1988;11:549–557. doi: 10.1016/0166-2236(88)90184-1. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. intersubject validation of linear and nonlinear models. Journal of Computer Assisted Tomography. 1998;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]


