Introduction
The clinical diagnosis of psychopathy describes individuals who suffer from a profound affective deficit, including shallow emotion and inability to experience empathy, guilt or remorse. These behavioral deficits are believed to predispose psychopaths to high rates of criminal transgression and recidivism (Hare, 1999). It is estimated that 1% of the general population meet criteria for the disorder, whereas psychopaths constitute 15–25% of the prison population and commit over 50% more criminal offenses than non-psychopathic prisoners (Hare, 1996, Hare, 1999). In light of this inclination to violence and criminality, the ability to use neuroimaging techniques to delineate the neurobiology of psychopathy has profound implications for improved management of the disorder. Importantly, two somewhat distinct groups of traits underlie psychopathy: one involving blunted affect and reduced concern for others (Factor 1), and the other involving impulsivity and antisocial actions (Factor 2) (Hare, Clark, Grann, & Thornton, 2000; Hare, 1996; Kiehl, 2006). Thus, identifying how regional changes in brain activity differentially relate to Factor 1 or Factor 2 psychopathy scores could help inform more targeted treatment, rehabilitation, and risk management techniques for psychopaths.
Over the past decade, several studies have linked activity differences in the brain’s default mode network (DMN) to several clinical disorders, including schizophrenia (Calhoun, Kiehl, & Pearlson, 2008; Garrity, Pearlson, McKiernan, Lloyd, Kiehl, & Calhoun, 2007), attention deficit hyperactivity disorder (ADHD) (Castellanos et al., 2008; Uddin et al., 2008), and major depression (Greicius et al., 2007; Sheline et al., 2009). The DMN is a large-scale network composed of spatially distinct brain regions, including the posteromedial cortex and medial prefrontal cortex, that exhibit coherent fluctuations of activity at low frequencies (Raichle et al., 2001). In healthy individuals, the DMN is predominantly active during rest—particularly when the subject is engaged in self-referential processing (Buckner & Carroll, 2007; Northoff et al., 2006). In contrast, during externally-focused tasks, the DMN generally deactivates, a process that is thought to enable “task-positive” networks (i.e., the dorsal attention network) to become engaged with minimal interference (Raichle et al., 2001; Greicius, Krasnow, Reiss, & Menon, 2003; Fox et al., 2005). Notably, several of the aforementioned studies localized the DMN dysfunction to particular sub-nodes of the DMN (e.g., Garrity et al., 2007; Greicius et al., 2007). For example, using resting state functional connectivity, Greicius et al., (2007) found that the subgenual cingulate was a prominent part of the DMN network in depressed, but not control, subjects. Such studies suggest that a particular node of the DMN can play a key role in a psychological disorder, which may have broader ramifications for cognitive processing within the larger network.
In recent years, DMN dysfunction has also been linked to individuals with psychopathic tendencies (Juárez, Kiehl, & Calhoun, 2012; Pujol et al., 2012; Sheng, Gheytanchi, & Aziz-Zadeh, 2010). Specifically, studies have shown that the DMN in individuals with psychopathic tendencies remains relatively more active during externally-focused tasks (Pujol et al., 2012; Sheng et al., 2010), suggesting a possible failure to down-regulate intrinsic, self-referential brain activity (i.e., activity not directly related to identifiable sensory or motor events). In spite of this initial evidence for task-related DMN differences in individuals with psychopathic tendencies, the nature and extent of this dysfunction remains poorly characterized. For example, a previous functional magnetic resonance imaging (fMRI) study found that the medial prefrontal cortex (mPFC) sub-region of the DMN failed to deactivate in individuals with mild psychopathy (Pujol et al., 2011), whereas a separate fMRI study found that more posterior DMN sub-region (i.e., posterior cingulate) most strongly predicted psychopathy scores (Juarez et al., 2012). It is therefore unclear how extensive the attenuated deactivation is among psychopaths within the different DMN sub-regions. Moreover, Sheng et al. (2010) demonstrated that activity in these key DMN sub-regions was related to personality measures of self-concern and indifference towards consequences—both of which are hallmark psychopathic traits (Hare, 1996). However, they only examined healthy, non-psychopathic individuals, thereby limiting their claims regarding the relationship between DMN activity and psychopathy. Thus, fMRI studies have yet to determine how, in an externally-focused task, activity in various sub-regions of the DMN relates to psychopathic traits in a population of clinical psychopaths. To address these issues, we aimed to determine: 1) the extent to which specific nodes of the DMN are dysfunctional in psychopaths, and 2) how activity in the various DMN nodes relates to Factor 1 and Factor 2 sub-scores in psychopaths.
In the present study, we acquired fMRI data from prison inmates with varying degrees of psychopathy to examine regional brain activity differences during a cognitive task that is known to deactivate the DMN in healthy individuals (Liddle et al., 2011; Stawarczyk et al., 2011). During scanning, each participant completed a standard visual Go/NoGo task involving simple motor response inhibition. A group independent component analysis (ICA) was used to identify the DMN across all participants, which was then parcellated into six primary nodal regions-of-interest (ROIs), including the left/right posteromedial cortex (mPC), left/right medial prefrontal cortex (mPFC), and left/right lateral parietal cortex (LP). By sub-dividing the DMN, we were able to test for differences in nodal DMN activation between high psychopathic and low psychopathic prisoners, and how differences in activity within these DMN nodes were related to the affective versus behavioral traits that are characteristic of psychopathy.
Methods
Participant characteristics and group identification
The data used in this study was part of a larger project examining brain differences in 91 male prisoners. All participants completed an informed consent procedure approved by the University of New Mexico Human Research Review Committee, The New Mexico Corrections Department, and the Office of Human Research Protections (OHRP). We utilized the Hare Psychopathy Checklist-Revised (PCL-R) scale to quantify the degree of psychopathy in each participant, which is considered the “gold standard” for the clinical diagnosis of psychopathy (Hare, 1996; Hare, 1999; Hare, Clark, Grann, & Thornton, 2000). The PCL-R is an expert-rated scale based on information collected during a 2–4 hour interview and extensive collateral file review. The PCL-R includes 20 items, all of which are rated on a 0, 1, or 2 point system corresponding to absent, partial fit, or reasonably good fit for levels of the trait being found in most areas of the client’s life. This interview yields a total PCL-R score ranging from 0, being low-psychopathy, to 40, being extreme psychopathy. A PCL-R score of 30 is generally considered to be an appropriate cut-off score for psychopathy. However, it is noteworthy that several studies have used lower cut-off scores to classify psychopaths (e.g., Kiehl et al., 2001; Müller et al., 2008; Pujol et al., 2012). In order to maximize power for the group spatial ICA, we used a more liberal cut-off score of 281. A total of 22 participants had scores meeting or exceeding this threshold (M = 31.34, SD = 2.62, range: 28–37.6), and we henceforth refer to this group as the “High Psychopathy” group. Since we aimed to compare psychopathic prisoners with the typical individual in a prison population, we constructed a control group by selecting 22 participants with PCL-R scores centered on the sample median (Median = 20; M = 20.08, SD = 1.24, range: 18–22). All individuals in this group had a PCL-R score at or below the mean PCL-R from North American incarcerated samples, which is centered at 22. Henceforth, we refer to this group as the “Low Psychopathy” group. Individuals were excluded for the study if they reported any event that resulted in loss of consciousness for more than 10 minutes. Further exclusion criteria included: individuals with current axis I disorders, individuals with a history of psychosis psychopathology as assessed with the SCID, and individuals with a first degree relative with a history of psychosis.
To further control for potential confounds, we examined potential group differences in age, Wechsler Adult Intelligence Test (WAIS) IQ, number of years addicted to illegal substances, and ADD scores using the Brown Attention Deficit Disorder (ADD) Scale for Adults (Table 1). For years of addiction, data was not collected for 3 High Psychopathy and 4 Low Psychopathy subjects. For ADD scores, data was not collected for 7 High Psychopathy and 7 Low Psychopathy subjects. Independent-samples t-tests indicated that High and Low Psychopathy groups did not differ on WAIS-IQ, years of addiction, or Brown ADD scores (all Ps > .3), though they did significantly differ on age, t(42) = 2.34, p = .02. Thus, age was used as a covariate in all analyses.
Table 1.
Group characteristics for High Psychopathy and Low Psychopathy groups
| PCL- R Score |
Age | WAIS- IQ |
Years of Substance Abuse |
Brown ADD |
Factor 1 Scores |
Factor 2 Scores |
|
|---|---|---|---|---|---|---|---|
| High Psychopathy | 31.34 (2.6) | 28.73 (7.9) | 96.82 (11.5) | 19.61 (19.6) | 43.80 (22.8) | 10.5 (1.9) | 17.59 (1.4) |
| Low Psychopathy | 20.08 (1.2) | 34.77 (8.9) | 95.73 (14.7) | 18.41 (13.6) | 52.67 (24.9) | 5.95 (2.2) | 12.68 (1.8) |
Numbers outside parentheses represent group means, with standard deviations listed in parentheses.
Finally, we verified our results by re-analyzing the data after excluding subjects with a PCL-R below the more conservative PCL-R cut-off score of 30 for the High Psychopathy group. This resulted in group sizes of n = 15 and n = 22 for High Psychopathy and Low Psychopathy groups, respectively. The new results were generally consistent with the original analyses that differentiated the High and Low Psychopathy group based on a PCL-R cut-off score of 28 (see Supplemental Materials).
Experimental design
We used a standard Go/NoGo task with a ratio of 84/16 Go to NoGo responses (Kiehl et al., 2000). The letter ‘X’ was displayed as the cue for a Go trial (412 total trials), while a ‘K’ was used as the cue for a NoGo trial (78 total trials). Each stimulus appeared for 250 ms in white text within a continuously displayed rectangular fixation box. The participants were instructed to press a button with their right index finger every time a Go trial was presented and to not respond when a NoGo trial was presented. There were a total of 412 Go trials and 78 NoGo trials in the experiment. To optimize later deconvolution of the hemodynamic response function (HRF), the ITI period was jittered for 1s, 2s, or 3s. Typical spacing between consecutive NoGo signals was 5 trials, with a range of 4 to 7 trials.
Imaging data acquisition
The fMRI data was acquired on the Mind Research Network mobile Siemens Avanto 1.5 Tesla whole-body MRI scanner with a 12-channel parallel acquisition head coil. Two event-related fMRI runs were completed, with each run consisting of 220 volumes. Each run lasted for approximately 7 minutes.
Scanning parameters for the whole-brain T2* echo-planar imaging (EPI) sequence were as follows: 27 axial slices (4mm thick, 1mm gap), TR = 2000ms, TE = 39ms, flip angle = 65°, and 64×64 acquisition matrix. The effective voxel resolution was 3.75mm × 3.75mm × 5mm. The six initial images from stabilization period were discarded before post-processing. Visual stimuli were projected onto a ground glass screen located at the rear of the magnet bore by a digital projector. A mirror above the head coil allowed the subject to observe the projected image. Stimulus presentation was performed by the experiment-scripting program Presentation (Neurobehavioral Systems, Albany, CA) and synchronized to a TTL voltage trigger from the scanner.
A high-resolution T1-weighted anatomical image was also collected to aid with functional image co-registration. The 3D-T1 MPRAGE sequence had the following parameters: 128 sagittal slices, TR = 2530ms, TE = 1.64ms, flip angle = 7°, 256×192 acquisition matrix, and voxel resolution = 1×1×1.33mm3. Four 3D volume averages were conducted in-sequence to increase the signal-to-noise ratio.
Image preprocessing
Preprocessing for all images was completed in SPM5 (Wellcome Trust Centre for Neuroimaging, London, UK, http://www.fil.ion.ucl.ac.uk/spm) with all available software updates installed as of January, 2010. Realignment of the fMRI volumes was completed using the INRIAlign toolbox and an affine rigid-body spatial transformations with a Geman-McClure similarity measure (Freire, Roche, & Mangin, 2002). The realigned functional volumes were then co-registered to the high-resolution T1 anatomical image using an affine rigid-body spatial transformation with a mutual information similarity measure. The resulting images were normalized into a standard 3D stereotaxic space defined by the International Consortium for Brain Mapping [ICBM]-152 atlas space and were resampled into 3 × 3 × 3 mm, resulting in 53 × 63 × 46 voxels. These normalized fMRI volumes were smoothed with a 6mm full-width-at-half-maximum (FWHM) Gaussian smoothing kernel. In addition, a high-pass filter with a frequency cutoff of 128 seconds was used to remove low frequency signal drift from the data. No autocorrelation correction was applied.
Independent component analysis (ICA)
A group ICA was carried out on the task data using the MELODIC toolbox in the FSL software suite (Beckmann & Smith, 2004). This data-driven approach allowed us to delineate the DMN, which should intermittently deactive in a structured way according to task conditions (Greicius et al., 2003). No large spikes were seen in the ICA time courses of interest; thus, time series spikes were not edited prior to running the ICA. The group ICA was conducted across all 44 participants and fMRI runs for a total of 88 inputs. These inputs were temporally concatenated, and spatial components that accounted for signal variance across the group were produced using a mixture model approach. The concatenated 4D dataset was decomposed into spatial maps of structured component signals in the data (Beckmann & Smith, 2004), with no constraints on the number of dimensions estimated by the algorithm. All components were thresholded at an alternative hypothesis level of 0.95.
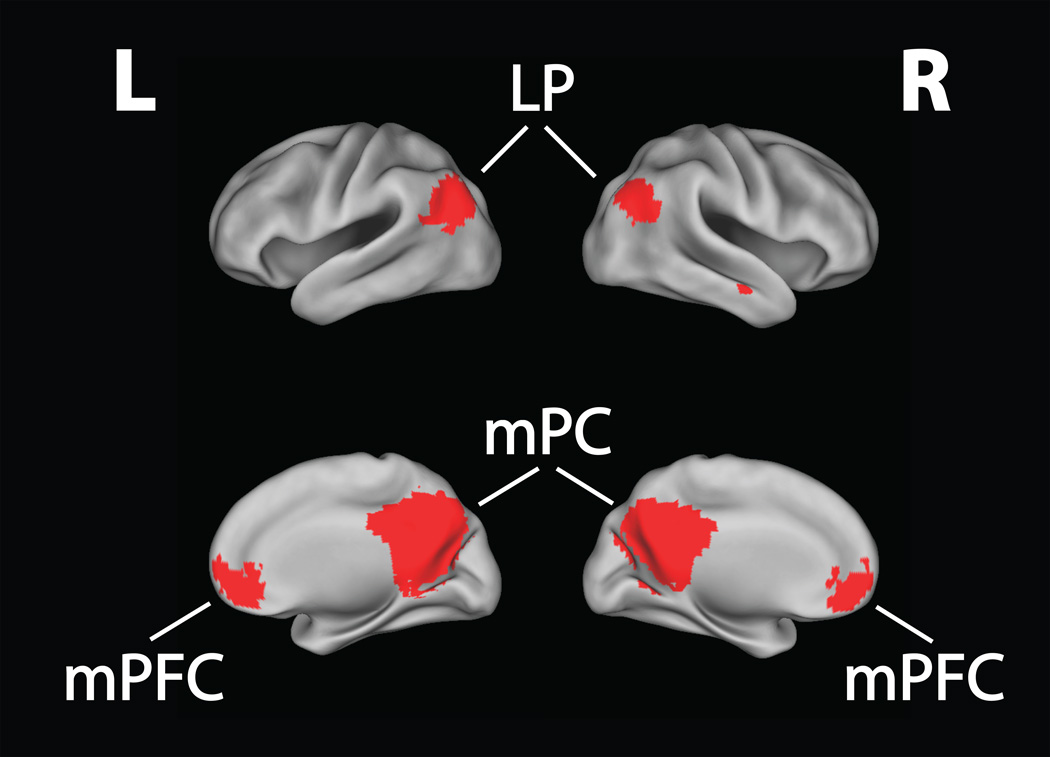
The ICA yielded a total of 32 components, with the first 10 components comprising 75% of the total explained variance. The range of explained variance was 16.09% (1st component) to 4.27% (32th component). To identify the DMN in our dataset, we performed a goodness-of-fit analysis by spatially cross-correlating each group component with a DMN template delineated by Smith and colleagues (2009). The best-fitting component had a correlation coefficient of .85, indicating very high overlap between our selected DMN and the Smith et al. (2009) DMN template (see Figure 1). Notably, this DMN component also explained the largest amount of variance among all 32 ICA components. In addition, the next highest correlation coefficient for a component was .38, which suggests that our selected DMN component had far higher overlap with the standard DMN template than any other ICA component.
Figure 1. Default Mode Network.
An independent components analysis (ICA) was used to identify the default mode network across our prison sample. The DMN was divided into six major nodes for a region-of-interest analysis. These nodes were: 1) right posteromedial cortex; 2) left posteromedial cortex; 3) right medial prefrontal cortex; 4) left medial prefrontal cortex; 5) right lateral parietal; 6) left lateral parietal. mPC = posteromedial cortex; mPFC = medial prefrontal cortex; LP = lateral parietal.
Default Mode Network region-of-interest (ROI) analysis
To dissociate task-induced differences in activation within different nodes of the DMN, we parcellated the entire network into six nodal ROIs: left/right mPC, which included the posterior cingulate cortex, precuneus, and retrosplenial cortex; left/right mPFC; and left/right lateral parietal (LP) regions. For each participant, mean Task-Baseline parameter estimates (betas) were extracted from each ROI using the MarsBaR toolbox (Brett, Anton, Valabregue, & Poline, 2002). ‘Task’ included Go and NoGo trials, while ‘Baseline’ represented the pre-stimulus interval. Before comparing mean ROI activity across groups, we examined mean task activity in relation to Baseline within each sub-group. The purpose of examining sub-groups was to replicate earlier findings that the DMN in healthy individuals deactivates during externally-focused tasks in the Low Psychopathy group and to examine whether or not similar deactivation occurred in the High Psychopathy group. To test this, we used one-sample t-tests comparing mean beta values for each ROI against zero for both the High Psychopathy and Low Psychopathy groups separately. We applied a Bonferroni correction for twelve comparisons (six regions × two groups), yielding an adjusted alpha of p < .0042.
Significant group differences in sub-regional DMN activity were assessed using a mixed-model Analysis of Covariance (ANCOVA) with Group (High Psychopathy/Low Psychopathy) as a between-subjects factor, DMN ROI (the six sub-regions) as a within-subjects factor, and Age as a covariate. Sub-regional group differences were then assessed using separate ANCOVA models for each ROI. To correct for multiple comparisons, we applied a Bonferroni correction for six statistical tests for an adjusted alpha of p < .0083.
Associations between psychopathic traits and task-induced DMN nodal activity
To examine whether activity in specific DMN sub-regions related to behavioral characteristics of psychopathy, PCL-R factor scores were calculated for each subject. The Factor 1 PCL-R score includes PCL-R items related to affective and interpersonal traits of psychopaths, while the Factor 2 score includes PCL-R items related to antisocial behavior and lifestyle (Table 2). Multiple linear regression analyses were run to determine which DMN sub-regions made significant variance contributions to Factor 1 and Factor 2 scores. Specifically, mean Task-Baseline beta values for the six DMN sub-regions and Age were modeled as predictor variables, while the Factor scores were modeled as the dependent variable in separate regressions. Left and right hemisphere regions were also modeled separately to minimize multicollinearity effects in the multiple regressions. Thus, multiple regression models were run for each hemisphere and each factor score, yielding four total regressions.
Table 2.
PCL-R list items broken down by factor
| Factor 1: Affective/Interpersonal |
| Glibness/superficial charm |
| Grandiose sense of self-worth |
| Pathological lying |
| Cunning/manipulative |
| Lack of remorse or guilt |
| Emotionally shallow |
| Callous/lack of empathy |
| Failure to accept responsibility for own actions |
| Factor 2: Antisocial/Lifestyle |
| Need for stimulation/proneness to boredom |
| Parasitic lifestyle |
| Poor behavioral control |
| Promiscuous sexual behavior |
| Lack of realistic, long-term goals |
| Impulsiveness |
| Irresponsibility |
| Juvenile delinquency |
| Early behavioral problems |
| Revocation of conditional release |
Adapted from Juarez et al., 2012
Results
Behavioral data
Performance differences on the Go/NoGo task were assessed using percent correct (PC) for Go and NoGo trials, and reaction time (RT) for Go trials. Separate independent-samples t-tests determined that Go PC, NoGo PC, and Go-RT did not significantly differ between High Psychopathy and Low Psychopathy groups (all Ps > .3) (see Table 3 for means and SDs).
Table 3.
Behavioral results
| Go Percent Correct |
NoGo Percent Correct |
Go RT | |
|---|---|---|---|
| High Psychopathy | 97.9 (3.6) | 74.2 (14.3) | 441.5 (55.6) |
| Low Psychopathy | 98.7 (1.6) | 76.2 (12.0) | 454.8 (55.8) |
Numbers outside parentheses represent group means, with standard deviations listed in parentheses.
Task versus baseline activity in the DMN regions-of-interest (ROIs)
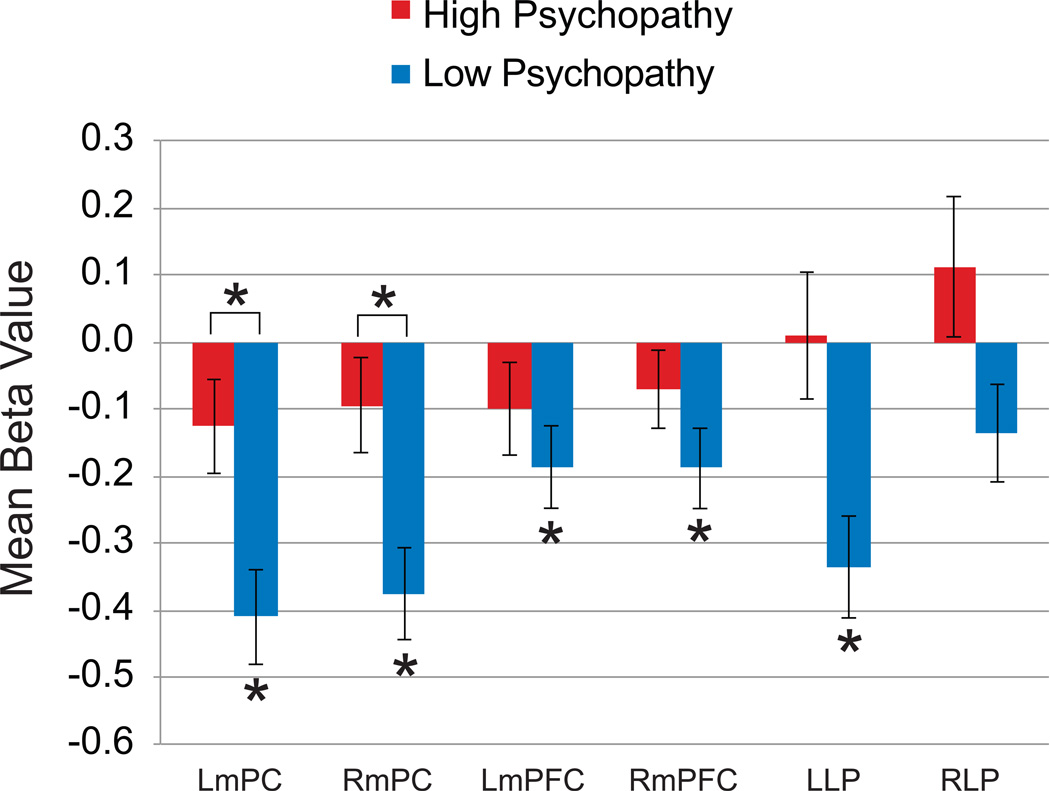
For the Low Psychopathy group, one-sample t-tests comparing mean activity in each ROI to a baseline of zero revealed significant task-induced deactivations in 5 of the 6 DMN nodes (all Ps < .003), with the right lateral parietal region showing significant deactivation at an uncorrected threshold, p = 0.04 (Fig. 2). In contrast, no DMN nodes showed significant task-induced deactivation in the High Psychopathy group. This finding agrees with previous studies reporting attenuated DMN deactivation in psychopaths compared with non-psychopaths and healthy individuals (Juárez et al., 2012; Pujol et al., 2012; Sheng et al., 2010).
Figure 2. Default Mode Network (DMN) Region-of-Interest Analysis.
The bar graph depicts mean beta values for the High Psychopathy and Low Psychopathy groups for each region of interest in the Task vs. Baseline contrast. The Low Psychopathy group showed typical deactivations beneath baseline, while the High Psychopathy group did not deactivate in any of the six DMN sub-regions. When directly comparing mean activity differences between the High Psychopathy and Low Psychopathy groups, the attenuated deactivation pattern was most pronounced in the left and right posteromedial regions of the DMN. Error bars represent standard errors of the means. *p < .05, Bonferroni corrected. RmPC = right posteromedial cortex; LmPC = left posteromedial cortex; RmPFC = right medial prefrontal cortex; LmPFC = left medial prefrontal cortex; RLP = right lateral parietal; LLP = left lateral parietal.
When we directly compared mean Task-Baseline ROI activity between High Psychopathy and Low Psychopathy groups, we found that only the mPC node (bilaterally) was significantly less deactivated in the Low Psychopathy group at a corrected threshold [right mPC: F(1,41) = 7.66, p = .0078 ηp2 = .16; left mPC: F(1,41) = 7.58, p = .0083, ηp2 = .16] (Fig. 2). These results indicate that, compared to the Low Psychopathy group, a lack of DMN deactivation during the externally-focused task in the High Psychopathy group was predominantly localized to the mPC.
Association between task-induced DMN deactivation and factors scores
For the right hemisphere, the 4 predictor variables explained 24% of the total variance in PCL-R Factor 1 scores, F(4,39) = 3.08, p = .027, and 21% of the total variance in PCL-R Factor 2 scores, F(4,39) = 2.56, p = .053. Partial correlation results showed that only the mPC significantly predicted PCL-R Factor 1 scores, β = 4.1, p = .02, R2 = .11, whereas none of the sub-regions significantly predicted Factor 2 scores. For the left hemisphere, the 4 predictors variables explained 31% of the total variance in PCL-R Factor 1 scores, F(4,39) = 4.44, p = .005, and 22% of the total variance in PCL-R Factor 2 scores, F(4,39) = 2.75, p = .04. Partial correlation results showed that only the mPC significantly predicted PCL-R Factor 1 scores, β = 4.48, p = .038, R2 = .08, whereas none of the sub-regions significantly predicted Factor 2 scores (see Table 4 for full results). These results demonstrate that, bilaterally, the mPC region is specifically associated with Factor 1 scores of psychopathy.
Table 4.
Multiple regressions relating DMN sub-region deactivation to psychopathy factor scores for left and right hemispheres
| Factor 1 | Factor 2 | |||
| Predictor | β | P-value | β | P-value |
| Right mPC | 4.1 | *.02 | 2.7 | .11 |
| Right mPFC | −1.9 | .44 | .16 | .95 |
| Right LP | −.16 | .93 | .42 | .82 |
| Age | −.09 | .08 | −.07 | .13 |
| Factor 1 | Factor 2 | |||
| Predictor | β | P-value | β | P-value |
| Left mPC | 4.5 | *.04 | 1.8 | .41 |
| Left mPFC | −3.7 | .07 | −1.7 | .41 |
| Left LP | .96 | .64 | 2.1 | .31 |
| Age | −.08 | .11 | −.06 | .27 |
indicates p< 0.05
Discussion
The goal of the present study was to localize task-induced deficits in the DMN of psychopaths to specific sub-regions within this network. In healthy individuals, the DMN has been shown to be more active at rest or during self-referential processing, and consistently deactivates during an externally-focused task (Fox et al., 2005; Raichle et al., 2001; Raichle & Snyder, 2007; Cauda et al., 2010). Whereas previous studies have suggested that the DMN of psychopaths fails to deactivate during task-induced behavior (Pujol et al., 2012; Sheng et al., 2010), the extent of this attenuated deactivation has remained unclear. Using an externally-focused task that is known to deactivate the DMN in healthy individuals, we determined that the Low Psychopathy group exhibited DMN deactivation, just as non-prison healthy subjects do, while the High Psychopathy group failed to deactivate any DMN sub-regions. Furthermore, when compared to the Low Psychopathy group, this pattern of attenuated task-induced DMN deactivation in the High Psychopathy group was primarily localized to the posteromedial cortex (mPC).
While the exact function of the DMN is currently unknown, studies over the past decade have consistently demonstrated a relationship between increased DMN activity and self-referential processing (Buckner & Carroll, 2007; Northoff et al., 2006; Spreng & Grady, 2010). Among DMN sub-regions, the mPC region has been described as a central “hub” region of the DMN (Andrews-Hanna et al., 2010; Lynch et al., 2013; Raichle et al., 2001), as the mPC during rest consumes about 40% more glucose than the hemispheric mean (Andrews-Hanna et al., 2010; Fransson & Marrelec, 2008; Raichle et al., 2001) and is densely interconnected with other DMN sub-regions (Cauda et al., 2010). Thus, the mPC region is believed to play a key role in regulating intrinsic brain activity, highlighting its involvement in higher cognitive processes, such as self-referential processing (Fransson & Marrelec, 2008). In support of this idea, several fMRI studies have found that the mPC region was more active when subjects made judgments of trait adjectives about themselves compared to others (Heatherton et al., 2006; Kelley et al., 2002). Similarly, a recent fMRI study showed that areas within the mPC were more active when people made self-relevant, affective (emotionally-laden) decisions (Andrews-Hanna et al., 2010). It is therefore possible that an overly-active mPC region in psychopaths could contribute to psychopaths’ excessive self-focus (Hare, 1996).
This possibility is supported by an fMRI study showing that task-induced changes in DMN activity are associated with PCL-R Factor 1 scores, though this study did not distinguish between DMN sub-regions (Juárez et al., 2012). Furthermore, a separate fMRI study demonstrated that task-induced mPC activity is correlated with egocentric traits in normal individuals (Sheng et al., 2010). For the first time, we extend these findings by showing that only mPC activity was significantly predictive of PCL-R Factor 1—but not Factor 2—scores in a prison population. While strong interpretations of this association are limited due to reverse inference, one possible explanation for the attenuated mPC deactivation is that there is greater self-focus in prisoners that possess higher Factor 1 scores. It will be important for future studies to investigate this hypothesized link between psychopaths’ degree of self-referential processing and DMN activity during a task, perhaps through a design that explicitly manipulates and compares engagement of the DMN during self-focus and disengagement during external focus.
In addition to an increased tendency to self-focus, failure to deactivate key nodes of the DMN during cognitive tasks could also potentially affect attention-related processes (Corbetta et al., 2008; Pagnoni, 2012; Sonuga-Barke & Castellanos, 2007). This is due to the complementary roles of task-positive networks (e.g., ventral attention network, VAN, and dorsal attention network, DAN) and the DMN in adaptive behavior (Corbetta et al., 2008). Specifically, while VAN regions, such as the inferior frontal gyrus and temporo-parietal junction, correlate positively with conscious perception of an external stimulus, DMN activity is negatively correlated with attentional focus and has been directly linked to lapses in attention (Boly et al., 2007; Weissman, Roberts, Visscher, & Woldorff, 2006). In light of these findings, researchers have theorized that failing to deactivate the DMN during goal-directed tasks could lead to increased competition between the DMN and task-positive networks, possibly resulting in performance deficits in tasks that require focused attention (Pagnoni, 2012; Sonuga-Barke & Castellanos, 2007). Supporting this framework, Pagnoni (2012) demonstrated a positive relationship between down-regulating (deactivating) the ventral mPC region and higher performance on a sustained attention task.
Considering the results of the current study, this raises the possibility for attentional processing dysfunction in psychopaths. Interestingly, a previous study using an auditory oddball task found that, in response to oddballs, psychopaths exhibited reduced P300 responses, an event-related potential that is sensitive to changes in allocation of attentional resources (Kiehl et al., 1999). Moreover, a separate study using an auditory oddball task observed a correlation between task-induced DMN activity and psychopathy scores (Juárez et al., 2012). Similar to the results presented here, neither of these studies showed reduced behavioral performance among psychopaths in spite of differences in neural activity. Thus, it is possible that while psychopaths display different neural functioning, they are able to use compensatory mechanisms in these simple cognitive tasks. It is also possible that our simple Go/NoGo task is not sensitive enough to detect subtle differences in attention-related processes between our High and Low Psychopathy groups, particularly if compensatory mechanisms are being recruited. A third possibility is that, since task performance was not significantly different between our High and Low Psychopathy groups, the altered DMN activity was not related to the task, but was instead a manifestation of an underlying DMN dysfunction during rest. This is supported by one recent fMRI study that found altered DMN activity in psychopaths during wakeful rest (Pujol et al., 2011), suggesting that more baseline changes in the DMN represent a core dysfunction in psychopaths. A final possibility is that the DMN is actually playing an adaptive role to compensate for hypo-dysfunction in non-DMN regions that have been associated with psychopathy, such as the anterior cingulate cortex, anterior superior temporal gyrus, and parts of the orbitofrontal cortex (Kiehl, 2006). In this case, an overly active mPC region could be helping to improve behavioral performance in our High Psychopathy group, resulting in similar levels of performance. Future research would benefit from investigating the relationship between attenuated mPC deactivation and attention-related functioning in psychopaths using tasks specifically intended to test allocation of attentional resources.
It is noteworthy that mean activity differences in the DMN has been associated with other clinical disorders, including schizophrenia, ADHD, and major depression (e.g., Garrity et al., 2007; Greicius et al., 2007; Sheline et al., 2009). Interestingly, a similar reduction in task-induced DMN deactivation has been found in depressed patients, though the observed heightened activity occurred in a task involving emotional processing (Sheline et al., 2009). In contrast, positive symptoms of schizophrenia have been associated with greater task-induced deactivation in DMN sub-regions during, including the precuneus (part of the mPC) and the medial frontal gyrus (Garrity et al., 2007). Considering the increasing number of findings relating DMN activity to clinical disorders (Broyd et al., 2009), it will be important for future studies to provide a more mechanistic understanding of how various forms of DMN dysfunction relate to different clinical symptoms.
Conclusion
Compared with typical, low psychopathy prisoners, psychopathic prisoners showed less deactivation in the posteromedial cortical region of the DMN during an externally-focused task. These results suggest a potential mechanism underlying key clinical traits (e.g., excessive self-focus and reduced empathy) associated with psychopathy. Moreover, we found that mPC dysfunction relates specifically to Factor 1 scores, suggesting that a failure to deactivate this critical DMN node during an externally-focused task may be specifically linked to affective/interpersonal deficits in criminal psychopaths.
Supplementary Material
Acknowledgements
Supported by NIDA R01s DA020870/DA026964 (Kiehl), NIMH R01 MH070539 (Kiehl), and a grant from the MacArthur Law and Neuroscience Project. The authors would also like to thank Keith Harenski and Prashanth Nyalakanti for assistance with fMRI data collection and analysis and the Kiehl lab for collection of the clinical data. Finally, we thank the staff and inmates in the New Mexico Corrections Department (NMCD) for their assistance and cooperation.
Footnotes
Though we used a more liberal cut-off score for psychopathy, the mean PCL-R score for our psychopath group was above the more conservative threshold of 30 (see Table 1).
References
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. 2010;65(4):550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE transactions on medical imaging. 2004;23(2):137–52. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen a, Phillips C, et al. Baseline brain activity fluctuations predict somatosensory perception in humans. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(29):12187–12192. doi: 10.1073/pnas.0611404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. NeuroImage. 2002;16(2):497. Retrieved from https://cirl.berkeley.edu/mb312/abstracts/Marsbar/marsbar_abs.html. [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJS. Default-mode brain dysfunction in mental disorders: a systematic review. Neuroscience and biobehavioral reviews. 2009;33(3):279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends in cognitive sciences. 2007;11(2):49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biological psychiatry. 2008;63(3):332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, Geminiani G, D’Agata F, Sacco K, Duca S, Bagshaw AP, Cavanna AE. Functional connectivity of the posteromedial cortex. PloS one. 2010;5(9):1–11. doi: 10.1371/journal.pone.0013107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from enviroment to theory of mind. Nature neuroscience. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. NeuroImage. 2008;42(3):1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Freire L, Roche a, Mangin JF. What is the best similarity measure for motion correction in fMRI time series? IEEE transactions on medical imaging. 2002;21(5):470–484. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- Garrity AG, GD P, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant"default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological psychiatry. 2007;62(5):429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare RD. Psychopathy as a risk factor for violence. The Psychiatric quarterly. 1999;70(3):181–197. doi: 10.1023/a:1022094925150. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10457544. [DOI] [PubMed] [Google Scholar]
- Hare RD, Clark D, Grann M, Thornton D. Psychopathy and the predictive validity of the PCL-R: an international perspective. Behavioral sciences & the law. 2000;18(5):623–645. doi: 10.1002/1099-0798(200010)18:5<623::aid-bsl409>3.0.co;2-w. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11113965. [DOI] [PubMed] [Google Scholar]
- Hare RD. Psychopathy: A Clinical Construct Whose Time Has Come. Criminal Justice and Behavior. 1996;23(1):25–54. [Google Scholar]
- Heatherton TF, Wyland CL, Macrae CN, Demos KE, Denny BT, Kelley WM. Medial prefrontal activity differentiates self from close others. Social cognitive and affective neuroscience. 2006;1(1):18–25. doi: 10.1093/scan/nsl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juárez M, Kiehl Ka, Calhoun VD. Intrinsic limbic and paralimbic networks are associated with criminal psychopathy. Human brain mapping. 2012:1–10. doi: 10.1002/hbm.22037. 00(July 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of cognitive neuroscience. 2002;14(5):785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kiehl K, Hare RD, Liddle PF, McDonald JJ. Reduced P300 responses in criminal psychopaths during a visual oddball task. Biological psychiatry. 1999;45(11):1498–1507. doi: 10.1016/s0006-3223(98)00193-0. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10356633. [DOI] [PubMed] [Google Scholar]
- Kiehl K, Smith aM, Hare RD, Mendrek A, Forster BB, Brink J, Liddle PF. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biological psychiatry. 2001;50(9):677–684. doi: 10.1016/s0006-3223(01)01222-7. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11704074. [DOI] [PubMed] [Google Scholar]
- Kiehl Kent. A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system dysfunction. Psychiatry research. 2006;142(2–3):107–128. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle EB, Hollis C, Batty MJ, Groom MJ, Totman JJ, Liotti M, Scerif G, et al. Task-related default mode network modulation and inhibitory control in ADHD: effects of motivation and methylphenidate. Journal of child psychology and psychiatry, and allied disciplines. 2011;52(7):761–771. doi: 10.1111/j.1469-7610.2010.02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CJ, Uddin LQ, Supekar K, Khouzam A, Phillips J, Menon V. Default mode network in childhood autism: posteromedial cortex heterogeneity and relationship with social deficits. Biological psychiatry. 2013;74(3):212–219. doi: 10.1016/j.biopsych.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller JL, Gänssbauer S, Sommer M, Döhnel K, Weber T, Schmidt-Wilcke T, Hajak G. Gray matter changes in right superior temporal gyrus in criminal psychopaths. Evidence from voxel-based morphometry. Psychiatry research. 2008;163(3):213–222. doi: 10.1016/j.pscychresns.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, De Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. NeuroImage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Pagnoni G. Dynamical properties of BOLD activity from the ventral posteromedial cortex associated with meditation and attentional skills. 2012;32(15):5242–5249. doi: 10.1523/JNEUROSCI.4135-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Batalla I, Contreras-Rodríguez O, Harrison BJ, Pera V, Hernández-Ribas R, Real E, et al. Breakdown in the brain network subserving moral judgment in criminal psychopathy. Social cognitive and affective neuroscience. 2012;7(8):917–923. doi: 10.1093/scan/nsr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod aM, Snyder aZ, Powers WJ, Gusnard Da, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle Marcus E, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. NeuroImage. 2007;37(4):1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097–9. [DOI] [PubMed] [Google Scholar]
- Sheng T, Gheytanchi A, Aziz-Zadeh L. Default network deactivations are correlated with psychopathic personality traits. PloS one. 2010;5(9):e12611. doi: 10.1371/journal.pone.0012611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neuroscience and biobehavioral reviews. 2007;31(7):977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. Journal of cognitive neuroscience. 2010;22(6):1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Stawarczyk D, Majerus S, Maquet P, D’Argembeau A. Neural correlates of ongoing conscious experience: both task-unrelatedness and stimulus-independence are related to default network activity. PloS one. 2011;6(2):e16997. doi: 10.1371/journal.pone.0016997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Kelly aMC, Biswal BB, Margulies DS, Shehzad Z, Shaw D, Ghaffari M, et al. Network homogeneity reveals decreased integrity of default-mode network in ADHD. Journal of neuroscience methods. 2008;169(1):249–254. doi: 10.1016/j.jneumeth.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nature neuroscience. 2006;9(7):971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.