Abstract
Background
Microvascular dysfunction is a key event in the development of atherosclerosis, which predates the clinical manifestations of vascular disease including stroke and myocardial infarction. Dysfunction of the microvasculature can be measured as a decreased microperfusion in response to heat.
Objective
We sought to evaluate the microvasculature using heat among adolescents and young adults with type 1 diabetes (T1D) compared to healthy non-diabetic controls. We hypothesized that youth with T1D would have impaired microvascular function measured as decreased perfusion.
Methods
We studied 181 adolescents and young adults with T1D and 96 age-, race-, and sex-matched healthy controls (mean age 19 yr). Patients were seen at an in-person study visit where demographics, anthropometrics, and laboratory data was obtained. Skin microvascular perfusion was measured on the volvar surface of the right forearm using a standard laser flow Doppler. Measurements were taken at baseline and after heating to 44° C.
Results
Youth with T1D had decreased microvascular perfusion as measured by lower percent change of perfusion units (1870 ± 945 vs. 2539 ± 1255, p < 0.01) and percent change in area under the curve (1870 ± 945 vs. 2539 ± 1255, p < 0.01) compared to controls. Glycosylated hemoglobin A1c (HbA1c) was found to be an independent determinant of microvascular function (p < 0.05).
Conclusions
Adolescents and young adults with T1D have evidence of microvascular dysfunction that can be detected using heat, a non-invasive physiologic stimulus. HbA1c appears to play an independent role in determining microvascular perfusion suggesting tight glycemic control is probably important for the development of vascular disease.
Keywords: endothelial function, heat, pediatrics, type 1 diabetes
Reduced perfusion is a key event in the development of microvascular disease, which predates the clinical manifestations of stroke and myocardial infarction (1, 2). Microvascular disease is a common feature of type 1 diabetes (T1D) that results from the combination of hyperglycemia and oxidative stress (3).
Microvasculature function can be assessed by measuring the ability of the blood vessels to vasodilate or perfuse in response to physiologic and pharmacologic stimuli. Pharmacologic stimuli include acetylcholine and sodium nitroprusside (4–6) while nonpharmacologic agents include blood pressure occlusion (7, 8) and heat. Pharmacologic agents require cannulation of an artery or percutaneous iontophoresis adding risk to the procedure and blood pressure occlusion can cause discomfort. Heat, however, is a non-invasive and painless physiological stimulus that is a more attractive alternative in the pediatric population. Heat causes an initial peak in cutaneous blood flow via a local sensory nerve reflex (9) followed by a long-term plateau phase that is predominantly regulated by nitric oxide (9, 10). Heat also induces key chemicals involved in regulating blood flow such as endothelium-derived relaxing factor/nitric oxide, prostacylin, and endothelin (11) that become impaired as microvascular endothelial dysfunction develops. In adults, impaired vascular response to heat has been associated with increased cardiovascular risk (12) and with end organ damage (13).
Recently, the first paper utilizing a heating protocol in the forearm of youth with T1D was published in Germany. The authors found children with T1D (mean age 12.8 ± 3.3 yr) showed impaired vasodilation vs. controls (14). In this study we sought to validate these findings in an older group of adolescents and young adults in the USA. Additionally, we aimed to evaluate the relationship between glycosylated hemoglobin A1c (HbA1c) and impaired microvasculature vasodilation and establish the independent predictors of decreased microvascular perfusion in youth with T1D. We hypothesized that, compared to controls, youth with T1D would have impaired function measured as decreased microvascular perfusion and that the observed differences between T1D and controls would persist after adjustment for cardiovascular risk factors.
Methods
Participants for this study were recruited as part of the SEARCH Cardiovascular Disease (SEARCH CVD) Study, an ancillary study to the SEARCH for Diabetes in Youth Study. A detailed description of the SEARCH and SEARCH CVD has been previously published (15, 16). Laser flow Doppler studies were conducted in only Cincinnati, OH, one of the two clinical sites of SEARCH CVD. Participants included in this analysis had T1D for at least 5 yr and were age 11–26 yr at the time of the study visit. Characteristics of participants with provider diagnosed T1D have been examined previously and are consistent with the clinical diagnosis of T1D including the presence of diabetes autoantibodies (17). Non-diabetic (fasting glucose <100 mg/dL) control participants were recruited by phone from research clinics at Cincinnati Children's Hospital Medical Center and matched to T1D participants by age, race/ethnicity, and sex.
All participants were seen at an in-person study visit where fasting blood samples were collected, processed, and sent within 24 h to the SEARCH Central Laboratory at the University of Washington for analysis of total, low density lipoprotein (LDL), high density lipoprotein (HDL) cholesterol, triglycerides, glucose, HbA1c, and adiponectin (18). Measurements of total and HDL cholesterol and triglycerides were performed enzymatically on a Hitachi 917 autoanalyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN, USA). LDL cholesterol was calculated by the Friedewald equation for individuals with triglyceride levels <400 mg/dL and by Lipid Research Clinics Beta Quantification for those with triglyceride levels of >400 mg/dL. Hemoglobin A1c levels were measured by ion-exchange high-performance liquid chromatography (TOSOH Bioscience, South San Francisco, CA, USA). Adiponectin was measured using a radio-immunoassay kit (Linco Research, Inc., St. Charles, MO, USA). All medications including short-acting insulin were held until after the blood draw and vascular testing was complete.
Weight and height were measured in light clothing without shoes. Height was measured to the nearest 0.1 cm and weight to the nearest 0.1 kg. The average of the two anthropometric measures was used in the analyses. Both were compared with 2000 Centers for Disease Control and Prevention standards for the Unites States to calculate body mass index (BMI), which was then converted to z-scores (19). Waist circumference was measured to the nearest 0.1 cm using NHANES protocol (20). Seated, resting ausculatory blood pressure (BP) was measured three times and averaged according to the Fourth Report and z-scores were calculated (21). Informed consent and assent, where applicable, were obtained from all participants and their parent/guardian if aged <18 yr at the time of the visit. This study was approved by the local institutional review board.
Skin blood perfusion was measured on the volvar surface of the right forearm using a fiberoptic laser Doppler probe (Periflux 5000 device, Perimed, Stockholm, Sweden). Principals underlying this technique have been published previously (22). Simply, laser Doppler measures the total local microcirculatory blood perfusion (capillaries, arterioles, venules, and shunting vessels). The fiber optic probe emits a beam of laser light that is scattered and partly absorbed by the tissue being studied. Light hitting moving blood cells undergoes a change in wavelength (Doppler shift). The magnitude and frequency distribution of these changes are directly related to the number and velocity of the blood cells within the area of study.
Baseline perfusion of the skin was measured in perfusion units for 5 min and then the probe automatically heated to 44 °C and perfusion was measured for an additional 15 min. The mean value of the perfusion during rest was calculated over 1 min (from minutes 3 to 4) and after heating for 1 min during heating (from minutes 13 to 14). This procedure was automated by Perisoft (the Perimed analysis program for PeriFlux) to ensure all data was obtained and analyzed in the same way and to ensure steady state perfusion was reached for each participant. Investigation was conducted in a quiet temperature controlled room. Each subject rested for 5 min before measurements were obtained. Calibrations of the instrument were performed monthly as suggested by the manufacturer.
All analyses were performed using sas 9.3 (SAS Institute, Cary, NC, USA). T tests or non-parametric equivalent were used to compare group differences for continuous variables and chi-squared tests were used to compare categorical variables. Multiple linear regression models were constructed to explore the independent determinants of the major outcomes: percent change in perfusion units from baseline to heating and percent change in area under the curve from baseline to heating. The use of the area under the curve in the assessment of skin microvascular reactivity using laser Doppler flow is well-validated, because it represents the overall flux response to different physiological and pharmacological stimuli (23–26). Initial models for percent change in perfusion units or percent change in area under the curve included T1D group and were adjusted for skin temperature differences between groups and either baseline perfusion units or baseline area under the curve, respectively. Subsequent models added known cardiometabolic risk factors including age, race, sex, BMI z-score, waist circumference, systolic and diastolic blood pressure z-scores, heart rate, lipids (total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides), HbA1c, adiponectin, and pubertal stage (pubic hair for males and breast development and pubic hair for females). Given that cardiometabolic risk factors could have influenced the two groups differently, separate regression models were also created for the T1D group and controls using the variables above. Interactions between group and cardiometabolic risk factors were tested in all models. A p value of <0.05 was considered significant.
Results
Demographic and clinical data are shown in Table 1. The cohort consisted of 181 adolescents and young adults with T1D (mean age 19.4 ± 2.8, 53% male, 86% Caucasian, duration of diabetes 10.6 ± 3.9 years) and 96 age, race, and sex matched controls (mean age 19.1 ± 2.8, 48% male and 80% Caucasian). Youth with T1D had higher heart rates, HbA1c and adiponectin levels compared to controls, p < 0.05. There were no differences in BMI z-score, waist circumference, blood pressure, lipids, and puberty stages between groups.
Table 1.
Characteristics of the study population
| Variable | Type 1 diabetes n = 181 | Controls n = 96 | p value |
|---|---|---|---|
| Age (years) | 19.4 ± 2.8 | 19.1 ± 2.8 | 0.39 |
| Males, n (%) | 95 (53%) | 46 (48%) | 0.41 |
| Non-Caucasian, n (%) | 26 (14%) | 19 (20%) | 0.24 |
| Height z-score | 0.2 ± 1.0 | 0.5 ± 1.0 | 0.10 |
| Weight (kg) | 75.6 ± 15.8 | 79.3 ± 24.0 | 0.83 |
| Waist circumference (cm) | 88.8 ± 13.1 | 92.0 ± 18.6 | 0.64 |
| BMI (kg/m2) | 25.7 ± 5.0 | 26.7 ± 7.5 | 0.79 |
| BMI z-score | 0.7 ± 0.8 | 0.8 ± 1.1 | 0.83 |
| Systolic BP (mmHg) | 111 ± 9 | 110 ± 10 | 0.52 |
| Diastolic BP (mmHg) | 67 ± 9 | 66 ± 8 | 0.30 |
| Heart rate (beats/min) | 69.9 ± 12.0 | 64.0 ± 9.9 | < 0.01 |
| Total cholesterol (mg/dL) | 170.4 ± 35.4 | 165.6 ± 30.2 | 0.41 |
| Triglycerides (mg/dL)* | 96.5 ± 65.5 | 107.7 ± 67.6 | 0.07 |
| LDL-cholesterol (mg/dL) | 98.6 ± 30.1 | 94.1 ± 25.8 | 0.47 |
| HDL-cholesterol (mg/dL) | 52.7 ± 14.6 | 49.9 ± 13.2 | 0.19 |
| HbA1c (%) | 8.8 ± 1.8 | 5.0 ± 0.3 | < 0.01 |
| Diabetes duration (years) | 10.6 ± 3.9 | NA | NA |
| Adiponectin (μg/mL)* | 12.9 ± 6.5 | 11.0 ± 5.1 | 0.045 |
| Tanner stage pubic hair, n (%) | 0.69 | ||
| Tanner 1–3 | 2 (2%) | 3(3%) | |
| Tanner 4 | 25 (14%) | 13 (14%) | |
| Tanner 5 | 152 (85%) | 80 (83%) | |
| Tanner stage, breast n (%) | 0.31 | ||
| Tanner 1–3 | 4 (5%) | 3 (6%) | |
| Tanner 4 | 16(19%) | 13(26%) | |
| Tanner 5 | 64 (76%) | 34 (68%) |
BP, blood pressure; BMI, body mass index; HDL, high density lipoprotein; LDL, low density lipoprotein; HbA1c, glycosylated hemoglobin.
Data are mean and standard deviation unless stated.
Compared by non-parametric test.
Table 2 lists the laser Doppler flow measurements for T1D and controls. There was no difference in baseline perfusion measured as either mean perfusion units or baseline area under the curve between groups. Maximum microvascular response to heat stimulus whether expressed as perfusion units or area under the curve was reduced in T1DM, p < 0.01. This resulted in a decrease in percent change in perfusion units and percent change in area under the curve among youth with T1D compared to controls, p < 0.01. We defined ‘abnormal microvascular perfusion’ as <10th percentile for percent change in perfusion units or percent change in area under the curve. Twenty-four percent of adolescents and young adults with T1D had abnormal microvascular perfusion compared to 9% of controls.
Table 2.
Laser Doppler measurements
| Variable | Type 1 diabetes | Controls | p value |
|---|---|---|---|
| Baseline temperature (°C) | 30.88 ± 0.96 | 30.63 ± 0.96 | 0.037 |
| Maximum temperature (°C) | 43.972 ± 0.012 | 43.966 ± 0.010 | < 0.01 |
| Temperature difference (°C) | 13.09 ± 0.96 | 13.34 ± 0.96 | 0.041 |
| Baseline perfusion units (PU/s) | 7.0 ± 3.5 | 6.0 ± 2.4 | 0.14 |
| Maximum perfusion units (PU/s) | 11388 ± 5396 | 14183 ± 6888 | < 0.01 |
| Percent change in perfusion units (%) | 1870 ± 945 | 2539 ± 1255 | < 0.01 |
| Baseline AUC (PU/s) | 419 ± 210 | 365 ± 144 | 0.14 |
| Maximum AUC (PU/s) | 682747 ± 323577 | 850318 ± 412915 | < 0.01 |
| Percent change in AUC (%) | 1870 ± 945 | 2539 ± 1255 | < 0.01 |
| Abnormal perfusion, n (%) | 43 (24%) | 9 (9%) | NA |
PU/s, perfusion units/second; AUC, area under the curve.
Data are mean and standard deviation.
In linear regression models that included T1D group and were adjusted for temperature differences between groups and either baseline perfusion or baseline area under the curve, T1D group was found to be a significant independent determinant of the two major outcomes, percent change in perfusion units (R2 = 0.41, p < 0.0001) and percent change in area under the curve (R2 = 0.41, p < 0.0001). When other risk factors including age, race, sex, BMI z-score, waist circumference, systolic and diastolic blood pressure z-scores, heart rate, lipids (total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides), HbA1c, adiponectin, and puberty stage were added to the models, HbA1c was the only variable found to be significantly associated with either outcome (p < 0.001) and T1D group was no longer significant. Of note, the interaction of T1D group and HbA1c group was also non-significant. The loss of significance by group in these models suggests that glycemic control or hyperglycemia measured by HbA1c probably has the largest contribution to microvascular response in T1D rather than an unmeasured characteristic.
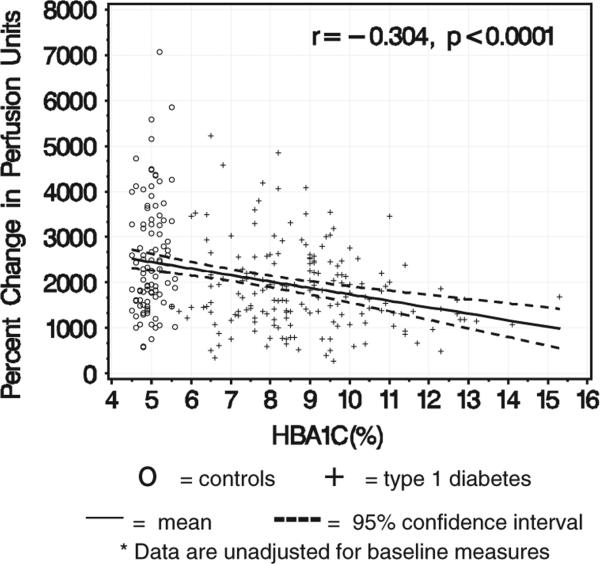
Figure 1 shows the mean and 95% confidence interval for the association between HbA1c and percent change in perfusion units by group. With increasing HbA1c in the T1D group, the percent change in perfusion units decreases (Figure 1).
Fig. 1.
The relationship between glycosylated hemoglobin A1c (HbA1c) and percent change in perfusion units*.
Separate models were also created for T1D and controls using the two major outcomes, percent change in perfusion units and percent change in area under the curve and were adjusted for baseline temperature differences and either baseline perfusion or area under the curve. In the model for controls only, triglycerides was the only significant independent determinant (p = 0.04). In the model for T1D group only, HbA1c remained the only independent predictor of both outcomes (p < 0.01).
Discussion
This study shows that heat, a non-invasive painless stimulus, can be used in adolescents to assess microvascular function. Using heat, we show nearly one-fourth of adolescents with T1D with a mean duration of T1D disease of 10 years have a significant decrease in forearm microvascular perfusion compared to similar aged matched controls. Furthermore, we show worse glycemic control, measured as HbA1c, is a significant independent determinant of microvascular response in T1D youth suggesting glycemic control is probably important to prevent early vascular complications.
Endothelial dysfunction which can be assessed by decreased microvascular perfusion is an early feature of atherosclerosis that predates the development of stroke and myocardial infarction (1, 2). Under normal conditions the endothelium releases nitric oxide in response to sheer stress which in turn stimulates smooth muscle cells resulting in relaxation and vasodilation. Nitric oxide also prevents adhesion of arterial wall leukocytes and prevents platelet activation, key steps in the development of thrombus formation (27). Thus, dysfunction of the endothelium through decreased nitric oxide release is associated with an increased risk to develop cardiovascular disease.
Prior work in T1D has assessed endothelial function using blood pressure occlusion in the arm (28–30). Heat has been used less often and earlier studies focused on the dorsum of the foot (31, 32). Shore et al. studied 50 adolescents with T1D and controls between the ages of 11–13 years and showed decreased maximum blood flow in those with T1D (32). Khan et al. later found maximum hyperemia was reduced in adolescents 14–15 yr with T1D compared to controls (31). Then in 2013 Hamriti et al. published the first study using the forearm in 68 German children with T1D (mean age 12.9 ± 3.3 yr) and found decreased microvascular perfusion in youth with T1D compared to controls (p < 0.01) (14). However, they did not evaluate the relationship with worsening glycemic control or established the independent determinants of decreased perfusion.
Our data show increasing HbA1c is associated with a decrease in microvascular perfusion and demonstrates that HbA1c above other risk factors is an independent determinant of forearm microperfusion in youth with T1D. In support of these findings, both the Diabetes Complications and Control Trial and follow up Epidemiology of Diabetes Interventions and Complications trial have shown improved glycemic control reduces the risk of microvascular disease in T1D (33–35). In contrast, prior cross sectional work in youth with T1D has not shown an association between endothelial dysfunction in the foot and glycemic control (31, 32, 36). Discrepant findings may be explained by a smaller sample size in this prior work or differences in the relationship between HbA1c and microvascular perfusion in the dorsum of the foot vs. the forearm. We conclude that HbA1c is an independent determinant of microvascular function in the forearm. These finding suggest the importance of metabolic control in microvascular health early in the course of T1D. To confirm our results repeated measures of HbA1c (representing long term glycemic control) are needed and whether these microvascular changes are reversible with improvements in HbA1c requires further study.
Youth with T1D had higher heart rates compared to controls. Increased heart rates among youth with T1D are thought to reflect parasympathetic withdrawal with sympathetic override and are suggestive of cardiac autonomic dysfunction (37). Impaired autonomic regulation is linked to both endothelial dysfunction (38) and arterial stiffness (39) in the peripheral vasculature and may be a potential mechanism that leads to increased cardiovascular disease. The magnitude of heart rate differences in this study was small and when heart rate was included in our regression models it was not significant. Therefore heart rate differences do not appear to be contributing to the case/control differences in microvascular perfusion. It is possible with longer duration of diabetes higher heart rates (reflecting autonomic dysfunction) may become more important.
A few limitations should be mentioned. We do not have perfusion data in the foot to compare our results thus we do not know if the observed microvascular perfusion changes occur globally or if one precedes the other. The forearm was chosen because there is no hair on the surface to shave and participants do not need to remove shoes or socks. However, prior work in adults with T1D of 18 yr duration has shown maximal hyperemic response to heat was not different in the forearm and the foot suggesting either location can be used and perhaps changes to the microvascular occur globally (40). Second, we do not have reproducibility data for our measures. However, if reproducibility was poor, it would diminish our ability to see case/control differences. Finally, since our population was older with a mean age of diabetes duration of 10 yr, further study is needed to determine if our findings are applicable to a younger pediatric population with similar duration of diabetes.
In conclusion, this study shows decreased microvascular perfusion in T1D adolescents and young adults who have no clinical evidence of vascular disease. Additionally, this study suggests that poor glycemic control has an adverse effect on microvascular function. The use of heat as a stimulus to assess microvascular function provides a simple, non-invasive, painless, safe alternative to assess endothelial health.
Acknowledgements
In part by SEARCH CVD grant R01 DK078542 to Dabelea and a T32DK063929 (PI: Scott Powers, PhD) to Shah.
Footnotes
Conflicts of interest
The authors have declared no conflicting interests.
References
- 1.Brunner H, Cockcroft JR, Deanfield J, et al. Endothelial function and dysfunction. Part II: association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:233–246. doi: 10.1097/00004872-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Turner J, Belch JJ, Khan F. Current concepts in assessment of microvascular endothelial function using laser Doppler imaging and iontophoresis. Trends Cardiovasc Med. 2008;18:109–116. doi: 10.1016/j.tcm.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Schalkwijk CG, Stehouwer CD. Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin Sci (Lond) 2005;109:143–159. doi: 10.1042/CS20050025. [DOI] [PubMed] [Google Scholar]
- 4.Chan NN, Vallance P, Colhoun HM. Endothelium-dependent and -independent vascular dysfunction in type 1 diabetes: role of conventional risk factors, sex, and glycemic control. Arterioscler Thromb Vasc Biol. 2003;23:1048–1054. doi: 10.1161/01.ATV.0000072968.00157.6B. [DOI] [PubMed] [Google Scholar]
- 5.Johnstone MT, Creager SJ, Scales KM, Cusco JA, Lee BK, Creager MA. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation. 1993;88:2510–2516. doi: 10.1161/01.cir.88.6.2510. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Manas L, Lopez-Doriga P, Petidier R, et al. Effect of glycaemic control on the vascular nitric oxide system in patients with type 1 diabetes. J Hypertens. 2003;21:1137–1143. doi: 10.1097/00004872-200306000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Hu J, Norman M, Wallensteen M, Gennser G. Dynamic properties of the aorta and of the foot microcirculation in adolescents with diabetes mellitus. Acta Paediatr. 1997;86:620–625. doi: 10.1111/j.1651-2227.1997.tb08945.x. [DOI] [PubMed] [Google Scholar]
- 8.Schlager O, Hammer A, Willfort-Ehringer A, et al. Microvascular autoregulation in children and adolescents with type 1 diabetes mellitus. Diabetologia. 2012;55:1633–1640. doi: 10.1007/s00125-012-2502-8. [DOI] [PubMed] [Google Scholar]
- 9.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 10.Gooding KM, Hannemann MM, Tooke JE, Clough GF, Shore AC. Maximum skin hyperaemia induced by local heating: possible mechanisms. J Vasc Res. 2006;43:270–277. doi: 10.1159/000091736. [DOI] [PubMed] [Google Scholar]
- 11.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 12.Kruger A, Stewart J, Sahityani R, et al. Laser Doppler flowmetry detection of endothelial dysfunction in end-stage renal disease patients: correlation with cardiovascular risk. Kidney Int. 2006;70:157–164. doi: 10.1038/sj.ki.5001511. [DOI] [PubMed] [Google Scholar]
- 13.Strain WD, Chaturvedi N, Leggetter S, et al. Ethnic differences in skin microvascular function and their relation to cardiac target-organ damage. J Hypertens. 2005;23:133–140. doi: 10.1097/00004872-200501000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Heimhalt-El Hamriti M, Schreiver C, Noerenberg A, et al. Impaired skin microcirculation in paediatric patients with type 1 diabetes mellitus. Cardiovasc Diabetol. 2013;12:115. doi: 10.1186/1475-2840-12-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dabelea D, Talton JW, D’Agostino R, Jr, et al. Cardiovascular risk factors are associated with increased arterial stiffness in youth with type 1 diabetes: the SEARCH CVD study. Diabetes Care. 2013;36:3938–3943. doi: 10.2337/dc13-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Group SS. SEARCH for diabetes in youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials. 2004;25:458–471. doi: 10.1016/j.cct.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Dabelea D, Pihoker C, Talton JW, et al. Etiological approach to characterization of diabetes type: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2011;34:1628–1633. doi: 10.2337/dc10-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials. 2004;25:458–471. doi: 10.1016/j.cct.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145:439–444. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 21.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 22.Nilsson GE, Tenland T, Oberg PA. Evaluation of a laser Doppler flowmeter for measurement of tissue blood flow. IEEE Trans Biomed Eng. 1980;27:597–604. doi: 10.1109/TBME.1980.326582. [DOI] [PubMed] [Google Scholar]
- 23.Gomes MB, Matheus AS, Tibirica E. Evaluation of microvascular endothelial function in patients with type 1 diabetes using laser-Doppler perfusion monitoring: which method to choose? Microvasc Res. 2008;76:132–133. doi: 10.1016/j.mvr.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Leslie SJ, Affolter J, Denvir MA, Webb DJ. Validation of laser Doppler flowmetry coupled with intra-dermal injection for investigating effects of vasoactive agents on the skin microcirculation in man. Eur J Clin Pharmacol. 2003;59:99–102. doi: 10.1007/s00228-003-0577-3. [DOI] [PubMed] [Google Scholar]
- 25.Opazo Saez AM, Mosel F, Nurnberger J, et al. Laser Doppler imager (LDI) scanner and intradermal injection for in vivo pharmacology in human skin microcirculation: responses to acetylcholine, endothelin-1 and their repeatability. Br J Clin Pharmacol. 2005;59:511–519. doi: 10.1111/j.1365-2125.2004.02344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossi M, Matteucci E, Gaddeo C, Giampietro O, Santoro G. Preserved endothelial-dependent and endothelial-independent skin vasodilator responses in relatives of type 1 diabetes patients. Microvasc Res. 2009;78:253–255. doi: 10.1016/j.mvr.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Forstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers Arch. 2010;459:923–939. doi: 10.1007/s00424-010-0808-2. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman RP, Dye AS, Huang H, Bauer JA. Effects of glucose control and variability on endothelial function and repair in adolescents with type 1 diabetes. ISRN Endocrinol. 2013;2013:876547. doi: 10.1155/2013/876547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nadeau KJ, Regensteiner JG, Bauer TA, et al. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab. 2010;95:513–521. doi: 10.1210/jc.2009-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newkumet KM, Goble MM, Young RB, Kaplowitz PB, Schieken RM. Altered blood pressure reactivity in adolescent diabetics. Pediatrics. 1994;93:616–621. [PubMed] [Google Scholar]
- 31.Khan F, Elhadd TA, Greene SA, Belch JJ. Impaired skin microvascular function in children, adolescents, and young adults with type 1 diabetes. Diabetes Care. 2000;23:215–220. doi: 10.2337/diacare.23.2.215. [DOI] [PubMed] [Google Scholar]
- 32.Shore AC, Price KJ, Sandeman DD, Green EM, Tripp JH, Tooke JE. Impaired microvascular hyperaemic response in children with diabetes mellitus. Diabet Med. 1991;8:619–623. doi: 10.1111/j.1464-5491.1991.tb01667.x. [DOI] [PubMed] [Google Scholar]
- 33.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 34.Epidemiology of Diabetes Interventions and Complications (EDIC) Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22:99–111. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The Diabetes Control and Complications Trial/ Epidemiology of Diabetes Interventions and Complications Research Group Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381–389. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Golster H, Hyllienmark L, Ledin T, Ludvigsson J, Sjoberg F. Impaired microvascular function related to poor metabolic control in young patients with diabetes. Clin Physiol Funct Imaging. 2005;25:100–105. doi: 10.1111/j.1475-097X.2004.00596.x. [DOI] [PubMed] [Google Scholar]
- 37.Jaiswal M, Urbina EM, Wadwa RP, et al. Reduced heart rate variability among youth with type 1 diabetes: the SEARCH CVD study. Diabetes Care. 2013;36:157–162. doi: 10.2337/dc12-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris KF, Matthews KA. Interactions between autonomic nervous system activity and endothelial function: a model for the development of cardiovascular disease. Psychosom Med. 2004;66:153–164. doi: 10.1097/01.psy.0000116719.95524.e2. [DOI] [PubMed] [Google Scholar]
- 39.Jaiswal M, Urbina EM, Wadwa RP, et al. Reduced heart rate variability is associated with increased arterial stiffness in youth with type 1 diabetes: the SEARCH CVD study. Diabetes Care. 2013;36:2351–2358. doi: 10.2337/dc12-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arora S, Smakowski P, Frykberg RG, et al. Differences in foot and forearm skin microcirculation in diabetic patients with and without neuropathy. Diabetes Care. 1998;21:1339–1344. doi: 10.2337/diacare.21.8.1339. [DOI] [PubMed] [Google Scholar]